Abstract
Glutamate cycling is critically important for neurotransmission, and may be altered in schizophrenia. The excitatory amino acid transporters (EAATs) facilitate the reuptake of glutamate from the synaptic cleft and have a key role in glutamate cycling. We hypothesized that expression of the EAATs and the EAAT regulating proteins ARHGEF11, JWA, G protein suppressor pathway 1 (GPS1), and KIAA0302 are altered in the brain in schizophrenia. To test this, we measured expression of EAAT1, EAAT2, EAAT3, and EAAT interacting proteins in postmortem tissue from the dorsolateral prefrontal and anterior cingulate cortex of patients with schizophrenia and a comparison group using in situ hybridization and Western blot analysis. We found increased EAAT1 transcripts and decreased protein expression, increased EAAT3 transcripts and protein, and elevated protein expression of both GPS1 and KIAA0302 protein. We did not find any changes in expression of EAAT2. These data indicate that proteins involved in glutamate reuptake and cycling are altered in the cortex in schizophrenia, and may provide potential targets for future treatment strategies.
Keywords: GPS1, anterior cingulate cortex, dorsolateral prefrontal cortex, postmortem, Western blot, in situ hybridization
Introduction
Glutamate is rapidly removed from the synapse by plasma membrane excitatory amino acid transporters (EAATs), primarily localized to postsynaptic neurons and astrocytes (Masson et al., 1999). EAATs are critical for glutamate transmission, because it is reuptake and not enzymatic breakdown that is responsible for clearance of glutamate from the synapse (Danbolt, 2001). Of these transporters, EAAT1-3 are expressed abundantly throughout the CNS, while EAAT4 is expressed primarily in the cerebellum, and EAAT5 is found in the retina (Yamada et al., 1996; Arriza et al., 1997). EAAT1 and EAAT2 are generally localized to the plasma membranes of glial cells, and are responsible for the majority of glutamate reuptake in the forebrain (Lehre et al., 1995; Rothstein et al., 1996; Tanaka et al., 1997; Williams et al., 2005). EAAT3 is localized to pre- and postsynaptic neurons in the plasma membrane and cytoplasm, and has been implicated in the regulation of synaptic plasticity (Crino et al., 2002; Levenson et al., 2002).
The EAATs are regulated by a variety of mechanisms, including phosphorylation, glycosylation, enzymatic degradation, and protein-protein interactions. A number of EAAT interacting proteins have been identified, including G-protein suppressor pathway 1 (GPS1), JWA, ARHGEF11, and KIAA0302 (also called beta III spectrin). GPS1 interacts with GLT-1 (the rodent isoform of EAAT2), EAAC1 (EAAT3), and rodent EAAT4. GPS1 decreases GLT-1 mediated glutamate reuptake through a direct protein-protein interaction (Watanabe et al., 2003). JWA is the human homolog of GTRAP3-18, which interacts with and downregulates EAAC1 mediated glutamate reuptake (Lin et al., 2001). GTRAP41 (the rodent isoform of ARHGEF11) and GTRAP48 (the rodent isoform of KIAA0302) interact with rodent EAAT4, increasing glutamate transport (Jackson et al., 2001).
Disruptions in cortical functioning and glutamate transmission have both been implicated in schizophrenia (Itil et al., 1967; Perry, 1982; Bjerkenstedt et al., 1985; Gattaz et al., 1985; Aanonsen and Wilcox, 1986; Korpi et al., 1987; Alfredsson and Wiesel, 1989; Deutsch et al., 1989; Macciardi et al., 1990; Javitt and Zukin, 1991; Tamminga et al., 1992; Saransaari et al., 1993; Choe et al., 1994; Krystal et al., 1994; Lahti et al., 1995; Lehre et al., 1995; Coyle, 1996; Utsunomiya-Tate et al., 1996; Deicken et al., 1997; Goff and Wine, 1997; Omori et al., 1997; Bertolino et al., 1998; Kegeles et al., 1998; Ohnuma et al., 1998; Cecil et al., 1999; Tamminga, 1999; Callicott et al., 2000; Dracheva et al., 2001; Meador-Woodruff et al., 2001; Smith et al., 2001a; Steel et al., 2001; Varoqui et al., 2002; Burbaeva et al., 2003; Hisano, 2003; Manoach, 2003; Ghose et al., 2004; Quintana et al., 2004; Dracheva et al., 2005; Liu et al., 2006). A genetic variant of EAAT1 has been associated with schizophrenia, and expression of a high-risk for schizophrenia allele of the GRM3 metabotropic glutamate receptor is associated with decreased EAAT2 mRNA expression in human prefrontal cortex (Egan et al., 2004; Walsh et al., 2008). Because the glutamate transporters maintain extracellular glutamate, we hypothesized that EAAT1–3 expression is altered in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) in schizophrenia. We also predicted that expression of molecules regulating EAAT function, including ARHGEF11, JWA, GPS1, and KIAA0302, are altered in schizophrenia. To test these hypotheses, we investigated both the transcript and protein expression of these EAATs and EAAT interacting proteins in schizophrenia using in situ hybridization and Western blot analysis.
Materials and Methods
Subjects
Two groups of subjects provided separately from the Mount Sinai Medical Center Brain Bank were studied (Table 1 and Table 2) for mRNA and protein expression, respectively. 11 subjects (3 control subjects and 8 subjects with schizophrenia) were common to both groups. Neuropathological examination revealed no neurodegenerative disorders including Alzheimer’s Disease in any subject. Brain samples were prepared as previously described (Oni-Orisan et al., 2007). ACC was dissected at the level of the genu of the corpus callosum. Tissue blocks were dissected from the dorsal surface of the corpus callosum extending 12–15 mm dorsally and extending 12–15 mm laterally from the midline. DLPFC was dissected as described by Rajkowska and Goldman-Rakic (Rajkowska and Goldman-Rakic, 1995).
Table 1.
Subject Characteristics for In Situ Hybridizations
| Age (Years) | Sex | PMI (min) | pH | Cause of Death | |||
|---|---|---|---|---|---|---|---|
| Control Subjects | |||||||
| 79* | F | 181 | 6.3 | CPF | |||
| 96* | F | 195 | 6.7 | CPF | |||
| 90* | F | 250 | 6.0 | CPF | |||
| 69 | M | 255 | 6.3 | Unknown | |||
| 64 | F | 1145 | 6.1 | Pulmonary edema | |||
| 93 | M | 1140 | 6.4 | Congestive heart failure | |||
| 102 | F | 423 | 6.5 | Acute Myocardial Infarction | |||
| 73 | F | 203 | 6.3 | Acute Myocardial Infarction | |||
| 79 | F | 460 | 6.5 | Acute Myocardial Infarction | |||
| 84 | F | 1110 | 6.2 | Unknown | |||
| 101 | M | 280 | 6.8 | Coronary artery disease | |||
| mean ± SD: 85 ± 13 | 3M / 8F | 513 ± 407 | 6.4 ± 0.2 | ||||
| Subjects with Schizophrenia | Subtype | Medications | Weeks med free | ||||
| 84 | F | 935 | 6.2 | Unknown | par | thi/chl | 115 |
| 61* | M | 212 | 6.5 | CPF | undif | per/hal/ser | 1 |
| 69* | M | 270 | 6.4 | Cardiac infarction, renal failure | par | hal/flu/tri/chl | 6 |
| 63* | M | 372 | 5.9 | CPF | undif | hal | 0 |
| 69* | F | 820 | 6.2 | CPF | undiff | thi/tri | 4 |
| 87* | M | 670 | 6.5 | CPF | undif | thi/tri | 11 |
| 68* | M | 335 | 6.8 | CPF | par | pro | 2 |
| 85 | M | 320 | 6.3 | CPF | undiff | flu/chl | 0 |
| 73 | M | 475 | 6.5 | Cardiorespiratory failure | disorg | hal | 0 |
| 66 | M | 725 | 6.5 | Acute cardiac failure | undif | unknown | unknown |
| 76 | F | 1270 | 6.1 | Cardiogenic shock | par | flu/tri/chl | 0 |
| 97 | M | 555 | 6.5 | CPF | undif | hal | 0 |
| 66 | M | 504 | 6.7 | CPF | sa | hal | 0 |
| 82 | F | 1126 | 6.6 | CPF | res | ris | 124 |
| 79 | F | 595 | 6.8 | Cardiac Arrest | undif | thx | 364 |
| 68 | M | 1036 | 6.6 | CPF | cat | flu | 0 |
| 86 | F | 415 | 5.8 | Respiratory insufficiency, renal failure | cat | thx/chl | 36 |
| 65* | F | 350 | 5.9 | CPF | undif | unknown | unknown |
| 79* | F | 1225 | 7.1 | CPF, cancer of pancreas | undif | thi/thx | 9 |
| 84* | M | 372 | 6.5 | CPF | undif | hal/per/thi/chl | 106 |
| mean ± SD: 75 ± 10 | 12M / 8F | 629 ± 333 | 6.5 ± 0.3 |
Abbreviations: post-mortem interval (PMI), standard deviation (SD), female (F), male (M), cardiopulmonary failure (CPF), paranoid (par), undifferentiated (undif), disorganized (disorg), schizoaffective (sa), residual (res), catatonic (cat), medication (med), Thioridazine (thi), Chlorpromazine (chl), Perphenazine (per), Haloperidol (hal), Trifluoperazine (tri), Prochlorperazine (pro), Fluphenazine (flu), Thiothixene (thx), Risperidone (ris).
Asterisks indicate subject is present in both in situ hybridization and Western blot analysis cohorts.
Table 2.
Subject Characteristics for Western Blot Analyses
| Age (Years) | Sex | PMI (Min) | pH | Cause of Death | |||
|---|---|---|---|---|---|---|---|
| Control Subjects | |||||||
| 88 | M | 285 | 5.9 | Cardiac | |||
| 86 | F | 280 | 6.5 | Unknown | |||
| 55 | M | 600 | 5.7 | Cancer | |||
| 79* | F | 181 | 6.3 | CPF | |||
| 96* | F | 195 | 6.7 | CPF | |||
| 90* | F | 250 | 6.0 | CPF | |||
| 74 | F | 180 | 6.0 | CPF | |||
| 70 | M | 482 | 6.0 | ||||
| mean ± SD: 74 ± 13 | 3M / 5F | 306 ± 154 | 6.1 ± 0.3 | ||||
| Subjects with Schizophrenia | Subtype | Medications | Weeks med free | ||||
| 54 | M | 490 | 6.0 | Acute myelocytic leukemia | disorg | unknown | unknown |
| 61* | M | 212 | 6.5 | CPF | undif | per/hal/ser | 1 |
| 84* | M | 372 | 6.5 | CPF | undif | hal/per/thi/chl | 106 |
| 69* | M | 270 | 6.4 | Cardiac infarction, renal failure | failure par | hal/flu/tri/chl | 6 |
| 76 | F | 510 | 6.1 | CPF, breast cancer | par | thi | 0 |
| 87* | M | 670 | 6.5 | CPF | undif | thi/tri | 11 |
| 68* | M | 335 | 6.8 | CPF | par | pro | 2 |
| 86 | F | 330 | 6.2 | Cardiac, pneumonia | undif | unknown | unknown |
| 72 | M | 1235 | 6.6 | CPF | undif | neuroleptic | 12 |
| 65* | F | 350 | 5.9 | CPF | undif | unknown | unknown |
| 79* | F | 1225 | 7.1 | CPF, pancreatic cancer | undif | thi/thx | 9 |
| 63* | M | 372 | 5.9 | CPF | undif | hal | 0 |
| 69* | F | 820 | 6.2 | CPF | undif | thi/tri | 4 |
| mean ± SD: 72 ± 13 | 8M / 5F | 553 ± 342 | 6.4 ± 0.4 |
Abbreviations: post-mortem interval (PMI), standard deviation (SD), female (F), male (M), cardiopulmonary failure (CPF), paranoid (par), undifferentiated (undif), disorganized (disorg), medication (med), Thioridazine (thi), Chlorpromazine (chl), Perphenazine (per), Haloperidol (hal), Fluphenazine (flu), Trifluoperazine (tri), Prochlorperazine (pro), Thiothixene (thx).
Asterisks indicate subject is present in both in situ hybridization and Western blot analysis cohorts.
In Situ Hybridization
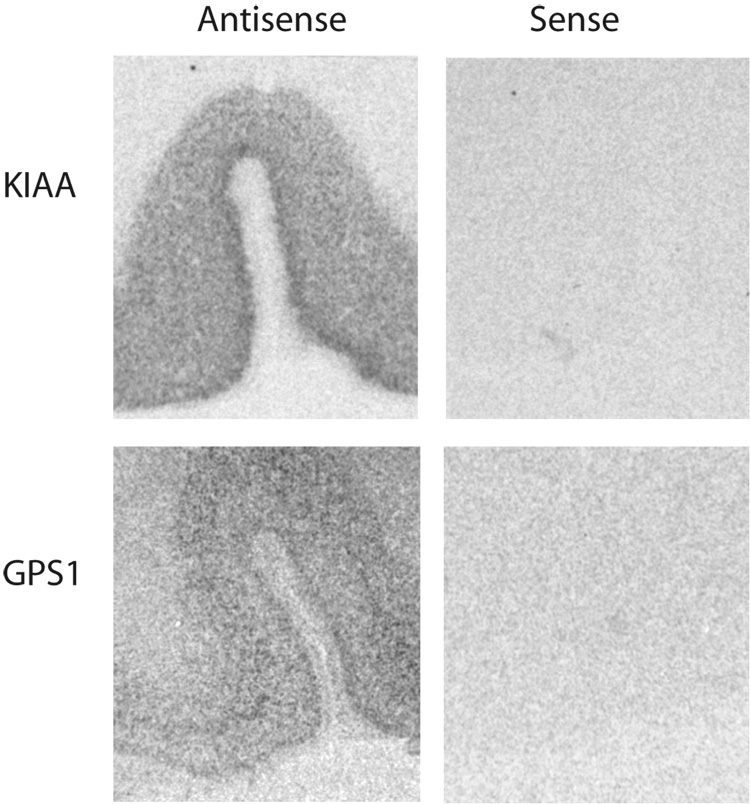
mRNA expression was measured using in situ hybridization for the following subclones: GPS-1 (Genebank accession no. BC064503; nucleotide coding region 1396-1602), ARHGEF11 (NM_014784; 1935-2309), JWA (NM_006407; 181-520), KIAA0302 (AB008567; 5958-6345), EAAT1 (U03504; 5260825), EAAT2 (NM004171; 601-1026), and EAAT3 (NM004170; 156-979), GLAST (rat EAAT1, X63744 S49018; 569-1001) as previously described (McCullumsmith and Meador-Woodruff, 2002; Huerta et al., 2006). We have previously shown specificity of the probes for EAAT1, EAAT2, and EAAT3 using sense and antisense probes (McCullumsmith and Meador-Woodruff, 2002). Specificity of the probes for GPS1 and KIAA0302 is shown in Figure 3.
Figure 3. Probe specificity of KIAA0302 and GPS1.
In situ hybridization analysis of KIAA0302 and GPS1 mRNA expression using sense and antisense probes in anterior cingulate cortex. Abbreviations: KIAA0302 (KIAA).
Western Blot Analysis
Western Blot analysis was performed as described previously (Oni-Orisan et al., 2007). For each assay, protein was transferred to membranes from multiple gels together in the same apparatus, and subsequent treatments were performed in parallel under identical conditions to minimize interblot variability. Membranes were incubated in blocking solution (5% milk powder in Tris buffered saline with 0.1% Tween (milk/TBST) for GPS1 and KIAA0302, 5% milk powder in phosphate buffered saline with 0.1% Tween (milk/PBST) for EAAT1 and EAAT2, and 3% milk/TBST for EAAT3) for 1 hour at room temperature. Membranes were incubated in primary antibody diluted 1:1000 for GPS1 (Abcam, ab4535), EAAT1 (Santa Cruz, sc-7758), and EAAT2 (Chemicon, AB1783), 1:200 for KIAA0302 (Santa Cruz, sc-28273) 1:250 for EAAT3 (Alpha Diagnostics, EAAC11-A), and 1:10,000 for beta-tubulin (Upstate, 05-661) in blocking solution overnight at 4°C. The GLT-1 antibody was raised to a peptide sharing 100% sequence homology with human EAAT2, and cross-reacts with human EAAT2 (Lauderback et al., 2001). The EAAC1 and spectrin β III antibodies were raised to peptides sharing 100% sequence homology with human EAAT3 and KIAA0302. Membranes were washed in TBST or PBST, then incubated for 1 hour with horseradish peroxidase (HRP) coupled secondary antibody diluted 1:5000 in blocking solution for EAAT1, GPS1, KIAA0302, and beta-tubulin, 1:4000 for EAAT2, and 1:400 for EAAT3. Membranes were washed in TBST or PBST followed by high purity water. Prior to examining protein expression, we tested our EAAT1, EAAT2, EAAT3, GPS1, KIAA0302, and beta-tubulin Western blot assays using varying concentrations of total protein of human cortical tissue homogenate. These control studies demonstrated that our assay was linear for the protein concentrations used in our studies.
Statistical Analysis
Values from two sections (for in situ hybridizations) or bands (for Western blots) per subject for each region were averaged and converted to optical density. For in situ hybridizations, background from white matter (EAAT1, EAAT2, EAAT3, ARHGEF11, KIAA0302, and JWA) or slide background (GPS1) was subtracted from cortical expression grayscale values before conversion to optical density. For Western blots, optical density was divided by optical density of beta-tubulin from the same lane as a loading control and analyzed as a ratio. Outliers, defined as values more than 2 standard deviations from the mean, were excluded. Because tissue pH is a predictor of RNA integrity, correlation analysis was performed to investigate possible associations between transcript expression and tissue pH (Stan et al., 2006). Protein integrity is generally not affected by pH (Stan et al., 2006). Diagnostic groups were matched for age, pH, and PMI, and t-tests revealed no statistically significant differences between groups for any of these variables. When significant associations between transcript expression and pH were found, analysis of covariance (ANCOVA) was utilized; otherwise analysis of variance (ANOVA) was utilized with diagnosis as the independent variable and optical density or optical density ratio as the dependent variable. For all tests α = 0.05.
Results
Transcript Studies
Using in situ hybridization, transcript expression of EAAT1-3, ARHGEF11, GPS1, JWA, and KIAA0302 were measured. There were no associations between EAAT1, EAAT2, ARHGEF11, or KIAA0302 transcript expression and pH in either the ACC or the DLPFC. Correlation analysis revealed an association between EAAT3 transcript expression and pH in ACC (R= 0.49, p < 0.05) but not DLPFC. Correlation analysis revealed an association between JWA transcript expression and pH in ACC (R = 0.45, p < 0.05) but not DLPFC.
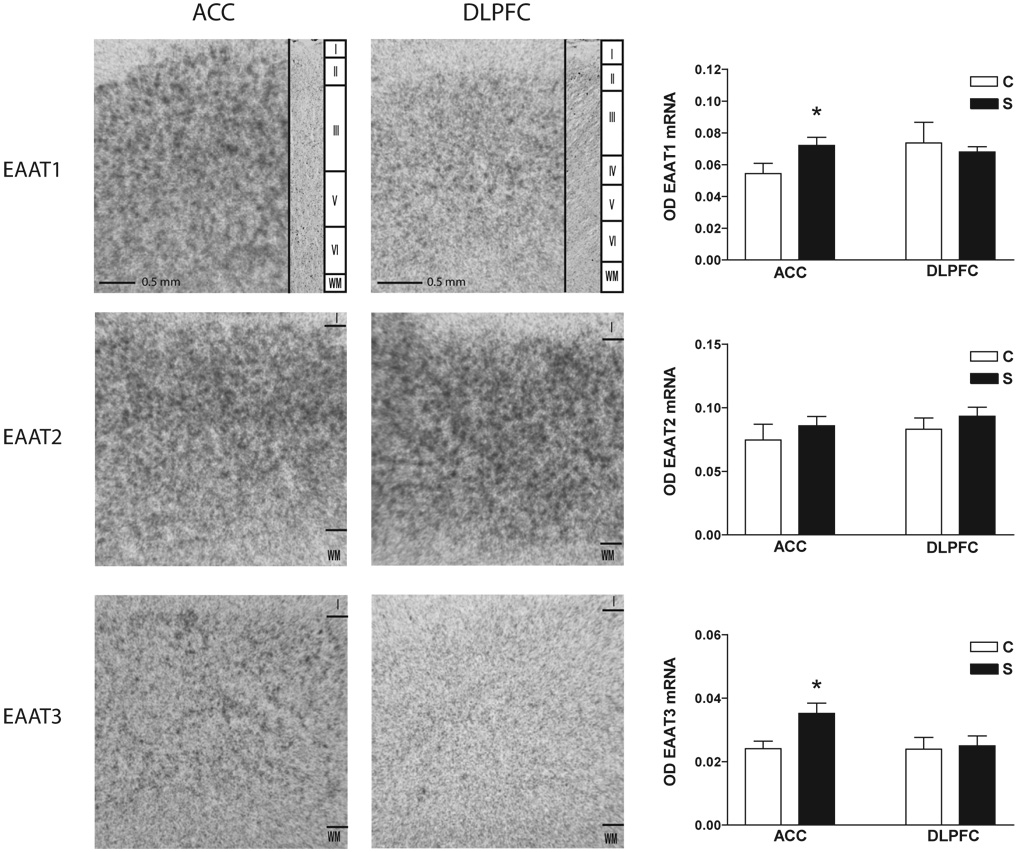
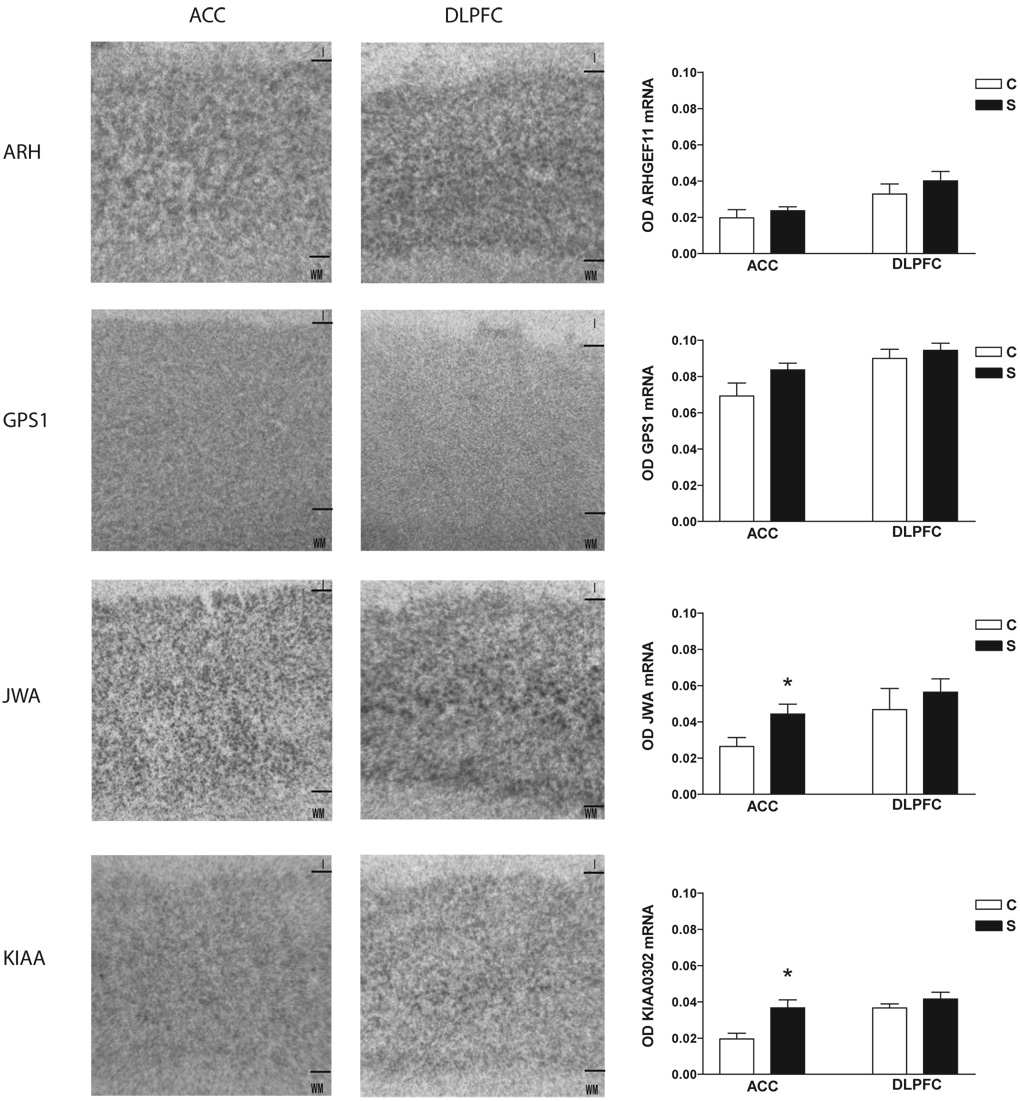
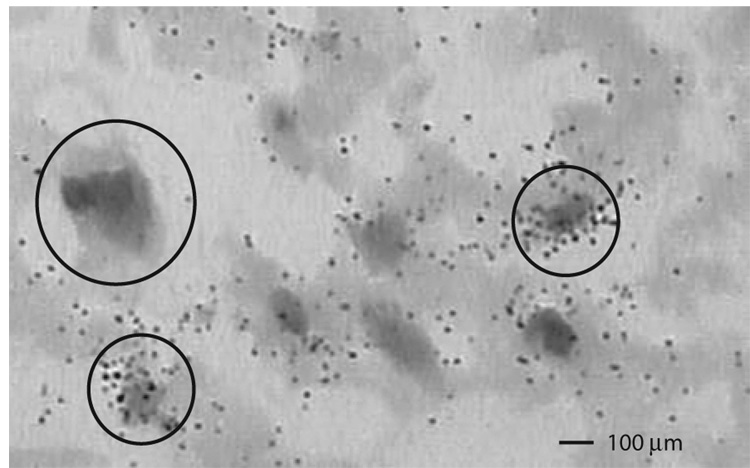
EAAT1 transcript expression was increased in schizophrenia in ACC (F(1,21) = 4.54, p < 0.05), but not DLPFC (Figure 1). EAAT3 transcript expression was increased in schizophrenia in ACC (F(1, 19) = 18.07, p < 0.05), but not DLPFC (Figure 1). JWA transcript expression was increased in schizophrenia in ACC (F(1,24) = 5.73, p < 0.05). KIAA0302 transcript expression was increased in schizophrenia in ACC (F(1,24) = 8.59, p< 0.05), but not DLPFC. There were no significant differences between groups for EAAT2, ARHGEF11, or GPS1 (Figure 1 and Figure 2). Sense controls confirmed the specificity of our antisense probes for KIAA0302 and GPS1 (Figure 3). Emulsion dipping in the vicinity of layer V of the ACC confirmed the specificity of EAAT2 expression to small cells, consistent with astrocytic expression (Figure 4).
Figure 1. EAAT transcript expression.
In situ hybridization analysis of EAAT1-3 mRNA expression in the anterior cingulate cortex and dorsolateral prefrontal cortex from control and schizophrenia subjects. Nissl stained sections are shown for comparison. Roman numerals indicate cortical layers. Data expressed as means +/− standard error of the mean. Asterisks indicate a significant difference between control and schizophrenia (p < 0.05). Abbreviations: Optical density (OD), control (C), schizophrenia (S), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), excitatory amino acid transporter (EAAT), layer I (I), white matter (WM).
Figure 2. EAAT interacting partner transcript expression.
In situ hybridization analysis of ARHGEF11, GPS1, JWA, and KIAA0302 mRNA expression in the anterior cingulate cortex and dorsolateral prefrontal cortex from control and schizophrenia subjects. Data expressed as means +/− standard error of the mean. Abbreviations: Optical density (OD), control (C), schizophrenia (S), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), ARHGEF11 (ARH), G-protein suppressor pathway 1 (GPS1), KIAA0302 (KIAA), layer I (I), white matter (WM).
Figure 4. Cellular expression pattern of EAAT2.
Emulsion dipped in situ hybridization of EAAT2 mRNA in nissl stained tissue from the vicinity of layer V of anterior cingulate cortex. Large circle indicates large cell, small circles indicate small cells.
Protein Studies
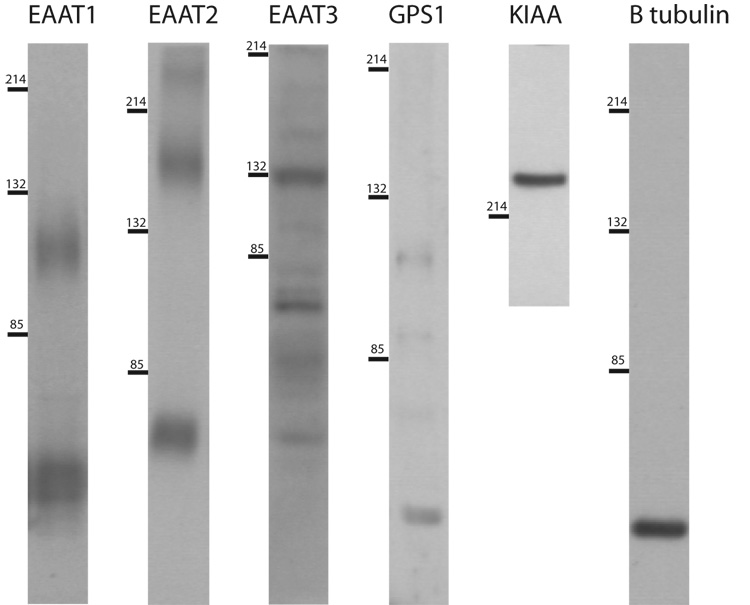
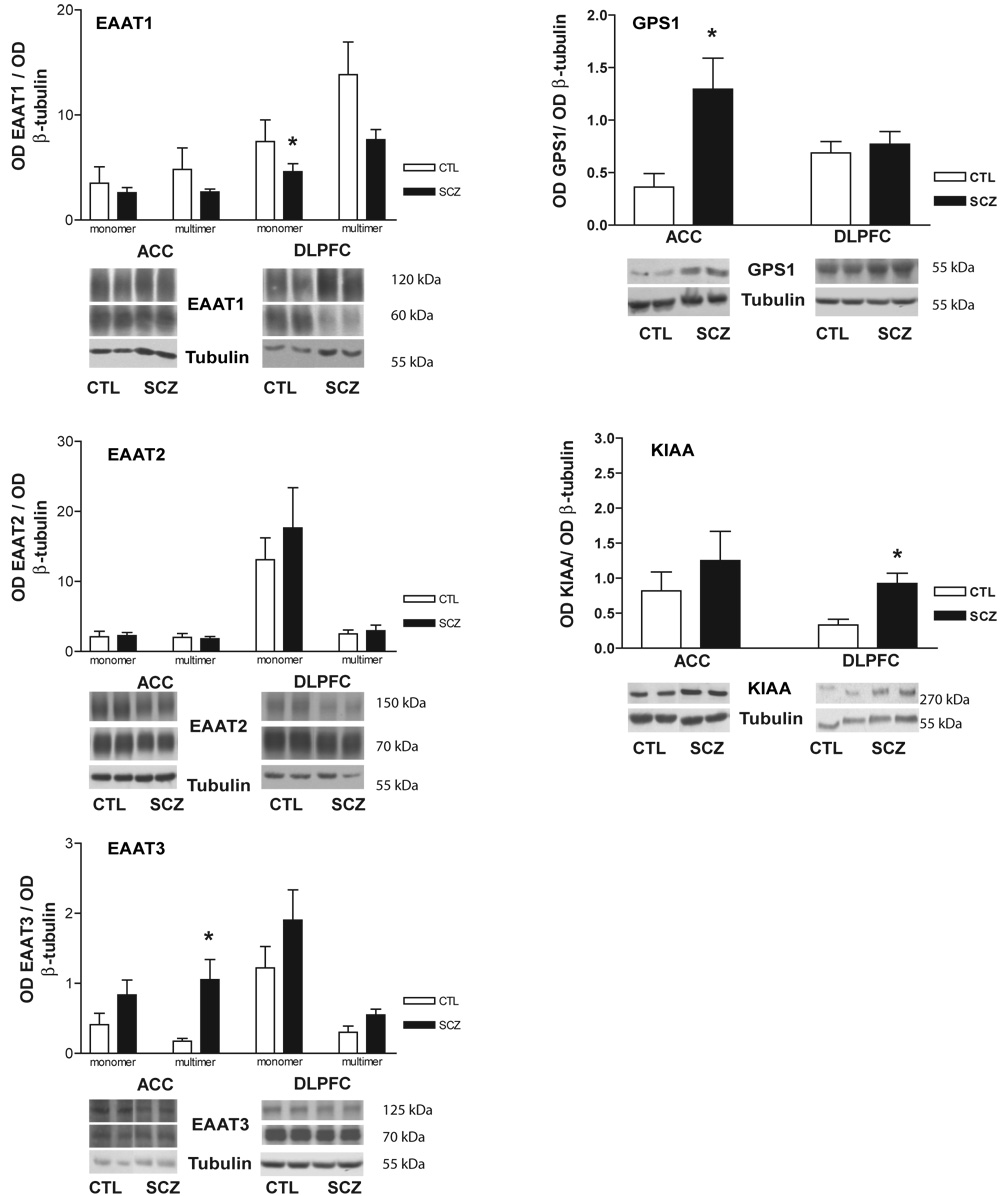
Using Western blot analysis, protein expression of EAAT1-3, GPS1, and KIAA0302 were measured. For EAAT1–3, protein bands were present for both monomeric and multimeric forms (Figure 5). Each of these bands was analyzed separately and in sum. Decreased total EAAT1 expression was found in the DLPFC (F(1,14) = 6.25, p < 0.05), associated with decreased expression of the monomer (F(1, 15) = 7.31, p < 0.05), but not the multimer (Figure 6). There were no changes in EAAT1 protein expression in the ACC. There were no changes in EAAT2 protein expression in the DLPFC or ACC (Figure 6). Increased expression of the EAAT3 multimer was found in the ACC (F(1, 16) = 4.92, p < 0.05) but not the DLPFC in schizophrenia (Figure 6). There were no changes in expression of the monomer or the total amount of EAAT3 protein.
Figure 5. Protein expression profiles.
Western blot analysis of all proteins studied. Numbers on left indicate molecular weight in kDa. Abbreviations: KIAA0302 (KIAA).
Figure 6. EAAT and EAAT interacting partner protein expression.
Western blot analysis in the anterior cingulate cortex and dorsolateral prefrontal cortex from control and schizophrenia subjects for EAAT1–3, GPS1, and KIAA0302. Values are normalized to tubulin. Asterisks indicate a significant difference between control and schizophrenia (p < 0.05). Data expressed as means +/− standard error of the mean. Abbreviations: Optical density (OD), control (CTL), schizophrenia (SCZ), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), excitatory amino acid transporter (EAAT).
GPS1 protein expression was increased in the ACC in schizophrenia (F(1, 17) = 6.41, p < 0.05) (Figure 3), but unaltered in the DLPFC. KIAA0302 protein expression was increased in the DLPFC (F(1, 16) = 10.56; p < 0.05), but not the ACC, in schizophrenia (Figure 6).
Sex Effects
There was a sex imbalance between diagnostic groups, so the dependent variables that differed by diagnosis were tested for sex effects. There were generally no sex effects for these variables with the exception of EAAT3 transcripts. EAAT3 transcript expression was increased in male subjects compared to female subjects in the ACC (F(1,20) = 11.07, p < 0.05). When split by sex, EAAT3 transcript expression was increased in males (F(1,8) = 15.04, p < 0.05) and females (F(1,10) = 5.04, p < 0.05) with schizophrenia compared to same sex controls.
Antipsychotic Effects
A number of patients with schizophrenia were medication-free for at least 6 weeks prior to death. No differences in transcript expression were detected between medicated and unmedicated patients for most of the transcripts found altered with the exception of JWA. An increase in JWA expression in the ACC was found in medicated versus unmedicated patients (F(1,4) = 13.13, p < 0.05). No changes in transcript expression detected between control subjects and unmedicated patients with schizophrenia for any of the transcripts found altered in schizophrenia. No differences in protein expression were detected between medicated and unmedicated patients with schizophrenia for any of the proteins found altered in schizophrenia. No changes in protein expression were detected between control subjects and unmedicated patients with schizophrenia for EAAT1, GPS1, or KIAA0302. EAAT3 protein expression was increased in the ACC in unmedicated patients with schizophrenia compared to controls 10 (F(1, 9) = 7.07, p < 0.05).
Discussion
We found alterations in expression of molecules involved in glutamate transport in schizophrenia, suggesting abnormal glutamate reuptake is involved in the pathophysiology of this illness. Of particular interest are the changes in protein expression of the glial transporter EAAT1, the neuronal transporter EAAT3, and the negative modulator of EAAT2 mediated glutamate reuptake, GPS1. These results suggest that neuronal glutamate reuptake may be increased in schizophrenia, while glial glutamate reuptake may be diminished.
We detected a decrease in total EAAT1 protein expression in the DLPFC, most likely attributable to decreased expression of the monomer. While the EAATs function natively as homomultimers (Haugeto et al., 1996), this result suggests that there may be a decrease in monomeric pools of EAAT1. Decreased EAAT1 protein expression may reflect decreased glutamate reuptake into glial cells or a decrease in the total number of EAAT1 expressing glia. In addition to decreased protein expression in the DLPFC, we detected increased EAAT1 transcript, but not protein, expression in the ACC. Unchanged protein expression with increased mRNA expression may be due to a number of factors including abnormal translation and folding, abnormal post-translational modifications, or increased protein turnover.
We did not detect changes in transcript or protein expression of EAAT2, the transporter responsible for approximately 90% of glutamate reuptake in the forebrain (Robinson, 1998). Previous studies of EAAT2 expression have yielded discrepant results. Two studies found increased EAAT2 transcript expression in the PFC, while another found no changes (Ohnuma et al., 1998; Matute et al., 2005; Lauriat et al., 2006). While we did not find changes in EAAT2, it is possible that EAAT2 mediated glutamate reuptake is regulated by processes other than simply increasing or decreasing gene expression. Phosphorylation of specific serine residues and degradation pathways are known to influence EAAT2 function (Kalandadze et al., 2002; Boston-Howes et al., 2006).
EAAT2 mediated glutamate reuptake may also be regulated by GPS1 which interacts with the C-terminus of GLT-1 (the rat homolog of EAAT2), regulating surface trafficking through a leucine zipper-like motif (Watanabe et al., 2003). Coexpression of GPS-1 with GLT-1 downregulates glutamate reuptake in HEK cells (Watanabe et al., 2004). We found increased GPS1 protein, but not mRNA, in the ACC, suggesting decreased EAAT2 mediated glutamate reuptake. GPS1 protein levels have not previously been examined in schizophrenia, but in a different study (Huerta et al., 2006), we found no changes in GPS1 mRNA expression in the thalamus, suggesting that changes in GPS1 may be region specific.
GPS1 may have other functions besides regulating GLT-1/EAAT2. GPS1 also associated with EAAC1 and rodent EAAT4 in an in vitro binding assay, and it might regulate these transporters in a manner similar to GLT-1 (Watanabe et al., 2003). It has also been suggested that GPS1 functions in humans as a suppressor of G-protein pathway signaling (Spain et al., 1996). Thus, it is possible that GPS1 could also have effects on G-protein linked receptors, including the mGluRs. mGluRs play a role in glutamate cycling because activation of group I mGluRs or group II-III mGluRs enhances and inhibits glutamate release, respectively (Cartmell and Schoepp, 2000).
While it appears that EAAT2 is not regulated simply by changes in gene expression, EAAT3 expression was increased at both transcript and protein levels. Animal and cell culture experiments have shown that the EAATs exist natively as homomultimers (Haugeto et al., 1996). EAAT trimers are formed immediately after biosynthesis, and are the only functioning forms of EAAT3 in native systems (Gendreau et al., 2004). It is unclear whether the multimer bands we measured are dimers or trimers, given that undissociated proteins do not migrate with a uniform surface area to charge ratio (van Holde, 1985). Our data suggest increased EAAT3 mediated glutamate reuptake in the ACC in schizophrenia, since we found an increase in multimer protein expression.
We propose that the changes in EAAT3 expression are secondary to changes in EAAT1 and GPS1 expression. Decreased EAAT1 and increased GPS1 expression may cause decreased glutamate reuptake in glial cells, leading to increased basal synaptic glutamate levels and synaptic spillover. EAAT3 is largely localized in perisynaptic regions and contributes to clearance of glutamate spillover (Diamond, 2001; Huang and Bergles, 2004). Thus, EAAT3 expression may be increased in neurons to compensate for these changes. Consistent with this notion, EAAT3 protein is localized to regulatable cytosolic pools that may be rapidly mobilized to the plasma membrane, suggesting a biological process that is highly responsive to changes in glutamate levels (Davis et al., 1998). It is also possible that increased EAAT3 expression is an effect of direct interaction with GPS1, or another EAAT regulating molecule. However, it is not known what effect, if any, GPS1 has on EAAT3 mediated glutamate reuptake.
In addition to the changes in GPS1, EAAT1, and EAAT3, we detected increased KIAA0302 protein expression in the DLPFC. This increase is difficult to interpret as it relates to glutamate transport because KIAA0302 is reported to interact only with EAAT4 (Jackson et al., 2001). Although both transcript and protein levels of KIAA0302 are highly expressed in the cortex, EAAT4 is expressed at very low levels, and is predominantly expressed in the cerebellum (Nagao et al., 1997; Lin et al., 1998). This anatomical mismatch suggests that KIAA0302 has other functions. KIAA0302 is a structural molecule involved in Golgi and vesicular membrane skeletons, binds to dynein and dynactin, and it has been implicated in vesicular trafficking along microtubules (Stankewich et al., 1998; Holleran et al., 2001; Jackson et al., 2001). Given the scarcity of EAAT4, it is likely that KIAA0302 serves structural, trafficking, or other roles in the prefrontal cortex.
We did not detect changes in transcript expression for any of the EAAT interacting proteins that we studied. This suggests that these molecules are not altered in schizophrenia at the mRNA level. We were unable to measure protein expression of JWA or ARHGEF11 with commercially available antibodies. It is therefore possible that, similar to GPS1 and KIAA0302, they are altered in schizophrenia at the level of protein, but not mRNA, expression.
Another point of interest from these data is that all significant changes were detected in the anterior cingulate but not the dorsolateral prefrontal cortex. Although the DLPFC has been a major focus of studies involving altered gene expression in schizophrenia, many other regions have also been implicated in this disease, and may have more robust changes. In a microarray study of multiple brain regions in schizophrenia, the anterior cingulate cortex had ~20 fold more altered genes than the dorsolateral prefrontal cortex. (Katsel et al., 2005) Thus, our findings are consistent with the hypothesis that the anterior cingulate cortex may be a particularly vulnerable site in the pathophysiology of schizophrenia.
There are several potential limitations of this study. One concern is the advanced age of the subjects. Disorder-specific alterations in gene expression could be masked by age effects on those genes. However, we have previously detected robust changes in glutamate receptor transcripts, proteins, and binding sites in a similar sample from the same brain bank, demonstrating significant changes in gene expression in older subjects (Smith et al., 2001a; Smith et al., 2001b; Clinton et al., 2003; Bruneau et al., 2005; Huerta et al., 2006). Sex effects may also be a confounding factor. In our entire sample, we found an increase in EAAT3 transcript expression in males compared to females in the ACC. However, EAAT3 expression was increased in schizophrenia compared to controls in both males and females when analyzed separately, suggesting that our diagnosis-related results are likely not attributable to sex differences.
Another limitation of this study is the potential effects of treatment with antipsychotic medications (De Souza et al., 1999; Melone et al., 2001). We generally did not detect any differences in expression between medicated and unmedicated patients for any transcripts or proteins we found increased in schizophrenia with one exception. We detected increased JWA transcript expression in the ACC in medicated versus unmedicated subjects. These analyses, however, are underpowered. The increased JWA transcript expression in schizophrenia may therefore be due to either disease specific or medication specific effects. However, we did find increased EAAT3 protein expression in the ACC in unmedicated patients with schizophrenia, compared to control subjects, suggesting that our EAAT3 findings are not due to a medication effect.
Our results shed light on several aspects of glutamate cycling in schizophrenia. Increased GPS1 protein and decreased EAAT1 protein suggest decreased glutamate reuptake capacity in glia, leading to increased synaptic glutamate levels and synaptic spillover. The increase in EAAT3 expression might be compensatory to decreased glutamate reuptake by glia. This scenario suggests a state of increased basal synaptic glutamate levels in the prefrontal cortex in schizophrenia. It remains unclear how KIAA0302 fits into this model, as the transporter subtype it regulates is expressed at very low levels in the cortex. These findings have important implications for neuronal plasticity in schizophrenia, and provide new targets for the development of novel treatments for this illness.
Acknowledgments
Supported by MH53327 (JMW), MH78378 (DEB), and MH074016 (REM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanonsen LM, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-D- aspartate in mice. Neurosci Lett. 1986;67(2):191–197. doi: 10.1016/0304-3940(86)90396-4. [DOI] [PubMed] [Google Scholar]
- Alfredsson G, Wiesel FA. Monoamine metabolites and amino acids in serum from schizophrenic patients before and during sulpiride treatment. Psychopharmacology (Berl) 1989;99(3):322–327. doi: 10.1007/BF00445551. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Nawroz S, Mattay VS, Duyn JH, Tedeschi G, Frank JA, Weinberger DR. Reproducibility of proton magnetic resonance spectroscopic imaging in patients with schizophrenia. Neuropsychopharmacology. 1998;18(1):1–9. doi: 10.1016/S0893-133X(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Bjerkenstedt L, Edman G, Hagenfeldt L, Sedvall G, Wiesel FA. Plasma amino acids in relation to cerebrospinal fluid monoamine metabolites in schizophrenic patients and healthy controls. Br J Psychiatry. 1985;147:276–282. doi: 10.1192/bjp.147.3.276. [DOI] [PubMed] [Google Scholar]
- Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281(20):14076–14084. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- Bruneau EG, McCullumsmith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Increased expression of glutaminase and glutamine synthetase mRNA in the thalamus in schizophrenia. Schizophr Res. 2005;75(1):27–34. doi: 10.1016/j.schres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):675–680. doi: 10.1016/s0278-5846(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Egan MF, Mattay VS, Langheim FJ, Weinberger DR. Selective relationship between prefrontal N-acetylaspartate measures and negative symptoms in schizophrenia. Am J Psychiatry. 2000;157(10):1646–1651. doi: 10.1176/appi.ajp.157.10.1646. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75(3):889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Lenkinski RE, Gur RE, Gur RC. Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999;20(2):131–140. doi: 10.1016/S0893-133X(98)00063-3. [DOI] [PubMed] [Google Scholar]
- Choe BY, Kim KT, Suh TS, Lee C, Paik IH, Bahk YW, Shinn KS, Lenkinski RE. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naive, chronic schizophrenia. Acad Radiol. 1994;1(3):211–216. doi: 10.1016/s1076-6332(05)80716-0. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Haroutunian V, Davis KL, Meador-Woodruff JH. Altered Transcript Expression of NMDA Receptor-Associated Postsynaptic Proteins in the Thalamus of Subjects With Schizophrenia. Am J Psychiatry. 2003;160(6):1100–1109. doi: 10.1176/appi.ajp.160.6.1100. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43(3):211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18(7):2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza IE, McBean GJ, Meredith GE. Chronic haloperidol treatment impairs glutamate transport in the rat striatum. Eur J Pharmacol. 1999;382(2):139–142. doi: 10.1016/s0014-2999(99)00589-0. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Weiner MW. Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr Res. 1997;27(1):65–71. doi: 10.1016/S0920-9964(97)00082-0. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB, Morihisa JM. A "glutamatergic hypothesis" of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12(1):1–13. [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21(21):8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158(9):1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79(6):868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101(34):12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattaz WF, Gasser T, Beckmann H. Multidimensional analysis of the concentrations of 17 substances in the CSF of schizophrenics and controls. Biol Psychiatry. 1985;20(4):360–366. doi: 10.1016/0006-3223(85)90038-1. [DOI] [PubMed] [Google Scholar]
- Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. J Biol Chem. 2004;279(38):39505–39512. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- Ghose S, Weickert CS, Colvin SM, Coyle JT, Herman MM, Hyde TM, Kleinman JE. Glutamate carboxypeptidase II gene expression in the human frontal and temporal lobe in schizophrenia. Neuropsychopharmacology. 2004;29(1):117–125. doi: 10.1038/sj.npp.1300304. [DOI] [PubMed] [Google Scholar]
- Goff DC, Wine L. Glutamate in schizophrenia: clinical and research implications. Schizophr Res. 1997;27(2–3):157–168. doi: 10.1016/S0920-9964(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. J Biol Chem. 1996;271(44):27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- Hisano S. Vesicular glutamate transporters in the brain. Anat Sci Int. 2003;78(4):191–204. doi: 10.1046/j.0022-7722.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276(39):36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14(3):346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59(7):394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- Itil T, Keskiner A, Kiremitci N, Holden JM. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr Assoc J. 1967;12(2):209–212. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410(6824):89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277(48):45741–45750. doi: 10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77(2–3):241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Humaran TJ, Mann JJ. In vivo neurochemistry of the brain in schizophrenia as revealed by magnetic resonance spectroscopy. Biol Psychiatry. 1998;44(6):382–398. doi: 10.1016/s0006-3223(97)00425-3. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kaufmann CA, Marnela KM, Weinberger DR. Cerebrospinal fluid amino acid concentrations in chronic schizophrenia. Psychiatry Res. 1987;20(4):337–345. doi: 10.1016/0165-1781(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6(6):869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1–42. J Neurochem. 2001;78(2):413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lauriat TL, Dracheva S, Chin B, Schmeidler J, McInnes LA, Haroutunian V. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience. 2006;137(3):843–851. doi: 10.1016/j.neuroscience.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Lin CI, Orlov I, Ruggiero AM, Dykes-Hoberg M, Lee A, Jackson M, Rothstein JD. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3–18. Nature. 2001;410(6824):84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- Lin CL, Tzingounis AV, Jin L, Furuta A, Kavanaugh MP, Rothstein JD. Molecular cloning and expression of the rat EAAT4 glutamate transporter subtype. Brain Res Mol Brain Res. 1998;63(1):174–179. doi: 10.1016/s0169-328x(98)00256-3. [DOI] [PubMed] [Google Scholar]
- Liu YL, Shen-Jang Fann C, Liu CM, Wu JY, Hung SI, Chan HY, Chen JJ, Lin CY, Liu SK, Hsieh MH, Hwang TJ, Ouyang WC, Chen CY, Lin JJ, Chou FH, Chueh CM, Liu WM, Tsuang MM, Faraone SV, Tsuang MT, Chen WJ, Hwu HG. Evaluation of RGS4 as a candidate gene for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141(4):418–420. doi: 10.1002/ajmg.b.30286. [DOI] [PubMed] [Google Scholar]
- Macciardi F, Lucca A, Catalano M, Marino C, Zanardi R, Smeraldi E. Amino acid patterns in schizophrenia: some new findings. Psychiatry Res. 1990;32(1):63–70. doi: 10.1016/0165-1781(90)90136-s. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;6(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Masson J, Sagne C, Hamon M, El Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacol Rev. 1999;51(3):439–464. [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49(3):451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26(3):368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Davis KL, Haroutunian V. Abnormal kainate receptor expression in prefrontal cortex in schizophrenia. Neuropsychopharmacology. 2001;24(5):545–552. doi: 10.1016/S0893-133X(00)00189-5. [DOI] [PubMed] [Google Scholar]
- Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, Perez-Samartin A, Matute C, Cozzi A, Pellegrini-Giampietro DE, Rothstein JD, Conti F. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry. 2001;6(4):380–386. doi: 10.1038/sj.mp.4000880. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kwak S, Kanazawa I. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience. 1997;78(4):929–933. doi: 10.1016/s0306-4522(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res Mol Brain Res. 1998;56(1–2):207–217. doi: 10.1016/s0169-328x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Omori M, Pearce J, Komoroski RA, Griffin WS, Mrak RE, Husain MM, Karson CN. In vitro 1H-magnetic resonance spectroscopy of postmortem brains with schizophrenia. Biol Psychiatry. 1997;42(5):359–366. doi: 10.1016/S0006-3223(96)00409-X. [DOI] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered Vesicular Glutamate Transporter Expression in the Anterior Cingulate Cortex in Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL. Normal cerebrospinal fluid and brain glutamate levels in schizophrenia do not support the hypothesis of glutamatergic neuronal dysfunction. Neurosci Lett. 1982;28(1):81–85. doi: 10.1016/0304-3940(82)90212-9. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Anterior cingulate dysfunction during choice anticipation in schizophrenia. Psychiatry Res. 2004;132(2):117–130. doi: 10.1016/j.pscychresns.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5(4):307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33(6):479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Lillrank SM, Oja SS. Phencyclidine treatment in mice: effects on phencyclidine binding sites and glutamate uptake in cerebral cortex preparations. J Neural Transm Gen Sect. 1993;93(1):47–59. doi: 10.1007/BF01244937. [DOI] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001a;158(9):1393–1399. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport. 2001b;12(13):2885–2887. doi: 10.1097/00001756-200109170-00026. [DOI] [PubMed] [Google Scholar]
- Spain BH, Bowdish KS, Pacal AR, Staub SF, Koo D, Chang CY, Xie W, Colicelli J. Two human cDNAs, including a homolog of Arabidopsis FUS6 (COP11), suppress G-protein- and mitogen-activated protein kinase-mediated signal transduction in yeast and mammalian cells. Mol Cell Biol. 1996;16(12):6698–6706. doi: 10.1128/mcb.16.12.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich MC, Tse WT, Peters LL, Ch'ng Y, John KM, Stabach PR, Devarajan P, Morrow JS, Lux SE. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci U S A. 1998;95(24):14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJ. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res. 2001;106(3):161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Tamminga C. Glutamatergic aspects of schizophrenia. Br J Psychiatry Suppl. 1999;(37):12–15. [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+- dependent neutral amino acid transporter. J Biol Chem. 1996;271(25):14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- van Holde KE. Physical Biochemistry. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1985. [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22(1):142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Robinson MB, Kalandadze A, Rothstein JD. GPS1, interacting protein with GLT-1. Program No. 372.16 2003 SFN Abstract. 2003 [Google Scholar]
- Watanabe M, Ueki T, Jackson M, Ruggiero AM, Sawa A, Kalandadze A, Robinson MB, Rothstein JD. CSN1 Downregulates GLT-1 activity. SFN Abstract. 2004 [Google Scholar]
- Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, Dodd PR, Pow DV. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49(4):520–541. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- Yamada K, Watanabe M, Shibata T, Tanaka K, Wada K, Inoue Y. EAAT4 is a post-synaptic glutamate transporter at Purkinje cell synapses. Neuroreport. 1996;7(12):2013–2017. doi: 10.1097/00001756-199608120-00032. [DOI] [PubMed] [Google Scholar]