Abstract
Background
Achieving transplantation tolerance remains an unresolved clinical challenge. Although bone marrow transplantation (BMT) induces mixed chimerism that establishes transplantation tolerance, the preconditioning regimens required for BMT to succeed are too prohibitive for routine use. Recently, embryonic stem (ES) cells have emerged as a potential alternative cell source to bone marrow cells. However, it remains difficult to efficiently differentiate these cells into hematopoietic cells. Here, we tested whether bone morphogenetic protein-4 (BMP-4)-treated or HOXB4-transduced ES-derived hematopoietic cells engraft permanently inducing long-term mixed chimerism.
Methods
Initially, 129 SvJ R1 ES cells (H-2b) were treated with BMP-4 for 36 hr. The cells were phenotyped and polymerase chain reaction studies were performed. The robustness of mixed chimerism was tested using mixed lymphocyte cultures and skin grafts. Chimeric MRL (H-2k) animals received grafts from 129SvJ (H-2b), Balb/c (H-2d) or class II−/− (H-2b) donor mice, and graft survival was monitored. Additionally, HOXB4-transduced ES cells were shown to more efficiently differentiate into hematopoietic progenitor cells that engrafted in allogenic and syngeneic recipient mice.
Results
BMP-4 treatment induced Sca-1 expression and up-regulated HOXB4, BMP-4, and BMP receptor gene expressions. The cells induced transient mixed chimerism, whereas cells derived from HOXB4-transduced ES cells engrafted long term (>100 days).
Conclusions
Although BMP-4 promotes hematopoiesis of ES cells, its impact is only transient, whereas permanent ectopic expression of HOXB4 significantly confers self-renewal and long-term engraftment of ES-derived hematopoietic cells. This strategy could facilitate the establishment of an alternative source of hematopoietic cells that could induce transplantation tolerance.
Keywords: BMP-4, HOXB4, Embryonic stem cells, Mice, Mixed chimerism, Skin transplantation
Induction of transplantation tolerance remains a major challenge in solid organ transplantation. Virtually all transplant recipients require life-long immunosuppression, which is associated with substantial risks including opportunistic infection, cardiovascular disease, and malignancy (1). Thus, there is an urgent need to develop alternative approaches for the induction of transplantation tolerance that will allow maximizing the use of the few available organs and decrease the adverse effects of chronic immunosuppression.
To date, establishing mixed chimerism has been the most effective approach to accomplish this goal (2). Bone marrow cells (BMC) have been extensively studied as a source of donor-derived hematopoietic stem cells. For example, studies by Hamawi et al. (3) demonstrated that patients with end-stage renal failure who had previously received a bone marrow transplant (BMT) were tolerant to subsequent renal transplants from their donors. These studies under-scored the clinical effectiveness of BMC in establishing transplantation tolerance. Unfortunately, for routine kidney transplants, the preconditioning regimens of BMT are too harsh, leading to severe infections and even death.
Embryonic stem (ES) cells have emerged as the ideal alternative to bone marrow as they poorly express major histocompatibility complex (MHC) antigens and form less immunogenic hematopoietic cells (4). Recently, our studies indicated that HOXB4 transduced ES cells efficiently form hematopoietic progenitor cells (HPCs) that engraft permanently in Rag2−/−γc−/− mice. Thus, ES cells seem to be a possible novel source of HPCs that can be used for transplantation. Alternatively, bone morphogenetic protein-4 (BMP-4), a member of the transforming growth factor β superfamily, has been shown to regulate the fate of ES cell differentiation into hematopoietic precursors by signaling through the Smad proteins (5). Some studies have implicated a pivotal role for BMP-4 in mesoderm induction and hematopoietic commitment (6). For example, studies using Xenopus embryos demonstrated that BMP-4 induced the expression of the hematopoietic-specific transcription factors GATA-1, GATA-2, stem-cell leukemia, and globin (6, 7). In mice, inactivation of the BMP-4 gene by homologous recombination led to embryo death in homozygous mutants characterized by minimal mesoderm differentiation and a reduction in extraembryonic mesoderm, including blood islands. When evaluating the differentiation of murine ES cells, BMP-4 was shown to mediate the formation of the ventral mesoderm including hematopoietic precursors, and inhibition of BMP-4 impaired hematopoiesis (5, 8). Importantly, treatment of human ES cells with hematopoietic cytokines and BMP-4 has also been shown to promote hematopoietic differentiation (9).
Here, we show that treatment of ES cells with BMP4 leads to partial differentiation of ES cells. However, engraftment of these cells was only transient and led to partial protection of skin allografts from cellular rejection. In contrast, HOXB4-transduced ES cells led to the formation of CD45+ HPCs that permanently engrafted in syngeneic, allogenic, and immunodeficient Rag2−/−γc−/− mice.
MATERIALS AND METHODS
Stem Cell Lines and Mouse Strains
The 129SvJ R1 ES cell line (10) was adapted to grow in feeder cell-free medium containing recombinant leukemia-inhibiting factor (LIF) as previously described (11). The CCE cell line transduced with HOXB4-green fluorescent protein (GFP) was a kind gift by Dr. Hannes Klump, Medizinische Hochschule Hannover, Germany. Briefly, the cells were maintained on gelatin-coated T25 flasks (Corning) in standard ES cells consisting of Dulbecco's modified Eagle's medium supplemented with 15% fetal calf serum (Gibco), 0.1 mmol l-glutamine, 150 mol monothioglycerol, 100 U/mL penicillin, 100 g/mL streptomycin, and 1000 U/mL LIF. Six- to 8-week-old MRL (H-2k), Balb/c (H-2d), Rag2−/−γc−/−, 129 SvJ (H-2b), and B6.129-H2dlAb1–Ea/J (class II null mice, H-2b) mice were purchased from Jackson Laboratories and maintained at the VA Medical Center Animal Facility, Iowa City. The animal studies were approved by the Board of Animal Care at the University of Iowa.
BMP-4 Primes ES Cells Toward Hematopoiesis
129SvJ R1 ES cells (H-2b) were cultured for 36 hr with 2 μg/mL BMP-4 (R&D Systems) in LIF-free medium. The cells were subsequently stained with fluorochromes-conjugated antibodies against Sca-1, CD45, H-2Kb, CD117, Gr-1, B220, CD3, and MHC class II (BD Biosciences). Similarly, HOXB4-transduced ES cells were differentiated as we previously described (4) and CD45+ HPCs purified using immunomagnetic bead separation. Briefly, to differentiate ES cells into HPCs, a two-stage culture strategy was adopted as previously described by Pilat et al. (12). EBs were dissociated into single-cell suspension using trypsin (2.75%), and 3.5×106 cells/mL cells were replated on another ultra-low attachment Petri dish in a serum-free hematopoietic differentiation medium that contained StemPro34 plus nutrient supplement (GIBCO/BRL, Carlsbad, CA) and a cocktail of hematopoietic cytokines including mouse stem cell factor (mSCF) (100 ng/mL, R&D system), mIL-3 (2 ng/mL), mIL-6 (5 ng/mL), Flt3-L (10 ng/mL), IGF-1 (40 ng/mL, Promega, US), and dexamethasone (1 μM, Sigma-Aldrich, US). Culture medium was changed every other day and cell density maintained below 4×106 cells/mL. Differentiation was complete after 26 days.
To determine the ability of the cells to engraft, HPCs derived from HOXB4-transduced ES cells or precursor cells derived from the BMP4-treated cultures were infused intravenously through the supraorbital vein of irradiated (850 cGy) MRL mice (H-2k), syngeneic 129SvJ, or the immunodeficient Rag2−/−γc−/− mice. To monitor mixed chimerism, peripheral blood was drawn from the supraorbital vein of recipient mice regularly and stained with anti-H-2b mAb (BD Biosciences). Animals were defined as chimeric if more than 2% of peripheral blood mononuclear cells were donor derived.
Determination of Gene Expression on ES Cells Treated With BMP-4
Total RNA was isolated from nontreated or BMP-4-treated 129 SvJ R1 ES cells using the Stratagene Absolutely RNA Miniprep kit, according to the manufacturer's recommended protocol. cDNA was synthesized from 1 μg total RNA using the Invitrogen SuperScript III synthesis system. The polymerase chain reaction (PCR) primers were as follows: GAPDH (5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′), Nanog (5′-ACCATGAGTGTGGGTCTTCCTGGTCC-3′ and reverse 5′-TATTTCACCTGGTGGAGTCACAGAGT-3′), BMP-4 (5′-TGTGAGGAGTTTCCATCACG-3′ and reverse 5′-TTATTCTTCTTCCTGGACCG-3′), BMP receptor (BMPR) (5′-TCGTCGTTGTATTACAGGAG-3′ and reverse 5′-TTACATCCTGGGATTCAACC-3′), and HOXB4 (5′-ATGGCTATGAGTTCTTTTTTGATCAAC-3′ and reverse 5′-CTAGAGCGCGCGGGGGCCTCCATTGG-3′). The PCR products were size fractionated by 1% agarose gel electrophoresis.
Skin Grafting
To induce mixed chimerism, sublethally irradiated mice were infused with 2×106 cells and mixed chimerism monitored regularly by flow cytometry. Between 7 and 10 days postinfusion, tail skin allografts from donor 129SvJ, third-party Balb/c, or class II null mice were transplanted dorsally as previously described (13). Bandages were removed after 5 days and skin allografts monitored for rejection. Allografts were classified as rejected when more than 75% of the graft was necrotic. Skin graft survival was calculated according to the Kaplan-Meier product limit method and compared between groups using the log-rank test. A P value less than 0.05 was considered statistically significant.
RESULTS
Characterization of BMP-4-Treated ES Cells
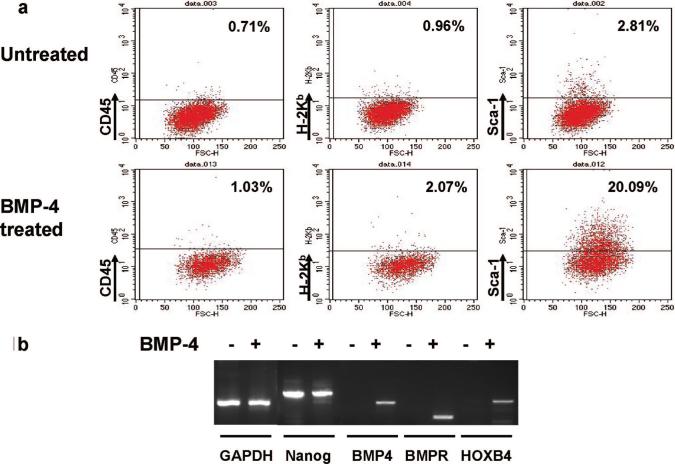
Undifferentiated 129 SvJ R1 ES cells were treated with BMP-4 for 36 hr to induce hematopoietic cell differentiation. Expression of hematopoietic markers CD45, H-2Kb, Sca-1, CD117, class II, CD11b, CD3, B220, and Gr-1 was determined by flow cytometry. In comparison with untreated 129 SvJ R1 ES cells, the only hematopoietic cell surface marker significantly up-regulated by BMP-4 treatment was Sca-1. Representative plots for CD45, H-2Kb, and Sca-1 are shown (Fig. 1a). To further characterize these cells, reverse-transcriptase polymerase chain reaction for hematopoietic genes and ES cell-specific genes was performed in BMP-4-treated and nontreated 129 SvJ R1 ES cells. Interestingly, mRNA transcripts for the hematopoietic-specific genes BMP4, BMPR, and HOXB4 were up-regulated in BMP-4-treated cells (Fig. 1b), suggesting their differentiation toward hematopoietic cells. Despite BMP-4 treatment, mRNA transcripts for the ES cells specific gene Nanog could still be detected, suggesting some nondifferentiated ES cells were still present (Fig. 1b). Taken together, these data suggest that the treatment of undifferentiated ES cells with BMP-4 modestly up-regulates Sca-1 and HOXB4, but however is not sufficient to lead to the full derivation of hematopoietic progenitors.
FIGURE 1.
BMP-4 induces modest expression of Sca-1 and HOXB4. (a) Flow cytometric analysis of CD45, H-2Kb, and Sca-1 expression on untreated or BMP-4-treated 129 SvJ R1 ES cells. BMP-4 modestly up-regulated Sca-1 expression. (b) Reverse-transcriptase polymerase chain reaction of the ES cell-specific gene Nanog and the hematopoietic-specific genes BMP-4, BMPR, and HOXB4 in untreated or BMP-4-treated 129SvJ R1 ES cells. BMP-4 treatment induced HOXB4 expression in ES cells.
BMP-4-Treated ES Cells Transiently Induce Mixed Chimerism
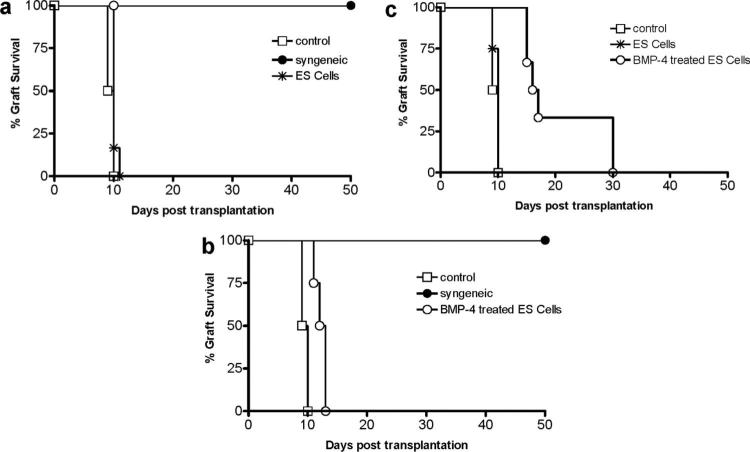
To determine whether BMP-4-treated ES cells induce mixed chimerism, 1.5 to 2×106 BMP-4-treated 129 SvJ R1 ES cells were infused intravenously into sublethally irradiated MRL recipient mice. Mixed chimerism was monitored by flow cytometry for the presence of donor-derived cells (H-2b) in peripheral blood. By day 7, peripheral blood mixed chimerism was observed in 88% of infused animals (7 of 8) with the amount of donor cells ranging between 2% and 4%. Having established that infusion of BMP-4-treated ES cells induced modest mixed chimerism, we sought to determine whether chimeric mice accepted donor-derived skin allografts. Seven days postinfusion with undifferentiated 129SvJ R1 ES cells or BMP-4-treated 129 SvJ R1 ES cells, MRL mice received tail skin allografts from 129SvJ donors. Skin allografts in MRL recipients that had been infused with undifferentiated ES cells (n=6) were rejected with similar kinetics to allografts in naïve MRL recipients (Fig. 2a, n=3). This finding suggested that ES cells neither sensitized the mice to the allografts nor did they tolerize them. In contrast, graft survival in MRL recipients that had been infused with BMP-4-treated ES cells (n=6) was significantly prolonged (P<0.005) compared with naïve MRL recipients, with mean graft survivals of 12.6±0.6 days and 9.6±0.8 days, respectively (Fig. 2b). The short but significant prolonged graft survival in mice infused with BMP-4-treated cells was not due to the sublethal irradiation administered before ES cell infusion because sublethally irradiated MRL mice that did not receive infused ES cells rejected 129 SvJ skin allografts similarly naïve MRL recipients (data not shown). Control syngeneic tail skin isografts were tolerated and survived more than 50 days (Fig. 2a, b). To determine whether less immunogenic allografts would be tolerated longer, we transplanted skin allografts from 129 SvJ class II-negative mice into chimeric MRL mice (n=8). In contrast to naïve MRL recipients (n=3), which rejected the class II-negative grafts with similar kinetics as class II wild-type grafts (10.7±0.6 days), the mean graft survival of class II negative grafts was significantly extended to 18.5±7.1 days (P<0.001) (Fig. 2C). Of the animals receiving BMP-4-treated ES cells, 25% tolerated the class II negative skin grafts for up to 30 days; however, we were unable to determine long-term graft survival because these recipients died at day-30, due to tumors that developed from the nondifferentiated ES cells that remained after BMP-4 treatment. Taken together, these data indicate that infusing BMP-4-treated ES cells into recipient mice before transplantation can lead to prolonged graft survival compared with nontreated recipients; however, infusion of BMP-4-treated ES cells is not sufficient to induce transplantation tolerance and confer long-term graft survival. Further, the data suggest that any manipulation of ES cells to differentiate into hematopoietic cells must be followed with a purification procedure to eliminate nondifferentiated cells that can be teratogenic.
FIGURE 2.
BMP-4-treated ES cells prolong skin graft survival. (a) Graft survival of 129SvJ tail skin grafts transplanted onto the dorsal flank of naïve MRL control mice, 129SvJ syngeneic mice, or MRL mice that had been infused with undifferentiated 129SvJ R1 ES cells seven days before transplant. ES cell infusion had no influence on skin graft survival. (b) Graft survival of 129 SvJ tail skin grafts transplanted onto the dorsal flank of naïve MRL mice, 129 SvJ syngeneic mice, or MRL mice that had been infused with BMP-4 treated-129 SvJ R1 ES cells 7 days before skin transplant. BMP-4-treated cells prolonged graft survival modestly. (c) Graft survival of tail skin allografts from 129 SvJ class II null mice transplanted onto the dorsal flank of naïve MRL mice, or MRL mice that had been infused with undifferentiated or BMP-4-treated ES cells. BMP-4-treated ES cells prolonged graft survival of class II-negative 129SvJ allografts.
HOXB4 Promotes Self Renewal of ES-Derived HPC
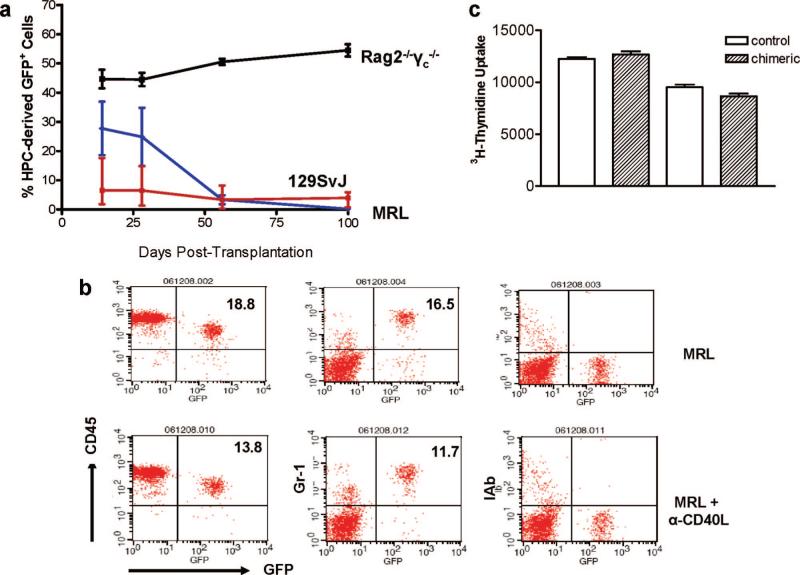
As observed in Figure 1, BMP4 up-regulated HOXB4 in ES cells. Here, we surmised that transduction of ES cells with HOXB4 promotes the self-renewal of ES-derived HPCs, as has been previously described for hematopoetic stem cells. HOXB4-transduced ES cells were differentiated as we previously described into HPCs (4). The purified CD45+ progenitor cells were transplanted into Rag2−/−γc−/−, syngeneic 129SvJ, or into allogenic MRL mice. Mixed chimerism was monitored regularly over 100 days by drawing venous whole blood and performing flow cytometric analysis to detect GFP. The Rag2−/−γc−/− mice showed high engraftment from the beginning that was maintained throughout the study period, Figure 3(a). In contrast, the MRL were high by day 14 declining significantly by day 56. In contrast, the syngeneic 129SvJ started low with approximately 8% of donor cells. These were maintained over 100 days. In fact mixed chimerism in this group was observed over 200 days. Interestingly, at day 28, all groups had high cell numbers as summarized in Table 1, which also shows that an additional group treated with an anti-CD40L antibody had no increased HPC engraftment. A typical flow cytometric analysis of CD45, Gr-1 and IAb (class II antigens) is shown in Figure 3(b), showing insignificant differences between allogenic MRL mice treated with an anti-CD40L antibody and untreated mice. We further performed a mixed lymphocyte culture whereby splenocytes from a control MRL mouse and those from a chimeric MRL mouse were used as responders to irradiated129SvJ or the Balb/c splenocytes (Fig. 3c). There was no difference in the T-cell responses in these cultures clearly indicating that our chimeric mice were not sensitized, but also that alloreactive T cells were not deleted as their responses were identical to those of the control mice. These results show for the first time that HPCs derived from ES cells engraft across MHC barriers and that blockage of costimulation has no impact on their engraftment. Our preliminary data seem to indicate that natural killer cells, not T cells, regulate the early phase of HPC engraftment in immunocompetent allogenic recipients.
FIGURE 3.
Hematopoietic progenitor cells derived from HOXB4-transduced ES cells engraft across major histocompatibility complex barriers. (a) HPCs were derived from HOXB4-transduced ES cells and infused in Rag2−/−γc−/−, the synge-neic 129SvJ or the allogenic MRL mice, and mixed chimerism was studied. Each group comprised six animals. All three mouse strains became chimeric, with the Rag2−/−γc−/− mice showing the highest degree in engraftment. The MRL showed higher engraftment than the 129SvJ, a factor we attribute possibly to different requirements for sublethal irradiation. However, it was the 129SvJ that maintained permanent engraftment over 100 days. The donor cells in the MRLs significantly decreased after 28 days and were nondetectable after 100 days in peripheral blood. (b) To determine whether blocking of costimulation protected transplanted HPCs from rejection, one group of MRL mice was left untreated. A second group was treated with an anti-CD40L antibody. Peripheral blood was analyzed after staining for GFP+CD45+, GFP+Gr-1+, and GFP+Ib+ cells 28 days posttransplantation. The anti-CD40L antibody did not improve significantly the degree of engraftment of the HPCs. (c) Chimeric mice show no altered responses to alloantigen. To determine whether alloresponses of chimeric mice to donor alloantigen were changed, splenocytes from donor-derived 129SvJ or third-party Balb/c mice were used as stimulator cells in a 4-day mixed lymphocyte reaction. The splenocytes from chimeric animals were used as responders. There was no difference of these mice's responses compared with that of control mice. These results showed no evidence of clonal deletion of alloreactive T cells. As expected, third-party responses were also the same in both controls and chimeric animals. This is a representative experiment of three different animals tested in triplicates.
TABLE 1.
HPCs induce mixed chimerism in allogenic recipients
| Strain of recipient mice | Total no. animals | No. chimeric mice | No. non-chimeric mice | Range of achieved mixed chimerism | Means of mixed chimerism (%) |
|---|---|---|---|---|---|
| 129SvJ | 6 | 6 | 0 | 2.0−15.1 | 8.9±5.1 |
| Balb/c | 6 | 4 | 2 | 3.0−4.1 | 3.6±0.8 |
| MRL | 6 | 5 | 1 | 8.6−31.9 | 16.1±14.0 |
| MRL+αCD40L | 6 | 6 | 0 | 20.7−21.7 | 21.1±0.6 |
| Rag2−/−γc | 10 | 10 | 0 | 45.0−69.2 | 55±6.5 |
Mixed chimerism was defined as >2% CD45+GFP+ cells in peripheral blood lymphocytes of recipient mice. The data are from mice at 28 day post-HPC transplantation. An anti-CD40L antibody did not lead to an increase in donor cells compared with the untreated MRL group of recipient mice.
HPC, hematopoietic progenitor cell.
DISCUSSION
In this study, we investigated whether BMP-4 directs the differentiation of ES cells into hematopoietic precursor cells and whether these cells induce mixed chimerism in allogeneic recipients. Treatment of ES cells with BMP-4 increased surface expression of Sca-1, a member of the Ly-6 antigen family expressed on primitive hematopoietic stem cells (14). In addition to Sca-1, BMP-4, and BMPR mRNA were up-regulated. This suggests that BMP-4 signaling in ES cells initiates cellular events leading to hematopoietic differentiation. Consistent with this idea, HOXB4 mRNA was also up-regulated. HOXB4 is a member of the HOX gene family transcribed during hematopoiesis and has been shown to promote hematopoiesis after ectopic expression (15, 16). In addition, Pilat et al. (12) most recently reported that ectopic expression of HOXB4 in murine ES cells enhances their hematopoietic development in vivo.
In organ transplantation, established mixed chimerism is the most effective approach to inducing transplantation tolerance (2). Some investigators have sought to differentiate ES cells into hematopoietic cells (17); however, there has been little success in achieving a high yield of hematopoietic cells capable of establishing stable mixed chimerism. A report by Potocnik et al. (18, 19) demonstrated that ES cells could reconstitute bone marrow of Rag 1−/− mice, but the nature of those BMC was not fully characterized. In another study, Palacios et al. (20) demonstrated the capacity of in vitro pre-differentiated ES cells to reconstitute the bone marrow of severe combined immuno-deficiency (SCID) mice. Although encouraging, these studies are limited because of their use of immunocompromised mice as recipients. One of the key findings of our study is that the hematopoietic precursors developed from BMP-4-treated ES cells were able to establish mixed chimerism in fully allogeneic recipients. However, the degree of mixed chimerism achieved was modest, ranging between 2% and 4% in peripheral blood 7 days post-ES cells infusion, and peaking at day 21 postinfusion with a range of 6% to 12%. A significant population of donor-derived cells was also detectable in the livers of ES cell-infused recipients. These results indicate that BMP-4-treated ES cells establish transient mixed chimerism in recipient mice. One major caveat of this approach is that the mice developed tumors and died after day 30, suggesting that better alternatives should be pursued.
Although BMP-4-treated ES cells induced transient mixed chimerism, HOXB4- transduced cells robustly differentiated into HPCs that engrafted across MHC barriers. However, the cells generated by HOXB4-expressing cells showed a high degree of myeloid cells and lower lymphoid cells as we and others have described in the past (4, 12). For organ transplantation purposes, this bias toward myeloid cells may not be a disadvantage for tolerance induction since myeloid cells generally express both class I and class II antigens. Indeed, we have recently shown that these chimeric animals are tolerant to donor-type cardiac allografts (21), but we have not yet examined skin grafts. Another problem that has not been clearly resolved in mice models is whether HOXB4-expressing cells might induce tumors due to the retroviral vectors. Although no-one has reported tumors in recipient mice of these cells, Zhang et al. (22) now report that there was a high incidence of leukemia in dogs that received HOXB4-expressing cells. These results indicate the need for alternative approaches to HOXB4. An example of such an approach might be to generate recombinant forms of HOXB4 and determine whether these are safer, but equally effective as they will contain no retroviruses. Unfortunately, our preliminary data indicate that recombinant HOXB4 seems to be poorly stable in solution. In other studies, we have observed that construction of a dimeric MHC class I molecule fused to the Fc region of IgG1 strongly enhanced stability and the half-life in vivo (23). Such an approach should be probed to stabilize HOXB4 eliminating the need to use retroviral vectors.
ACKNOWLEDGMENT
The authors thank Jessica Hook for performing skin transplantation.
This work was supported by NIH/NHLBI (R01 HLO7301502), a VA Merit Review Award, Roche Organ Transplantation Research Foundation, and T32 Training Grants (5T32AI00762602−0) (A.M.D.) and (T32AI007485) (W.B.T.).
REFERENCES
- 1.Lopez MM, Valenzuela JE, Álvarez FC, et al. Long-term problems related to immunosuppression. Transpl Immunol. 2006;17:31. doi: 10.1016/j.trim.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 3.Hamawi K, De Magalhaes-Silverman M, Bertolatus JA. Outcomes of renal transplantation following bone marrow transplantation. Am J Transplant. 2003;3:301. doi: 10.1034/j.1600-6143.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 4.Chan KM, Bonde S, Klump H, et al. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood. 2008;111:2953. doi: 10.1182/blood-2007-10-117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeno M, Mead PE, Kelley C, et al. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1965. [PubMed] [Google Scholar]
- 7.Huber TL, Zhou Y, Mead PE, et al. Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood. 1998;92:4128. [PubMed] [Google Scholar]
- 8.Park C, Afrikanova I, Chung YS, et al. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 10.Simpson E, Linder C, Sargent E, et al. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 11.Keller G, Kennedy M, Papayannopoulou T, et al. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilat S, Carotta S, Schiedlmeier B, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci USA. 2005;102:12101. doi: 10.1073/pnas.0505624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behrens D, Lange K, Fried A, et al. Donor-derived soluble MHC antigens plus low-dose cyclosporine induce transplantation unresponsiveness independent of the thymus by down-regulating T cell mediated alloresponses in a rat transplantation model. Transplantation. 2001;72:1974. doi: 10.1097/00007890-200112270-00018. [DOI] [PubMed] [Google Scholar]
- 14.Osawa M, Nakamura K, Nishi N, et al. In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/–) hemopoietic stem cells. J Immunol. 1996;156:3207. [PubMed] [Google Scholar]
- 15.Pineault N, Abramovich C, Ohta H, et al. Differential and common leukemogenic potentials of multiple NUP98-Hox fusion proteins alone or with Meis1. Mol Cell Biol. 2004;24:1907. doi: 10.1128/MCB.24.5.1907-1917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiedlmeier B, Klump H, Will E, et al. High-level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage on human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood. 2003;101:1759. doi: 10.1182/blood-2002-03-0767. [DOI] [PubMed] [Google Scholar]
- 17.Burt RK, Verda L, Kim DA, et al. Embryonic stem cells as an alternate marrow donor source: Engraftment without graft-versus-host disease. J Exp Med. 2004;199:895. doi: 10.1084/jem.20031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potocnik AJ, Nerz G, Kohler H, et al. Reconstitution of B cell subsets in Rag deficient mice by transplantation of in vitro differentiated embryonic stem cells 4. Immunol Lett. 1997;57:131. doi: 10.1016/s0165-2478(97)00089-8. [DOI] [PubMed] [Google Scholar]
- 19.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen and bone marrow. Immunity. 2000;12:653. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 20.Palacios R, Golunski E, Samaridis J. In vitro generation of hematopoietic stem cells from an embryonic stem cell line. PNAS. 1995;92:7530. doi: 10.1073/pnas.92.16.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonde S, Chan KM, Zavazava N. ES-cell derived hematopoietic progenitor cells induce transplantation tolerance. PLoS ONE. 2008;3:e3212. doi: 10.1371/journal.pone.0003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XB, Beard BC, Trobridge GD, et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J Clin Invest. 2008;118:1502. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rickert U, Welke J, Behrens D, et al. A divalent human leukocyte antigen-B7 fusion-protein up-regulates CD25 and CD69 in alloreactive CD8+ T cells bypassing CD28 costimulation. Transplantation. 2006;81:1337. doi: 10.1097/01.tp.0000205770.07196.e6. [DOI] [PubMed] [Google Scholar]