Abstract
Transient global ischemia induces selective, delayed neuronal death in the hippocampal CA1 and cognitive deficits. Physiological levels of 17β-estradiol ameliorate ischemia-induced neuronal death and cognitive impairments in young animals. In view of concerns regarding hormone therapy in postmenopausal women, we investigated whether chronic estradiol treatment initiated 14 days prior to ischemia attenuates ischemia-induced CA1 cell loss and impairments in visual and spatial memory, in ovariohysterectomized (OVX), middle-aged (9-11 months) female rats. To determine whether the duration of hormone withdrawal affects the efficacy of estradiol treatment, hormone treatment was initiated immediately (0 week), 1 week, or 8 weeks after OVX. Age-matched, OVX and gonadally intact females were studied at each OVX interval. Ischemia was induced 1 week after animals were pretested on a variety of behavioral tasks. Global ischemia produced significant neuronal loss in the CA1 and impaired performance on visual and spatial recognition. Chronic estradiol modestly but significantly increased the number of surviving CA1 neurons in animals at all OVX durations. However, in contrast with previous results in young females, estradiol did not preserve visual or spatial memory performance in middle-aged females. All animals displayed normal locomotion, spontaneous alternation and social preference, indicating the absence of global behavioral impairments. Therefore, the neuroprotective effects of estradiol are different in middle-aged than in young rats. These findings highlight the importance of using older animals in studies assessing potential treatments for focal and global ischemia.
Keywords: cognition, neuroprotection, hippocampus, estrogen, ischemia, object recognition
Introduction
Cerebral ischemia, arising from cardiac arrest in humans or induced experimentally in rodents, results in selective, delayed cell death, particularly of pyramidal neurons in the highly vulnerable CA1 area of the hippocampus (Kirino, 1982; Pulsinelli et al., 1982). The ovarian hormone estradiol affords histological protection in experimental models of global and focal ischemia (Alkayed et al., 2000; Chen et al., 1998; Glendenning et al., 2008; He et al., 2002; Jover-Mengual et al., 2007; Koh et al., 2006; Plahta et al., 2004; Rau et al., 2003) and ameliorates the cognitive deficits associated with ischemic cell death after global ischemia (Gulinello et al., 2006; Plamondon et al., 2006). The majority of these studies investigated estradiol’s neuroprotective properties in young animals (< 6 months), although there is evidence that estradiol retains its beneficial actions in focal ischemia (middle cerebral artery occlusion, MCAO) in middle-aged (9-11 months) (Dubal and Wise, 2001; Wise and Dubal, 2000) and reproductively senescent (16 months) (Alkayed et al., 2000; Toung et al., 2004) female rats. Stroke and cardiac arrest occur more frequently in older individuals, and in women, the risk increases after menopause (Lobo, 2007). In view of recent controversies over the benefits of hormone therapy for postmenopausal women (Espeland et al., 2004; Wassertheil-Smoller et al., 2003), neuroprotection studies after global ischemia should examine whether estradiol is also neuroprotective in the aging brain.
In the single study examining global ischemia in older females, estradiol pretreatment did not protect middle-aged female gerbils from ischemia-induced CA1 pyramidal cell death (De Butte-Smith et al., 2007). Whether the failure of estradiol to protect against ischemia-induced cell death in gerbils is species specific, a reflection of age-related changes in brain responsiveness to estradiol, or a consequence of prolonged hormone withdrawal prior to treatment is unknown. The critical period hypothesis proposes that there is an opportune time window for estradiol to retain its beneficial actions following ovarian hormone withdrawal and that following extended periods of hormone deprivation, estradiol may no longer be efficacious in protecting the brain against various insults such as stroke. Support for this hypothesis comes from both human (MacLennan et al., 2006; Rossouw et al., 2002; Viscoli et al., 2001) and animal (Daniel et al., 2006; Selvamani and Sohrabji, 2008; Suzuki et al., 2007) studies. In large clinical trials such as the Women Estrogen Stroke Trial (WEST) and the Women’s Health Initiative (WHI), no beneficial effects of estradiol (WEST) or premarin (WHI) on stroke incidence were reported (Rossouw et al., 2002; Viscoli et al., 2001). In the WEST trials, postmenopausal women with estradiol treatment had an increased risk of stroke and worse neurological outcomes (Viscoli et al., 2001). Similarly, the WHI study reported an increase in the risk for stroke following estrogen and/or progestin treatment (Wassertheil-Smoller et al., 2003). Notably, many of the women in these clinical trials were postmenopausal for many years prior to the initiation of the hormone treatment. These unexpected negative results might indicate that beneficial effects of estrogen treatment may be lost or reversed with increasing duration of hormone withdrawal.
Research in rodents also suggests that the timing of estradiol administration may be critical. Estradiol treatment ameliorates age-related impairments in working memory in middle-aged female rats (12 months) when initiated immediately following ovariectomy but not when initiated 5 months later (Daniel et al., 2006). As well, estradiol protects young mice from MCAO-induced injury when initiated immediately following OVX but not when initiated after 10 weeks of ovarian steroid deprivation (Suzuki et al., 2007). In a recent study, chronic estradiol reduced cortical infarct volume in young adult females (7-8 weeks old) subjected to MCAO by endothelin-1 application but increased cortical and striatal infarct volumes in middle-aged (9-11 months), reproductively senescent females (Selvamani and Sohrabji, 2008). Thus it remains unclear whether the effectiveness of estradiol treatment in older females depends on age per se, the timing of treatment initiation with respect to reproductive status, or the type of insult (global versus focal ischemia).
The current study investigated whether long-term estradiol at physiological levels attenuates ischemia-induced CA1 cell loss and impairments in recognition memory in middle-aged female rats. We used the same duration and dose of hormone treatment previously shown to decrease ischemia-induced hippocampal neuron loss and associated cognitive dysfunction in young (6-8 week old) females (Gulinello et al., 2006). To test the critical period hypothesis, estradiol treatment was initiated either immediately (0 week), 1 week, or 8 weeks following hormone withdrawal.
Materials and Methods
Animals
This study was conducted in accordance with the National Institutes of Health guidelines for care and use of animals in research, and the protocol was approved by the Animal Care and Use Committee of the Albert Einstein College of Medicine. Female Sprague-Dawley rats (retired breeders, 9-11 months of age) obtained from Charles Rivers (Wilmington, DE) were group housed (3 per cage), maintained in a temperature- and light-controlled environment with a 14h light, and 10h dark cycle, and given food and water ad libitum. We used retired breeders rather than virgin females because the majority of middle-aged women have had at least one pregnancy; therefore, the use of retired breeders is more clinically relevant.
A total of 225 rats entered the study. Of these, 26 died during ischemia surgery, and two animals were excluded because large abdominal cysts were found during ischemia surgery. Twenty-four additional rats were excluded because they failed to show neurological signs of ischemia, either awakening (n=14) or failing to show both complete loss of righting reflex and dilation of the pupils from 1 min after occlusion was initiated until the end of occlusion (n=10). Rats that died during ischemia or failed to show neurological signs of ischemia were not disproportionately represented in any of the age or hormone groups. One cohort of 18 animals from the 8 week OVX interval underwent behavioral testing, but problems with perfusion prevented us from carrying out histological analysis. One cohort of 27 animals from the 1 week OVX interval (13 sham-operated and 14 ischemic) did not undergo behavioral testing but were used for histological analysis. After surgical deaths and the exclusions described above, we collected neuronal survival data from 155 rats. For behavior tests, animals that exhibited pre-ischemia deficits on any behavioral task were excluded from post-ischemia analysis of that task (see description of each task for exclusion criteria and number of animals excluded). Excluded rats were not disproportionately represented in any of the different age or hormone groups.
Ovariohysterectomy (OVX) and estradiol treatment
Daily vaginal smears, monitored for 1 week prior to OVX or sham surgery to verify that animals were not in constant estrus or constant diestrus, revealed that rats had normal 4-5 day estrous cycles. Rats then underwent either OVX or sham surgery under isoflurane (4% induction, 2% maintenance in 70% N2O:30% O2) anesthesia. They received subcutaneous implants (dorsal neck) of either 17β-estradiol pellets (0.1 mg; 60-day time release; Innovative Research of America Inc., Sarasota, FL) or no pellet (sham surgery) either immediately (0 week), 1 week or 8 weeks following OVX. Estradiol pellets were present for two weeks before ischemia and remained in place until the animals were euthanized. These pellets were used so that we could compare the results to numerous reports from our laboratory (Jover-Mengual et al., 2007; Jover et al., 2002; Miller et al., 2005) as well as others (Koh et al., 2006; Plahta et al., 2004; Shughrue & Merchanthaler, 2003) demonstrating that they are effective in ameliorating ischemia-induced hippocampal injury in young male and female rodents. Each OVX interval contained 3 groups of rats (intact [sham OVX], OVX, and OVX+estradiol). Figure 1 illustrates a timeline of experimental procedures and behavioral testing schedule.
Figure 1.
Timeline of experimental procedures and behavioral tests. Day 0 (D0) refers to the day of estradiol or placebo pellet implantation irrespective of OVX duration.
Behavioral Testing
One week following pellet implantation, rats underwent pre-ischemia testing in an open field, object placement, object recognition, spontaneous alternation, and social preference task. Tests were carried out in the order indicated below. All animals were videotaped and tracked using software (Viewer; Biobserve, Bonn, Germany). The experimenter was always blind to the condition of the animals. One week following ischemia or sham surgery, surviving rats were retested. All tasks and the order of tests were identical to those given during pre-ischemia sessions (see Figure 1).
Open field
Open field testing was conducted first, in a square grey box (27 × 27 in) with sides that were 3 ft high. The floor of the box was divided into 9 equal squares. Rats were habituated for 30-45 min to the testing room and placed in the open field for 6 min. The behaviors measured included locomotor activity, assessed by the number of grid crosses and defined as crossing all four paws across one of the grid lines; rearing, a measure of exploration defined as lifting of the upper body and forepaws off the ground; and amount of time (sec) spent in the center square, an indicator of anxiety levels, and defined as having all four paws in the square.
Object placement and object recognition
Two and three days after open field testing, rats were tested on the object placement and object recognition tasks. These memory tests are based on the natural propensity of animals to spend more time exploring a novel rather than a formerly encountered object. The advantage of these tasks is that they are one-trial tasks that do not require food or water deprivation or exposure to stressful conditions (for review, see Dere et al., 2007) and can be repeated in the same subjects in longitudinal tests. Recently, both the object recognition (Gulinello et al., 2006; Plamondon et al., 2006) and object placement (Gulinello et al., 2006) task were used to assess both the functional outcome after global ischemia and potential cognitive benefits of estradiol in young rats.
To assess spatial recognition, rats were placed in the open field with two identical objects, and the duration of exploration of each object (sec) was recorded for 3 min (Trial 1). Exploration was defined as physical contact with the object (rearing toward or on object, placing paws on object, whisking or sniffing object) or orientation and looking at the object from a distance of 2 cm or less. Following a retention interval of 3 min, rats were then placed in the same open field for 3 min (Trial 2) with the same 2 objects except that the location of one of the objects was displaced in space. The time spent exploring both objects was recorded (sec). For each animal, a preference score was calculated as the time spent exploring the displaced object divided by the total time spent exploring both objects multiplied by 100. This preference ratio has been used by us (Gulinello et al., 2006) as well as others (Frye and Walf, 2008) to measure recognition memory. A preference score of 50% corresponds to chance levels and a higher score reflects intact spatial recognition.
To assess object recognition, rats were placed in the open field with two identical objects, and the duration of exploration of each object (sec) was recorded for 3 min (Trial 1). Following a retention interval of 30 min, rats were then placed in the same open field for 3 min (Trial 2) with one of the familiar objects and a novel object. Time spent exploring both objects was recorded. Similar to the spatial task, a preference score was calculated as the time spent exploring the novel object divided by the total time spent exploring both objects multiplied by 100. For both tasks, the arena and objects were cleaned with 70% ethanol before each trial to eliminate olfactory cues.
To be included in data analysis for a particular task, rats had to demonstrate a preference score of 53% or greater on the pre-ischemia test. We chose this criterion for several reasons. First, we conducted extensive validation of the object placement and object recognition tasks, which demonstrated that animals with higher than 53% preference scores consistently demonstrated novel object and placement preferences when retested, whereas animals with lower scores did not. Very few animals fell into the borderline category (51%-53%), in that the majority of excluded animals exhibited preferences <50%. Second, we analyzed the data with a more stringent pre-ischemia criterion (e,g., 58 or 60%); this resulted in more animals being excluded, reducing some groups sizes to the point where statistical analyses could no longer be performed. Animals were also excluded if they were unable to complete the task (did not explore at all) or showed insufficient exploration times (< 2 sec total exploration time) during either sample or test trials of the visual or spatial object recognition tasks. Of the rats that survived ischemia surgery, 136 received post-ischemia testing on the visual and spatial recognition task. Among rats that explored the objects, none were excluded because they had exploration times less than 2 sec. In total, 59 rats were excluded from the visual task (12 of these did not explore on the pre-ischemia test, precluding comparison of pre- and post-ischemia scores), and 74 rats were excluded from the spatial test (4 of these did not explore on the pre-ischemia test, precluding comparison of pre- and post-ischemia scores). There was overlap in exclusions as some rats were excluded from both tasks.
Spontaneous alternation
Rats were tested for spontaneous alternation using a Y-maze one day after object recognition. This task measures exploratory behavior (Deacon et al., 2002; Dember & Fowler, 1959; Lalonde 2002) and requires rats to remember which arms they previously entered in order to alternate successfully. Spontaneous alternation performance was assessed by placing each rat in a grey Y-maze composed of three equally spaced arms (44 cm long × 40 cm high). All rats were placed in the same starting arm of the Y-maze and allowed to explore the maze for 15 min. The sequence and number of arms entered were recorded. The maze was cleaned with 70% ethanol after each rat. For each consecutive triplet entry, it was determined if the rat exhibited a spontaneous alternation pattern, an alternate arm return, or a same arm return. A spontaneous alternation pattern was recorded if the rat entered three different arms consecutively. A same arm return error was recorded if the rat entered two different arms consecutively and then re-visited the first arm or if a rat re-entered an arm immediately after exiting that arm. A percent alternation score was computed by dividing the number of spontaneous alternations made by each rat by the total number of triplets then multiplying that quotient by 100.
One rat was excluded from analysis because she did not complete the task (i.e., stayed in one arm for the entire duration of the test). An additional 7 rats were excluded because they showed a percent alternation score less than 50% at pre-test.
Social preference
One day after the spontaneous alternation task, rats were tested on a social preference task adapted from Engelmann and colleagues (1995). This task measures preference for a conspecific over an inanimate object and is useful for assessing sickness behavior and anxiety-like behavior, as reduced or abolished social preference reliably measures sickness behavior (Bluthé et al., 1992; 1995) and anxiety (File, 1978; 1980). A wire mesh was placed at the end of two of the arms of the Y-maze. Enclosed at the end of one arm was a juvenile female rat while at the other end was a small clear bottle. Because of the wire mesh, rats could not physically touch either the juvenile animal or the small clear bottle. A rat was placed in the start arm and allowed to explore the maze freely for 5 min. The amount of time exploring either the juvenile animal or clear bottle was recorded (sec). Because rats could not physically touch the juvenile or the object, exploration in this case was defined as sniffing, whisking, or rearing toward or on the wire mesh located at the end of the arms. For each animal, a preference score was defined as the time spent exploring the juvenile rat divided by the total time spent exploring the juvenile rat and the object multiplied by 100. A preference score of 50% indicates chance performance while a higher preference score is indicative of intact social preference. To be included in data analysis for this task, rats had to demonstrate a preference score of 53% or greater on the pre-ischemia test. Four rats were excluded from post-test analysis because their pre-test preference score was below 53%.
Global ischemia
Two weeks following pellet implantation or sham surgery, most rats were subjected to global ischemia by four vessel occlusion as previously described by Pulsinelli and Brierley (1979). Briefly, rats were deeply anesthetized under isoflurane (4% induction, 2% maintenance in 70% N2O:30% O2), and the vertebral arteries were irreversibly occluded by electrocoagulation to prevent collateral blood flow to the forebrain during the subsequent occlusion of the common carotid arteries. A 3-0 silk thread was also looped around the carotid arteries to facilitate subsequent occlusion. Twenty-four hr later, the animals were anesthetized again, the wound was reopened and both carotid arteries were occluded with microarterial clamps for 10 min. During clamping, the animals were awake and spontaneously ventilating. During both surgeries, rectal temperature was monitored and maintained at 36.5-37.5°C with a rectal thermistor and heat lamp until recovery from anesthesia. For animals subjected to global ischemia, those that failed to show complete loss of righting reflex and dilation of the pupils from 1 min after occlusion was initiated until the end of occlusion were excluded from the study.
Some rats from the 1 (n=13) and 8 (n=34) week OVX interval were subjected to sham surgery. Sham operated animals were subjected to the same anesthesia and surgical procedures as animals subjected to global ischemia, except the carotid arteries were not occluded. These sham animals included intact, OVX, and OVX+estradiol conditions.
Hippocampal cell counts
After the completion of behavioral testing, on day 12 following ischemia or sham surgery, rats were deeply anesthetized with isoflurane, and blood was collected by cardiac puncture for the measurement of serum estradiol levels (see below). Rats were trascardially perfused using 0.9% saline with heparin followed by ice cold 10% phosphate buffered formalin (Fisher Scientific, Pittsburgh, PA). Brains were removed, placed in formalin at 4°C overnight, fixed in 30% sucrose in phosphate buffered saline at 4°C for 48 hr and then frozen at -80.
Coronal sections (20 μm) were cut at the level of the dorsal hippocampus (3.3-4.0 mm posterior from bregma) and 4 sections per animal at 140 μm intervals were mounted and stained with toluidine blue. The dorsal hippocampus is more vulnerable to ischemic damage than the ventral hippocampus (Akai et al., 2003; Ashton et al., 1989); hence, cells counts were performed only at the dorsal level. Medial, middle, and lateral sectors from the CA1 region of the left and right hippocampus were photographed at 40X magnification using a Nikon microscope and digital camera. As previously described by Colbourne and Corbett (1995) and shown in Figure 1A, a microscope counting grid (250 μm × 250 μm) was positioned a few cells medial from CA2 neurons (lateral sector), at the apex of the CA1 (middle sector) and on the upswing of CA1 in an area clearly distinct from subiculum (medial sector). Digital images were opened in Adobe Photoshop and the number of viable pyramidal neurons in this 250 μm × 250 μm region of interest was counted. Viable neurons had rounded cell bodies and clearly visible nucleoli. Pyknotic and shrunken neurons were not counted. Counts were summated over right and left hemispheres. All cell counts were carried out by an investigator who was blind to the animals’ treatment.
Estradiol assay
Cardiac blood samples from animals at each of the OVX durations were obtained at euthanasia to assess serum estradiol levels. The samples were left to clot overnight at 4°C and then centrifuged (800 × g for 5 min) to obtain the serum. Serum was stored at -20°C and later analyzed using a Coat-a-Count radioimmunoassay kit (Siemens Medical Solutions Diagnostics; Los Angeles, CA). All assays were performed in duplicate, and the mean value was reported. The sensitivity of detection was 8 pg/ml. The inter- and intra-assay coefficients of variance were 8.1% and 7.0%, respectively. Serum estradiol levels are reported from a subset of the animals in this study. We found that the assay previously used successfully in young rats (Jover-Mengual et al., 2007; Gulinello et al., 2006; Miller et al., 2005) did not provide valid results in older animals. Therefore, we only include values from the Coat-a-Count estradiol assay in the statistical analysis.
Statistical analysis
Serum estradiol levels and body weights were analyzed using Statview® with a two-way ANOVA (OVX duration × hormone condition) followed by Newman-Keuls post-hoc test. Body weight (g) was measured on the day of pellet implantation and one week later, at the start of the pre-ischemia behavioral tests. A difference score (in g) was calculated and these difference scores were analyzed by two-way ANOVA.
Cell count data from sham animals were analyzed using a two-way ANOVA (OVX duration × hormone condition) followed by Newman-Keuls. Because there was no effect of hormone condition on cell counts in the sham group, we next compared sham-operated to ischemic animals using a two-way ANOVA (ischemia × hormone condition) followed by Newman-Keuls. After verifying significant cell loss in all ischemic animals, a two-way ANOVA (OVX duration × hormone condition) was performed to determine differences among ischemic groups under different OVX intervals and hormone conditions.
Open field, object recognition(exploration time on training and test trials and DI scores), spontaneous alternation, and social preference data were analyzed with a three-way ANOVA (OVX duration × hormone condition × pre/post) with repeated measures on pre/post, followed by Newman-Keuls. For animals at the 8-week OVX interval only, which included sham-operated animals, a three-way ANOVA (ischemia × hormone condition × pre/post) with repeated measures on pre/post was performed to determine whether there were differences between sham and ischemic groups. Associations between memory performance (preference score) and cell survival were analyzed using Pearson’s correlation coefficient. For all tests, differences were considered significant at p < .05. Because sham-operated animals that were assigned to the intact, OVX, or OVX+estradiol groups were not statistically different in either the histological or behavioral measures, these groups were combined in the graphs and are labeled as “sham”.
Results
Serum estradiol levels and body weight
Serum estradiol levels at the time of euthanasia in animals from each treatment group are shown in Table 1. Estradiol levels were significantly lower in the OVX compared to the intact and OVX+estradiol groups [F(2,101)= 9.2, p<.001] and were commensurate with our previous reports (Miller et al., 2005; Gulinello et al., 2006). Serum estradiol was in the physiological range in both the intact and OVX + estradiol animals, and these two groups did not differ significantly. There was no effect of OVX duration [F(2,101)=1.8, p>.05] or interaction between hormone condition and OVX duration [F(4,101)=1.3, p>.05].
Table 1.
Serum estradiol levels in the intact, OVX, and OVX+ estradiol treated groups at 0, 1, and 8 week OVX durations.
| Group | 0 week (n) | 1 week (n) | 8 week (n) |
|---|---|---|---|
| Intact | 44.6 ± 7.4 (11) | 23.4 ± 4.6 (9) | 39.7 ± 5.2 (18) |
| OVX | 19.5 ± 2.4(13)* | 17.1 ± 2.6 (8)* | 22.8 ± 2.4 (12) * |
| OVX+E | 34.0 ± 4.4 (12) | 41.0 ± 8.4 (10) | 48.4 ± 8.3 (17) |
Values are mean serum estradiol levels in pg/ml ± S.E.M.
p<.01 compared to OVX+E and intact groups, ANOVA followed by Newman-Keuls.
Estradiol reduces food intake and body weight in both intact and OVX female rats (McElroy et al., 1987; Santallo et al., 2007). Therefore, to confirm the physiological effects of hormone treatment, body weight was measured on the day of pellet implantation and one week later, at the start of the pre-ischemia behavioral tests. Therefore, a positive difference score reflects weight gain, while a negative score reflects weight loss. Difference scores were analyzed by ANOVA. There was a significant main effect of hormone condition [F(2,102) = 66.7, p<.0001]. As shown in Table 2, OVX rats treated with estradiol lost weight after pellet implantation, as evidenced by negative difference scores, while non-treated OVX rats maintained or gained weight (Newman-Keuls, p<.01). Although intact rats showed a small negative difference score at 0 and 8 weeks post-OVX, indicative of a loss of weight in these animals after sham surgery, the extent of weight loss was significantly less compared to the OVX+estradiol rats (Newman-Keuls, p<.01).
Table 2.
Impact of OVX and estradiol (E) treatment on body weight at 0, 1, and 8 week OVX durations.
| OVX duration | W1 (g) | W2 (g) | Difference (W2-W1) |
|---|---|---|---|
| 0 week | |||
| Intact (n=11) | 382.3 ± 8.2 | 379.1 ±8.6 | -3.1 ±3.1 |
| OVX* (n=13) | 374.8 ± 8.7 | 382.85 ± 9.1 | 8.0 ± 2.7 |
| OVX+E**(n=12) | 383.0 ± 8.5 | 360.75 ± 7.6 | -22.3 ± 4.2 |
| 1 week | |||
| Intact (n=9) | 402.5 ± 8.3 | 404.6 ± 10.6 | 2.1 ± 3.5 |
| OVX* (n=8) | 392.3 ± 11.6 | 407.6 ± 13.0 | 15.2 ± 2.5 |
| OVX+E**(n=10) | 388.3 ± 12.9 | 377.0 ± 13.1 | -11.3 ± 2.8 |
| 8 week | |||
| Intact (n=18) | 397.2 ± 17.4 | 393.5 ± 17.0 | -3.7 ± 3.4 |
| OVX* (n=12) | 450.0 ± 19.3 | 450.1 ± 20.9 | .08 ± 2.7 |
| OVX+E**(n=17) | 479.5 ± 7.9 | 446.9 ± 7.0 | -32.5 ± 1.9 |
Values are mean weights ± S.E.M. at time of pellet implant (W1) and Day 1 of pre-test (W2) for intact, OVX, and OVX+E rats at the 0, 1, and 8 week OVX duration. Difference scores represent W2-W1 and these scores were subjected to statistical analysis (ANOVA followed by Newman-Keuls).
p< .01 compared to intact and OVX+E rats.
p< .01 compared to intact and OVX rats
Estradiol replacement modestly reduces CA1 cell loss after global ischemia
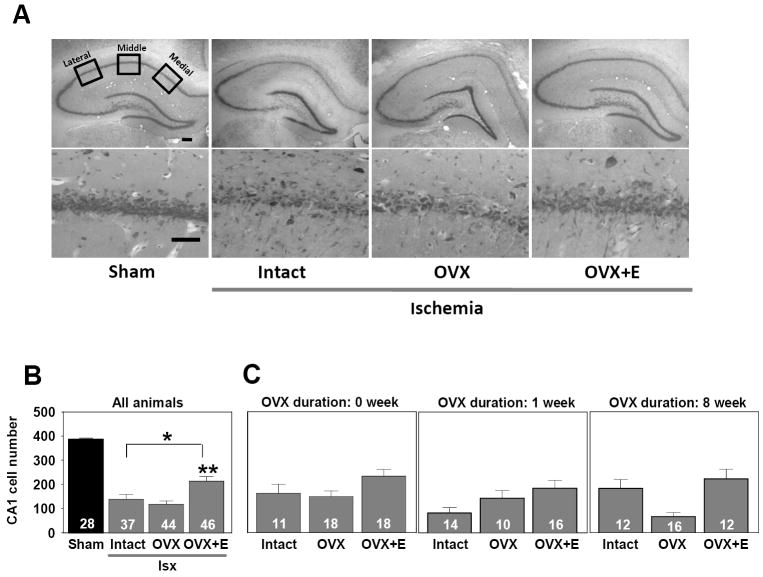
Hippocampal CA1 cell counts were performed after completion of behavioral testing following perfusion at 13 days post-ischemia. Sham-operated controls were included at the 1 and 8 week OVX durations; 0 week animals were not included as it is unlikely that there would be significant differences in pyramidal cell number between animals given pellets immediately after OVX or 1 week later. As noted previously, the sham-operated rats in the intact, OVX, and OVX+ estradiol groups were analyzed statistically as separate groups, but because they did not differ [F(2,22)=.44, p>.05], the data for all sham-operated animals at all OVX durations were combined on the figure.
As previously reported, ischemia induces substantial (50-70%) CA1 cell death as indicated by a significant main effect of ischemia on CA1 cell counts [F(1,149) = 111.2, p<.0001]. As illustrated in Figure 2, ischemia induced significant loss of CA1 pyramidal neurons in all animals at all OVX durations and hormone treatments when compared to sham-operated controls (Newman-Keuls, p<.01 for each post hoc comparison). As compared to sham-operated rats, a 64%, 70%, and 45% loss of CA1 neurons was observed following global ischemia in the intact, OVX, and OVX+estradiol rats, respectively. There was a significant main effect of estradiol administration on cell loss [F(2,118)=8.6, p< .001], with OVX+ estradiol animals exhibiting significantly more surviving cells than OVX or intact rats. Estradiol increased cell survival by 46% and 36% relative to the OVX and intact groups subjected to ischemia, respectively (Newman-Keuls, p<.01 for both comparisons). The number of surviving pyramidal neurons in CA1 did not differ between the intact and OVX animals (Newman-Keuls, p>.05), despite the fact that endogenous levels of estradiol in the intact rats were higher than in the OVX rats. There was neither a significant main effect of OVX duration [F(2,118)=1.8, p>.05] nor a significant interaction between hormone condition and OVX duration [F(4,118)=2.2, p>.05] on CA1 pyramidal neuron survival.
Figure 2.
Estradiol replacement modestly reduces CA1 cell loss in OVX, middle-aged females after global ischemia (Isx). (A) Representative photomicrographs from the 1 week OVX interval of intact, OVX, and OVX+estradiol (E) middle-aged female rats subjected to global ischemia or sham surgery. At 12 days after reperfusion, viable neurons in 4 toluidine blue stained sections containing the dorsal hippocampal CA1 were counted in 3 sectors (lateral, middle, and medial). Scale bars, Lower magnification (4X), 400 μm; higher magnification (40X), 60 μm. (B) Data represent a grand sum across 3 counting sectors and over right and left hemispheres. Because no statistical difference was found among the different sham groups (intact, OVX, OVX+E) at 1 and 8 weeks after OVX), these animals were pooled into one sham group. Global ischemia induced CA1 cell loss vs sham-operated animals. Treatment with E significantly increased CA1 cell survival. * p< .01 compared to sham-operated rats, ** p < .01 compared to intact and OVX rats. (C) There was no impact of OVX duration on CA1 cell survival.
Visual and spatial recognition memory were not preserved by chronic estradiol treatment
To examine ischemia-induced deficits in cognition and the ability of estradiol to ameliorate these deficits, we tested animals for spatial and visual memory using the object placement and object recognition tasks, respectively. Rats were pre-tested one week before ischemia or sham surgery and were then retested one week after ischemia. Approximately 44% and 28% of the middle-aged rats were unable to perform above chance level (preference score >53%) in the spatial and visual memory task, respectively, even before ischemia or sham surgery. Animals that could not perform these tasks were distributed among all OVX durations and hormone treatments. To evaluate ischemia-induced memory impairments, only animals that could perform the task prior to ischemia or sham surgery were included in the statistical analysis of ischemia effect. Importantly, the pattern of results does not change if post-ischemia behavioral performance is analyzed statistically without excluding these animals (see Supplemental Figure 1). However, because our objective was to use the rats’ own baseline to determine if ischemia induced deficits and if estradiol could prevent these deficits, inclusion of rats that were unable to do the task prior to ischemia could have been misleading.
Object recognition
Neither OVX duration [F(2,55)=1.7, p>.05] nor hormone treatment [F(2,55)=.4, p>.05] altered the extent of object exploration during training Trial 1 (see Table 3). All rats explored both objects for similar amounts of time during this trial (data not illustrated). However, ischemia had a significant impact on mean object exploration time. The mean total time spent exploring the objects during training post-ischemia was significantly greater than the time spent exploring the objects pre-ischemia (ANOVA, [F(1,55)=33.6, p<.0001]) (Table 3).
Table 3.
Total exploration time (s) during trial 1 and test trial 2 at pre and post test on the visual working memory task.
| OVX Duration | Trial 1 | Trial 2 | ||
|---|---|---|---|---|
| Pre | Post* | Pre | Post* | |
| 0 week | ||||
| Intact | 14.8 ± 1.7 | 22.1 ± 4.1 | 18.4 ± 2.0 | 25.6 ± 3.7 |
| OVX | 14.9 ± 2.4 | 22.0 ± 1.6 | 19.3 ± 1.9 | 27.6 ± 2.7 |
| OVX+E | 17.9 ± 2.2 | 22.3 ± 2.6 | 22.4 ± 2.6 | 25.2 ± 3.8 |
| 1 week | ||||
| Intact | 13.6 ± 4.9 | 26.6 ± 1.7 | 17.4 ± 4.3 | 22.4 ± 7.7 |
| OVX | 18.0 ± 1.5 | 22.1 ± 3.0 | 18.9 ± 3.3 | 23.8 ± 4.4 |
| OVX+E | 11.0 ± 3.3 | 14.6 ± 2.5 | 13.0 ± 2.8 | 21.3 ± 7.7 |
| 8 week | ||||
| Sham | 17.0 ± 1.8 | 24.9 ± 2.8 | 19.3 ± 1.7 | 27.4 ± 2.4 |
| Intact | 12.9 ± 1.6 | 20.3 ± 2.9 | 19.3 ± 2.3 | 21.8 ± 1.7 |
| OVX | 10.4 ± 1.7 | 16.7 ± 3.8 | 15.3 ± 3.9 | 22.5 ± 5.7 |
| OVX+E | 7.8 ± 1.0 | 23.1 ± 5.2 | 7.7 ± 2.0 | 22.9 ± 2.4 |
Data represent mean exploration times ± S.E.M. All rats showed significantly greater exploration times at post-test compared to pre-test during both trial 1 [ F(1,55)= 33.6, p<.0001] and trial 2 [F(1,55)=19.2, p<.0001]
denotes p<.001
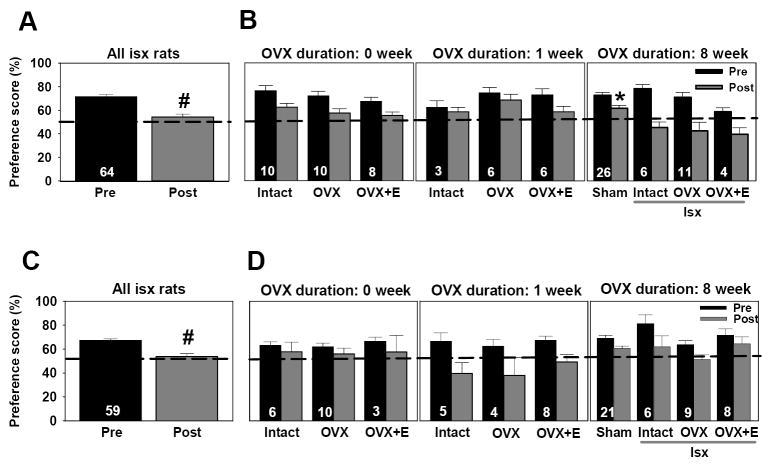
We next compared pre-test and post-test visual preference scores for ischemic and sham operated rats to determine the impact of ischemia and sham surgery on visual memory at the 8 week OVX duration (Figure 2B). Ischemia significantly impaired visual working memory performance (pre/post [F(1,41) = 24.4, p<.0001]) indicated by the fact that all groups showed lower preference scores at post-test compared to pre-test. There was also a significant interaction between ischemia and pre/post [F(1,41) = 4.6, p<.04]. While the pre-test performance of animals subjected to ischemia did not differ from sham-operated rats, they differed on the post-test (Newman-Keuls, p<.01). As illustrated in Figure 3B, sham-operated rats showed modestly lower preference scores on the post-test than on the pre-test, but the preference scores of 79% of these animals were still above chance (>53%). This contrasts with the 55% of ischemic animals that had post-test preference scores that were below chance (<53%).
Figure 3.
Visual and spatial recognition memory performance was not preserved after chronic estradiol treatment. (A) Visual memory was assessed by the object recognition task. Data are reported as preference scores (novel object exploration/total object exploration, %, X ± S.E.M.) for 3-min trials. There was a retention interval of 30-min between training and test trials. Black dotted line at 50% represents equal exploration of both objects (chance performance). Only animals that were able to do the task pre-ischemia (preference >53%) were included in post-test analysis. Ischemic rats showed significantly lower preference scores at post-test compared to pre-test. # p< .05. (B) Ischemic rats exhibited significantly impaired visual recognition memory relative to sham-operated controls. Ischemia-induced deficits in visual memory were significantly greater at the 8 week OVX duration compared to either the 0, or 1 week OVX duration (p<.01). * p< .05 compared to all ischemic rats. (C) Ischemic rats showed significantly lower preference scores at post-test compared to pre-test. # p<.05. (D) There was no impact of estradiol treatment on spatial memory.
To determine whether hormone condition and OVX duration modified visual memory performance on the post-test in ischemic animals, we compared preference scores of intact, OVX, and OVX+estradiol rats at all OVX intervals. As illustrated in Figure 3A and described above, all ischemic groups showed a significant decline in visual memory performance at post-test compared to pre-test [F(1,55)= 22.1, p<.0001]. Duration of OVX also impacted working memory performance [F (2,55)= 6.1, p<.01]. Ischemic rats from the 8 week OVX interval exhibited significantly lower preference scores in the visual memory task compared to rats at either the 0 or 1 week OVX durations (Newman-Keuls, p<.05 for both durations). There was no effect of hormone condition [F(2,55)=1.8, p>.05] or an interaction between OVX duration and hormone condition [F(4,55)=1.4, p>.05] (Figure 3B). Thus, estradiol administration did not significantly preserve visual memory performance in middle-aged, OVX rats subjected to global ischemia.
We also assessed whether CA1 pyramidal cell survival correlated with visual memory performance on the post-test in sham-operated and ischemic rats. No correlation was found between the number of surviving neurons and preference scores (r= -.04, p>.05). As well, no correlation was found between total exploration times and preference score for either the training (r=.09, p>.05) or test (r=.05, p>.05) trials. Thus, the absolute amount of time rats spent exploring the objects did not affect their preference for a novel object.
Object placement
The overall pattern of results in the spatial memory test (object placement task) (Figure 3C, 3D) was very similar to that for object recognition. To determine whether ischemia, hormone condition and OVX duration modified spatial memory performance on the post-test in ischemic animals, we compared preference scores of intact, OVX, and OVX+estradiol rats at all OVX intervals. As illustrated in Figure 3C, all ischemic groups showed a significant decline in spatial memory performance at post-test compared to pre-test [F(1,50)= 22.4, p<.0001]. Duration of OVX also impacted spatial memory performance [F (2,50)= 4.9, p<.05]. Ischemic rats from the 8 week OVX interval exhibited significantly higher preference scores in the spatial memory task compared to rats at the 1 week OVX duration (Figure 3D; Newman-Keuls, p<.05). There was no effect of hormone condition [F(2,50)=2.1, p>.05] or an interaction between OVX duration and hormone condition [F(4,50)=.7, p>.05] (Figure 3D). Although it appeared that estradiol might have attenuated the effects of ischemia on spatial memory in the 8 week OVX group, a separate three-way ANOVA on the entire 8 week cohort, which included sham-operated animals, showed that there was no significant difference in performance between sham-operated and ischemic animals [F(2,38) = .2, p > .05] nor was there an interaction between ischemia status and post-test performance [F(2,38)=.7, p>.05]. Thus, estradiol administration did not significantly preserve spatial memory performance in middle-aged, OVX rats subjected to global ischemia.
Similar to the visual memory task, no correlation was found between preference scores on the spatial memory task and the number of surviving CA1 cells (r=.186, p=.13). Similar to the visual task, no correlation was found between total exploration times and preference score during either training (r=.04, p>.05) or testing (r=.01, p>.05) trials.
Exploration but not locomotion in the open field test is affected by ischemia in middle-aged female rats
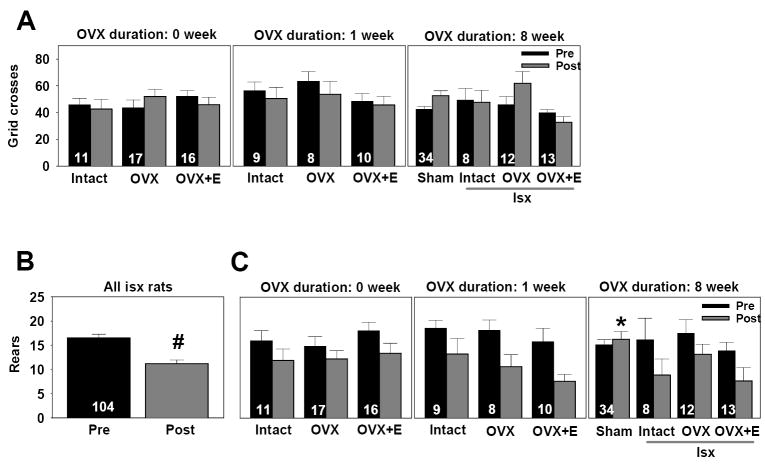
To test whether chronic estradiol treatment and/or ischemic damage produces global behavioral deficits, we assessed locomotor activity and exploration in the open field. We first compared locomotor activity (grid crosses) at pre-test and post-test of ischemic and sham-operated rats at the 8 week OVX interval. There was no impact of hormone condition [F(2,61)=2.3, p>.05], or ischemia [F(1,61)=.09, p>.05] on locomotor activity. As illustrated in Figure 4A, regardless of hormonal status, both sham-operated and ischemic rats showed similar locomotor activity. Further comparisons of the intact, OVX, and OVX+estradiol groups at all OVX durations did not reveal any significant differences in locomotor activity as a function of hormonal condition [F(2,94)=2.3, p>.05] or OVX duration [F(2,94)=.03, p>.05], and there were no significant interactions [F(4,94)=1.1, p>.05]. As well, there was no significant main effect of pre/post [F(1,94)=.57, p>.05] or any significant interactions between pre/post and hormone condition [F(2,94)=2.0, p>.05] or OVX duration [F(2,94)=.03, p>.05]. Hence, there was no impact of long-term OVX, hormone condition, or ischemia on locomotor activity.
Figure 4.
Exploration but not locomotor activity is affected by ischemia in middle-aged female rats. (A) Locomotor activity was assessed as the number of grid crosses in the open field. Data are presented as X ± S.E.M. Neither global ischemia, OVX duration, nor estradiol altered locomotor activity in the open field. (B) Exploration of the open field was assessed as the number of rears in the open field. Data are presented as X ± S.E.M. All ischemic rats reared significantly less at post-test compared to pre-test. # p<.01 (C) Ischemic rats reared significantly less relative to sham-operated controls at the 8 week OVX interval. Regardless of hormonal status or OVX duration, all rats subjected to global ischemia reared less often at post-test compared to pre-test.* p<.05 compared to ischemic rats.
Analysis of exploration (rears) at pre-test and post-test of ischemic and sham-operated rats at the 8-week OVX interval, the only duration in which sham subjects were represented, revealed a significant main effect of pre/post [F(1,61) = 5.8, p<.02] and a significant interaction between ischemia and pre/post [F(1,61) = 12.3, p<.001]. While sham-operated and ischemic rats did not differ in the number of rears on the pre-test, ischemic rats exhibited significantly fewer rears than sham-operated rats during the post-test (Newman-Keuls, p<.05). We further compared pre and post-ischemia exploration as a function of hormone condition and OVX duration in ischemic rats at all hormone durations. Ischemia significantly reduced rearing [F(1,95) = 44.6, p<.0001], but there was no significant effect of hormone condition [F(2,94)=.70, p>.05] or OVX duration [F(2,94)=.54, p>.05] and no interactions between pre/post and either hormone condition [F(2,94)=.23, p>.05] or OVX duration [F(2,94)=1.2, p>.05]. As illustrated in Figure 4B, regardless of hormonal status or OVX duration, all rats subjected to global ischemia reared less often at post-test than at pre-test (Newman-Keuls, p<.01).
Because object recognition involves exploratory behavior (i.e., object exploration), a correlational analysis was performed to determine whether the number of rears on the post-ischemia test was associated with visual memory performance (preference score) on the post-test. No significant relationship was found (r=.19, p=.14), indicating that impaired performance was unlikely to be due to inadequate object exploration. This conclusion is strengthened by the observation that the time animals spent exploring the objects on the post-ischemia test was greater than the time spent exploring the objects on the pre-test (Table 3). Similar to the visual task, no correlation was found between total exploration times and preference score during either training (r=.04, p>.05) or testing (r=.01, p>.05) trials.
Spontaneous alternation and social preference are not altered by global ischemia or chronic estradiol treatment in middle-aged female rats
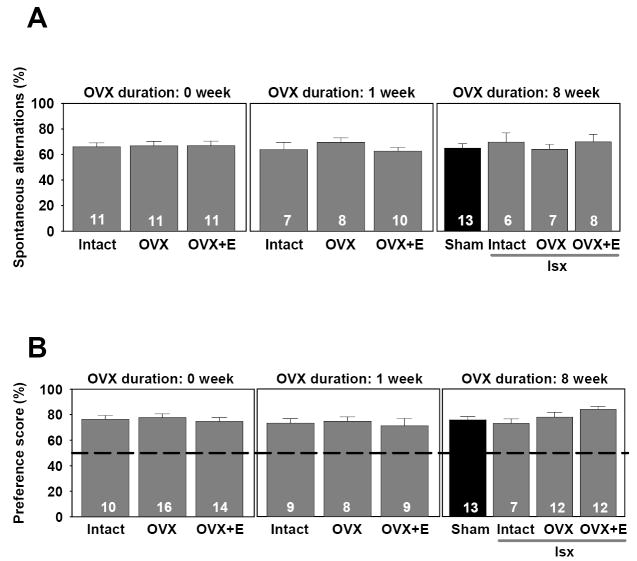
Because global ischemic injury could conceivably alter other behaviors, we tested animals on a spontaneous alternation task using a Y-maze as well as a social preference task. Spontaneous alternation deficits are not reliably induced observed after selective CA1 damage, but rather are evident only when more widespread damage occurs, involving the prefrontal cortex, septum, and basal forebrain (Lalonde, 2002). Neither ischemia, OVX duration nor hormone condition altered the high spontaneous alternation predictably exhibited by rats, a finding that is consistent with specific and restricted cell death in the CA1. ANOVAs comparing pre-ischemia and post-ischemia spontaneous alternation and social preference scores revealed no significant main effects of pre/post, hormone condition or OVX duration and no interactions between pre/post and either OVX duration or hormone condition. Therefore, only post-test data are presented in the figure for both spontaneous alternation and social preference.
Figure 5 shows the percentage of spontaneous alternation patterns exhibited on the Y-maze by sham-operated (8 week OVX) and ischemic rats at all OVX durations. Both sham-operated and ischemic rats exhibited similar, high levels of spontaneous alternation after surgery. Hormone treatment and OVX duration had no impact on spontaneous alternation as there was no significant main effect of ischemia [F(1,28)=.56, p>.05], hormone treatment [F(2,70)=1.0, p>.05] or OVX duration [F(2,70)=.23, p>.05] and no interactions [F(4,70]=.62, p>.05].
Figure 5.
Spontaneous alternation and social preference are not altered by global ischemia or chronic estradiol treatment in middle-aged female rats. (A) For spontaneous alternation, rats were placed on the Y-maze for 15 min and the sequence and number of arms entered were recorded. For each consecutive triplet entry, it was determined if the rat exhibited a spontaneous alternation pattern. Data are reported as percentage scores (± S.E.M.) that were computed by dividing the number of alternations made by each rat by the total number of triplets then multiplying that quotient by 100. Because no differences were found between pre-test and post-test scores for either sham-operated or ischemic groups, only post-test data are illustrated. (B) For social preference tests, rats were placed in the start arm of a Y-maze and were allowed to freely explore for 5 min. Enclosed at the end of one arm was a juvenile female rat while at the other end was a small clear bottle. The amount of time exploring either the juvenile animal or clear bottle was recorded (sec). For each animal, a preference score was defined as the time spent exploring the juvenile rat divided by the total time spent exploring the juvenile rat and the object. Data are reported as mean percentage ± S.E.M. Because no significant differences were found between pre- and post-test scores for either sham-operated or ischemic rats, only post-test data are illustrated.
Social preference scores of sham-operated (8 week OVX) and ischemic rats at all OVX durations were compared to determine the impact of ischemia on social preference. Here, there was no significant main effect of ischemia [F(1,38)=.80, p>.05] or a significant interaction between ischemia and hormone condition [F(2,38)=1.1, p>0.5] on preference scores. As shown in Figure 5, both sham operated and ischemic rats exhibited a strong preference for a juvenile animal compared to an inanimate object. Further comparisons of intact, OVX, and OVX+estradiol rats at all OVX durations did not reveal a significant main effect of hormone condition [F(2,88)=.44, p>.05], OVX duration [F(2,88)=1.5, p>.05] or interaction between hormone condition and OVX duration [F(4,88)=1.1, p>.05], indicating that social preference, like spontaneous alternation, was not influenced by either hormone status or OVX duration.
Discussion
Estradiol increases neuron survival but not cognitive performance in middle-aged rats
Our results demonstrate that one cannot extrapolate findings in young females pretreated with estradiol and subjected to transient global ischemia to middle-aged females given similar hormone therapy. Pretreatment with physiological levels of estradiol provided a statistically significant rescue of CA1 pyramidal neurons in middle-aged females assessed approximately 2 weeks after global ischemia. However, the increase in neuron survival was much more variable than in most reports in young females (e.g., Jover-Mengual et al., 2007; Miller et al., 2005; Plamondon et al., 2006). About 50% of CA1 pyramidal neurons survived in estradiol-treated rats when compared to sham-operated females, which is somewhat lower than the approximately 60% survival rate in most of our young estradiol-treated animals (Miller et al., 2005; Jover-Mengual et al., 2007). Additionally, Plamondon and colleagues (2006) reported that estradiol-treated animals showed 73.5% CA1 neuronal survival compared to shams as long as 6 months after ischemia. Thus, it is plausible that age may reduce the ability of estradiol to attenuate global ischemia-induced hippocampal cell death in female rats. However, estradiol continues to provide significant histological preservation of CA1 neurons in middle-aged female rats, which stands in contrast to our recent report that estradiol failed to preserve CA1 neurons in middle-aged gerbils subjected to 2-vessel occlusion (De Butte-Smith et al., 2007).
Of perhaps greater clinical relevance is the current finding that the significant increase in CA1 pyramidal neuron survival is not correlated with improved performance on spatial and visual recognition memory tests. These results are in stark contrast with reports from our own (Gulinello et al., 2006) and other (Kondo et al., 1997; Plamondon et al., 2006) laboratories that estradiol pretreatment significantly attenuates ischemia-induced deficits in visual and spatial memory in young animals, even in rats with massive loss of CA1 pyramidal neurons (Gulinello et al., 2006; Kondo et al., 1997). The failure of estradiol to improve recognition memory in middle-aged rats after ischemia is unlikely to be attributable to gross behavioral impairments caused by damage to brain structures other than the hippocampus as these animals showed similar locomotor activity and social preference when compared to shams or to their own pre-ischemia test performance. As well, they demonstrated normal levels of spontaneous alternation. The middle-aged rats in this study also showed similar object exploration times (Table 3) as younger females of the same strain (Gulinello et al., 2006), suggesting that lower preference scores were not a result of lower exploration or lack of interest in the objects. Likewise, there was no correlation between the number of surviving CA1 pyramidal neurons and performance on recognition memory tasks. This latter observation agrees with previous reports that cell number does not correlate with behavioral outcome after global ischemia (Gulinello et al., 2006; Kondo et al., 1997; Nunn et al., 1994; Wahl et al., 1992).
We cannot be certain that age is the only factor that contributed to the failure of estradiol to preserve hippocampal-dependent cognitive function after global ischemia. To more closely model the human situation, wherein the majority of middle-aged women have had at least one pregnancy, the present experiments used retired breeders. However, the young females in our earlier study were all virgins (Gulinello et al., 2006). Parity can affect hippocampal function (Kinsley et al., 2006; Tomizawa et al., 2003) and cognition (Kinsley et al., 1999; Macbeth et al., 2007; Paris and Frye, 2008) in rodents. Therefore, we cannot discern what effects, if any, parity may have had on the behavioral and hippocampal responses to estradiol and ischemia.
Present findings reinforce our previous suggestion that distinct mechanisms may underlie the neuroprotective effects of estradiol in global and focal ischemia. In agreement with observations in middle-aged females subjected to MCAO (Wise and Dubal, 2000), estradiol reduces the extent of neuron loss after global ischemia in middle-aged animals. However, studies with knockout mice implicate estrogen receptor-α as the exclusive mediator of neuroprotection in animals subjected to MCAO (Dubal et al., 2001). In contrast, our data (Miller et al., 2005) and other laboratories (Carswell et al., 2004; Shughrue & Merchanthaler 2003) suggest that both estrogen receptor isoforms contribute to increased neuron survival after global ischemia. The ability of physiological levels of estradiol to reduce infarct size after MCAO is lost when young animals are OVX for 10 weeks prior to insult (Suzuki et al., 2007). Our results indicate that estradiol retains a significant ability to reduce global ischemia-induced hippocampal cell death in middle-aged rats even when hormone administration does not begin until 8 weeks after hormone withdrawal.
Cognitive performance in middle-aged females
On the pretests performed prior to global ischemia or sham surgery, 28% and 44% of middle-aged rats showed poor performance in the visual and spatial recognition tasks, respectively. This finding is consistent with previous reports that both visual (de Lima et al., 2005; 2007; Wallace et al., 2007) and spatial (Cavoy & Delacour, 1993; Soffie et al., 1992; Shukitt-Hale et al., 2001) object recognition may decline with age. Our finding that some but not all rats exhibited behavioral deficits at pre-test is consistent with reports by Gallagher (1988) that older rats can be subdivided into cognitively impaired and unimpaired groups. Although animals in some of the earlier studies were much older (18-24 months) than our rats, the pattern was similar. That is, some aged rats exhibit age-related impairments in learning and memory, whereas other aged rats are comparable to their younger counterparts. Pre-test performance in animals at the 8 week OVX duration was equal to or even better than in animals at the 0 or 1 week OVX intervals. The 8 week animals were, on average, two months older than rats in the other groups, suggesting that age is not the only explanation for the observed deficits. It is possible that poor performance of middle-aged females on these tasks reflects their slow recovery from the OVX or sham surgery. If so, then the object recognition and placement tests may be especially sensitive to subclinical inflammation or other aspects of surgical stress. However, the animals were healthy enough to perform well in a social preference test, a reliable indicator of sickness behavior (Bluthé et al., 1992). The greater vulnerability of older animals to surgical stress may also explain the reduced levels of rearing in the open field after ischemia and modestly lower preference scores of shams on the visual test after ischemia.
Both aging and global ischemia can modulate important features of hippocampal circuitry required for intact cognitive function. Long-term potentiation is impaired in aged rodents (Barnes, 2003; Deupree et al., 1993; Rex et al., 2005; Rosenzweig & Barnes, 2003; Shankar et al., 1998) and in young rodents subjected to global ischemia (Aoyagi et al., 1998; Dai et al., 2007). Theta rhythm is also important for normal hippocampal functioning and is impaired in both aged (Watabe & O’Dell, 2003) and ischemic (Mariucci et al., 2003; Monmaur et al., 1986) rodents. It is plausible that because many anatomical, endocrine and neurophysiological changes occur independently as a result of age and global ischemia, the combination of the two results in cognitive injury that is difficult to reverse in older females.
Exogenous but not endogenous estradiol reduces ischemia-induced CA1 neuron loss
An unexpected finding was that estradiol levels were similar in gonadally intact and OVX+ estradiol animals, but that only OVX animals supplemented with exogenous estradiol showed significantly greater cell survival than OVX controls. One possible explanation is that in OVX rats implanted with pellets, estradiol is continuously released so that cells are constantly exposed to the hormone for the duration of the experiment. Intact rats, in contrast, are likely to experience greater fluctuations in their estradiol levels over time. This possibility is supported by the significant difference in weight loss between intact and OVX, estradiol-treated rats. Female rats continue to produce significant levels of ovarian estradiol as they age (Chakraborty & Gore, 2004; LeFevre & McClintock, 1988), but little is known about the pattern of estradiol release in this population. Estradiol is also synthesized in adult mammalian hippocampal neurons (Hojo et al., 2008; Mukai et al., 2006) and may be altered in older rodents. We did not monitor vaginal cytology in the intact rats after they were assigned to treatment groups, so we cannot determine the degree of variation in endogenous hormones.
Additionally, it is possible that other ovarian or adrenal secretions such as progesterone may have impacted the response to endogenous estradiol in intact animals. A recent study suggests that progesterone may attenuate the beneficial actions of estradiol in middle-aged females. Carroll et al. (2008) reported that estradiol treatment protected hippocampal CA3 neurons from kainate-induced injury in middle-aged (9 months) female rats; however, this effect was blocked if progesterone was also administered. On the other hand, exogenous administration of progesterone alone (Alkayed 2000; Cervantes et al., 2002; Gonzalez-Vidal et al., 1998; Frye and Walf, 2008) or in conjunction with estradiol (Dohanich et al., 1994; Harburger et al., 2007; Sato et al., 2003; Frye et al., 2007; Vongher and Frye, 1999) provided neuroprotection and enhanced cognitive function in young and aged rodents. Progesterone can also protect against global ischemia-induced hippocampal damage in young male rats (Morali et al., 2005) and in female cats (Gonzalez-Vidal et al., 1998). Because hormone therapy in menopausal women often includes both estrogen and progestin, it will be important to evaluate the actions of progestins in future studies of global ischemia
Alternatively, our finding that endogenous estradiol does not afford histological protection in middle-aged females may suggest that the pattern of brain exposure to estradiol (constant versus episodic) dictates the downstream molecular events that determine whether estradiol can avert specific cell death cascades that lead to neuronal loss. In young females, global ischemia results in dephosphorylation and inactivation of extracellular signal regulated kinase (ERK) and its target, the transcription factor cyclic AMP response element binding protein (CREB), followed by down-regulation of the anti-apoptotic protein Bcl-2 and activation of caspase 3 (Jover-Mengual et al., 2007). In young rats, estradiol attenuates the deleterious effects of ischemia on ERK, CREB, Bcl-2 and caspase 3 activation, leading to a reduction in apoptotic neuronal death (Jover-Mengual et al., 2007). It will be of interest to determine in middle-aged rats whether exogenous estradiol intervenes at similar or different molecular levels in comparison to young rats.
Conclusion
This study investigated whether estradiol attenuates ischemia-induced CA1 cell loss and improves working memory in middle-aged female rats and if so, whether increasing the duration of ovarian hormone withdrawal modifies the ability of estradiol to protect against the histological and behavioral consequences of global ischemia. Our results demonstrate that one cannot easily extrapolate histological or behavioral findings in young females pretreated with estradiol and subjected to transient global ischemia to middle-aged females. Because the risk of stroke and cardiac arrest in women increases after menopause, and the WHI enrolled many women who were more than 10 years post-menopause before initiating hormone therapy, these results have important implications for evaluating the potential benefits of long-term hormone treatment in menopausal women. Our results show that responsiveness to the neuroprotective effects of estradiol is different in middle-aged than in young rodents. Estradiol attenuates ischemia-induced hippocampal cell loss in middle-aged females even after long periods of hormone withdrawal. However, estradiol does not preserve cognitive function in middle-aged females. These findings highlight the importance of using older animals in studies assessing potential treatments for focal and global ischemia.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge research support by DHHS grant R01 AG027702 to A.M.E. and by the D.P. Purpura Department of Neuroscience, Albert Einstein College of Medicine. The authors would also like to thank Dr. Takahiro Miyawaki and Mr. Fabrizio Pontarelli for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akai F, Yanagihara T. Identity of the dorsal hippocampal region most vulnerable to cerebral ischemia. Brain Res. 1993;603:87–95. doi: 10.1016/0006-8993(93)91302-9. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Aoyagi A, Saito H, Abe K, Nishiyama N. Early impairment and late recovery of synaptic transmission in the rat dentate gyrus following transient forebrain ischemia in vivo. Brain Res. 1998;799:130–137. doi: 10.1016/s0006-8993(98)00465-x. [DOI] [PubMed] [Google Scholar]
- Ashton D, Reempts JV, Haseldonckx M, Willems R. Dorsal-ventral gradient in vulnerability of CA1 hippocampus to ischemia: A combined histological and electrophysiological study. Brain Res. 1989;487:368–372. doi: 10.1016/0006-8993(89)90842-1. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995;677:171–176. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Pike CJ. Progesterone blocks estrogen neurprotection from kainate in middle-age female rats. Neurosci Lett. 2008 doi: 10.1016/j.neulet.2008.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Cavoy A, Delacour J. Spatial but not object recognition is impaired by aging in rats. Physiol Behav. 1993;53:527–530. doi: 10.1016/0031-9384(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Age-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Chen J, Adachi N, Liu K, Arai T. The effects of 17beta-estradiol on ischemia-induced neuronal damage in the gerbil hippocampus. Neuroscience. 1998;87:817–822. doi: 10.1016/s0306-4522(98)00198-5. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Chen L, Sokabe M. Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacology. 2007;52:1124–1138. doi: 10.1016/j.neuropharm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;142:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- De Butte-Smith M, Nguyen AP, Zukin RS, Etgen AM, Colbourne F. Failure of estradiol to ameliorate global ischemia-induced CA1 sector injury in middle-aged female gerbils. Brain Res. 2007;1153:214–220. doi: 10.1016/j.brainres.2007.03.082. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Laranja DC, Caldana F, Bromberg E, Roesler R, Schroder N. Reversal of age-related deficits in object recognition memory in rats with 1-deprenyl. Exp Gerontol. 2005;40:506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Dias CP, Torres JP, Dornelles A, Garcia VA, Scalco FS, Guimaraes MR, Petry RC, Bromberg E, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Reversion of age-related recognition memory impairment by iron chelation in rats. Neurobiol aging. 2008;29:1052–1059. doi: 10.1016/j.neurobiolaging.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. Spontaneous alternation after free and forced trials. Can J Psychol. 1959;13:151–154. doi: 10.1037/h0083776. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy, and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments conteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J Women’s Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2007;24:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JRG. Can social interaction be used to measure activity? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Pelleymounter MA. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol aging. 1988;9:549–556. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- Glendenning ML, Lovekamp-Swan T, Schreihofer DA. Protective effect of estrogen in endothelin-induced middle cerebral artery occlusion in female rats. Neurosci Lett. 2008;445:188–192. doi: 10.1016/j.neulet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Vidal MD, Cervera-Gavira M, Ruelas R, Escobar A, Morali G, Cervantes M. Progesterone: protective effects on the cat hippocampal neuronal damage due to acute global cerebral ischemia. Arch Med Res. 1998;29:117–124. [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effect of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and independent mechanisms. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain-Role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148:1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GN, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Koh PO, Cho GJ, Choi WS. 17-β-estradiol pre-treatment prevents the global ischemia injury-induced decrease of Akt activation and Bad phosphorylation in gerbils. J Vet Med Sci. 2006;68:1019–1022. doi: 10.1292/jvms.68.1019. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki K, Sakuma Y. Estrogen alleviates cognitive dysfunction following transient brain ischemia in ovariectomized gerbils. Neurosci Lett. 1997;238:45–48. doi: 10.1016/s0304-3940(97)00847-1. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Menopause and stroke and the effects of hormonal therapy. Climacteric. 2007;Suppl. 2:27–31. doi: 10.1080/13697130701550903. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm and Behav. 2008 doi: 10.1016/j.yhbeh.2007.08.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;28:111–114. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Mariucci G, Stasi MA, Taurelli R, Nardo P, Tantucci M, Pacifici L, Carminati P, Ambrosini MV. EEG power spectra changes and forebrain ischemia in rats. Can J Neurol Sci. 2003;30:54–60. doi: 10.1017/s0317167100002444. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39:361–375. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Monmaur P, Thomson MA, M’Harzi M. Temporal changes in hippocampal theta activity following twenty minutes of forebrain ischemia in the chronic rat. Brain Res. 1986;378:262–273. doi: 10.1016/0006-8993(86)90929-7. [DOI] [PubMed] [Google Scholar]
- Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Marales L, Cervantes M. Post ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neursci Lett. 2005;382:286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: Influence on synaptic plasticity of memory. Neuroendocrinology. 2006;84:255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Nunn JA, LePeillet E, Netto CA, Hodges H, Gray JA, Meldrum BS. Global ischemia: hippocampal pathology and spatial deficits in the water maze. Behav Brain Res. 1994;62:41–54. doi: 10.1016/0166-4328(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plahta WC, Clark DL, Colbourne F. 17β-Estradiol pretreatment reduces CA1 sector cell death and the spontaneous hyperthermia that follows forebrain ischemia in the gerbil. Neuroscience. 2004;129:187–193. doi: 10.1016/j.neuroscience.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Plamondon H, Morin A, Charron C. Chronic 17β-estradiol pretreatment and ischemia-induced hippocampal degeneration and memory impairments: A 6-month survival study. Horm Behav. 2006;50:361–369. doi: 10.1016/j.yhbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–596. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principle results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behav Brain Res. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Casadesus G, Cantuti-Castelvetri I, Joseph JA. Effect of age on object exploration, habituation, and response to spatial and nonspatial change. Behav Neurosci. 2001;115:1059–1064. doi: 10.1037//0735-7044.115.5.1059. [DOI] [PubMed] [Google Scholar]
- Soffie M, Buhot MC, Poucet B. Cognitive and noncognitive processes involved in selective object exploration: comparison between young adult and old rats. Physiol Behav. 1992;52:1029–1035. doi: 10.1016/0031-9384(92)90387-h. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and anti-inflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyanmoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nature Neuroscience. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Wahl F, Allix M, Plotkine M, Boulu RG. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. Impaired object recognition and decreased prefrontal cortex spine density in aged female rats. Ann N Y Acad Sci. 2007;1097:54–57. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ WHI investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Watabe AM, O’Dell TJ. Age-related changes in theta frequency stimulation-induced long-term potentiation. Neurobiol aging. 2003;24:267–272. doi: 10.1016/s0197-4580(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB. Estradiol protects against ischemic brain injury in middle-aged rats. Biol Reprod. 2000;63:982–985. doi: 10.1095/biolreprod63.4.982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.