Abstract
Introduction
Female sexual arousal disorder (FSAD) is a major component of female sexual dysfunctions, affecting 25–70% of women. The mechanisms of FSAD are poorly understood. Estrogen contributes to the control of genital blood flow during the sexual response. Vascular effects of estrogen are mostly attributed to its regulation of endothelial nitric oxide (NO) production. However, the role of endothelial NO synthase (eNOS) and the mechanisms that regulate eNOS in female genital tract structures are largely unknown.
Aim
To review available evidence of the mechanisms of eNOS regulation in female genital tract structures.
Methods
This article reviews the literature that relates to the role of NO and eNOS in female sexual arousal and its modulation by estrogen.
Main Outcome Measures
Association between female sexual arousal, NO, and eNOS.
Results
The NO/cyclic guanosine monophosphate pathway is believed to have a primary role in the regulation of clitoral and vaginal blood flow, and smooth muscle relaxation during sexual arousal. Estrogen is critical for maintaining vaginal and clitoral blood flow and vaginal transudate production. Estrogen regulates eNOS by genomic mechanisms, involving augmented mRNA transcription and protein synthesis, and by non-genomic mechanisms, which occur without alterations in gene expression. However, limited studies have evaluated the physiological role of endothelial NO and the molecular mechanisms of eNOS regulation in the female genital tract.
Conclusions
The effects of estrogen on increasing genital blood flow and smooth muscle relaxation have been attributed mostly to regulation of eNOS. However, the exact mechanisms of eNOS regulation in female genital tract structures and the molecular basis for the eNOS defect with aging and vascular diseases warrant further investigation.
Keywords: Vagina, Clitoris, Female Sexual Arousal, Caveolin
Introduction
Genital arousal, an early physiologic event in the overall female sexual response, is a neurophysiological process comprised of central and peripheral components. The peripheral component is characterized by an increase in genital blood flow coordinated with clitoral and vaginal smooth muscle relaxation, engorgement of the clitoris and vaginal wall, vaginal lubrication and lengthening [1]. Studies conducted in the past several years suggest that increased clitoral and vaginal blood flow during sexual arousal is primarily mediated by the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway [2]. Female sexual arousal disorder (FSAD) is the inability to attain or maintain sufficient sexual excitement and pertains to the impairment in hemodynamic changes during sexual response, resulting in decreased clitoral engorgement, lack of vaginal wall relaxation, and diminished vaginal lubrication [3].
Although psychosocial factors contribute to FSAD, physiological factors—including vascular insufficiency and altered hormonal environment—may be the primary basis [1]. Diabetic women develop FSAD more often than nondiabetic women [4]. Diabetic rats have reduced vaginal blood flow in response to pelvic nerve stimulation [5]. Atherosclerosis in rabbits is associated with decreased vaginal and clitoral blood flow and insufficiency in vaginal and clitoral engorgement [6]. Common to these risk factors is a decreased capacity of vascular endothelium to generate NO and endothelial dysfunction.
Female genital function is assumed to be closely related to the hormonal milieu. Various hormones may influence female sexual function. Estrogen has a significant role in maintaining vaginal mucosal epithelium and genital blood flow [7], and medical management of FSAD so far is primarily based on hormone replacement therapy. Many studies report an increase in the prevalence of FSAD with aging and menopause related to a decline in circulating levels of estrogen [2]. Estrogen stimulation of blood flow and genital arousal during sexual responses has been mostly attributed to endothelial NO production [2].
NO in Female Sexual Arousal Response
NO is a potent vasodilator of clitoral tissue, reminiscent of its effects on penile cavernosal tissue. The clitoral cavernosal tissue consists of 40–45% smooth muscle and blood-filled sinusoids [8]. The increase in clitoral blood supply is a result of arterial vasodilation and smooth muscle relaxation in the corpus cavernosum. Clitoral tumescence occurs mainly because of the arterial inflow over-whelming blood drainage in the absence of veno-occlusion. Several studies have shown that stimulation of the pelvic nerve in rabbits [6,9] and clitoral cavernous nerve in rats [10] and dogs [11] increases clitoral intracavernosal pressure and blood flow. Relaxation of rabbit [12,13] and human [14] clitoral corpus cavernosum is induced by electrical field stimulation, enhanced by the NO donor sodium nitroprusside, reduced by constitutive NOS inhibition, and facilitated by phosphodiesterase type 5 inhibitors. The pathway down-stream from NO, including protein kinase G-dependent activation of calcium-activated potassium channels, has also been suggested to contribute to increased blood flow in the clitoris [14].
The wall of the vagina is lined with an inner epithelium, a lamina propria, the muscularis, and an outer supportive layer. The lamina propria contains extensive network of blood vessels, which become engorged during sexual arousal. Dilation of the arteries in the vaginal wall increases the blood flow, resulting in increased transudation through the vaginal epithelium. Studies with rabbit vaginal strips in organ bath have suggested that neurogenic relaxation of the rabbit vaginal wall is partially mediated by NO [15]. However, organ bath studies with rat vagina suggested a key role for the NO/cGMP pathway in vaginal smooth muscle relaxation [16]. In rabbits [17], dogs [11], and rats, [10,18,19] pelvic nerve stimulation and arginase inhibition [20] increased vaginal blood flow, whereas inhibition of NOS and soluble guanylate cyclase reduced vaginal blood flow. Topical application of the slow-releasing NO donor DS1 increased vaginal blood flow in rats [21]. Human and rabbit vaginal smooth muscle cells treated with the NO donor sodium nitroprusside in the presence of sildenafil exhibited enhanced intracellular cGMP synthesis and accumulation [22].
The vagina embryologically, morphologically, and functionally differs according to its region. Compared with the proximal part, the distal part of the rat vagina is richly innervated and has numerous vascular sinusoids lined by endothelial cells scattered among muscles [16]. Although the distal vagina plays a major role in sexual function, the proximal vagina is thought to serve as a reservoir for spermatozoa and in maintaining their viability. Different anatomical parts of the vagina exhibit differences in the expression of NOS isoforms and smooth muscle functional responses. Total NOS activity was found to be higher in the rabbit proximal compared with the distal vagina [23]. On the contrary, the activity of arginase was higher in distal than in proximal vagina [23]. Arginase competes with NOS for the common substrate L-arginine, and in addition may regulate cell growth [24]. In rabbits [25] and rats [16,26] relaxation responses of distal vagina to electrical field stimulation were greater compared with morphologically distinct proximal vagina, and were inhibited by NOS [26] and guanylate cyclase [16] inhibitors. These findings suggest a tissue-specific role for NOS in the vagina.
eNOS in Female Sexual Arousal Response
Endothelial NOS (eNOS) is the NOS isoform playing a key role in vascular function and vasodilation. Information regarding eNOS in the female genital tract is very limited. eNOS has been localized to endothelium of the rat, rabbit, and human clitoris and vagina [13,14,27–31]. Spontaneously hypertensive rats exhibit decreased immunoexpression of eNOS in sinusoidal endothelium of the clitoris [32]. Furthermore, diabetic rats exhibit reduced vaginal blood flow in response to pelvic nerve stimulation and reduced vaginal levels of eNOS [5]. Given the importance of endothelial/vascular function in blood flow and vascular homeostasis, it is conceivable that constitutive activation of eNOS in female genital tract structures is important. Endothelial NO may lead to vasodilatation and increases in blood flow involved in perfusion and engorgement of sexual organs in response to a sexual stimulus, and ultimately to the improvement of vascular homeostasis in the female genital tract.
Sex Steroid Hormones in Female Genital Blood Flow
Sex steroid hormones regulate the structure and function of female genital tissues. Several clinical studies reported improvement in the arousal response of surgically postmenopausal women who received estrogen and testosterone supplementation [2]. It is currently believed that the effects of sex steroid hormones in female genital organs are mediated, at least in part, by cytoplasmic and nuclear receptors [33,34].
Androgen Effect on eNOS
Limited data are available regarding the role of androgens in female genital arousal. It has been postulated that androgens may maintain the structure and function of peripheral nerves in the vagina and thus enhance blood flow to the vagina in response to sexual stimulation [33]. In rats, ovariectomy decreased vaginal blood flow in response to pelvic nerve stimulation, which was increased by testosterone [33]. Relaxation of vaginal smooth muscle of ovariectomized rabbits in response to electrical field stimulation was also enhanced by testosterone treatment [35]. Decreased vaginal lubrication in rabbits following ovariectomy was, however, not normalized by testosterone [36]. Treatment of ovariectomized rabbits with test-osterone increased NOS activity and down-regulated arginase activity in the proximal vagina, whereas treatment with dihydrotestosterone increased NOS activity in both the proximal and distal vagina [23]. However, the effect of androgen on eNOS in female genital tract has not been evaluated.
Estrogen Effect on eNOS
Estrogen is critical for maintaining clitoral and vaginal blood flow and vaginal lubrication. Organ bath and in vivo studies showed that chronic ovariectomy decreased NO-dependent relaxation of the rabbit clitoris, which was reversed by estrogen replacement [37,38]. In rats and rabbits, ovariectomy decreased vaginal blood flow in response to pelvic nerve stimulation, while estradiol increased vaginal blood flow [34,38]. Decreased vaginal lubrication following ovariectomy was also normalized by estrogen [39]. In contrast to these findings, organ bath studies with rabbit vaginal tissue demonstrated that estradiol diminished the relaxatory response caused by electrical field stimulation [35].
Estrogen regulates eNOS by genomic mechanisms, involving augmented mRNA transcription and protein synthesis, and by non-genomic mechanisms, which occur without alterations in gene expression.
Genomic Regulation of eNOS by Estrogen in Female Genital Tract Structures
Studies evaluating the effects of estrogen on eNOS protein expression have produced controversial data. In vaginas of intact cycling rats eNOS expression was found to be lowest during diestrus and highest during proestrus, coinciding with low and high levels of circulating estrogens, respectively [30]. In the rat vagina, eNOS expression declined substantially with ovariectomy and increased with estrogen replacement [30]. However, opposite findings have been reported in the rabbit vagina and clitoris: ovariectomy increased, whereas estrogen decreased total NOS activity and eNOS protein expression [27,29,40]. Further studies indicated that this effect of estrogen was limited to the rabbit proximal vagina [23]. The findings imply that the observed differences in NOS activity may be species-specific. In contrast to eNOS activity, vaginal arginase activity was reduced by ovariectomy, and upregulated by estrogen replacement in the distal vagina [23], suggesting differential regulation of NOS and arginase by estrogen in different anatomical regions of the rabbit vagina. The significance of down-regulation of NOS activity and up-regulation of arginase activity by estrogens remains to be determined.
Posttranslational Modification of eNOS by Estrogen Across Vascular Beds
In the past several years complex mechanisms of posttranslational regulation of eNOS activity have emerged, including calcium–calmodulin binding, fatty acid modification, alterations in intracellular translocation, substrate and cofactor availability, dimerization of the enzyme subunits, binding to other proteins (such as caveolin-1 and heat shock protein [Hsp90]), and phosphorylation [41]. They modulate endothelial NO bioavailability without changes in the enzyme’s protein expression. Posttranslational effects of estrogen on eNOS are mediated by the traditional receptors α and β or truncated receptor α, which associate with plasma membrane caveolae [42–44]. In aortic, umbilical, and pulmonary artery endothelial cells estrogen-induced NO release includes activation of eNOS by phosphorylation on Ser-1177 via phosphati-dylinositol 3-kinase/Akt [45–47] and mitogen-activated protein kinase extracellular signal-regulated protein kinase ½ [48,49] signaling pathways. These in vitro findings were further substantiated by in vivo studies showing that both short- and long-term estrogen treatment of ovariectomized rats increased phosphorylation of Akt (Ser-473) and eNOS (Ser-1177), and increased NO production in cerebral blood vessels [50]. These data suggest that estrogen signaling via eNOS phopshorylation may have both short- and long-term homeostatic consequences on vascular function.
Another mechanism by which estrogen may posttranslationally activate eNOS involves stimulation of the binding of the enzyme to its positive regulator Hsp90 and inhibition of the binding of the enzyme to its negative regulator caveolin-1. In human umbilical vein endothelial cells and thoracic aortic rings, estrogen stimulates eNOS interaction with Hsp90, which enhances the enzyme’s activity [51,52]. In COS-7 cells transfected with estrogen receptor a and eNOS, activation of eNOS by estrogen involves decreased binding of eNOS to caveolin-1 [42]. The effect of estrogen on eNOS interaction with allosteric proteins in vivo and its physiologic significance is, however, not known.
Posttranslational Regulation of eNOS by Estrogen in the Vagina
Although multiple modes of eNOS posttranslational regulation by estrogen have been demonstrated mostly in vitro in nongenital endothelial cells, it remains unclear whether these mechanisms have physiologic relevance in female genital structures. We conducted preliminary studies to evaluate whether estrogen regulates eNOS postranslationally in the vagina. We specifically evaluated eNOS phosphorylation on its positive (Ser-1177) and negative (Ser-114) regulatory sites, and eNOS interaction with its major negative regulatory protein caveolin-1 in the vagina of female rats in response to ovariectomy (7 days) and after short-term (2 days) replacement with physiologic-doses (15 µg) of estradiol. We localized phosphoeNOS (Ser-1177) and total eNOS to the sinusoidal and vascular endothelium of the vagina [53]. Estrogen withdrawal decreased phosphoeNOS (Ser-1177) (but not total eNOS) levels in the vagina by approximately 35%, and it was normalized by estrogen replacement [53]. In addition, estrogen withdrawal increased approximately twofold eNOS binding to caveolin-1 (but not caveolin-1 protein expression) in the vagina, whereas estrogen replacement normalized it (Musicki et al., unpublished data). One of the mechanisms of increased association of eNOS with caveolin-1 and consequent inhibition of eNOS activity has been attributed to eNOS phosphorylation at Ser-114 [54]. We found that eNOS phosphorylation on Ser-114 was increased approximately threefold in the vagina by estrogen withdrawal and normalized by estrogen replacement, implying that decreased phosphorylation of eNOS on Ser-114 by estrogen may limit eNOS interaction with its negative regulator caveolin-1 in the vagina (Musicki et al., unpublished data). Decreased eNOS interaction with caveolin-1 is a molecular basis for increased eNOS activation and improved vascular function.
These preliminary data demonstrate that estrogen posttranslationally modulates eNOS in the rat vagina through phosphorylation and by limiting its interaction with caveolin-1 (Figure 1). Estrogen-stimulated eNOS function would conceivably lead to vasodilatation in the vagina in response to a sexual stimulus. Our findings indicate that specific eNOS regulatory mechanisms can be targeted posttranslationally in the female genital tract.
Figure 1.
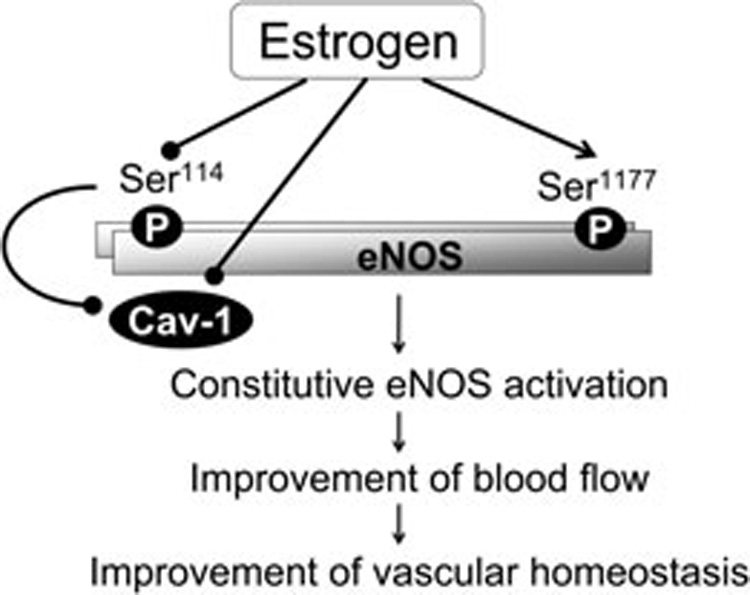
Estrogen posttranslationally modulates endothelial NO synthase (eNOS) in the vagina through phosphorylation and by limiting its interaction with caveolin-1. Estrogen-stimulated eNOS function and constitutive endothelial NO production conceivably leads to vasodilatation in the vagina in response to a sexual stimulus, improvement of blood flow, and improvement of vascular homeostasis. P = phosphorylation.
Conclusions
Modulation of genital tissue hemodynamics and genital arousal responses by sex steroid hormones, and molecular mechanisms by which hormones regulate vaginal and clitoral blood flow via eNOS physiologically and pathophysiologically, are areas that remain poorly defined. Elucidation of molecular mechanisms of action of estrogens in the vagina and clitoris is crucial for better understanding of female sexual function and dysfunction and the overall beneficial effects of estrogen. The importance is further potentiated by a recent controversy regarding the benefits of hormone replacement therapy with respect to risks of stroke and cardio-vascular disease. The exact mechanisms of eNOS regulation in the female genital tract structures warrant further investigation. This includes, among others, elucidation of the role of estrogen plasma membrane receptors α and β, the interaction between estrogen receptors, eNOS, and caveolin-1 in caveolae, the interplay with other sex hormones, and the physiologic relevance of eNOS posttranslational regulation in female sexual arousal.
In addition, scientific data proving the molecular basis for the eNOS defect with aging-associated estrogen deficiency in the vascular bed of the female genital organs is limited. Considering that estrogen increases genital blood flow in postmenopausal women and ovariectomized animals, it is crucial to determine whether eNOS activation mechanisms are impaired by aging-associated estrogen withdrawal, and whether estrogen replacement may rescue the function of eNOS and endothelial function. It is conceivable that estrogen may maintain increased eNOS activity through posttranslational mechanisms, allowing sexual arousal responses to sexual stimuli and improving vascular homeostasis. Furthermore, additional studies are needed to delineate the molecular basis for defective eNOS function in the female genital organs in vascular diseases such as diabetes, hypertension, and hypercholesterolemia. In view of the key role of endothelial NO in smooth muscle relaxation and vasodilation, it is conceivable that deranged eNOS posttranslational modifications may play important roles in the vascular patho-physiology of female genital tract structures.
Acknowledgment
This work was supported by NIH/NIDDK grant DK074826 to B. Musicki.
Footnotes
Conflict of Interest: None declared.
References
- 1.Goldstein I, Berman JR. Vasculogenic female sexual dysfunction: Vaginal engorgement and clitoral erectile insufficiency syndromes. Int J Impot Res. 1998;10(2 suppl):S84–S90. [PubMed] [Google Scholar]
- 2.Giuliano F, Rampin O, Allard J. Neurophysiology and pharmacology of female genital sexual response. J Sex Marital Ther. 2002;28(1 suppl):101–121. doi: 10.1080/00926230252851230. [DOI] [PubMed] [Google Scholar]
- 3.Berman JR. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17:S44–S51. doi: 10.1038/sj.ijir.3901428. [DOI] [PubMed] [Google Scholar]
- 4.Nappi R, Salonia A, Traish AM, van Lunsen RHW, Vardi Y, Kodiglu A, Goldstein I. Clinical biologic pathophysiologies of women’s sexual dysfunction. J Sex Med. 2005;2:4–25. doi: 10.1111/j.1743-6109.2005.20102.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006;6:4–13. doi: 10.1186/1472-6793-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, Azadzoi KM. Vasculogenic female sexual dysfunction: The hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int J Impot Res. 1997;9:27–37. doi: 10.1038/sj.ijir.3900258. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein I, Traish A, Kim N, Munarriz R. The role of sex steroid hormones in female sexual function and dysfunction. Clin Obstet Gynecol. 2004;47:471–484. doi: 10.1097/00003081-200406000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Tarcan T, Park K, Goldstein I, Maio G, Fassina A, Krane RJ, Azadzoi KM. Histomorphometric analysis of age-related structural changes in human clitoral cavernosal tissue. J Urol. 1999;161:940–944. [PubMed] [Google Scholar]
- 9.Min K, Kim NN, McAuley I, Stankowicz M, Goldstein I, Traish AM. Sildenafil augments pelvic nerve-mediated female genital sexual arousal in the anesthetized rabbit. Int J Impot Res. 2000;12(3 suppl):S32–S39. doi: 10.1038/sj.ijir.3900610. [DOI] [PubMed] [Google Scholar]
- 10.Vachon P, Simmerman N, Zahran AR, Carrier S. Increases in clitoral and vaginal blood flow following clitoral and pelvic plexus nerve stimulations in the female rat. Int J Impot Res. 2000;12:53–57. doi: 10.1038/sj.ijir.3900480. [DOI] [PubMed] [Google Scholar]
- 11.Angulo J, Cuevas P, Cuevas B, Bischoff E, Saenz de Tejada I. Vardenafil enhances clitoral and vaginal blood flow responses to pelvic nerve stimulation in female dogs. Int J Impot Res. 2003;15:137–141. doi: 10.1038/sj.ijir.3900985. [DOI] [PubMed] [Google Scholar]
- 12.Cellek S, Moncada S. Nitrergic neurotransmission mediates the non-adrenergic non-cholinergic responses in the clitoral corpus cavernosum of the rabbit. Br J Pharmacol. 1998;125:1627–1629. doi: 10.1038/sj.bjp.0702278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JK, Kim JU, Lee SO, Hwang PH, Yi HK, Kim YG, Nitric Cho KW. oxide-cyclic GMP signaling pathway in the regulation of rabbit clitoral cavernosum tone. Exp Biol Med. 2002;227:1022–1030. doi: 10.1177/153537020222701111. [DOI] [PubMed] [Google Scholar]
- 14.Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, Bonnet S, Haromy A, Archer SL. The neurovascular mechanism of clitoral erection: Nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J. 2004;18:1382–1391. doi: 10.1096/fj.04-1978com. [DOI] [PubMed] [Google Scholar]
- 15.Ziessen T, Moncada S, Cellek S. Characterization of the non-nitrergic NANC relaxation responses in the rabbit vaginal wall. Br J Pharmacol. 2002;135:546–554. doi: 10.1038/sj.bjp.0704481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14:271–282. doi: 10.1038/sj.ijir.3900886. [DOI] [PubMed] [Google Scholar]
- 17.Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. Role of the nitric oxide-cyclic GMP pathway in regulation of vaginal blood flow. Int J Impot Res. 2003;15:355–361. doi: 10.1038/sj.ijir.3901038. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004;171:1357–1361. doi: 10.1097/01.ju.0000109868.19569.d7. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano F, Allard J, Compagnie S, Alexandre L, Droupy S, Bernabe J. Vaginal physiological changes in a model of sexual arousal in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R140–R149. doi: 10.1152/ajpregu.2001.281.1.R140. [DOI] [PubMed] [Google Scholar]
- 20.Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, Traish AM, Ash DE, Christianson DW. Human arginase II: Crystal structure and physiological role in male and female sexual arousal. Biochem. 2003;42:8445–8451. doi: 10.1021/bi034340j. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Southan GJ, Abdelkarim GE, Szabo C, Salzman AL. Topical administration of a novel nitric oxide donor, linear polyethylenimine-nitric oxide/nucleophile adduct (DS1), selectively increases vaginal blood flow in anesthetized rats. Int J Impot Res. 2003;15:461–464. doi: 10.1038/sj.ijir.3901045. [DOI] [PubMed] [Google Scholar]
- 22.Traish A, Moreland RB, Huang YH, Kim NN, Berman J, Goldstein I. Development of human and rabbit vaginal smooth muscle cell cultures: Effects of vasoactive agents on intracellular levels of cyclic nucleotides. Mol Cell Biol Res Commun. 1999;2:131–137. doi: 10.1006/mcbr.1999.0164. [DOI] [PubMed] [Google Scholar]
- 23.Traish AM, Kim NN, Huang YH, Min K, Munarriz R, Goldstein I. Sex steroid hormones differentially regulate nitric oxide synthase and arginase activities in the proximal and distal rabbit vagina. Int J Impot Res. 2003;5:397–404. doi: 10.1038/sj.ijir.3901097. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Meininger CJ, Kelly KA, Hawker JR, Jr, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 25.Oh SJ, Hong SK, Kim SW, Paick JS. Histological and functional aspects of different regions of the rabbit vagina. Int J Impot Res. 2003;15:142–150. doi: 10.1038/sj.ijir.3900986. [DOI] [PubMed] [Google Scholar]
- 26.Onol FF, Ercan F, Tarcan T. The effect of ovariectomy on rat vaginal tissue contractility and histo-morphology. J Sex Med. 2006;3:233–241. doi: 10.1111/j.1743-6109.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoon HN, Chung WS, Park YY, Shim BS, Han WS, Kwon SW. Effects of estrogen on nitric oxide synthase and histological composition in the rabbit clitoris and vagina. Int J Impot Res. 2001;3:205–211. doi: 10.1038/sj.ijir.3900687. [DOI] [PubMed] [Google Scholar]
- 28.Burnett AL, Calvin DC, Silver RI, Peppas DS, Docimo SG. Immunohistochemical description of nitric oxide synthase isoforms in human clitoris. J Urol. 1997;158:75–78. doi: 10.1097/00005392-199707000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Batra S, Al-Hijji J. Characterization of nitric oxide synthase activity in rabbit uterus and vagina: Down-regulation by estrogen. Life Sci. 1998;62:2093–2100. doi: 10.1016/s0024-3205(98)00184-2. [DOI] [PubMed] [Google Scholar]
- 30.Berman JR, McCarthy MM, Kyprianou N. Effect of estrogen withdrawal on nitric oxide synthase expression and apoptosis in the rat vagina. Urology. 1998;51:650–656. doi: 10.1016/s0090-4295(97)00683-3. [DOI] [PubMed] [Google Scholar]
- 31.Uckert S, Oelke M, Waldkirch E, Stief CG, Albrecht K, Troger HD, Jonas U, Andersson KE, Hedlund P. Cyclic adenosine monophosphate and cyclic guanosine monophosphate-phosphodiesterase isoenzymes in human vagina: Relation to nitric oxide synthase isoforms and vaso-active intestinal polypeptide-containing nerves. Urology. 2005;65:604–610. doi: 10.1016/j.urology.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Toblli JE, Cao G, Casabé AR, Bechara AJ. Effects of ACE inhibition and beta-blockade on female genitalstructures in spontaneously hypertensive rats. J Sex Med. 2007;4:1593–1603. doi: 10.1111/j.1743-6109.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 33.Traish AM, Kim SW, Stankovic M, Goldstein I, Kim NN. Testosterone increases blood flow and expression of androgen and estrogen receptors in the rat vagina. J Sex Med. 2007;4:609–619. doi: 10.1111/j.1743-6109.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim SW, Kim NN, Jeong S-J, Munarriz R, Goldstein I, Traish AM. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. J Urol. 2004;172:1538–1543. doi: 10.1097/01.ju.0000137744.12814.2e. [DOI] [PubMed] [Google Scholar]
- 35.Kim NN, Min K, Pessina MA, Munarriz R, Goldstein I, Traish AM. Effects of ovariectomy and steroid hormones on vaginal smooth muscle contractility. Int J Impot Res. 2004;16:43–50. doi: 10.1038/sj.ijir.3901138. [DOI] [PubMed] [Google Scholar]
- 36.Min K, Munarriz R, Kim NN, Goldstein I, Traish A. Effects of ovariectomy and estrogen and androgen treatment on sildenafil-mediated changes in female genital blood flow and vaginal lubrication in the animal model. Am J Obstet Gynecol. 2002;187:1370–1376. doi: 10.1067/mob.2002.126641. [DOI] [PubMed] [Google Scholar]
- 37.Kim HW, Kim S-C, Seo KK, Lee MY. Effects of estrogen on the relaxation response of rabbit clitoral cavernous smooth muscle. Urol Res. 2002;30:26–30. doi: 10.1007/s00240-002-0235-8. [DOI] [PubMed] [Google Scholar]
- 38.Park K, Ahn K, Lee S, Ryu S, Park Y, Azadzoi KM. Decreased circulating levels of estrogen alter vaginal and clitoral blood flow and structure in the rabbit. Int J Impot Res. 2001;13:116–124. doi: 10.1038/sj.ijir.3900655. [DOI] [PubMed] [Google Scholar]
- 39.Min K, Munarriz R, Kim NN, Choi S, O’Connel L, Goldstein I, Traish AM. Effects of ovariectomy and estrogen replacement on basal and pelvic nerve stimulated vaginal lubrication in an animal model. J Sex Marital Ther. 2003;29(1 suppl):77–84. doi: 10.1080/713847131. [DOI] [PubMed] [Google Scholar]
- 40.Al Hijji J, Larsson B, Batra S. Nitric oxide synthase in the rabbit uterus and vagina: Hormonal regulation and functional significance. Biol Reprod. 2000;62:1387–1392. doi: 10.1095/biolreprod62.5.1387. [DOI] [PubMed] [Google Scholar]
- 41.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 42.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 43.Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor α46-kDa isoform in human endothelial cells. Relationship to acute activation of nitric oxide synthase. Circ. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- 44.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 45.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 47.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 48.Joy S, Siow RC, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, Mann GE. The isoflavone Equol mediates rapid vascular relaxation: Ca2+independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006;281:27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 49.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 50.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: Rapid and long-term effects. Mol Pharmacol. 2005;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 51.Russell KS, Haynes MP, Caulin-Glaser T, Rosneck J, Sessa WC, Bender JR. Estrogen stimulates heat shock protein 90 binding to endothelial nitric oxide synthase in human vascular endothelial cells. Effects on calcium sensitivity and NO release. J Biol Chem. 2000;275:5026–5030. doi: 10.1074/jbc.275.7.5026. [DOI] [PubMed] [Google Scholar]
- 52.Bucci M, Roviezzo F, Cicala C, Pinto A, Cirino G. 17-beta-oestradiol-induced vasorelaxation in vitro is mediated by eNOS through hsp90 and akt/pkb dependent mechanism. Br J Pharmacol. 2002;135:1695–1700. doi: 10.1038/sj.bjp.0704641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musicki B, Lagoda G, Burnett AL. The effect of estrogen withdrawal on eNOS phosphorylation in the female genital tract vasculature. J Sex Med. 2007;4(1 suppl):12. [Google Scholar]
- 54.Li C, Ruan L, Sood SG, Papapetropoulos A, Fulton D, Venema RC. Role of eNOS phosphorylation at Ser-116 in regulation of eNOS activity in endothelial cells. Vascul Pharmacol. 2007;47:257–264. doi: 10.1016/j.vph.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


