Abstract
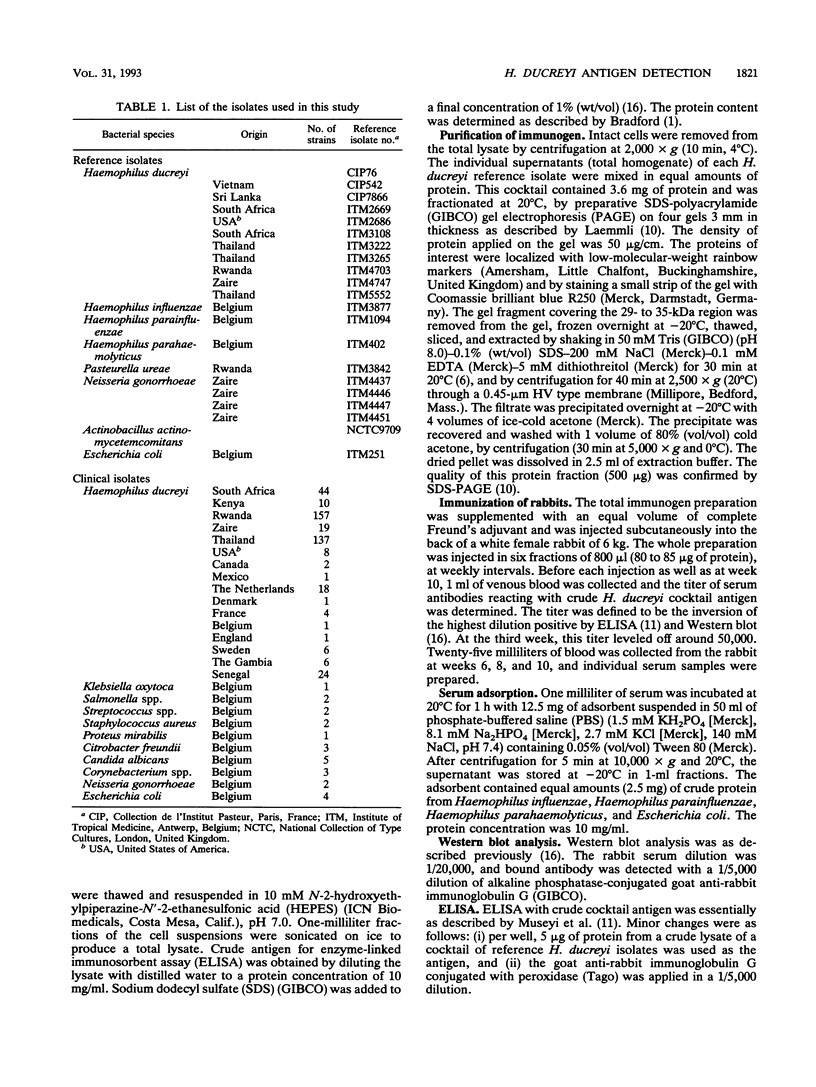
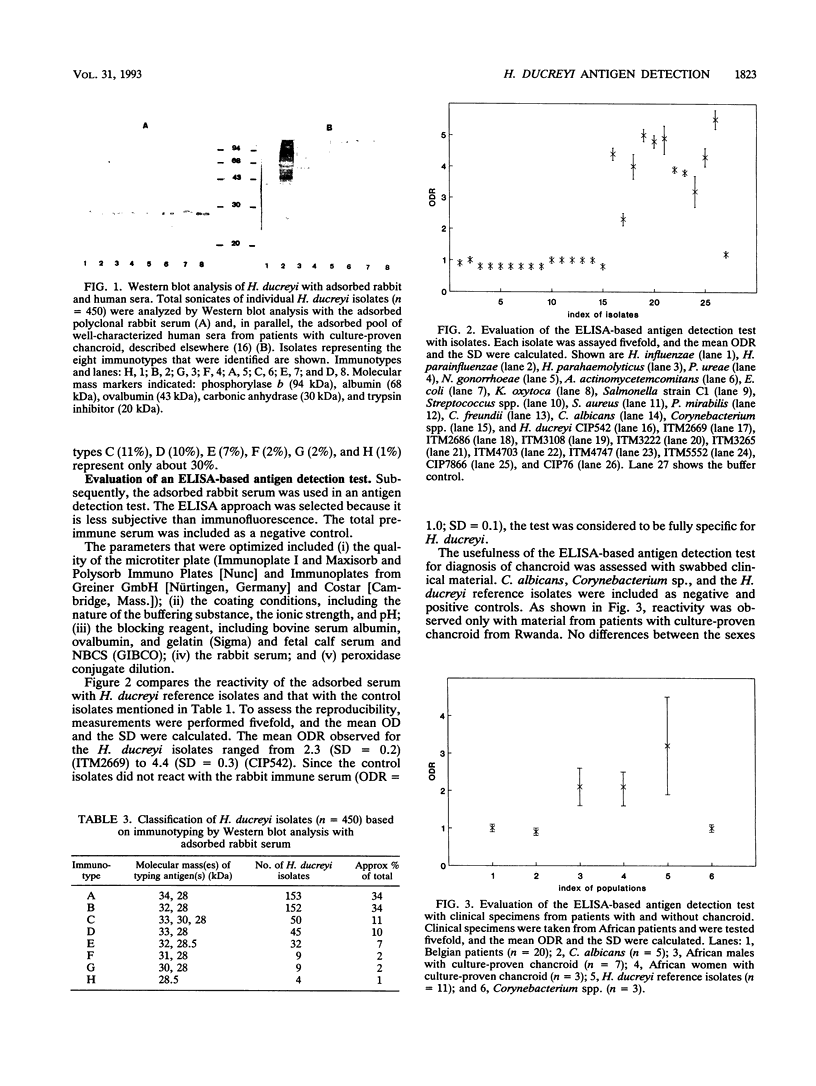
A rabbit polyclonal serum was raised against the 29-kDa species-specific marker, as well as the 30- to 34-kDa immunotype-specific markers of Haemophilus ducreyi described elsewhere (E. Roggen, S. De Breucker, E. Van Dyck, and P. Piot, Infect. Immun. 60:590-595, 1992). These antigens were purified from a cocktail of H. ducreyi isolates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The immune serum reacted in enzyme-linked immunosorbent assay (ELISA) preferentially with H. ducreyi, at a titer as high as 50,000. To make it specific to H. ducreyi, nonspecific antibodies were removed by adsorption on a mixture of Haemophilus spp., Escherichia coli, Candida albicans, and Corynebacterium spp. In the 29- to 34-kDa region of immunoblot profiles from H. ducreyi isolates (n = 450), the adsorbed serum revealed essentially the same antigens as did a pool of well-characterized human sera. Yet, eight different immunotypes were observed. With this rabbit polyclonal serum, an ELISA-based antigen detection test was developed. The adsorbed serum reacted specifically with all H. ducreyi isolates tested (n = 450), but not with other bacterial species (n = 15). This test was evaluated with a limited number of clinical specimens from African patients with culture-proven chancroid and no evidence for any other ulcerating etiology (n = 10) and a number of chancroid-negative control patients from Belgium (n = 20). Within this context, the test yielded a sensitivity and specificity of 100%.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chapel T., Brown W. J., Jeffries C., Stewart J. A. The microbiological flora of penile ulcerations. J Infect Dis. 1978 Jan;137(1):50–56. doi: 10.1093/infdis/137.1.50. [DOI] [PubMed] [Google Scholar]
- Choudhary B. P., Kumari S., Bhatia R., Agarwal D. S. Bacteriological study of chancroid. Indian J Med Res. 1982 Sep;76:379–385. [PubMed] [Google Scholar]
- Denys G. A., Chapel T. A., Jeffries C. D. An indirect fluorescent antibody technique for Haemophilus ducreyi. Health Lab Sci. 1978 Jul;15(3):128–132. [PubMed] [Google Scholar]
- Greenblatt R. M., Lukehart S. A., Plummer F. A., Quinn T. C., Critchlow C. W., Ashley R. L., D'Costa L. J., Ndinya-Achola J. O., Corey L., Ronald A. R. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988 Feb;2(1):47–50. doi: 10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hira S. K., Kamanga J., Macuacua R., Mwansa N., Cruess D. F., Perine P. L. Genital ulcers and male circumcision as risk factors for acquiring HIV-1 in Zambia. J Infect Dis. 1990 Mar;161(3):584–585. doi: 10.1093/infdis/161.3.584. [DOI] [PubMed] [Google Scholar]
- Karim Q. N., Finn G. Y., Easmon C. S., Dangor Y., Dance D. A., Ngeow Y. F., Ballard R. C. Rapid detection of Haemophilus ducreyi in clinical and experimental infections using monoclonal antibody: a preliminary evaluation. Genitourin Med. 1989 Dec;65(6):361–365. doi: 10.1136/sti.65.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto D. Y., Slaney L., Koss J., D'Costa L. J., Plummer F. A., Ndinja-Achola J. O., Ronald A. R. Field testing of modified Bieling media for the isolation of Haemophilus ducreyi in Kenya. Eur J Clin Microbiol. 1986 Dec;5(6):673–675. doi: 10.1007/BF02013300. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Museyi K., Van Dyck E., Vervoort T., Taylor D., Hoge C., Piot P. Use of an enzyme immunoassay to detect serum IgG antibodies to Haemophilus ducreyi. J Infect Dis. 1988 May;157(5):1039–1043. doi: 10.1093/infdis/157.5.1039. [DOI] [PubMed] [Google Scholar]
- Piot P., Laga M. Genital ulcers, other sexually transmitted diseases, and the sexual transmission of HIV. BMJ. 1989 Mar 11;298(6674):623–624. doi: 10.1136/bmj.298.6674.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P., Plummer F. A., Rey M. A., Ngugi E. N., Rouzioux C., Ndinya-Achola J. O., Veracauteren G., D'Costa L. J., Laga M., Nsanze H. Retrospective seroepidemiology of AIDS virus infection in Nairobi populations. J Infect Dis. 1987 Jun;155(6):1108–1112. doi: 10.1093/infdis/155.6.1108. [DOI] [PubMed] [Google Scholar]
- Roggen E. L., De Breucker S., van Dyck E., Piot P. Antigenic diversity in Haemophilus ducreyi as shown by western blot (immunoblot) analysis. Infect Immun. 1992 Feb;60(2):590–595. doi: 10.1128/iai.60.2.590-595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalla W. O., Sanders L. L., Schmid G. P., Tam M. R., Morse S. A. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986 May;153(5):879–887. doi: 10.1093/infdis/153.5.879. [DOI] [PubMed] [Google Scholar]
- Slootmans L., Vanden Berghe D. A., Piot P. Typing Haemophilus ducreyi by indirect immunofluorescence assay. Genitourin Med. 1985 Apr;61(2):123–126. doi: 10.1136/sti.61.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E., Handsfield H. H., Rompalo A. M., Ashley R. L., Roberts P. L., Corey L. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA. 1988 Sep 9;260(10):1429–1433. [PubMed] [Google Scholar]
- Sturm A. W., Stolting G. J., Cormane R. H., Zanen H. C. Clinical and microbiological evaluation of 46 episodes of genital ulceration. Genitourin Med. 1987 Apr;63(2):98–101. doi: 10.1136/sti.63.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Duangmani C., Suvongse C., O'Connor R., Pitarangsi C., Panikabutra K., Echeverria P. The role of Haemophilus ducreyi in penile ulcers in Bangkok, Thailand. Sex Transm Dis. 1984 Jul-Sep;11(3):148–151. doi: 10.1097/00007435-198407000-00005. [DOI] [PubMed] [Google Scholar]



