Abstract
Combined exposures to maternal lead (Pb) and prenatal stress (PS) can act synergistically to enhance behavioral and neurochemical toxicity in offspring. Maternal Pb itself causes permanent dysfunction of the body’s major stress system, the hypothalamic pituitary adrenal (HPA) axis. The current study sought to determine the potential involvement of altered negative glucocorticoid feedback as a mechanistic basis of the effects in rats of maternal Pb (0, 50 or 150 ppm in drinking water beginning 2 mos prior to breeding), prenatal stress (PS; restraint on gestational days 16–17) and combined maternal Pb + PS in 8 mo old male and female offspring. Corticosterone changes were measured over 24h following an i.p. injection stress containing vehicle or 100 or 300 μg/kg (females) or 100 or 150 μg/kg (males) dexamethasone (DEX). Both Pb and PS prolonged the time course of corticosterone reduction following vehicle injection stress. Pb effects were non-monotonic, with a greater impact of at 50 vs. 150 ppm, particularly in males, where further enhancement occurred with PS. In accord with these findings, the efficacy of DEX in suppressing corticosterone was reduced by Pb and Pb+PS in both genders, with Pb efficacy enhanced by PS in females, over the first 6h post-administration. A marked prolongation of DEX effects was found in males. Thus, Pb, PS and Pb+PS, sometimes additively, produced hypercortisolism in both genders, followed by hypocortisolism in males, consistent with HPA axis dysfunction. These findings may provide a plausible unifying biological mechanism for the reported links between Pb exposure and stress-associated diseases and disorders mediated via the HPA axis, including obesity, hypertension, diabetes, anxiety, schizophrenia and depression. They also suggest broadening of Pb screening program to pregnant women in high stress environments.
Keywords: lead, prenatal stress, corticosterone, dexamethasone suppression, hypothalamic-pituitary- adrenal axis, gender
Introduction
Recent studies demonstrate that exposure to lead (Pb) even below the currently designated 10 μg/dl blood Pb (PbB) level of concern can have detrimental consequences for both children and adults. No obvious threshold has been found for the IQ reductions associated with Pb in children. Indeed, the magnitude of IQ reduction at PbBs<10 μg/dl is actually steeper than corresponding reductions at higher PbBs (Canfield et al., 2003; Lanphear et al., 2005). In studies of adults, links between environmental Pb exposure and hypertension indicate that cumulative exposures to even low levels of Pb can increase risk (Cheng et al., 2001; Gerr et al., 2002) and at PbBs<10 μg/dl have been associated with increased all-cause and cardiovascular mortality (Menke et al., 2006). At the same time, the profile of diseases and disorders associated with low level environmental Pb exposures has broadened to include obesity, diabetes, anxiety, schizophrenia and depression (Kim et al., 1995; Cheng et al., 2001; Gerr et al., 2002; Rhodes et al., 2003; Opler et al., 2004; Rajan et al., 2007).
In the U.S., the highest Pb exposures are sustained by low socioeconomic status (SES) communities, i.e., inner city medically-underserved households with income <$25,000 renting homes built before 1950. A recent report noted that 5% of children screened in New York City had PbBs>10 μg/dl. In some upstate cities (Buffalo, Rochester, Syracuse, Albany and Schenectady), corresponding figures were >20% (Haley and Talbot, 2004). The potential for adverse consequences of long-term Pb exposure are unfortunately only further enhanced by the low rate of follow up of such children after their initial identification (Kemper et al., 2005).
In addition to elevated Pb exposures, low SES communities also sustain higher levels of a variety of diseases and disorders (Dohrenwend, 1973; Starfield, 1982; Pappas et al., 1993; Anderson and Armstead, 1995; Stipek and Ryan, 1997), many of which correspond to those associated with Pb exposure (Signorello et al., 2007; Werner et al., 2007; Lawes et al., 2008; Margellos-Anast et al., 2008; Stansfeld et al., 2008). The increased incidences of diseases and disorders associated with low SES has been hypothesized to arise from higher levels of stress associated with such environments resulting in a chronic elevation of cortisol (Munck et al., 1984; Lupien et al., 2000; Lupien et al., 2001; Cohen et al., 2006). One study, for example, found that the greater the number of years spent living in poverty, the greater the elevation in overnight cortisol (Evans and Kim, 2007). Thus, Pb and stress show both congruence as risk factors in low SES populations, and commonalities in diseases and disorders with which each has been associated.
This is notable because Pb and stress can also both act on brain mesocorticolimbic dopamine systems (Diorio et al., 1993; Lowy et al., 1993; Piazza et al., 1996; Pokora et al., 1996; Zuch et al., 1998; Moghaddam, 2002), and on the hypothalamic-pituitary-adrenal (HPA) axis, the major stress response system of the body (Vyskocil et al., 1991c; Cory-Slechta et al., 2004a). This co-occurrence of Pb and stress as risk factors, and the commonality of their biological targets served as the rationale for investigations by our laboratory of their interactive and potentially synergistic effects in experimental models (Cory-Slechta et al., 2004a; Virgolini et al., 2005; Virgolini et al., 2006; Cory-Slechta et al., 2008; Virgolini et al., 2008). Among other findings, these have revealed an enhancement by prenatal and offspring stress of maternal Pb-induced behavioral toxicity in females and potentiated changes in frontal cortical and nucleus accumbens catecholamines and serotonin levels in males and females, and permanent effects of Pb itself on HPA axis function at PbBs in dams averaging only 11 μg/dl at the time of weaning (Cory-Slechta et al., 2004a; Cory-Slechta et al., 2008; Virgolini et al., 2008). Consistent with these findings, PbBs<10 μg/dl have also now been shown to be associated with altered adrenocortical responses to acute stress in children (Gump et al., 2008).
Corticosterone (cortisol in humans) is released from the adrenal cortex in response to stressors. Although the stress response has evolved to protect the organism against potential danger, prolonged exposure to a stressor and/or an inability to rapidly return to basal cortisol levels after the stressor has been removed, can be detrimental (McEwen, 2007). Indeed, elevated cortisol levels are associated with a variety of SES-associated diseases and disorders, including Cushing’s disease, diabetes, metabolic syndrome, hypertension, depression and schizophrenia. In addition, chronic elevation of corticosterone can result in hippocampal neuronal loss and impaired cognitive function (McEwen and Sapolsky, 1995).
Release of glucocorticoids is terminated by a classic negative feedback loop, whereby the unbound form inhibits any further adrenocorticotrophic hormone (ACTH) release from the pituitary gland. This inhibition is further facilitated by glucocorticoid binding to specific brain regions rich in glucocorticoid receptors, such as the hippocampus and other structures of the limbic system. This cascade of events is under tight control in a healthy organism, but aberrations of this system may have pathophysiological consequences (McEwen, 2007). The current study sought to evaluate the potential involvement of altered glucocorticoid negative feedback as a mechanistic basis of the observed behavioral and neurochemical consequences of maternal Pb, prenatal stress, and the combination, by measuring the time course of corticosterone changes over 24h after a saline or dexamethasone injection stressor (DEX suppression test). DEX is a synthetic, high affinity glucocorticoid ligand that induces negative feedback by acting on the pituitary to suppress secretion of ACTH, and consequently, of corticosterone.
Methods and Materials
Pb Exposure
Three week old female Long Evans rats (Charles River, Germantown, NY) were randomly assigned to one of the following drinking solutions: 0 ppm (tap water), 50 or 150 ppm Pb acetate dissolved in distilled deionized water. Previous studies from our laboratory show 50 ppm to produce PbBs in our more recent studies of young adult males when exposure is initiated postweaning averaging 7–12 μg/dl (Brockel and Cory-Slechta, 1998; Cory-Slechta et al., 1998), just at and above the CDC’s currently designated level of concern for children. These PbBs are also associated with similar behavioral deficits in rodents and children (Cory-Slechta, 1995). The 150 ppm exposure was associated with mean PbBs in dams ranging from 32.6 ± 4.4 to 42.7 ± 4.0 μg/dl and were used because our previous study demonstrated synergistic effects of this Pb exposure level with stress (Cory-Slechta et al., 2004b).
Pb exposure of dams was initiated two months prior to breeding, and was continued throughout lactation to ensure elevated bone Pb levels to produce a Pb body burden in dams (Cory-Slechta et al., 1987), consistent with human exposure. When females reached 3 mos of age, they were paired with 3 mos old male Long-Evans rats for breeding over a period of up to 8 days (two estrous cycles). Animals were housed in a vivarium room with a 12h light-dark cycle (lights on at 0700h) that was maintained at 22 ± 5 °C. Standard rat chow diet was provided ad libitum. Pb exposure of the offspring of these dams ended at weaning. All procedures and treatments were in accord with NIH guidelines and were approved by the University of Rochester’s University Committee on Animal Resources.
Breeding and Prenatal Stress
At pro-estrus, as determined by vaginal smears, female rats were mated with males (2:1) across two estrous cycles. The presence of vaginal plugs or sperm in vaginal smears collected in the early morning indicative of pregnancy was considered gestational day 1 (GD1). Pregnant females in each Pb-treated group were weighed and further randomly subdivided to a non-stress (NS) or prenatal stress (PS) sub-group (see below) and individually housed for the remainder of pregnancy and lactation. This resulted in six groups of Pb-stress conditions with the following sample sizes: 0NS (n=20), 0PS (n=23), 50NS (n=21), 50PS (n=31), 150NS (n=19) and 150PS (n=33).
On gestational days 16 and 17, dams assigned to PS groups were weighed and subjected to three 45 min restraint sessions (0900, 1200 and 1500h) in plastic cylindrical devices using procedures modified from Ward and Weisz (Ward and Weisz, 1984). This procedure, or an even more protracted restraint stress, has been widely employed (Igosheva et al., 2007; Mairesse et al., 2007; Pallares et al., 2007; Baker et al., 2008; Darnaudery and Maccari, 2008). NS dams were weighed and subsequently left undisturbed in their home cages. The choice of days 16 and 17 was timed to correspond to the development of key brain regions associated with the endpoints under study. This protocol, as used in our previous study of Pb and stress, elevated corticosterone levels and altered catecholamine levels in frontal cortex and nucleus accumbens of dams (Cory-Slechta et al., 2004b). At the end of the last restraint session on GD16, blood was collected for corticosterone determinations. GD16 rather than GD17 was chosen for corticosterone determination to prevent potential habituation effects from obscuring any treatment-related differences.
At delivery (postnatal day 1: PND1), litter size was recorded and number of pups culled to 8 per litter, maintaining equal numbers of males and females wherever possible. Crossfostering was not performed, as the intent of the study was to model the human environment and culture. Pups were weaned at PND21, separated by gender, and housed in pairs for the duration of the experiment. Only one pup/gender was used from each litter for each outcome measure to preclude any litter-specific effects.
From weaning, pups were provided with unrestricted access to tap water (0 ppm) and food (Laboratory Rodent Diet 5001, POMI Foods Inc.) until male pups reached approximately 300 g and females 220 g. At this point, caloric intake was restricted to maintain the above-stated body weights. Animals were paired in same gender combinations for the duration of the experiment.
Experimental Procedures
At 5–6 months of age, blood was collected from a tail nick in littermates of these offspring from all NS and PS groups for determination of basal corticosterone levels. Littermates were used to avoid a potential handling of offspring to be used for injection stress challenge.
At 8 months of age, offspring from each group were randomly assigned to a group designated to receive a single i.p. injection stress challenge of dexamethasone phosphate (DEX; Sigma, St. Louis, MO) dissolved in physiological saline at a dose of 0 (vehicle), 100 or 150 μg/kg (males) or 0 (vehicle), 100 or 300 μg/kg (females). Because these animals had minimal handling during the 8 month period following birth, the associated handling and DEX injection served as a stressor, as in prior studies (Lukic and Haldar, 1993; Persico et al., 1993; Johnson and Glick, 1994; Persico et al., 1995). The differential dosing by gender and the time points for blood collection were based both on pilot studies carried out prior to this experiment in our laboratory and on a report of the higher dose levels required to suppress corticosterone in females (Osborn et al., 1996). Only one pup/gender was used from each litter for each DEX dose to preclude any litter-specific effects. The DEX injections began at approximately 8 a.m. and were followed approximately 15 min later by a tail phlebotomy designated as 0h time point. Additional tail phlebotomies were performed at 3 and 6h after DEX administration. At 24h post DEX administration, trunk blood was collected after decapitation without anesthesia. To prevent any additional stress, animals to be sacrificed were kept in a separate room until the time of decapitation. Serum was separated by centrifugation and stored at −20° C until assayed.
Changes in Fixed Interval schedule controlled behavior, response to stress challenges, basal and final corticosterone levels and neurochemical changes following the termination of behavioral testing in littermates of the offspring used in the current experiments have been reported previously (Virgolini et al., 2008).
PbB Determinations
PbB levels were measured by anodic stripping voltammetry according to methods described previously (Cory-Slechta et al., 1987; Cory-Slechta et al., 2004a) in dams (n=7–8 for each group) and pups (n=3–9) at the time of offspring weaning (PND21) from each treatment group. For each sample, 100 μl of blood was collected in heparinized microtubes and transferred to tubes containing pre-assembled reagents. The limit of sensitivity of the assay is 5 μg/dl. Pup PbBs decline after weaning following maternal Pb exposure, such that they are below detection limits by 40 days of age (Widzowski and Cory-Slechta, 1994). Since animals in this experiment were 8 mos of age at the beginning of the procedures, pup PbBs were not followed after weaning.
Radioimmunoassay
Serum corticosterone concentrations were measured by radioimmunoassay (RIA) kits from MP Biomedical (Irvine, CA) with I125 as a tracer. The minimal detectable level of corticosterone was 7.7 ng/ml.
Statistical Analyses
PbB Levels
Overall analysis of PbBs of dams and pups, from blood collected at the time of weaning, were analyzed using ANOVAs with Pb, stress and group (dam, male offspring, female offspring) as between group factors. Subsequent lower order ANOVAs, based on the outcome of the overall ANOVA, were done separately comparing male offspring to dams and female offspring to dams, as well as ANOVAs that included all groups but a single Pb exposure concentration.
Basal Corticosterone Levels
For comparisons of basal corticosterone levels, three way ANOVAs (Pb, stress, gender) were initially carried out, followed, for each gender, by lower order ANOVAs with appropriate post-hoc tests depending upon outcomes as initially reported (Virgolini et al., 2008).
Time Course of Corticosterone Changes
Given the differences in basal corticosterone (Figure 1) in relation to Pb (males, females) and PS (females), all corticosterone determinations were calculated as a percent of basal values, as specified below for each analysis to address specific effects as listed.
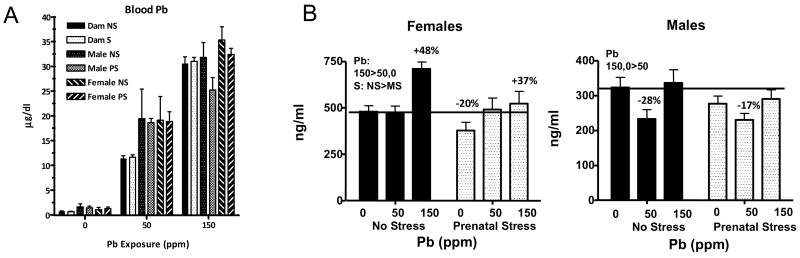
Figure 1.
Panel A: PbBs (μg/dl) of dams and male and female offspring in relation to Pb exposure concentration and stress condition (NS and PS). Each value represents a group mean ± S.E. Sample sizes ranged from 7–9 per treatment group in dams and from 2–8 in male offspring and 3–9 in female offspring (modified from (Virgolini et al., in press). Panel B: Basal corticosterone values (ng/ml) of littermates of rats used in the current study as reported previously (Virgolini et al., 2008). Each bar represents a group mean ± S.E. value for the groups as indicated. ❚ = no stress groups,  = prenatal stress groups. Pb= main effect of Pb; S=main effect of stress; PbxS=interaction of Pb and stress in statistical analyses. The horizontal line across values extends the 0NS control group mean values for comparative purposes. Sample sizes ranged from 8–11 for male and female groups.
= prenatal stress groups. Pb= main effect of Pb; S=main effect of stress; PbxS=interaction of Pb and stress in statistical analyses. The horizontal line across values extends the 0NS control group mean values for comparative purposes. Sample sizes ranged from 8–11 for male and female groups.
Effects of Pb Exposure on the Corticosterone Response Following Injection Stress
Effects of maternal Pb per se on time course of corticosterone changes following stress challenge were evaluated using data obtained following the 0 μg/kg DEX dose. Data were first calculated as percent of mean basal corticosterone levels (Figure 1) determined from littermates (Virgolini et al., 2008). For this purpose, values in each PbNS group at each time point were calculated as a percent of the basal PbNS values for each gender (i.e., 0NS values at 0, 3, 6 and 24h as a percent of the group mean 0NS basal value, 50NS values at 0, 3, 6 and 24h as a percent of the group mean 50NS basal value, etc.). An overall RMANOVA was then carried out with Pb and gender as between-group variables and time as a within-group variable. This analysis confirmed significant main effects of Pb and gender, as well as a Pb × gender and all time and Pb and gender interactions (including Pb × gender by time). RMANOVAs were then carried out separately for each gender. Significant interactions of Pb × time were confirmed for both genders. Subsequent analyses included lower order RMANOVAs for each Pb group or ANOVAs at each time point and post-hoc tests as appropriate.
Effects of PS on the Corticosterone Response Following Injection Stress
Effects of PS alone and in conjunction with Pb exposure on time course of corticosterone changes following injection stress were evaluated using data obtained following the 0 μg/kg DEX dose. Data were first calculated as percent of the corresponding group mean basal corticosterone levels determined from littermates (Figure 1; e.g., 50NS at 0, 3, 6 and 24h as a percent of the group mean 50NS basal value; 50PS at 0, 3, 6 and 24h as a percent of 50PS group mean basal value). An overall RMANOVA was then carried out that included Pb, gender and stress as between-group variables and time as a within-group variable. These confirmed significant main effects of Pb, stress, gender as well as lower and higher order interactions that included Pb × stress × gender × time. Subsequent RMANOVAs were then carried out separately for each gender that included Pb and stress as between-group variables and time as a within-group variable. These resulted in significant main effects of Pb and stress for both genders, and a Pb × stress interaction in females. Subsequently, lower order RMANOVAs were carried out for each Pb group with subsequent ANOVAs and post hoc tests as appropriate.
Effects of Pb and PS on Corticosterone Levels Following DEX Administration
Two approaches were used to evaluate the efficacy of DEX to suppress corticosterone following Pb, PS and combined Pb+PS. First, an overall RMANOVA was carried out using ng/ml corticosterone values that included Pb, stress, gender, and DEX as between-group variables and time as a within-group variable. Absolute ng/ml corticosterone values were used for these analyses as they focused on changes resulting from DEX doses relative to saline vehicle. Based on the interactions confirmed by that analysis, RMANOVAs that included Pb, stress and DEX as between-group variables and time as a within-group variable were carried out separately for each gender. Based on the confirmed interactions, lower order RMANOVAs were then carried out for each Pb-stress group separately with post-hoc tests as appropriate.
An additional analysis was run to compare the percent changes at each DEX dose in Pb- PS groups to the corresponding percent change observed in the 0NS control group at each DEX dose/time point. This allowed an assessment of how Pb, PS and Pb+PS altered the efficacy of DEX in relation to its effects in 0NS controls. For this purpose data for each DEX dose/time point were first calculated as a percent of the corresponding Pb-stress 0 μg/kg DEX value (e.g., 50NS 100 μg/kg at 0h was calculated as a percent of 50NS 0 μg/kg 0h; 150PS 100 μg/kg at 3h as a percent of 150PS 0 μg/kg at 3h) to provide a percent change from 0 μg/kg vehicle-associated corticosterone levels for each Pb-stress group. Post-hoc tests were then used to compare the resulting percent changes at a given DEX dose in the 50 and 150 ppm NS and PS groups and the 0PS group to the corresponding 0NS DEX dose change (e.g., percent change at 50NS, 50PS, 150NS and 150PS 100 μg/kg values to percent change at 0NS 100 μg/kg). These analyses were done separately for each time point (0, 3, 6 and 24h) and provided a statistical comparison of DEX efficacy across experimental groups.
Potentiated effects were defined as an effect in a Pb+PS group in the absence of significant effects in the corresponding PbNS and 0PS groups alone. Synergistic effects were defined as an effect level in a Pb+PS group that was greater than that of the corresponding PbNS and 0PS groups. A p value of ≤ 0.05 was considered statistically significant for all analyses.
Results
Pb-related Increases in PbB Levels
PbB levels of dams and offspring measured at the time of pup weaning (n=7–9 dams per group and 3–9 offspring per group) increased in a concentration-related fashion, as previously reported (Virgolini et al., 2008; Virgolini et al., in press) are shown in Figure 1A. Group mean PbBs (μg/dl) of all groups increased with increasing Pb exposure concentration. Overall ANOVA revealed significant main effects of Pb and group (both p values <0.0001) as well as a group × Pb interaction (p<0.0001), but no main effects or interactions that included stress. Subsequent lower-order ANOVAs confirmed that these effects were due to significantly higher PbBs of male and female offspring at 50 ppm, where levels of dams were approximately 12 μg/dl and those of pups approximately 19 μg/dl. At 150 ppm, PbBs of neither males nor female pups differed from offspring, but those of female offspring were higher than those of male offspring, an effect largely due to the slightly lower PbBs of the 150PS males relative to other groups, although there was no significant interaction with stress. Our prior studies demonstrate that pup PbBs decline after weaning following maternal Pb exposure, such that they are below detection limits by 40 days of age (Widzowski et al., 1994).
Lack of Pb or Stress Effects on Litter Parameters
No differences in numbers of pregnancies or length of pregnancies were found in relation to Pb and/or PS. Neither litter size nor sex ratios differed in relation to Pb, PS or combined Pb + PS. Given the numbers of litters generated, only a subset of litters, randomly chosen across treatment conditions, was systematically weighed. No differences in body weights were detected (Virgolini et al., 2008).
Basal Corticosterone Levels
Basal corticosterone levels, used to evaluate responses to injection stress in the current study, were determined in littermates of these offspring, as previously reported (Virgolini et al., 2008). Given their use in calculation of percent changes here, basal values are presented in Figure 1B. ANOVAs confirmed a main effect of Pb in females, with 150 ppm > than either 50 or 0 ppm. This outcome reflects the 48% and 37% increases in the 150NS and 150PS groups, respectively relative to their 0 ppm counterparts. In addition, a main effect of PS derived from the lower corticosterone levels in PS offspring (collapsed across Pb groups). Values of the 0PS females were approximately 20% lower than those of 0NS females.
In males, an inverse U-shaped Pb concentration effect curve was found, with 50 ppm corticosterone levels reduced by approximately 20–30% as compared to 0 and 150 ppm levels, with the latter two not differing. These effects were observed in both NS and PS groups. No effects of PS or interaction of Pb × PS was noted in males.
Maternal Pb Exposure Prolongs the Corticosterone Response to Vehicle Injection Stress in a Non-Linear Manner in Both Genders
To evaluate the time course of corticosterone changes following a stressor as a function of maternal Pb and PS per se, data from the 0 μg/kg DEX dose were used, as hereafter referred to as the injection stressor, with results of those analyses presented in Figure 2 for females and males as percent of basal corticosterone levels.
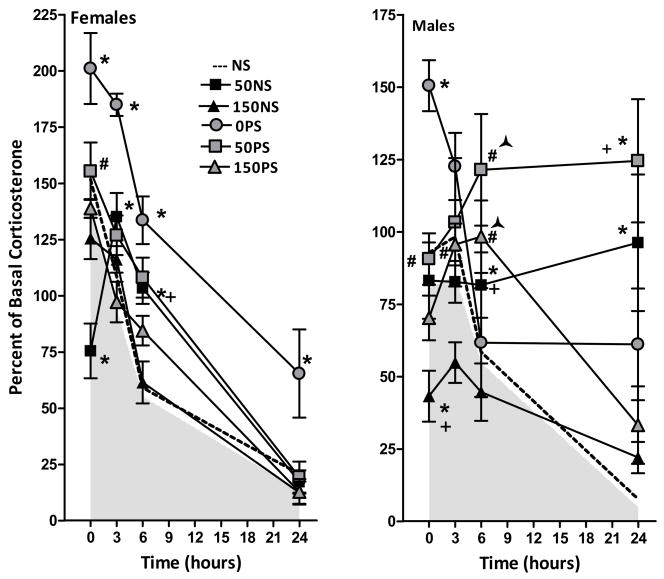
Figure 2.
Corticosterone levels (percent of basal levels) at 0, 3, 6 and 24h post injection stress in females (left panel) and males (right panel) treated with 0, 50 or 150 ppm Pb exposure alone (NS, no stress) or with prenatal stress (PS). Each data point represents a group mean ± S.E. value. Sample sizes were n= 7–8 for female groups and n=8 for male groups. The shaded area in each plot represents the area under the mean values, such that the upper boundary is group mean values; the dashed line indicates the upper standard error value. * indicates difference from corresponding 0 ppm value; + indicates difference from corresponding 150NS value; # indicates a difference from corresponding PbNS value.
In control (0NS) females (F(6,63)=8.86, p<0.0001), corticosterone levels were increased by approximately 35% at 0 hr relative to basal values, and declined thereafter over 24 hr. The 50 ppm Pb exposure, but not 150 ppm, significantly prolonged the time course of corticosterone reduction. Although 50 ppm initially reduced corticosterone levels by 25%, to values that differed significantly from 0 and 150 ppm groups, levels subsequently increased above basal values at 3 and 6h, before declining to 0 ppm levels at 24h. Thus 50 ppm levels were significantly higher than 0 ppm values at 3h and higher than 0 and 150 ppm values at 6h, representing increases of 52% and 84%, respectively in absolute corticosterone levels. Corticosterone changes of the 150 ppm group did not differ from 0 ppm levels at any time point.
A more pronounced effect of 50 ppm Pb was also found in males (Pb × time [F(6,63) = 4.29, p = .0011]. A time-related reduction over 24h was seen in 0NS males without any initial increase above basal levels. In 50NS males, however, virtually no decline in corticosterone occurred over the 24h period. At 24h, absolute 50 ppm corticosterone values were elevated 14- fold relative to 0NS values. Initial reductions in corticosterone levels were observed in 150NS males to levels significantly below 0NS and 50NS values, but values were thereafter comparable to 0NS values at all subsequent time points.
Prenatal Stress Also Prolongs the Corticosterone Response to Vehicle Injection Stress and Enhances Maternal Pb Effects in Males but Not Females
In 0 ppm females, PS produced a significant right-shift of the corticosterone time course curve (main effect of Pb(1,14)=41.54, p<0.0001) due to initially higher corticosterone levels (approximately 60%) at 0 hr, but the subsequent rate of decline in corticosterone was similar in 0NS and 0PS females. For that reason, corticosterone levels remained higher (by almost 4-fold in absolute values) in 0PS females even at 24 hrs. In 50 ppm females, PS increased corticosterone levels at 0 hr, but resulted in reductions equivalent to those seen with 50NS thereafter. Corticosterone levels of 150NS and 150 PS females were similar at all time points.
In males, (main effect of Pb (F(2,42)=16.2, p<0.0001) and of stress (F(1,42)=33.4, p<0.0001) and an interaction of Pb × time (F(6,126)=7.82, P<0.0001), PS significantly increased corticosterone levels across all Pb groups (p=0.001, 0.0498 and 0.0009 for the 0, 50 and 150 ppm groups, respectively). Further, when collapsed across NS and PS conditions, higher corticosterone levels were evident at 50 ppm as compared to either 0 or 150 ppm, and at 0 ppm relative to 150 ppm.
Summarized across Pb and PS, Figure 2 shows that in females, the greatest prolongation of corticosterone reduction post injection stress occurred in 0PS females, with smaller reductions in both the 50NS and 50PS groups, indicating that the increase in the 50NS group is not further enhanced by PS. No changes relative to control were found in 150NS or 150PS groups. In males, delays in corticosterone reduction are apparent for all but the 150NS group, and are particularly striking in the 50PS and 50NS groups. Moreover, evidence of synergistic effects were evident at 50 ppm at 6h, where 50PS levels were elevated by approximately 75%, while 50NS values were only 15% greater than 0NS, and 0PS values did not differ from controls. Potentiated effects were noted at 150 ppm at 6h, with 150PS values increased approximately 40% above 0NS controls, while neither the values of the 0PS or the 150 NS groups differed from controls.
Maternal Pb and Combined Maternal Pb and Prenatal Stress Initially Reduce DEX Efficacy in Both Genders, Then Markedly Prolong Corticosterone Suppression in Males
Dose–effect curves showing changes in corticosterone levels (ng/ml) over the 24h period following DEX administration are shown for each Pb-stress group in Figure 3 for females and Figure 4 for males.
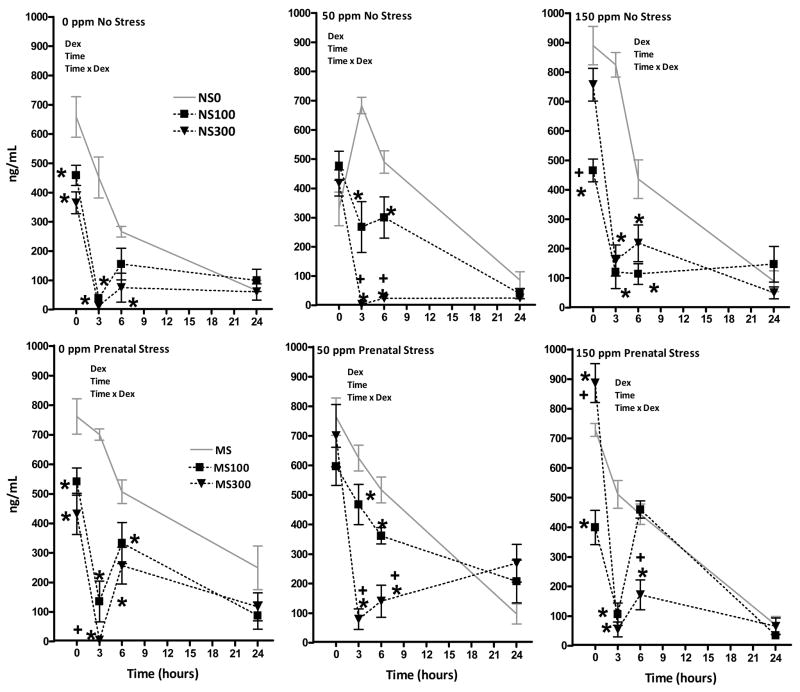
Figure 3.
Corticosterone levels (ng/ml) of female rats at 0, 3, 6 and 24h post administration of 0, 100 or 300 μg/kg DEX in Pb-treated non-stressed (NS; top row) and prenatally-stressed groups (PS; bottom row). Each data point represents a group mean ± S.E. value. Sample sizes were n=7– 8. * indicates difference from corresponding 0 ppm value; + indicates difference from corresponding 50 ppm value. Dex = significant main effect of DEX; time = significant main effect of time; and Dex × time = indicates significant interaction of Dex × time in corresponding RMANOVA.
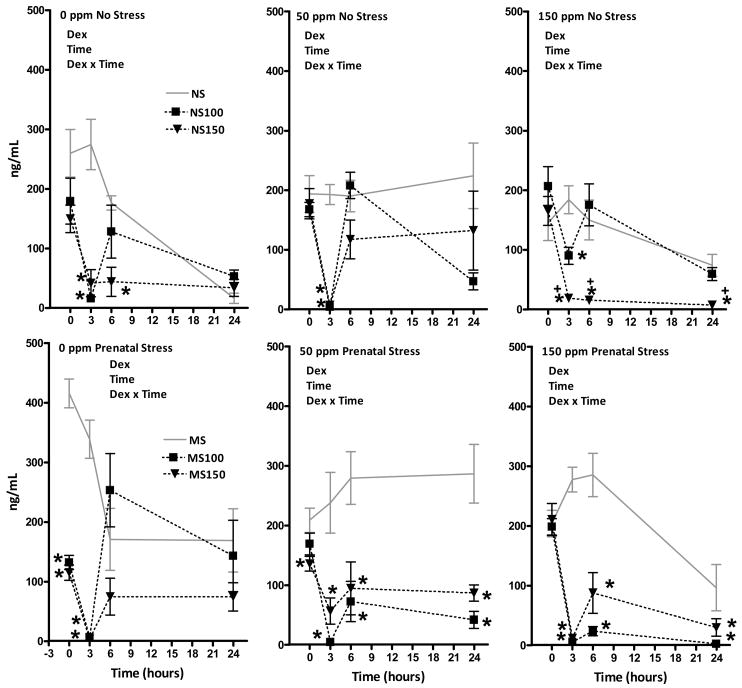
Figure 4.
Corticosterone levels (ng/ml) of male rats at 0, 3, 6 and 24h post administration of 0, 100 or 300 μg/kg DEX in Pb-treated non-stressed (NS; top row) and prenatally stressed groups (PS; bottom row). Each data point represents a group mean ± S.E. value. Sample sizes were n=8. * indicates difference from corresponding 0 ppm value; + indicates difference from corresponding 50 ppm value. Dex = significant main effect of DEX; time = significant main effect of time; and Dex × time = indicates significant interaction of Dex × time in corresponding RMANOVA.
In females, RMANOVAs confirmed significant main effects of DEX and time, as well as DEX × time interactions in all Pb-stress groups (0NS: F(6,63) = 6.84, p<0.0001; 50NS: 19.26, p<0.0001, 150NS: 13.7, p<0.0001; 0PS: 7.16, p<0.0001; 50PS: 9.13, p<0.0001; 150PS: 25.8, p<0.0001. In 0NS females (top left), similar reductions in corticosterone were produced by both 100 and 300 μg/kg DEX. These reductions were evident at 0h, maximal at 3h, began to recover at 6h, and were equivalent to vehicle dose levels at 24h. Dose-effects were evident only at the 6h time point, where significant corticosterone suppression was still evident at 300 μg/kg, but not at 100 μg/kg DEX. Highly similar outcomes were observed in the 0PS group, although the 100 ug/kg DEX dose retained its efficacy at 6h in this group. In both the 50NS and 50PS groups, the onset of corticosterone reduction following DEX was delayed to 3 hr. Unlike the changes seen at 0 ppm, dose-dependent effects were seen in both the 50NS and 50PS groups at 3 and 6h. In both the 150NS and 150 PS groups, the 300 μg/kg, but not the 100 μg/kg DEX dose was associated with a delayed suppression of corticosterone, consistent with an inverse U-shaped concentration effect curve for Pb at 0 hr. Maximal suppression occurred at 3h where the two doses were equally effective. By 6h, 100 μg/kg DEX was no longer effective in 150PS females, whereas it continued to suppress corticosterone at levels comparable to 300 μg/kg DEX in 150NS females. No residual suppression was apparent by 24h.
Significant main effects of DEX and of time, as well as significant DEX × time interactions were also observed in all male Pb-stress groups (Figure 4; 0NS: F(6,63) = 6.3, p<0.0001; 50NS: 3.94, p=0.002, 150NS: 6.23, p<0.0001; 0PS: 7.7, p<0.0001; 50PS: 2.38, p=0.039; 150PS: 11.51, p<0.0001). In 0NS males (top left), both the 100 μg/kg and the 150 μg/kg DEX doses reduced corticosterone levels beginning at 3h. At 6h, only the higher dose still suppressed corticosterone. At 24h, all groups displayed equivalent corticosterone levels. In 0PS males, significant corticosterone suppression occurred at 0h and was maximal at 3h; recovery was evident in both doses by 6h. No dose-related differences in potency were observed in 0PS males. DEX suppression of corticosterone was limited to the 3h time point in 50NS males, where the two doses produced a comparable and virtually complete suppression. In 50PS males, both DEX doses equally reduced corticosterone levels beginning at 3h and persisting across the entire 24h period. At the final time point, reductions of 86 and 70% were still present at the 100 μg/kg and 150 μg/kg doses, respectively. In 150NS males, dose-related suppression of corticosterone was not observed until the 3h time point, at which time dose-related differences were observed. At the 150 μg/kg dose, reductions were sustained through the 24h measurement period. DEX effects were particularly notable in 150PS males, where significant reductions of corticosterone levels were present at both doses from 3–24h, with reductions of 30–98% still evident at 24h.
To provide a comparison of the magnitude of DEX effects in relation to treatment, data were first calculated as a percent of corresponding Pb-stress 0 μg/kg group/time point values (e.g., 50NS 100 μg/kg and 50NS 300 μg/kg at 3h as a percent of 50NS 0 μg/kg at 3h; 150PS 100 μg/kg and 150PS 300 μg/kg at 0h as a percent of 150 PS 0 μg/kg at 0h, etc) based on the assumption that the 0 μg/kg data at each time point represented the ‘normal’ response to the injection stress. Post hoc tests were then carried out comparing the magnitude of changes at each dose/time point in the Pb-PS conditions to that of the 0NS group, e.g., comparisons of 50NS 100 μg/kg and 150NS 100 μg/kg at 0h to 0NS 100 μg/kg effect at 0h, etc., to provide comparison of percent DEX changes in 50 and 150 ppm NS and PS groups and the 0PS group in relation to percent changes in the 0NS control group. Results are shown in Figure 5 and summarized across all conditions in Table 1.
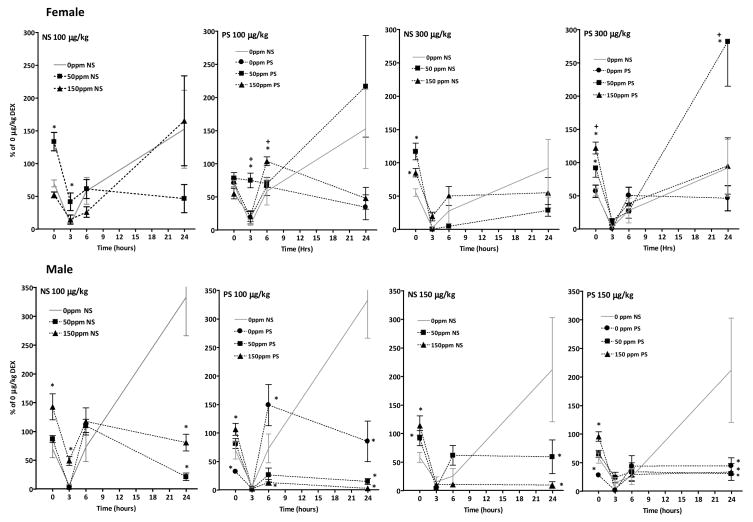
Figure 5.
Corticosterone levels as a percent of corresponding Pb-stress 0 μg/kg group/time point values (e.g., 50NS 100 μg/kg and 50NS 300 μg/kg as a percent of 50NS 0 μg/kg; 150PS 100 μg/kg and 150PS 300 μg/kg as a percent of 150 PS 0 μg/kg) of female (top row) and male (bottom row) offspring exposed to no stress (NS, left column) or prenatal stress (PS, right column) in response to DEX administration (100 or 300 μg/kg for females and 100 or 150 μg/kg for males). Sample sizes as defined above. * indicates difference from corresponding 0NS DEX dose/time point value.
Table 1.
Summary of Differences in DEX Efficacy in Pb and PS Groups Relative to DEX effects at the Corresponding 0NS Dose and Time Point control.
| Group | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 hr | 3 hr | 6 hr | 24 hr | 0 hr | 3 hr | 6 hr | 24 hr | |
| 0PS1001 | ↑ | ↓ | ↑ | |||||
| 0PS300/1502 | ↑ | ↑ | ||||||
| 50NS1001 | ↓3 | ↓ | ↑4 | |||||
| 150NS1001 | ↑ | ↓ | ↓ | ↑ | ||||
| 50NS300/1502 | ↓ | ↓ | ↑ | |||||
| 150NS300/1502 | ↓ | ↓ | ↑ | |||||
| 50PS1001 | ↓+ | ↑ | ||||||
| 150PS1001 | ↓+ | ↓ | ↓ | ↑ | ||||
| 50PS300/1502 | ↓ | ↓+ | ↑ | |||||
| 150PS300/1502 | ↓+ | ↓ | ↑ | |||||
compared to 0NS 100 μg/kg DEX dose as percent change
compared to 0NS 300/150 (female/male) μg/kg DEX dose as percent change
Indicates reduced efficacy relative to 0NS DEX dose
Indicates greater efficacy relative to 0NS DEX dose
Indicates effects greater than corresponding NS group
These analyses demonstrated examples of significantly reduced DEX efficacy in both genders over the first 6h post-administration. For females, this was found at 0 hr for the 100 μg/kg dose in the 50NS group, and for the 300 μg/kg dose in the 50NS, 150NS, 50PS and 150PS groups, with the magnitude of effect in the 150PS group greater than that in the 150NS group, indicative of enhancement by PS. Reductions in DEX efficacy at 3h were seen at the 100 μg/kg dose in both the 50NS and 50PS groups, with significantly greater magnitude of effect in the 50PS group, and at 6 hr in the 150PS group whereas no effect was seen in the 150NS group, again indicating PS enhancement. The 50PS group showed a markedly greater rebound following 300 μg/kg than did the 0PS group at 24h, and no such reduction was noted in the 50NS group.
In males, significant reductions in DEX suppression at 0h were observed at the 100 μg/kg DEX dose in the 150NS and 150PS groups, and at the 150 μg/kg dose in the 50NS, 150NS and 150PS groups. Reduced efficacy was also found at 3 hr for the 100 μg/kg dose in the 150NS group. 0PS males, by contrast, showed enhanced DEX suppression at both the 100 μg/kg and 150 μg/kg doses at 0h, and reduced suppression at the 100 μg/kg dose at 6h. By 24h, however, DEX potency was found to be significantly greater in all 50 and 150 ppm NS and PS groups than in corresponding 0NS controls as well as in the 0PS groups. At this time point, corticosterone values of the 0NS males dosed with 100 μg/kg and 150 μg/kg DEX had rebounded to mean levels of approximately 350% and 200% of the 0NS 0 μg/kg value. In contrast, corresponding values of the Pb-stress groups ranged from only 2–90% of the 0NS 0 μg/kg values.
Discussion
These studies demonstrate that both maternal Pb and PS alone delay corticosterone reduction following an injection stress challenge, with non-linear changes observed with Pb that were more pronounced at 50 ppm than at 150 ppm in both genders. This non-linearity was particularly striking in males, where PS further enhanced the effects of maternal Pb. In accord with these findings, the efficacy of DEX to suppress corticosterone was attenuated by Pb during the first 6h post-administration, effects showing evidence of enhancement by PS in females. However, DEX suppression was also markedly prolonged by both Pb and PS, although not additively, in males. Collectively, these findings demonstrate a hypo-responsive HPA axis, and consequently hypercortisolism, in males and females, with evidence of later HPA hyperresponsiveness and hypocortisolism in males, findings that may explain the corresponding disease profiles associated with Pb and elevated stress/cortisol levels.
PS Diminishes Glucocorticoid Negative Feedback in Females and Males
Both maternal Pb and PS alone prolonged the reduction in corticosterone following injection stress. Injection stress challenge increased corticosterone levels to a greater extent in 0PS males and females than in 0NS counterparts, and these groups also sustained higher corticosterone levels over the subsequent 24 hr measurement period (Figure 2). For 0PS males in particular, the decline in corticosterone was virtually negligible between 6 and 24 hr. These findings replicate previous reports of delayed reduction of corticosterone following stress challenge in PS offspring (e.g., (Szuran et al., 2000; Maccari et al., 2003; Darnaudery and Maccari, 2008). The current study, however, extends those findings, demonstrating a protracted duration of the effect, lasting at least 24h in males.
Maternal Pb Also Diminishes Negative Glucocorticoid Feedback in Both Genders, but Produces an Inverse U-Shaped Concentration Effect Function
Pb exposure also prolonged the post-injection stress reduction in corticosterone over 24h (Figure 2), but did so in a non-monotonic fashion in both genders, with a more pronounced impact at 50 than 150 ppm exposure. HPA hypo-responsiveness was particularly striking in 50NS males, where virtually no reduction in corticosterone occurred at any time point over 24h. While our prior studies have revealed preferential vulnerability of females to behavioral consequences of Pb and stress (Virgolini et al., 2008), the current data suggest a greater impact of Pb and PS and the combination on HPA negative feedback in males.
The greater impact in males is interesting in light of the report that PS actually produces greater in utero corticosterone levels in females than males (Montano et al., 1993), and points to the likelihood of different operative mechanisms by gender. As also appears to be the case for PS (Darnaudery and Maccari, 2008), the HPA axis dysfunction produced by maternal Pb apparently represents a permanent change, since offspring were already 8 mos of age when experiments were initiated and occurred following exposures of dams associated at weaning with PbBs averaging only 11 μg/dl, i.e., just above the CDC’s currently designated level of concern for children.
The non-monotonic dose-effect curve for Pb is also particularly notable, given accumulating evidence of greater effects of Pb on IQ in children at lower as compared to higher PbBs (Canfield et al., 2003). Additionally, these findings add to an accumulating literature reporting non-linearity of Pb effects for such varied endpoints as locomotor activity, cerebral cortex growth, operant behavior, maze performance, age at vaginal opening, serum urea and urate and body weight and height (Davis and Svendsgaard, 1990; Leasure et al., 2008). Moreover, inverse U-shaped concentration curves were noted in male offspring for basal corticosterone as well (Figure 1; (Virgolini et al., 2008). The mechanism(s) underlying this nonlinearity remain unknown as of yet, but the U-shaped curves point to different operative mechanisms at differing levels of exposure, e.g., engagement of homeostatic mechanisms at lower levels that may be overwhelmed at higher exposure levels.
Pb-Associated Reduction of Glucocorticoid Negative Feedback was Enhanced by PS in Males, but Attenuated in Females
Corticosterone levels were further increased when Pb was combined with PS in males at both 50 and 150 ppm (Figure 2). Indeed, 50PS male offspring had the highest corticosterone levels of all male groups following vehicle injection; and at 6h, corticosterone levels of the 50PS and 150PS groups were significantly higher than their PbNS counterparts, consistent with enhanced effects of Pb+PS. In contrast, Pb generally appeared to reduce the effects of PS in females, given the smaller increases in corticosterone in the 50NS and 50PS groups, and the lack of any effects in the 150NS and 150PS groups relative to 0NS. It is not currently possible to ascertain the mechanism(s) underlying these gender differences. At the very least, they suggest that Pb effects predominate for glucocorticoid negative feedback in females. Therefore, if Pb and PS act by similar or overlapping mechanisms in females, then the peak magnitude of effect that occurs is defined by the Pb concentration effect function. In the case of males, it is conceivable that Pb and PS act through different mechanisms, both of which ultimately block glucocorticoid feedback and thereby additively prevent reductions in corticosterone. Surprisingly, the impact of Pb exposure on different components of HPA axis function is virtually unexplored, with the single exception of an in vitro investigation reporting that Pb exposure reduced ACTH-induced steroid production with a site of action likely at the plasma membrane (Nishiyama et al., 1985) and early reports of corticosterone changes (Vyskocil et al., 1991a; Vyskocil et al., 1991b; Vyskocil et al., 1991c). Clearly, additional experiments will be required to determine associated mechanisms and the impact of Pb on the different components of the HPA axis, but the current findings clearly caution against any generalizations across genders.
Pb Exposure Initially Diminished DEX Suppression of Corticosterone in Both Genders with Some PS Enhancement Observed in Females
HPA axis hypo-responsivity associated with Pb and PS was concordant with the diminished efficacy of DEX-induced suppression of corticosterone in Pb and Pb+PS treated offspring observed initially following DEX injection (Figures 3–5). Direct comparisons of the magnitude of suppression produced by DEX doses across Pb-stress groups relative to 0NS controls (Figure 5 and Table 1) confirmed a reduced efficacy of DEX ranging from 15–65% in females and 34–73% in males during the first 6h post DEX administration in Pb and Pb+PS groups of both genders, with Pb effects further enhanced by PS in females.
Altered pharmacokinetics or pharmacodynamics of DEX in response to Pb, PS or Pb+PS is one potential basis for this attenuation, but cannot be directly ascertained in the absence of direct measures of tissue DEX levels. But that potential mechanism may be hard to disentangle from the alternative, but related explanation, that the attenuated DEX efficacy is a function of the higher corticosterone values seen in Pb and Pb+PS groups following stress challenge, since hypercortisolemia itself can actually decrease DEX half-life through the induction of hepatic enzymes by circulating corticosterone levels (Stokes et al., 2002). Such a possibility would be consistent with the higher corticosterone levels seen with PS and Pb, and the fact that the most consistent attenuation of DEX efficacy was observed at the first time point of measurement (Table 1). However, that explanation is inconsistent with our observations that despite the pronounced increases in corticosterone levels in 0PS females at all time points (Figures 3 and 4), no corresponding attenuation of DEX efficacy was found in this group. Moreover, attenuated DEX efficacy was found in 150NS and 150PS females, despite minimal evidence of increased corticosterone levels in these groups. Finally, at 24h, as noted above, enhanced, rather than attenuated DEX suppression was observed in all male groups relative to the 0NS controls.
Other potential mechanisms include increases in ACTH secretion and/or in corticotropin releasing hormone, vasopressin and/or oxcytocin that could indirectly lead to increased cortisol release (Cole et al., 2000), or indirect effects mediated through alterations in CNS glucocorticoid receptor function. DEX administration effectively produces a hypocortisolism in brain, and prior studies have described reductions in glucocorticoid receptors in response to PS (Koehl et al., 1999; Szuran et al., 2000; Maccari et al., 2003). In addition, mice under-expressing glucocorticoid receptors sustain higher corticosterone levels in DEX suppression tests (Ridder et al., 2005).
Both Maternal Pb and PS Markedly Prolong the Effects of DEX on Corticosterone Suppression in Males but Not Females
DEX was associated with reduction of corticosterone even out to 24h in males, whereas females showed full recovery by this time, notable findings given that male rats actually show more rapid DEX clearance than females (Lamiable et al., 1991). Nevertheless, male rats do appear to be more sensitive than females to DEX. A previous study noted a requirement for higher DEX doses to suppress corticosterone in females than in males (Osborn et al., 1996), an observation that served as the rationale for the use of a 300 μg/kg DEX dose for females and a 150 μg/kg DEX dose for males in this study; these did appear to produce comparable levels of suppression here, with the exception of the final 24h point, when males exhibited a greater rebound effect relative to basal levels than females. It may also be the case that maternal Pb/PS differentially influences corticosterone synthesis in males vs. females. Gender differences in corticosterone synthesis and metabolism have been long known (Glenister and Yates, 1961; Kitay, 1961; Gala and Westphal, 1965). Rats in these experiments were also food restricted to equate conditions with those of littermates undergoing behavioral testing, and the current experiments did not explicitly control for phase of the estrous cycle, parameters that can also affect corticosterone levels (Anderson et al., 1996; Stamp et al., 2008).
HPA Alterations as a Biological Unifying Mechanism for Commonalities in Pb- and PS- Associated Diseases and Disorders
The hypo-responsivity of the HPA axis shown here is consistent with hypercortisolism, which, if occurring in humans exposed to Pb, would be expected to have significant public health implications, since increased cortisol levels have been implicated in numerous disease states, including Type 2 diabetes (Taniguchi et al., 2008), depression (Carroll et al., 2007), obesity (Dallman et al., 2003) and schizophrenia (Gallagher et al., 2007). Cushing syndrome, a hormonal disorder characterized by prolonged exposure to high levels of cortisol, is characterized by upper body obesity, weakened bones, hypertension (metabolic syndrome) and anxiety and depression. It is notable that Pb exposure has indeed been linked to obesity, hypertension, diabetes, anxiety, schizophrenia and depression in epidemiological studies (Kim et al., 1995; Cheng et al., 2001; Gerr et al., 2002; Rhodes et al., 2003; Opler et al., 2004; Menke et al., 2006; Rajan et al., 2007). In male Pb and PS offspring, a delayed hyper-responsive HPA axis was also found. Hypocortisolism likewise has adverse health consequences. Chronic primary adrenal insufficiency in Addison’s disease, for example, is associated with malaise, anorexia, diarrhea, weight loss and joint and back pain (Nieman and Chanco Turner, 2006).
Our experimental studies demonstrating that Pb, PS and combined Pb and PS produce permanent, sometimes additive HPA axis dysfunction may, therefore, provide a biologically plausible unifying mechanism for the overlapping disease profiles associated with Pb and with stress in humans. Moreover, while current screening programs for elevated Pb burden focus on children, the fact that the permanent HPA axis effects arose from maternal only Pb exposure in this study suggests the need to screen pregnant women, particularly those with high-stress lifestyles. Indeed, PbBs measured in children are not necessarily reflective of the greater exposure of the fetus prenatally, given the increases in PbB during pregnancy (Rothenberg et al., 1994; Hertz-Picciotto et al., 2000; Manton et al., 2003; Gulson et al., 2004), exposures which have been linked more strongly than postnatal PbBs to poorer infant mental development (Hu et al., 2006). Similar increases during pregnancy are seen in rats (Dearth et al., 2002; Dearth et al., 2004) which may in part explain the increased PbBs of the 50 ppm offspring at weaning, although the lack of a similar effect in 150 ppm offspring suggests the potential for additional operative mechanism. Results from such studies may have particular significance for understanding the true health risks posed by Pb for human populations, especially since the cycles of poverty and elevated Pb are so congruous.
Acknowledgments
Supported by NIH ES05017 to D. Cory-Slechta. The authors declare no financial conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosom Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Saviolakis GA, Bauman RA, Chu KY, Ghosh S, Krant GJ. Effects of chronic stress on food acquisition, plasma hormones, and the estrous cycle of female rats. Physiol Behav. 1996;60:325–329. doi: 10.1016/0031-9384(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Brockel BJ, Cory-Slechta DA. Lead, attention, and impulsive behavior: Changes in a fixed-ratio waiting-for-reward paradigm. Pharmacol Biochem Behav. 1998;60:545–552. doi: 10.1016/s0091-3057(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 ug per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand Suppl. 2007:90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. American journal of epidemiology. 2001;153:164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic medicine. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annual review of pharmacology and toxicology. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates effects of developmental lead exposure. Environ Health Perspect. 2004a;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004b;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Mobilization and redistribution of lead over the course of calcium disodium ethylenediamine tetracetate chelation therapy. J Pharmacol Exp Ther. 1987;243:804–813. [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain research reviews. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Davis JM, Svendsgaard DJ. U-shaped dose-response curve-shaped doseresponse curves: Their occurrence and implications for risk assessment. J Toxicol Environ Health. 1990;30:71–83. doi: 10.1080/15287399009531412. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL. Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reproductive toxicology (Elmsford, NY) 2002;16:343–352. doi: 10.1016/s0890-6238(02)00037-0. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Srivastava V, Les Dees W, Bratton GR. Low level lead (Pb) exposure during gestation and lactation: assessment of effects on pubertal development in Fisher 344 and Sprague-Dawley female rats. Life Sci. 2004;74:1139–1148. doi: 10.1016/j.lfs.2003.07.033. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Social status and stressful life events. J Pers Soc Psychol. 1973;28:225–235. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Gala RR, Westphal U. Corticosteroid-binding globulin in the rat: studies on the sex difference. Endocrinology. 1965;77:841–851. doi: 10.1210/endo-77-5-841. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophr Res. 2007;90:258–265. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Gerr F, Letz R, Stokes L, Chettle D, McNeill F, Kaye W. Association between bone lead concentration and blood pressure among young adults. American journal of industrial medicine. 2002;42:98–106. doi: 10.1002/ajim.10096. [DOI] [PubMed] [Google Scholar]
- Glenister DW, Yates FE. Sex difference in the rate of disappearance of corticosterone-4-C14 from plasma of intact rats: further evidence for the influence of hepatic Delta4-steroid hydrogenase activity on adrenal cortical function. Endocrinology. 1961;68:747–758. doi: 10.1210/endo-68-5-747. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Palmer JM, Korsch MJ, Taylor AJ, Mahaffey KR. Blood lead changes during pregnancy and postpartum with calcium supplementation. Environ Health Perspect. 2004;112:1499–1507. doi: 10.1289/ehp.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Parsons PJ, Granger DA. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ Health Perspect. 2008;116:249–255. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley VB, Talbot TO. Geographic analysis of blood lead levels in New York State children born 1994-1997. Environ Health Perspect. 2004;112:1577–1582. doi: 10.1289/ehp.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and determinants of blood lead during pregnancy. American journal of epidemiology. 2000;152:829–837. doi: 10.1093/aje/152.9.829. [DOI] [PubMed] [Google Scholar]
- Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, Schwartz J, Schnaas L, Mercado-Garcia A, Hernandez-Avila M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igosheva N, Taylor PD, Poston L, Glover V. Prenatal stress in the rat results in increased blood pressure responsiveness to stress and enhanced arterial reactivity to neuropeptide Y in adulthood. J Physiol. 2007;582:665–674. doi: 10.1113/jphysiol.2007.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Glick SD. Handling and/or saline injections alter basal and morphine-evoked changes in dopamine metabolites in the striatum and nucleus accumbens of rats. Pharmacology, biochemistry, and behavior. 1994;47:765–768. doi: 10.1016/0091-3057(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Kemper AR, Cohn LM, Fant KE, Dombkowski KJ, Hudson SR. Follow-up testing among children with elevated screening blood lead levels. Jama. 2005;293:2232–2237. doi: 10.1001/jama.293.18.2232. [DOI] [PubMed] [Google Scholar]
- Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ Health Perspect. 1995;103:952–957. doi: 10.1289/ehp.95103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Lamiable D, Vistelle R, Fay R, Bensussan B, Millart H, Wiczewski M, Choisy H. Influence of sex and oestrogen replacement on the disposition of dexamethasone in rats. Fundam Clin Pharmacol. 1991;5:733–740. doi: 10.1111/j.1472-8206.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy M, Gault L, Yammamato B. Adrenalectomy attenuates stress induced elevation in extracellular glutamate concentration in hippocampus. J Neurosci. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Lukic D, Haldar J. Isotonic and hypertonic saline act as stressful stimuli for oxytocinergic system of the pituitary, hypothalamus and spinal cord. Life Sci. 1993;53:579–584. doi: 10.1016/0024-3205(93)90715-f. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- Manton WI, Angle CR, Stanek KL, Kuntzelman D, Reese YR, Kuehnemann TJ. Release of lead from bone in pregnancy and lactation. Environmental research. 2003;92:139–151. doi: 10.1016/s0013-9351(03)00020-3. [DOI] [PubMed] [Google Scholar]
- Margellos-Anast H, Shah AM, Whitman S. Prevalence of obesity among children in six Chicago communities: findings from a health survey. Public Health Rep. 2008;123:117–125. doi: 10.1177/003335490812300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang MH, vom Saal FS. Sex differences in plasma corticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. J Reprod Fertil. 1993;99:283–290. doi: 10.1530/jrf.0.0990283. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nieman LK, Chanco Turner ML. Addison’s disease. Clin Dermatol. 2006;24:276–280. doi: 10.1016/j.clindermatol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Nishiyama S, Nakamura K, Ogawa M. Effects of heavy metals on corticosteroid production in cultured rat adrenocortical cells. Toxicology and applied pharmacology. 1985;81:174–176. doi: 10.1016/0041-008x(85)90132-2. [DOI] [PubMed] [Google Scholar]
- Opler MG, Brown AS, Graziano JH, Desai M, Zheng W, Schaefer C, Factor-Litvak P, Susser ES. Prenatal lead exposure, delta-aminolevulinic acid, and schizophrenia. Environ Health Perspect. 2004;112:548–552. doi: 10.1289/ehp.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Yu W, Herbert L, Weinberg J. Fetal ethanol exposure alters pituitary-adrenal sensitivity to dexamethasone suppression. Psychoneuroendocrinology. 1996;21:127–143. doi: 10.1016/0306-4530(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Pallares ME, Scacchi Bernasconi PA, Feleder C, Cutrera RA. Effects of prenatal stress on motor performance and anxiety behavior in Swiss mice. Physiol Behav. 2007;92:951–956. doi: 10.1016/j.physbeh.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Pappas G, Queen S, Hadden W, Fisher G. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med. 1993;329:103–109. doi: 10.1056/NEJM199307083290207. [DOI] [PubMed] [Google Scholar]
- Persico AM, Schindler CW, O’Hara BF, Brannock MT, Uhl GR. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res Mol Brain Res. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Persico AM, Schindler CW, Zaczek R, Brannock MT, Uhl GR. Brain transcription factor gene expression, neurotransmitter levels, and novelty response behaviors: alterations during rat amphetamine withdrawal and following chronic injection stress. Synapse (New York, NY) 1995;19:212–227. doi: 10.1002/syn.890190309. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokora MJ, Richfield EK, Cory-Slechta DA. Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: Time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem. 1996;67:1540–1550. doi: 10.1046/j.1471-4159.1996.67041540.x. [DOI] [PubMed] [Google Scholar]
- Rajan P, Kelsey KT, Schwartz JD, Bellinger DC, Weuve J, Sparrow D, Spiro A, 3rd, Smith TJ, Nie H, Hu H, Wright RO. Lead burden and psychiatric symptoms and the modifying influence of the delta-aminolevulinic acid dehydratase (ALAD) polymorphism: the VA Normative Aging Study. American journal of epidemiology. 2007;166:1400–1408. doi: 10.1093/aje/kwm220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D, Spiro A, 3rd, Aro A, Hu H. Relationship of bone and blood lead levels to psychiatric symptoms: the normative aging study. J Occup Environ Med. 2003;45:1144–1151. doi: 10.1097/01.jom.0000094995.23808.7b. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, Zink M, Hortnagl H, Flor H, Henn FA, Schutz G, Gass P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SJ, Karchmer S, Schnaas L, Perroni E, Zea F, Fernandez Alba J. Changes in serial blood lead levels during pregnancy. Environ Health Perspect. 1994;102:876–880. doi: 10.1289/ehp.94102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, Hargreaves MK, Blot WJ. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97:2260–2267. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Clark C, Rodgers B, Caldwell T, Power C. Childhood and adulthood socio-economic position and midlife depressive and anxiety disorders. Br J Psychiatry. 2008;192:152–153. doi: 10.1192/bjp.bp.107.043208. [DOI] [PubMed] [Google Scholar]
- Starfield EL. Child health and social status. Pediatrics. 1982;69:550–557. [PubMed] [Google Scholar]
- Stipek JD, Ryan RH. Economically disadvantaged preschoolers: ready to learn but further to go. Dev Psychol. 1997;33:711–723. doi: 10.1037//0012-1649.33.4.711. [DOI] [PubMed] [Google Scholar]
- Stokes PE, Stoll PM, Schluger JH, Lasley B. Hypercortisolemia decreases dexamethasone half-life in rabbit. J Psychiatr Res. 2002;36:423–428. doi: 10.1016/s0022-3956(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Hamasaki A, Okamoto M. Subclinical Hypercortisolism in Hospitalized Patients with Type 2 Diabetes Mellitus. Endocr J. 2008 doi: 10.1507/endocrj.k07e-045. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicol. 2006;27:11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87:469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston D, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicol. 2008 doi: 10.1016/j.neuro.2008.03.003. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. INfluence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicol. doi: 10.1016/j.neuro.2008.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil A, Fiala Z, Lacinova V, Ettlerova E. A chronic study with lead acetate in female rats. J Appl Toxicol. 1991a;11:385–386. doi: 10.1002/jat.2550110517. [DOI] [PubMed] [Google Scholar]
- Vyskocil A, Fiala Z, Tejnorova I, Tusl M. Stress reaction in developing rats exposed to 1% lead acetate. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1991b;34:287–295. [PubMed] [Google Scholar]
- Vyskocil A, Smejkalova J, Lacinova V. Dose-related stress reaction in male rats chronically exposed to lead acetate. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1991c;34:393–401. [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Werner S, Malaspina D, Rabinowitz J. Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophr Bull. 2007;33:1373–1378. doi: 10.1093/schbul/sbm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widzowski DV, Cory-Slechta DA. Homogeneity of regional brain lead concentrations. Neurotoxicol. 1994;15:295–308. [PubMed] [Google Scholar]
- Widzowski DV, Finkelstein JN, Pokora MJ, Cory-Slechta DA. Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. Neurotoxicol. 1994;15:853–865. [PubMed] [Google Scholar]
- Zuch CL, O’Mara DJ, Cory-Slechta DA. Low-level lead exposure selectively enhances dopamine overflow in nucleus accumbens: An in vivo electrochemistry time course study. Toxicol Appl Pharmacol. 1998;150:174–185. doi: 10.1006/taap.1998.8396. [DOI] [PubMed] [Google Scholar]