Abstract
During renal ischemia-reperfusion, local and distant tissue injury is caused by an influx of neutrophils into the affected tissues. Here we measured the kinetics of margination and transmigration of neutrophils in vivo in the kidney and lungs following renal ischemia-reperfusion. After bilateral renal injury, kidney neutrophil content increased threefold at 24 h. The neutrophils were found primarily in the interstitium and to a lesser degree marginated to the vascular endothelium. These interstitial neutrophils had significantly lower levels of intracellular IFN-γ, IL-4, IL-6, and IL-10 a tendency for decreased amounts of IL-4 and TNF-α compared to the marginated neutrophils. Localization of the neutrophils to the kidney interstitium was confirmed by high resolution microscopy and these sites of transmigration were directly associated with areas of increased vascular permeability. Activation of the adenosine 2A receptor significantly decreased both kidney neutrophil transmigration by about half and vascular permeability by about a third. After unilateral renal ischemia-reperfusion, the unclipped kidney and lungs did not accumulate interstitial neutrophils or have increased vascular permeability despite a marked increase of neutrophil margination in the lungs. Our findings suggest there is a sequential recruitment and transmigration of neutrophils from the vasculature into the kidney interstitium at the site of tissue injury following renal ischemia-reperfusion.
Keywords: A2A-agonists, acute lung injury, flow cytometry, ischemia/reperfusion, neutrophil migration
Following kidney ischemia-reperfusion injury (IRI), the kidney content of immune cells increases and contributes to apoptosis and/or necrosis.1 Neutrophils, T and B lymphocytes, and macrophages are important in the pathogenesis of kidney IRI.1,2 Neutrophils marginate to vascular endothelium, plug the microvasculature and subsequently release oxygen-free radicals and proteases. These marginated cells are unlikely to have direct effects on renal interstitial cells and/or tubular function.2 Neutrophils secrete cytokines including interferon (IFN)-γ,3,4 interleukin (IL)-4,5 IL-6,6 IL-10,7 and tumor necrosis factor-α (TNF-α).4 To date, studies have not addressed whether neutrophils transmigrate following kidney IRI. Furthermore, IRI is exacerbated8 or attenuated9–11 by experimental maneuvers that increase or reduce neutrophil infiltration, respectively. Thus, determining the localization of polymorphonuclear cell (PMN) in tissue compartments (intravascular vs marginated vs extra-vascular) may improve our understanding of the pathogenesis of kidney IRI.
We have shown earlier that adenosine 2A receptor (A2AR) agonists reduce inflammation by reducing adhesion molecule expression and neutrophil adherence to endothelial cells following renal IRI.12 Blocking adhesion of neutrophils may be the first step in preventing neutrophil transmigration, a mechanism that is most likely to be important in the mechanism of A2A-agonist-mediated renal tissue protection.
Acute kidney injury (AKI) contributes to acute lung injury (ALI), factors which increase the overall morbidity and mortality associated with AKI.13 Defining the mechanism of AKI-induced ALI could reduce the high mortality of combined AKI and ALI, which approaches 80%.14 Compartmentation of neutrophils has been clearly described in other tissues including lungs15 but not in the kidney due to methodological limitations. Therefore, understanding the differential kinetics of kidney neutrophil accumulation in tissue compartments following kidney IRI using refined methodologies and in response to novel compounds is necessary.
We report neutrophil transmigration from the vascular to the interstitial compartments following renal IRI. Our present method distinguishes between marginated and interstitial compartments in the kidneys and lungs as well as the direct role of A2A-agonists on neutrophil distribution in tissue compartments. We show that a non-vascular, interstitial neutrophil population has direct effects on vascular permeability mediating kidney IRI and that the kidney interstitial neutrophil population, whose cytokine content is reduced, is functionally distinct from the marginated neutrophil population. This study highlights the potential importance of leukocyte transmigration across vascular endothelium to the pathogenesis of renal IRI and shows that compounds such as A2A-agonists may ameliorate injury by blocking leukocyte transmigration.
RESULTS
Renal IRI increases total kidney neutrophil content
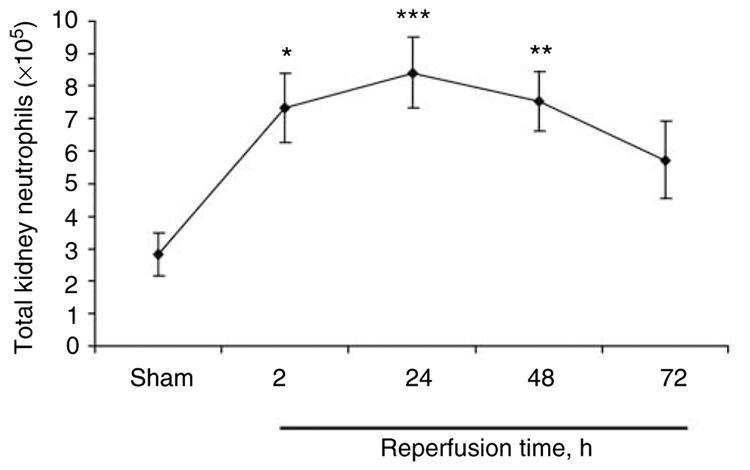
We assessed the kinetics of neutrophil accumulation and compartmentalization to either marginated or non-vascular (interstitial) compartments following kidney IRI. Total kidney content of neutrophils (sum of marginated and interstitial PMNs) was 2.8 ± 0.7×105 cells in sham, increased by 159% after 2 h following IRI (7.3 ± 1.1×105 cells; P<0.05), peaked at 24 h (8.4 ± 1.1×105 cells; a 200% increase above sham; P<0.005) and persisted at 48 and 72 h after IRI (7.5 ± 0.9×105 cells; P<0.01 and 5.7 ± 1.2×105 cells, a 165 and 102% increase above sham), respectively, following renal IRI (Figure 1).
Figure 1. Total kidney neutrophil content.
Mouse kidneys were subjected to sham or 32 min ischemia and reperfusion for 2, 24, 48, or 72 h. Kidney suspensions were prepared for flow cytometry. Neutrophils were identified by (1) their typical appearance in the forward-side scatter, (2) their CD45+ expression, and (3) two independent neutrophil markers, GR-1 APC and 7/4 FITC. N = 3–8 for all groups. *P<0.05, **P<0.01, ***P<0.005 compared with sham.
In vivo neutrophil compartmentation following kidney IRI
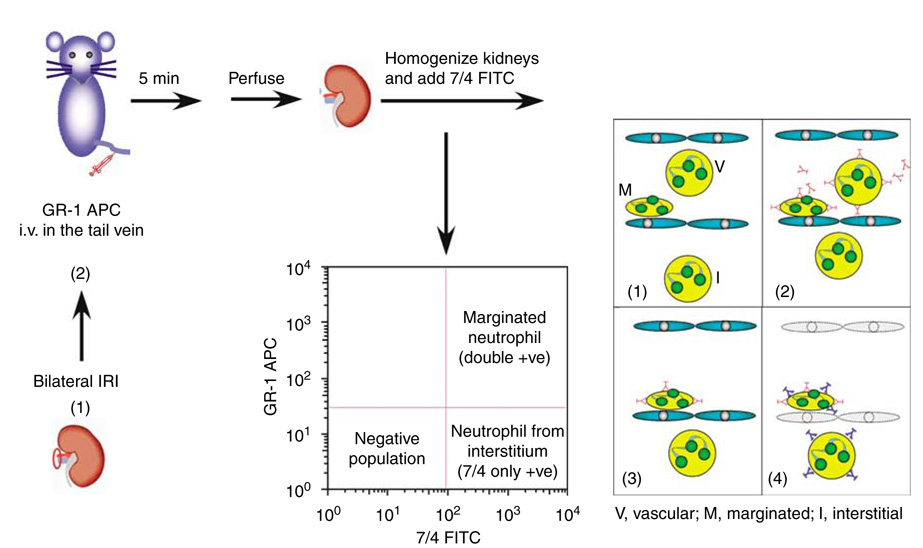
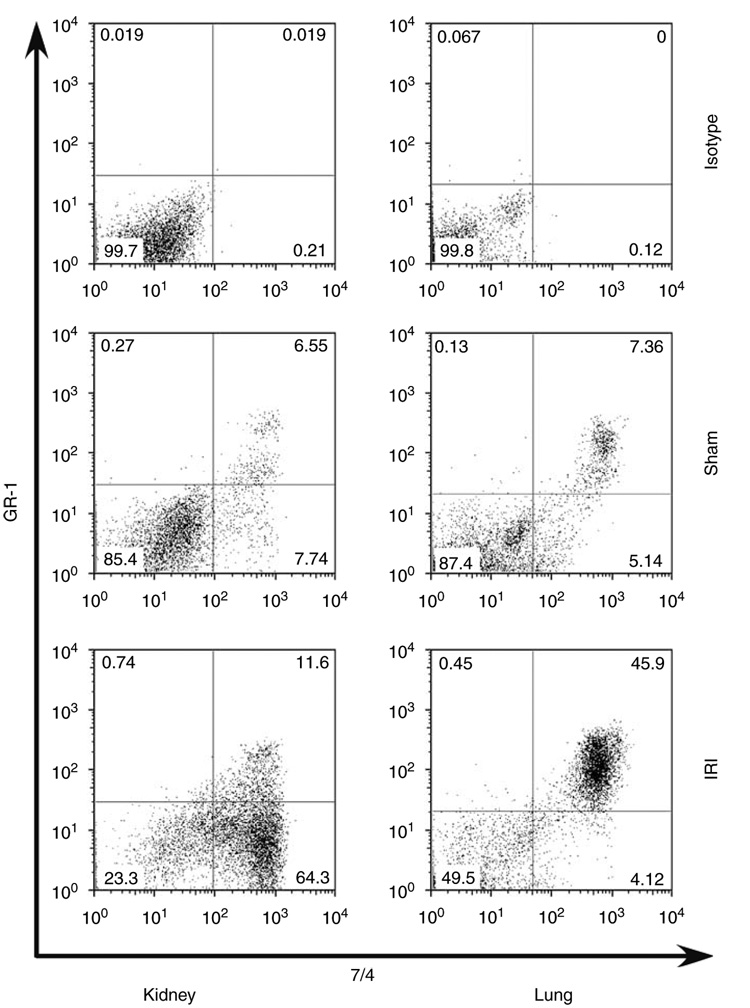
As the total kidney content of neutrophils peaked at 24 h, we sought to distinguish between the marginated (7/4+ GR-1+) and interstitial (7/4+ GR-1−) compartments of neutrophils in the kidneys and lungs, following renal IRI as shown in schematic Figure 2. We assessed compartmentation of neutrophils by harvesting kidneys 5 min after injection of anti-GR-1 antibody to selectively labeled neutrophils in the vascular and marginated compartments. It was shown earlier that 99.2% of blood neutrophils were stained without detectable vascular leakage 5 min after anti-GR-1 antibody injection.15 In our study, it was important to systemically perfuse mice at the end of the study to eliminate all non-adherent vascular cells, including neutrophils, and any free antibody. Thus, we could not draw an accurate conclusion about vascular neutrophil concentration. Kidney IRI followed by 24 h reperfusion increased kidney neutrophil interstitial content (7/4+ GR-1−) by 375% above sham, whereas there was a minimal increase (123% above sham) in marginated cells (7/4+ GR-1+) (Figure 3). In contrast, lung neutrophil interstitial content did not change despite a marked increase in marginated neutrophils (261% above sham).
Figure 2. Schematic diagram for analysis of kidney and lung neutrophils using flow cytometry.
Twenty-four hours following renal ischemia-reperfusion injury (IRI) (1), mice were injected with APC-conjugated GR-1 anti-mouse Ly-6G antibody or isotype control through tail vein (2). Five minutes later, mice were anesthetized and perfused (3), and the kidneys and lungs were excised and processed for FACS analysis (4). CD45 mAb was used to identify total leukocyte population and 7-AAD was used to distinguish live from dead cells. Neutrophil populations were sorted according to either 7/4+ GR-1+ (marginated neutrophils) or 7/4+ GR-1− (neutrophils from the interstitium).
Figure 3. Compartmentalization of neutrophils following kidney ischemia-reperfusion injury.
We harvested kidneys and lungs 5 min after injection of anti-GR-1 antibody or isotype control in sham or 24 h renal ischemia-reperfusion injury (IRI) mice. Marginated neutrophil content was identified as 7/4+GR-1+ and interstitial neutrophil content was identified as 7/4+GR-1−. Graphs show representative dot plots after 24 h of isotype control, sham, or IRI.
Our results also indicate that there were no differences between normal (non-operated) and sham-operated mice for both the kidney and lung marginated and interstitial neutrophil content (data not shown).
To exclude the possibility that poorly perfused areas of the vasculature that might be inaccessible for an intravenously GR-1 injected antibody, we used the monoclonal antibody to TER-119, an antigen associated with cells of the erythroid lineage.16 Five minutes after injection, blood, kidneys, and lungs were harvested from both sham and 24 h renal IRI. Erythrocytes were defined by their typical appearance in the forward-side scatter, and the TER-119+ erythrocytes in each organ were expressed as percentage of total erythrocytes by flow cytometry. We found 98 ± 0.2% of all blood erythrocytes to be TER-119+. At the same time, 83 ± 3 and 96 ± 0.6% of all erythrocytes were TER-119+ in the kidney and lung homogenates, respectively (n = 6–11 each group). This could result in an overestimation of the interstitial neutrophil concentration in the kidney by 17%. Because of the heterogeneity of kidney injury following IRI, the overestimation of neutrophil concentration resides primarily in the outer medulla, as this is the region with the greatest extent of injury. Therefore, we took this into consideration and corrected for this value in all subsequent experiments.
Histological and immunofluorescence localization of neutronphils in the kidney and lung
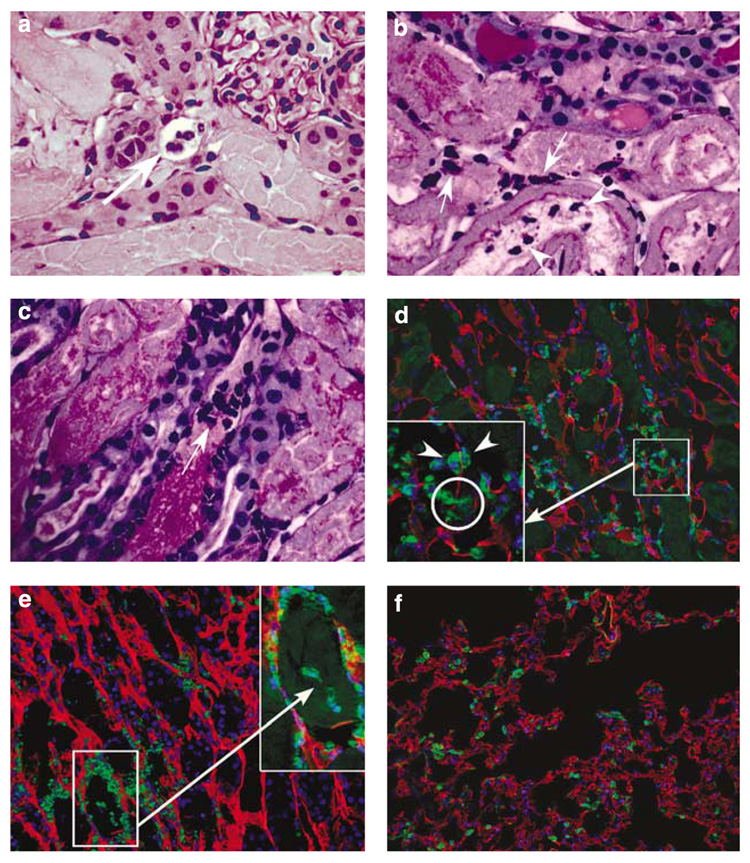
Interstitial neutrophils in the kidney identified by flow cytometry were confirmed by Periodic acid-Schiff stain and immunofluorescence labeling (Figure 4) using monoclonal antibody to 7/4 (neutrophils) and CD31 (to demarcate the vascular endothelium). Histological staining shows neutrophils in the peritubular capillary, vascular (Figure 4a), and interstitial (Figure 4b and c) compartments as well as within the tubular lumen (Figure 4b), indicating transmigration of neutrophils across the interstitial compartment. Immuno-fluorescence labeling confirmed these results and revealed neutrophils in both marginated and non-vascular (interstitial) compartments as well as a neutrophil transmigrating through the vascular wall in the kidney (Figure 4d). We also combined Z-stack images of 12 optical slices to identify several groups of kidney interstitial neutrophils (square; Figure 4e).Combined Z-stack images identify the borders of a vascular structure and improve our precision to distinguish morphologically vascular and non-vascular neutrophils. In contrast to the kidney, there were no interstitial neutrophils in the lung (Figure 4f).
Figure 4. Histological and immunofluorescence localization of neutrophils to interstitial and marginated compartments in the kidney and lung.
Histological staining of kidney outer medulla of mice subjected to 24 h renal ischemia-reperfusion injury (IRI) showing peritubular capillary neutrophils (a), interstitial neutrophils (b and c: arrows), and intraluminal neutrophils (b: arrowhead). Immunofluorescence staining of kidney outer medulla (d and e) and lung (f) using antibodies to 7/4 (green; for neutrophils) and CD31 (red; for vascular endothelium). Nuclei are depicted by DAPI labeling (blue). Neutrophils in both sides of the vascular endothelial wall, and circle shows a neutrophil transmigrating through the vascular wall of the kidney (d: arrowhead). Z-stack image (7.0 mm) of 12 optical slices of the kidney at 0.6 mm intervals (e). Kidney interstitial neutrophils (arrows) are shown in inset (e). Lung tissue shows all neutrophils in the vasculature (f).
Differential analysis of intracellular cytokine content between marginated and interstitial neutrophils
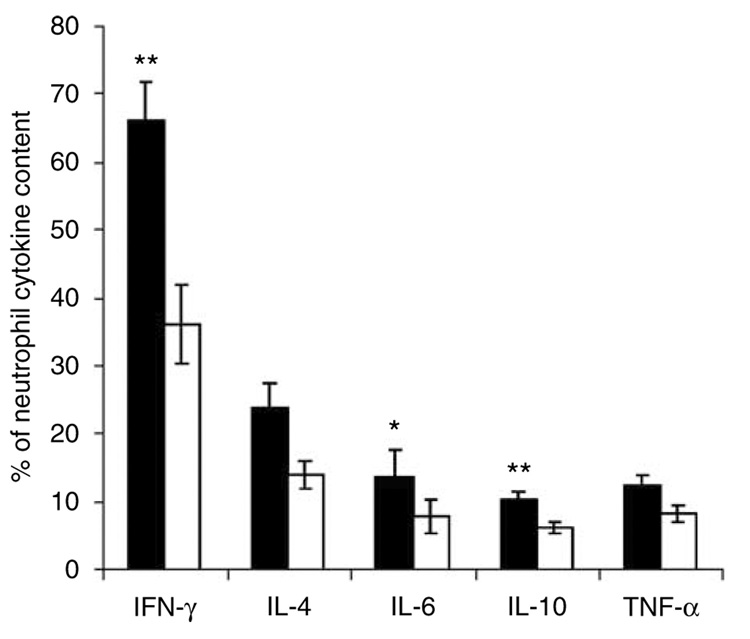
Once we were able to confirm the existence of two kidney neutrophil populations (marginated and interstitial), we further assessed their functional kinetics in vivo following renal IRI. In this experiment, we used flow cytometry to distinguish between the marginated and interstitial neutron-phil intracellular cytokine content in the kidney after 24 h following renal IRI. Kidney interstitial neutrophils showed a decrease in intracellular cytokine content relative to interstitial neutrophils for IFN-γ (−82%; P<0.005), IL-4 (−69%), IL-6 (−71%; P<0.05), IL-10 (−70%; P<0.005), and TNF-α (−52%) (Figure 5).
Figure 5. Differential analysis of intracellular cytokine content between marginated and interstitial neutrophils.
Twenty-four hours following renal ischemia-reperfusion injury (IRI), mice were injected with APC-conjugated GR-1 anti-mouse Ly-6G antibody through tail vein. Five minutes later, mice were anesthetized and perfused, and kidneys were excised and processed for FACS analysis for intracellular cytokine content. Values are mean ± s.e.m.; n = 5 all groups. *P<0.05, **P<0.005 compared with interstitial neutrophils. Open bars, interstitial neutrophils; filled bars, marginated neutrophils.
Time course of in vivo neutrophil trafficking following renal IRI
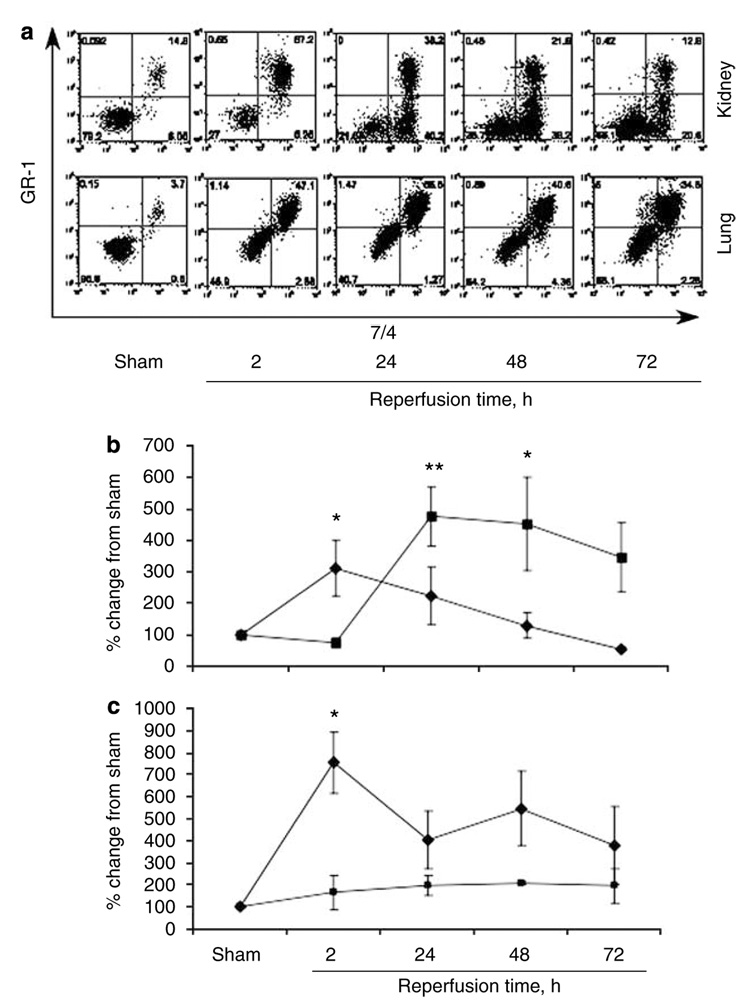
We used flow cytometry to distinguish between the marginated and interstitial neutrophil content of the kidney and lung in sham and at 2, 24, 48, and 72 h following renal IRI. Kidney neutrophil interstitial content increased significantly, peaked at 24 h (376% above sham; P<0.005) and persisted at 48 and 72 h following renal IRI (Figure 6a and b). Kidney neutrophil marginated cells peaked at 2 h (210% above sham; P<0.05) with a minimal increase at 24 h (123% above sham; P=NS) following renal IRI. In contrast, there was a marked increase in lung marginated cells in all ischemic groups with no significant difference in interstitial neutrophil content (Figure 6a and c).
Figure 6. Time course of in vivo neutrophil trafficking following renal IRI.
Representative dot plot graphs for both the kidney and lung (a) and quantitative analysis of neutrophil transmigration following renal ischemia-reperfusion injury (IRI) in the kidney (b) and lung (c). Values are mean ± s.e.m.; n = 6–10 all groups. *P<0.05, **P<0.005 compared with sham. Filled squares, interstitial neutrophils; filled diamonds, marginated neutrophils.
Effect of A2A-agonist on kidney and lung interstitial neutrophil content after renal IRI
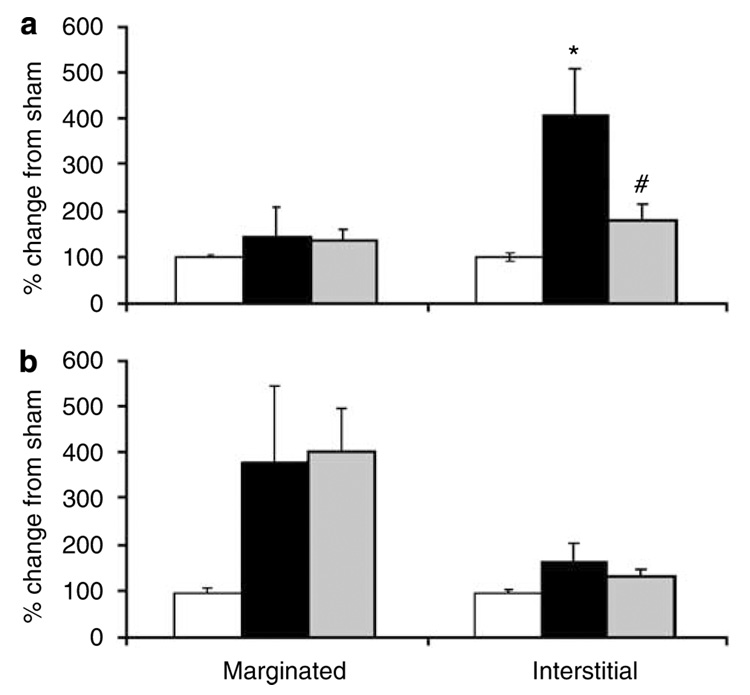
We have shown earlier that ATL146e and ATL313, selective A2A-agonists, reduce inflammatory cell recruitment in renal IRI;17–19 however, we did not distinguish between marginated and/or interstitial neutrophil compartments at that time. Using flow cytometry, we were now able to determine the effects of A2A agonist on kidney neutrophil compartments following 24 h of reperfusion. ATL313 administration reduced kidney interstitial neutrophils (56% reduction from IRI; P<0.05) to a level that was not significantly different from sham (Figure 7a). In contrast, ATL313 failed to reverse the induction of marginated PMNs in the lung (Figure 7b) following renal IRI (sham, 100 ± 8%; IRI, 380 ± 169%; IRI + ATL313, 405 ± 95%).
Figure 7. Effect of ATL313 on kidney and lung interstitial neutrophil content after renal IRI.
Mouse kidneys were subjected to 32 min ischemia and 24 h reperfusion and treated with vehicle or ATL313 (1 ng/kg per min) through osmotic pump. The kidney (a) and lung (b) neutrophil trafficking was determined using flow cytometry. Values are mean ± s.e.m.; n = 6–9 all groups. *P<0.005 compared with sham and #P<0.05 compared with 24 h ischemia-reperfusion injury (IRI) groups. Open bars, sham; filled bars, 24 h renal IRI; light gray bars, 24 h renal IRI + ATL313.
Compartmentation of neutrophils following bilateral vs unilateral renal IRI
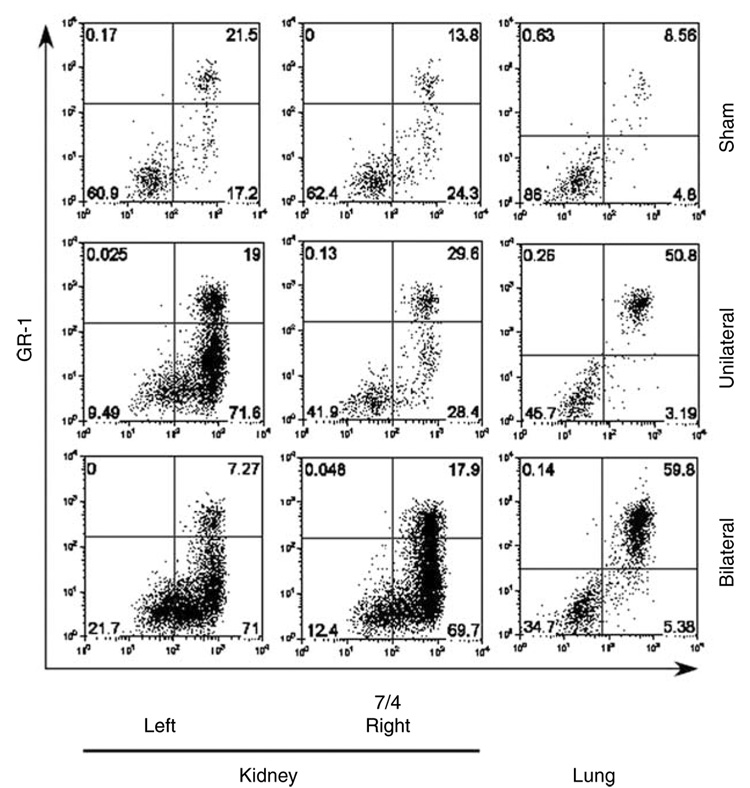
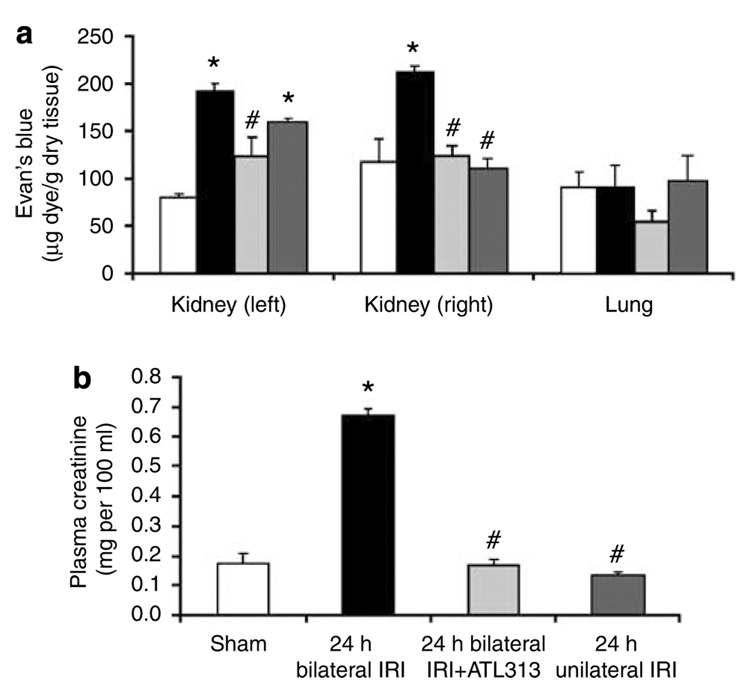
We studied the differences in neutrophil transmigration between bilateral and unilateral renal IRI. In these experiments, we clamped both renal pedicles in bilateral renal IRI, while only the left renal pedicle was clamped in unilateral renal IRI. Clipped kidneys in unilateral or bilateral IRI showed markedly increased interstitial neutrophil recruitment following renal IRI. Interestingly, only the unclipped kidney of unilateral IRI was not associated with increased interstitial neutrophil transmigration and was similar to sham control (Figure 8). Furthermore, the dissociation between the clipped and unclipped kidneys in the unilateral renal IRI provides an internal control for our technique and may signify the importance of interstitial neutrophil compartments after renal IRI. This conclusion was based on our observation of a similar trend using Evans Blue Dye (EBD) for the assessment of kidney and lung vascular permeability. Only the clipped kidney of unilateral renal IRI was associated with increased kidney tissue EBD content (158 ± 6 µg EBD/g dry kidney weight; P<0.005) relative to sham (79 ± 4 µg EBD/g dry kidney weight), whereas the unclipped kidney tissue EBD content was not different from sham (110 ± 11 µg EBD/g dry kidney weight, and 117 ± 23 µg EBD/g dry kidney weight, respectively) despite a minimal increase of marginated PMNs content (Figure 9a). Furthermore, there was no change in lung interstitial neutrophil content (although marginated neutrophil content increased) or tissue EBD content among all groups (Figure 8 and Figure 9a). These results show that ischemic injury to one kidney does not increase neutrophil interstitial accumulation or vascular permeability. Although lung content of neutrophils increased, they primarily localized to the marginated compartment and there was no associated increase in EBD content, suggesting the potential role of transmigration of PMNs to the interstitial compartment in mediating an increase in vascular permeability.
Figure 8. Compartmentation of neutrophils following bilateral vs unilateral renal ischemia-reperfusion injury (IRI).
Representative dot plot graphs of sham, unilateral (only left kidney was clipped), or bilateral (both kidneys were clipped) kidney and lung after renal IRI (n = 4 each groups). Neutrophils were labeled 5 min after injection of anti-GR-1 antibody. Marginated neutrophil content was identified as 7/4+ GR-1+ and interstitial neutrophil content was identified as 7/4+ GR-1−
Figure 9. Functional analysis following renal IRI.
(a) Mouse kidneys were subjected to 32 min ischemia and 24 h reperfusion. Two percent Evans Blue Dye (EBD) was administered through tail vein 1 h before harvesting the kidneys. EBD was extracted in formamide and the amount of extravasated EBD in each kidney and the lung was calculated against a standard curve. Values are mean ± s.e.m.; n = 4 all groups. *P<0.005 compared with sham, #P<0.005 compared with bilateral renal ischemia-reperfusion injury (IRI). (b) Blood was obtained following 24 h of renal IRI and plasma creatinine was assayed. Values are mean ± s.e.m.; n = 4 all groups. **P<0.005 compared with sham; #P<0.005 compared with bilateral renal IRI. Open bars, sham; filled bars, 24 h bilateral renal IRI; light gray bars, 24 h bilateral renal IRI + ATL313; dark gray bars, 24 h unilateral renal IRI.
Functional analysis following renal IRI
We measured plasma creatinine 24 h after renal IRI in both unilateral and bilateral IRI along with ATL313 in bilateral IRI. Bilateral renal IRI was associated with a significant increase in plasma creatinine (0.67 ± 0.02 mg/100 ml; P<0.005) relative to sham (0.17 ± 0.03 mg/100 ml) and unilateral renal IRI (0.13 ± 0.01 mg/100 ml) (Figure 9b). ATL313 administration significantly reduced plasma creatinine levels to 0.17 ± 0.02 mg/100 ml (P<0.005 from bilateral renal IRI). Compared with vehicle treatment, following bilateral IRI ATL313 reduced kidney vascular permeability in both the left (122 ± 20 vs 192 ± 7 µg EBD/g dry kidney weight; P<0.005) and right kidneys (124 ± 10 vs 212 ± 7 µg EBD/g dry kidney weight; P<0.005), respectively (Figure 9a). In the lung, there was a tendency of a lowering effect of ATL313 on lung permeability in comparison with sham (Figure 9b), but this was not significant.
DISCUSSION
This study shows sequential recruitment and transmigration of neutrophils from the vasculature to kidney interstitium following renal ischemia. Transmigration of PMNs from the vascular to the interstitial compartment in the kidney correlated with increased vascular permeability. The process of transmigration led to a decrease in intracellular cytokine levels of interstitial PMNs relative to vascular PMNs. In contrast, neither the lung nor the unclipped kidney in unilateral IRI showed significant changes in interstitial neutrophil recruitment or vascular permeability, despite increased neutrophil margination. A2AR activation reduced kidney neutrophil transmigration and hence prevented vascular permeability in renal IRI. Taken together, we conclude that the process of transmigration of PMNs led to a reduction of intracellular proinflammatory cytokines (presumably from their release) and was associated with tissue injury as assessed by histology, vascular permeability, and kidney function.
Accumulation of neutrophils in the kidney has been identified and thought to be the primary cellular mediator of local tissue injury following IRI. In renal IRI, margination of neutrophils to the vascular endothelium may migrate into renal tissue. Induction of adhesion molecules on endothelial surfaces is the initial step for neutrophil recruitment following renal ischemia.12,20 In particular, ICAM-1, E-selectin, l-selectin, and integrins are essential for the onset of post-ischemic renal injury.21 Neutrophil-endothelial cell adhesion may play an important role in nephron destruction 11,20 through capillary plugging and vascular congestion2 hindering oxygen and nutrient delivery. Whether neutrophils marginate and infiltrate the kidney interstitium is not known.
Using a new and quantitative flow cytometry technique, we showed neutrophil transmigration from the vasculature into ‘non-vascular’ interstitial kidney compartments. The peak of interstitial neutrophils was reached at 24 h and continued to remain elevated up to 72 h following ischemia. These data were confirmed by histological and immunohistochemical localization and showed the existence of a neutrophil population within the kidney interstitial compartment as well as the tubular lumen. Furthermore, interstitial neutrophils appear to be functionally different from vascular or marginated neutrophils as they showed reduced content of IFN-γ, IL-4, IL-6, IL-10, and TNF-α. The reduced intracellular cytokine content of interstitial neutrophils relative to marginated neutrophils suggests that the process of transmigration may lead to cytokine release. This in turn may contribute to increased neutrophil transmigration into the interstitium or indirectly by mediating changes across the vascular endothelial layer. Future experiments are needed to explore these possibilities.
Using a unilateral IRI model, only the clipped kidney showed neutrophil transmigration into the kidney interstitium, whereas the unclipped kidney did not, despite increased marginated PMNs content. We further questioned whether interstitial kidney neutrophils are important and play a direct role in ischemic injury. To test this question, we measured the vascular permeability of EBD in kidney tissue in both unilateral and bilateral renal IRI models. EBD has been used routinely by our laboratory and others to measure vascular permeability in various tissues including kidney.22,23 Our results showed that IRI led to an increase in kidney EBD content in both kidneys of bilateral renal IRI as well as the clipped kidney of unilateral renal IRI. In contrast, the unclipped kidney in unilateral renal IRI did not show increased vascular permeability despite increased neutrophil margination. It is possible that a small contribution of EBD measured in ischemic kidney tissue may be attributed to retention in the vasculature due to capillary plugging by neutrophils and other leukocytes cells. However, it should be emphasized that leukocyte plugging of peritubular capillaries is minimal early in reperfusion (4–6 h).12,24 In addition, we perfused kidneys in all of our experiments to eliminate all vascular EBD content. Thus, our data clearly show a direct association between interstitial neutrophil infiltration-mediated extravasation and vascular permeability following renal ischemia.
Migration of neutrophils has been described in other tissues including the lung15 but not in the kidney due to methodological limitations. By using flow cytometry and high-resolution microscopy, we were able to overcome this limitation and show both a marginated and interstitial kidney neutrophil population. These data combined with changes in intracellular cytokine content suggest that the process of transmigration may determine changes in vascular permeability and leukocyte extravasation.
Anti-inflammatory effects of A2ARs have been clearly established following IRI. However, the direct role and/or contribution of A2AR activation on neutrophil transmigration is unknown. A2ARs are abundantly expressed on activated neutrophils25 and reduce the release of reactive oxygen metabolites.25–27 Work from our laboratory indicated that A2A-agonists reduced inflammation in part by reducing adhesion molecule expression and neutrophil adherence to endothelial cells.12 We now were able to extend our earlier observations using our flow cytometry technique. Our study provides direct evidence of A2AR activation on reducing kidney interstitial neutrophils. Furthermore, the effect of A2AR activation was primarily on kidney interstitial neutrophils and was associated with reduced vascular permeability.
Recruitment of neutrophils into the lung is a key event in the early development of ALI. Earlier studies identified that kidney IRI causes lung edema, alveolar hemorrhage, increased leukocyte trafficking, and abnormalities of salt and water transporters,28–30 all of which are hallmarks of ALI. Therefore, defining the mechanism by which AKI causes ALI is important. Earlier studies showed increased pulmonary vascular permeability and leukocyte trafficking using traditional techniques, following ischemic renal injury in rats28,29 or in mice with 40-min ischemia.30 However, our data with 32-min ischemic injury in mice showed increased lung marginated neutrophils without significant changes in neutrophil migration or interstitial neutrophil content. Our result was supported by neutrophil immunolocalization only within the vasculature. Furthermore, there was no change in the alveolar vascular permeability. Because there was absence of significant injury, we cannot conclude form our data that the opposite, that is, the accumulation of interstitial neutrophils, is important in vascular permeability and lung injury. Showing the relationship between transmigration of neutrophils and lung injury following kidney IRI as well as the effect of adenosine 2A agonists on cellular constituents of the lung would be important in follow-up studies.
In summary, we have showed for the first time, to our knowledge, a sequential recruitment of neutrophils and provided evidence for their kidney transmigration from the vascular to interstitial compartments after renal IRI. The process of transmigration of neutrophils into the kidney interstitial compartment may be pathophysiologically important, as they increased kidney vascular permeability that was prevented by A2AR activation.
MATERIALS AND METHODS
Renal ischemia-reperfusion protocol
All animals were handled and procedures were performed in adherence to the NIH Guide for the Care and Use of Laboratory Animals. C57BL/6 mice (7–8 weeks of age, Charles River Laboratories, Wilmington, MA, USA) were allowed free access to food and water until the day of surgery. Mice were anesthetized with a regimen that consisted of ketamine (100 mg/kg intraperitoneally), xylazine (10 mg/kg intraperitoneally), and acepromazine (1 mg/kg intramuscular) and were placed on a thermoregulated pad to maintain body temperature at 37°C. Both kidneys (bilateral) or the left kidney was cross-clamped for 32 min. Surgical wounds were closed, and mice were returned to cages for up to 2, 24, 48, or 72 h. In selected protocols, we administered ATL313, a selective A2A agonist (1 ng/kg per min), or vehicle through osmotic mini pumps at the time of bilateral renal ischemia for 24 h.
Antibodies for flow cytometry
We used the following antibodies: (1) PE-conjugated anti-mouse CD45, clone 30-F11 (Pharmingen, San Diego, CA, USA), (2) FITC-conjugated anti-mouse 7/4, recognizing the 7/4 antigen on murine neutrophils (Caltag, Burlington, CA, USA), (3) APC-conjugated anti-mouse GR-1, clone RB6-8C5, recognizing the GR-1 antigen on murine neutrophils (eBioscience, San Diego, CA, USA), (4) GR-1 antibody, clone RB6-8C5 (hybridoma from ATCC), (5) anti-TER- 119 recognizing glycophorin A on cells of the erythroid lineage (eBioscience), and (6) PE-conjugated anti-mouse for intracellular staining for IFN-γ, IL-4, IL-6, IL-10, and TNF-α (eBioscience). Appropriate rat anti-mouse IgG2a and IgG2b (Pharmingen) were used as isotype controls.
In vivo neutrophil transmigration
Five minutes before the end of the study, mice were injected through tail vein with a 300 µl solution containing 10 µg APC-conjugated GR-1 anti-mouse Ly-6G antibody or isotype control. Mice were anesthetized as described above and were perfused through the heart with 10 ml phosphate-buffered saline at a rate of 3 ml/min. Mouse kidneys and lungs were excised and processed for flow cytometry FACS analysis.
In a separate experiment, a monoclonal antibody targeting the TER-119 antigen was used to assess its ability to bind erythrocytes in potential non-perfused or occluded blood vessels as a result of injury. Five minutes after tail vein injection of the TER-119 antibody, blood was harvested retroorbitally from sham and 24 h renal IRI mice, and both the kidneys and lungs were removed for further assessment.
Analysis of kidney and lung neutrophil content by flow cytometry
We used flow cytometry to analyze the differential kinetics of kidney and lung neutrophils between vascular and interstitial compartments (Figure 2) and as described earlier.3 In brief, the kidneys and lungs were extracted, weighed, minced in 100 µl unlabeled GR-1 (clone RB6-8C5, University of Virginia) to prevent possible binding of excess APC-conjugated anti-mouse GR-1 to the non-vascular neutrophils, digested and then passed through a filter and a cotton wool column.
After blocking nonspecific Fc binding with anti-mouse CD16/32 (2.4G2), fresh kidney and lung suspensions were incubated with anti-mouse CD45-PE (30-F11) for 30 min on ice. Sample volume was adjusted based on the kidney and lung weight (g/ml). The kidney and lung suspensions (100 µl) were mixed thoroughly with 30 µl of Caltag Counting Beads (994 beads per ml) before acquisition by BD FACSCalibur (BD Biosciences, San Jose, CA, USA) to normalize for differences in cell recovery among samples. Neutrophils were identified by (1) their typical appearance in the forward-side scatter, (2) their CD45+ expression, and (3) two independent neutrophils markers, GR-1 APC and 7/4 FITC, as described earlier.15 Neutrophil populations were sorted by being defined as either 7/4+ GR-1+ or 7/4+ GR-1− (Figure 2).
For intracellular staining, kidney cells were fixed, permeabilized, and then incubated with anti-mouse IFN-γ IL-4, IL-6, IL-10, and TNF-á as described earlier.3
Flow cytometry data acquisition was performed on FACSCalibur (Becton Dickinson, San Jose, CA, USA) and analyzed by Flowjo software 6.0 (Tree Star Inc., Ashland, OR, USA). PE-CD45 (FL2) was used to separate kidney and lung leukocytes from other cells. We used 7-AAD (FL3) to gate CD45 negative, viable cells. Lastly, the gated population was analyzed in FL4 (APC) vs FL1 (FITC). Values are expressed as % increase relative to sham.
Analysis of the kidney and lung vascular permeability
Changes in vascular permeability were assessed by quantitating extravasation of EBD into the kidney and lung tissues.23
Kidney histology
The kidneys were fixed in 4% periodate-lysine-paraformaldehyde and embedded in paraffin, and 3-µm sections were cut. Sections were subjected to Periodic acid-Schiff stain and viewed by light microscopy (Zeiss AxioSkop, Thorn Wood, NY, USA), and digital images were taken using a SPOT RT Camera (software version 3.3; Diagnostic Instruments, Sterling Heights, MI, USA).
Immunofluorescence and high-resolution microscopy
The kidneys and lungs were fixed in 1% periodate-lysine-paraformaldehyde, cryoprotected in 30% sucrose, and frozen in Tissue-tek optimal cutting temperature compound (Fisher Scientific, Pittsburgh, PA, USA). Twenty µm cryostat sections were rinsed with phosphate-buffered saline and then permeabilized with 0.3% Triton X-100. Nonspecific binding was blocked with 10% donkey serum and anti-mouse CD16/32 (5 µg/ml; clone 2.4G2; StemCell Technologies Inc., Vancouver, Canada). Sections were incubated with primary antibodies for 60 min at room temperature. We used: Alexa Fluor 647-labeled CD31 (5 µg/ml; PECAM-1 clone MEC 13.3; BioLegend, San Jose, CA, USA) and FITC-labeled neutrophil antibody (5 µg/ml; clone 7/4; Cedarlane, Ontario, Canada). Specimens were mounted with ProLong Gold Antifade with DAPI (Molecular Probes, Eugene, OR, USA). Images were acquired using the Zeiss Axiovert 200 microscopy system with ApoTome using a Plan-Apochromat 20×/0.80 objective (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA) and processed using Zeiss AxioVision 4.6 software.
Plasma creatinine
Plasma creatinine was determined using a colorimetric assay (Sigma Chemical Co., St Louis, MO, USA).23
Statistical analysis
Comparisons between groups were examined by one-way analysis of variance or t-test (for intracellular staining) using SPSS version 16.0 software for Windows (SPSS Inc., Chicago, IL, USA). Multiple comparisons of individual pairs of effect means were conducted by using the least squares methods of pooled variance. Data are expressed as mean ± s.e.m. Statistical significance was identified at P<0.05.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr Klaus Ley (La Jolla Institute for Allergy and Immunology) for reviewing the manuscript and his helpful discussion; Adenosine Therapeutics, LLC, Charlottesville, VA, USA, for the generous gift of ATL313; Drs Li Li and Konstantine Khutsishvili (University of Virginia) for helpful discussions; and Hong Ye for expert technical assistance. This study was supported by NIH Grants 1K99DK077444-01, DK56223, DK58413, DK62324, HL37942, and 1 PO1 HL073361.
Footnotes
DISCLOSURE
M.D.O. and J.L. owned equity in Adenosine Therapeutics, LLC, Charlottesville, VA, USA, which provided ATL313 during the course of this study.
REFERENCES
- 1.Li L, Okusa MD. Blocking the Immune respone in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat Clin Pract Nephrol. 2006;2:432–444. doi: 10.1038/ncpneph0238. [DOI] [PubMed] [Google Scholar]
- 2.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Huang L, Sung SJ, et al. NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol. 2007;178:5899–5911. doi: 10.4049/jimmunol.178.9.5899. [DOI] [PubMed] [Google Scholar]
- 4.Ethuin F, Gerard B, Benna JE, et al. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 5.Brandt E, Woerly G, Younes AB, et al. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. 2000;68:125–130. [PubMed] [Google Scholar]
- 6.Retini C, Vecchiarelli A, Monari C, et al. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman SJ, Polack FP, Hauer DA, et al. Measles virus infection of rhesus macaques affects neutrophil expression of IL-12 and IL-10. Viral Immunol. 2003;16:369–379. doi: 10.1089/088282403322396163. [DOI] [PubMed] [Google Scholar]
- 8.Linas SL, Shanley PF, Whittenburg D, et al. Neutrophils accentuate ischemia-reperfusion injury in isolated perfused rat kidneys. Am J Physiol. 1988;255:F728–F735. doi: 10.1152/ajprenal.1988.255.4.F728. [DOI] [PubMed] [Google Scholar]
- 9.Riera M, Torras J, Herrero I, et al. Neutrophils accentuate renal cold ischemia-reperfusion injury. Dose-dependent protective effect of a platelet-activating factor receptor antagonist. J Pharmacol Exp Ther. 1997;280:786–794. [PubMed] [Google Scholar]
- 10.Hellberg PO, Kallskog TO. Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int. 1989;36:555–561. doi: 10.1038/ki.1989.230. [DOI] [PubMed] [Google Scholar]
- 11.Klausner JM, Paterson IS, Goldman G, et al. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- 12.Okusa MD, Linden J, Huang L, et al. A2a-Adenosine receptor mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 13.Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. In Sem in Nephrol. 2000;20:4–19. [PubMed] [Google Scholar]
- 14.Mehta RL, Pascual MT, Gruta CG, et al. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 15.Reutershan J, Basit A, Galkina EV, et al. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 17.Day Y-J, Huang L, Ye H, et al. Renal ischemia-reperfusion and adenosine 2A receptor-mediated tissue protection: The role of CD4+ T cells and inteferon gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- 18.Day Y-J, Huang L, McDuffie MJ, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awad AS, Huang L, Ye H, et al. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 20.Lauriat S, Linas SL. The role of neutrophils in acute renal failure. Semin Nephrol. 1998;18:498–504. [Review] [37 refs] [PubMed] [Google Scholar]
- 21.Kelly KJ, Williams WW, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrero ME. In vivo vascular leakage assay. Methods Mol Med. 2004;98:191–198. doi: 10.1385/1-59259-771-8:191. [DOI] [PubMed] [Google Scholar]
- 23.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 (S1P1) receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chiao H, Kohda Y, McLeroy P, et al. a-Melanocyte-stimulating hormone protects against renal injury after Ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronstein BN, Rosenstein ED, Kramer SB, et al. Adenosine; a physiological modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985;135:1366–1371. [PubMed] [Google Scholar]
- 26.Cronstein BN, Kramer SB, Weissman G, et al. Adenosine: a physiological regulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts PA, Newby AC, Hallett MB, et al. Inhibition by adenosine of reactive oxygen metabolite production byhuman polymorphonuclar leucocytes. Biochem J. 1985;227:669–674. doi: 10.1042/bj2270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer AA, Postler G, Salhab KF, et al. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Hu X, Yuen PS, et al. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]