Abstract
The fresh water snail Biomphalaria glabrata (2n = 36) belongs to the taxonomic class Gastropoda (family Planorbidae) and is integral to the spread of the human parasitic disease schistosomiasis. The importance of this mollusc is such that it has been selected as a model molluscan organism for whole genome sequencing. In order to understand the structure and organisation of the B. glabrata’s genome it is important that gene-mapping studies are established. Thus, we have studied the genomes of two B. glabrata embryonic (Bge) cell line isolates 1 and 2 grown in separate laboratories, but both derived from Eder L. Hansen’s original culture from the 1970s. This cell line continues to be an important tool and model system for schistosomiasis and B. glabrata. Using these cell line isolates, we have investigated the genome content and established a revised karyotype based on chromosome size and centromere position for these cells. Unlike the original karyotype (2n = 36) established for the cell line, our investigations now show the existence of extensive aneuploidy in both cell line isolates to the extent that the total complement of chromosomes in both greatly exceeds the original cell line’s diploid number of 36 chromosomes. The isolates, designated Bge 1 and 2, had modal chromosome complements of 64 and 67, respectively (calculated from 50 metaphases). We found that the aneuploidy was most pronounced, for both isolates, amongst chromosomes of medium metacentric morphology. We also report, to our knowledge for the first time using Bge cells, the mapping of single copy genes peroxiredoxin (BgPrx4) and P-element induced wimpy testis (piwi) onto Bge chromosomes. These B. glabrata genes were mapped onto pairs of homologous chromosomes using fluorescence in situ hybridization (FISH). Thus, we have now established a FISH mapping technique that can eventually be utilized for physical mapping of the snail genome.
Keywords: Biomphalaria glabrata, Schistosomiasis, Chromosomes, Karyotyping, Bge cell line, Fluorescence in-situ hybridization (FISH), Bacterial Artificial Chromosomes (BACs), Mapping
1. Introduction
The fresh water snail Biomphalaria glabrata (diploid, 2n = 36) is a major intermediate host for the platyhelminth parasite Schistosoma mansoni that causes schistosomiasis. Humans are the obligate definitive host of the parasite. This disease is endemic in 74 tropical countries, (in regions of Africa, the Caribbean, the Middle East and South America) and causes vast morbidity and debilitation in terms of public health and socio-economic importance (LoVerde et al., 2004; Friedman et al., 2005).
The work of Eder L. Hansen (1976) in establishing the B. glabrata embryonic (Bge) cell line aided the efforts that led to most of what we currently know today about the molecular genetic interactions between trematode and the intermediate snail host in vitro. Before the cell line was established, much of the work focused on maintaining molluscan organs in vitro (Benex, 1961, 1965). Development of cell lines from other molluscs, such as the oyster Crassostrea gigas and the hard clam, Meretrix lusoria, have been attempted and primary cultures were successfully maintained for only up to 5 months (Chen and Wen, 1999) thus making Bge cells the only established cell line from molluscs. Despite this overall advantage these cells are an underutilized resource. Bge cells’ competence as a model for the in vitro development of S. mansoni was demonstrated when in the presence of these cells, miracidia were able to transform and, most significantly, complete the intramolluscan cycle from miracidium to cercaria (Ivanchenko et al., 1999; Coustau and Yoshino, 2000). By co-culturing these cells with the helminth parasites, it has been possible to examine the in vitro response to parasitic antigens and excretory-secretory (ES) products (Coustau and Yoshino, 2000). Indeed, some have shown that ES products from S. mansoni can stimulate the p38 signalling pathway of Bge cells, a response that is associated with stress factors, such as u.v. light, osmotic changes and heat shock (Sano et al., 2005; Humphries and Yoshino, 2006).
The importance of B. glabrata as an intermediate host of a major human pathogen is such that a proposal submitted by the snail genome project to the National Human Genome Research Institute (NHGRI) was accepted and its genome is currently being sequenced by the Genome Sequencing Center (GSC, Washington University in St. Louis, USA) (reviewed by Raghavan and Knight, 2006). The AT content of B. glabrata is estimated to be ~64% based on the analysis of Bacterial Artificial Chromosome (BAC) end sequence data (Adema et al, 2006) and trace reads of B. glabrata genome sequences currently deposited in GenBank. The genome size of B. glabrata is approximately 931 Mb and is based on Feulgen image analysis densitometry of haemocyte samples (Gregory, 2003). This is approximately three times smaller than that of the 3,000 Mb human genome (Venter et al., 2001) and three times larger than that of the 270 Mb S. mansoni genome (El-Sayed et al., 2004). Yet, compared with other molluscs, the genome is relatively small, e.g. Aplysia calfornica at 1,800 Mb, and Lymnaea stagnalis at 1,195 Mb (Raghavan and Knight, 2006). Currently in the GenBank database there are 808 nucleotide sequences, 633 protein sequences, 619 genome survey sequences (GSS), and 52,624 expressed sequence tags (ESTs), from B. glabrata, in addition to the 139,839 trace reads that have been deposited by the GSC. To date, numerous gene libraries have been constructed, e.g. cosmid (Knight et al., 1999), cDNA (Raghavan et al., 2003; Lockyer et al., 2007), two BAC libraries (B. glabrata BB02; Arizona Genomics Institute; Adema et al., 2006 and B. glabrata BS-90; Raghavan et al., 2007) and a fully sequenced B. glabrata mitochondrial genome of 13,670 nucleotides (DeJong et al., 2004).
The complete genome sequence of B. glabrata will be of great importance to further understand how host-parasite relationships are elicited and may be controlled. An additional feature of the snail genome project is to develop techniques to analyse B. glabrata on a biochemical, genomic and chromosomal level. The latter will be crucial in constructing a physical, cytogenetic map (via the use of fluorescence in-situ hybridisation (FISH) for physical mapping) of this organism (Langer et al., 1981).
Research into B. glabrata chromosomes has somewhat stagnated in recent years. Patterson and Burch (1960) performed the pioneering work in this field. They identified the basic chromosome number of planorbidae snail genera (which includes B. glabrata) as 2n = 36. Another important schistosome intermediate host, genus Bulinus exhibit diploid, tetraploid, hexaploid and even octoploid levels of polyploidy (Goldman et al., 1984). Raghunathan (1976) described the karyotype of B. glabrata by organising chromosomes into groups of metacentric, submetacentric, acrocentric and telocentric (in accordance with centromere position as stipulated by Levan et al. (1964), as well as confirming a diploid number of 36 chromosomes (Levan et al., 1964). Subsequently, Goldman et al. (1984) produced another karyotype of B. glabrata. Both karyotypes were derived from the snail and not the Bge cell line. However, Bayne et al. (1978) performed a detailed analysis of the Bge cell line developed by Hansen (1976) with respect to its antigenic determinants, karyotype, behavioural and enzyme characteristics prior to depositing the cells at the American Type Culture Collection (ATCC®, Manassas, USA). The Bge cells that were deposited by Dr. C. Bayne are currently available from ATCC® (catalog no. CRL-1494™) where they are described as only being loosely adherent, in contrast to their original morphology, described as monolayer forming fibroblast-like cells (Hansen 1976; Bayne et al., 1978). During the past several, years we have independently purchased different vials of Bge cells from ATCC® and to date have failed to propagate these particular cells in our laboratories (Biomedical Research Institute, Rockville, USA and Brunel University, West London, UK). Personal communication with technical support at ATCC® indicated that the cells are currently not being actively propagated due to lack of demand and also low availability of their stocks. Because of the failed attempts to propagate commercially purchased Bge cells from ATCC®, we obtained Bge cells from two different sources, the original ATCC® depositor Dr. C. Bayne (Oregon State University, Corvallis, USA), and Dr. E.S. Loker (University of New Mexico, Albuquerque, USA). Our interest was to characterize the isolates from these two different sources prior to using them for any molecular analysis, since slight differences were observed in their physical characteristics, for example in their ability to adhere, form monolayers, and in their generation time.
In this study, we have analysed the chromosomes of Bge cell lines from these two different sources, named here as Bge 1 (E.S. Loker laboratory) and Bge 2 (C. Bayne laboratory) and constructed a revised karyotype that reveals extensive aneuploidy in the cell line isolates. Additionally we demonstrate, to our knowledge for the first time, chromosomal mapping of non-repetitive (single-copy) B. glabrata genes onto homologous chromosomes isolated from Bge cells.
2. Materials and methods
2.1. Bge cell culture
Bge cells used in this study were obtained from the laboratories of Dr E.S. Loker (Bge 1) and Dr. C. Bayne (Bge 2). Both cultures were derived from Hansen’s original Bge cell line (Hansen 1976), and were grown in the absence of carbon dioxide, at 26°C in medium which comprised of 22% Schneider’s Drosophila medium (Invitrogen, Paisley, UK), 0.13% galactose (Invitrogen, Paisley, UK), 0.45% lactalbumin hydrolysate (Invitrogen, Paisley, UK) and 14.1 μM phenol red. The medium was sterilised using a 0.22 μm pore filter (Fisher Scientific UK Ltd, Loughborough, UK) and the antibiotic gentamicin (Invitrogen, Paisley, UK) was added post-filtration at a concentration of 20 μg/ml. The Bge medium was made complete by adding 10% FBS (v/v, Hyclone, Cramlington, UK) which had previously been inactivated at 56°C for 30 min. The Bge cells were passaged when their confluence had reached approximately 80%, and then reseeded at a 1:12 dilution. Since trypsinization of either cell line isolates over 10-15 passages resulted in the loss of viability of the cells and ultimately led to cell death, we resorted to releasing the cells using either a cell scraper or by firm tapping. While propagating the Bge cells from the two different sources in our laboratory we observed differences in their characteristics such as doubling time, cell adherence and morphology.
2.2. Bge cellular fixation and slide preparation
Bge cells were arrested in metaphase using the mitotic inhibitor colcemid. Ten μg/ml of colcemid dissolved in Hank’s balanced salt solution (Invitrogen, Paisley, UK) was added to T75 flasks (Fisher Scientific UK Ltd, Loughborough, UK) of Bge cells (at the stage of 70-80% confluence). The cells were incubated with the colcemid for 1.5 h at 26°C and the cells were subsequently dislodged from the flasks via the application of either a cell scraper (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) or by firmly tapping the flask. The cells were then centrifuged at 400 g at 15°C. The cell pellet was resuspended by vigorously tapping the tube, followed by the addition of hypotonic potassium chloride solution (0.005 M) and subsequently fixed with methanol and acetic acid (3:1 v/v). Twenty μl of this cellular suspension was then applied onto a glass slide to achieve metaphase chromosome spreads. Glass slides of fixed Bge cellular suspensions were stained with the DNA intercalator DAPI. Ten μl of 2 μg/ml DAPI in Vectorshield antifade mountant (Vector Laboratories, Peterborough, UK) was applied to the slide and sealed with a 22 × 50 mm coverslip.
2.3. Fluorescence in-situ hybridisation (FISH)
2.3.1. Preparation of Bge genomic DNA
Cells were collected after centrifugation (400 g) at 15°C, incubated with a digestion buffer containing 100 mM Tris (pH 8.0), 5 mM EDTA, 200 mM NaCl and 0.2 % SDS for 16 h and centrifuged at 10,000 g for 5 min. The supernatant collected was subsequently precipitated with ethanol at -80°C. The DNA was dissolved for 24 h in 500 μl of sterile deionised distilled water. This solution was then sonicated until the size of the DNA was between 200 and 500 bp. The concentration of DNA was measured using the High Sensitivity (HS) Qubit machine (Invitrogen, Paisley, UK).
2.3.2. Probe and slide preparation
DNA was isolated from BAC clones isolated from the B. glabrata (BS90) BAC DNA library as previously described (Raghavan et al., 2007). BAC DNA containing the gene for B. glabrata peroxiredoxin (BgPrx4) and the P-element induced wimpy testis (piwi), respectively, were labelled with Biotin-14 - dATP via nick translation (Langer et al., 1981). A nick translation kit (BioNick™, Invitrogen, Paisley, UK) was used for DNA labelling and produced probes ranging from 200-500 bp in size. The slides of Bge metaphase spreads were aged for 2 days at room temperature. They were then dehydrated through a series of ethanol solutions of 70%, 90% and 100% (5 min each). The slides were subsequently denatured in a solution of 70% formamide and 2 X saline sodium citrate (SSC) at 70°C for 1.5 min. Immediately after denaturation, the slides were immersed in ice cold 70% ethanol for 5 min before an additional 90% and 100% ethanol cycle. The slides were allowed to dry on a hot block (37°C) before addition of the probe.
Five hundred ng of the labelled probe, combined with 3 μg of herring sperm DNA as carrier and 40 μg of sonicated Bge genomic DNA, were ethanol precipitated together at -80°C for 30 min. The DNA was subsequently dissolved in 12 μl of hybridisation buffer (50% formamide, 10% dextran sulphate, 2 X SSC and 1% Tween 20) at room temperature for 24 h. The probes were denatured at 75°C for 5 min and then incubated at 37°C between 30 and 120 min.
2.3.3. Hybridisation, washing and counterstaining
Eight μl of the biotin-labelled probe was placed onto the slide, covered with a 24 × 40 mm coverslip and sealed with rubber cement. The denatured slides and probes were hybridised overnight (12-16 h) in a humified chamber at 37°C. Following hybridisation, the rubber cement and coverslips were removed and the slide was washed three times for 5 min in a neutral buffered solution of 50% formamide and 2 X SSC at 45°C. A second wash followed. The slides were transferred to coplin jars containing pre-warmed 0.1 X SSC at 60°C, which were transferred to a 45°C water bath. The slides were washed three times for 5 min. Subsequently, the slides were placed in a solution of 4 X SSC at room temperature for 10 min after which 100 μl of blocking solution was added to each slide (4% BSA in 4 X SSC). A 22 × 50 mm coverslip was placed on the slide and these were left for 10 min at room temperature. The coverslips were then removed and 100 μl of streptavidin conjugated to cyanine 3 in 1% BSA/4 X SSC (1:200 dilution) was added to each slide and a coverslip applied. The slides were incubated at 37°C for 30 min in the dark. After this incubation, the slides were washed three times for 5 min in 4 X SSC with 0.1% Tween 20 (v/v) at 42°C in the dark. A brief rinse in deionised distilled water was followed by the addition of the counterstain. The slides were counterstained with DAPI in Vectorshield anti-fade mountant (Vectorlabs).
2.4. Image capture and analysis
2.4.1. DAPI karyotype images
The karyotyping of the Bge cells was performed using images of DAPI-stained metaphase chromosomes. The DAPI images were captured using an epifluorescence microscope and X100 oil immersion objective (Zeiss, Axioplan 2). The images were captured using a charged coupled device (CCD) camera (RS Photometrics Sensys camera model KAF1401E G2) and the program Smart capture 3.00 (Digital Scientific). The greyscale images of the chromosomes were inverted using IPLab software (Scanalytics, BD Biosciences, San Jose, CA). The images were subsequently saved as TIFF files and visualised using the program Adobe Photoshop 5.0.2.
2.4.2. Gene mapping via FISH
The FISH slides were analysed using an epifluorescence microscope, X100 oil immersion objective (Zeiss, Axioplan 2). The images were captured using a CCD camera (RS Photometrics Sensys camera model KAF1401E G2) and the program Smart capture 3.00 (Digital Scientific).
3. Results
3.1. Bge cell karyotypes
Metaphase chromosome spreads were stained with the DNA intercalating dye DAPI and imaged digitally. In order to determine banding in the chromosomes, the grayscale images were inversed. DAPI banding was chosen since G-banding proved to be unsatisfactory for these chromosomes (Odoemelam and Bridger, data not shown).
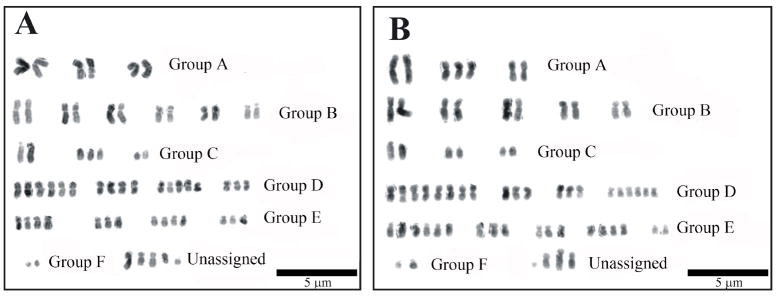
The chromosomes were digitally isolated and arranged initially according to size and then centromere position. Fifty images for both the Bge 1 and 2 cell line isolates were analysed in total. The chromosomes were organised into groups of large metacentric (group A), large submetacentric (group B), large acrocentric (group C), medium metacentric (group D), medium submetacentric (group E) and small acrocentric (group F). They were then grouped with other homologous chromosomes. Single chromosomes without a designated homologous copy were grouped as ‘unassigned’. Biomphalaria glabrata in vivo has a mitotic chromosome complement of 2n = 36. The results observed in this study reveal extensive aneuploidy in the Bge cell line. Both Bge 1 and 2 have a mean chromosome complement that vastly exceeds 36. Fig. 1 shows representative karyotypes for both Bge 1 cell line isolate (Fig. 1A) and Bge 2 (Fig. 1B) cell line isolate (representative from a total of 50 metaphase spreads of each cell line isolate).
Fig. 1.
DAPI karyotype images of Biomphalaria glabrata embryonic Bge cell line isolates. Chromosomes from Bge 1 (A) and Bge 2 (B) cell line isolates were stained with DAPI and then converted into grey scale image with subsequent inversion. There were variations between karyotypes for different metaphase spreads from the same isolate. The chromosomes were organised into groups according to size and centromere position (as indicated with notations). The unassigned group contains chromosomes which, given their unique morphology, were without a suitable homologue. Scale bar = 5 μm.
The modal total number of chromosomes in metaphases was 63 and 67 for Bge 1 and 2, respectively (see Table 1). These numbers surpass both B. glabrata’s natural diploid number of 36 and a theoretical triploid number of 54. Yet, both modal complements are lower than the theoretical tetraploid number of 72. These results indicate there is a non-uniform duplication of chromosomes within these cell line isolates.
Table 1.
Chromosomal mean and mode for each group within the karyotype. The mean and mode for each chromosomal group was calculated based on 50 reads for each of the Biomphalaria glabrata embryonic cell lines, Bge 1 and 2.
| Group | Bge 1
|
Bge 2
|
||
|---|---|---|---|---|
| Mean | Mode | Mean | Mode | |
| A | 6.18 | 6 | 6.4 | 6 |
| B | 10.36 | 10 | 11.04 | 10 |
| C | 6.24 | 6 | 6.8 | 6 |
| D | 19.42 | 18 | 20.58 | 21 |
| E | 13.76 | 16 | 14.54 | 17 |
| F | 2.8 | 2 | 2.14 | 2 |
| G | 4.04 | 5 | 3.7 | 5 |
|
| ||||
| Total | 62.8 | 63 | 65.2 | 67 |
The mean and modal numbers for groups A, B, C, D, E, F and G (unassigned) for two cell lines, Bge 1 and Bge 2 are shown. The mean and modal total chromosomal numbers derived from the groups for each isolate, Bge 1 and 2 are shown at the bottom of the table.
Table 1 displays both the chromosomal mean and mode for each group within the karyotypes. The modal numbers between the groups is identical for groups A, B and C (six, 10 and six, respectively). However, they deviate in groups D and E. The Bge 2 cell line isolate has 21 and 17 chromosomes for the groups D and E, respectively, which exceeds Bge 1’s groups D and E, respectively. The modal chromosome numbers for groups F and G (unassigned) are the same. Fig. 2 shows the frequency distribution graphs for groups A to G for both Bge 1 and 2. Compared with groups D and E, groups A, B, C, F and G display moderate distribution with integer ranges not exceeding 10. Groups D and E, however, show a more marked distribution, with chromosome integer ranges of 14-26 and 8-20, respectively. Hence, although there are duplications in the larger chromosomes, the duplications appear to be more pronounced in the medium sized chromosomes.
Fig. 2.
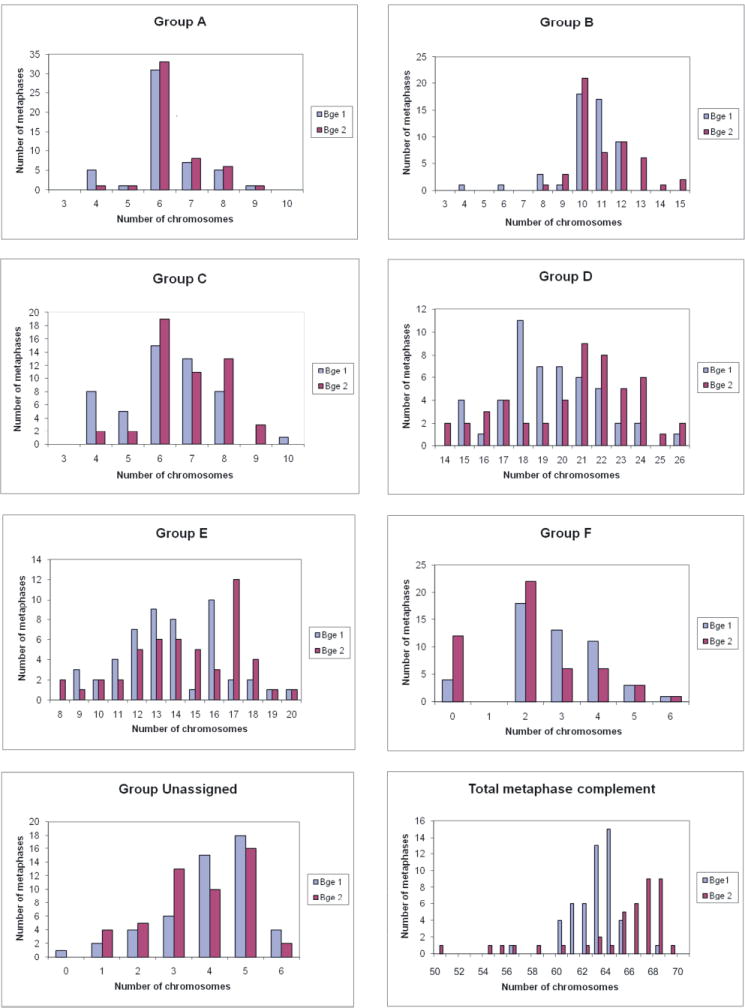
Frequency distribution of chromosomal groups in Biomphalaria glabrata. Chromosome groups A – F, the unassigned group and total metaphase complement for both B. glabrata embryonic Bge 1 and 2 cell lines. For groups A-F the chromosomes from 100 metaphases (50 each for Bge 1 and 2) were grouped according to size and centromere position.
3.2. Comparison of the karyotype of Bge 1 and 2 cell line isolates to the published karyotype of B. glabrata
The morphology of these chromosomes can be described in relation to other organisms such as Homo sapiens, as being comparatively small and monomorphic. Thus the size of these chromosomes restricted the clarity of banding that one would observe in human chromosomes. When we compared our chromosome grouping from the two cell line isolates to that originally reported by Bayne et al. (1978), we observed that only a loose similarity to the original chromosomal grouping exists for cell line isolates 1 and 2. These differences may be due to morphological changes that might have resulted from the extensive polyploidy/aneuploidy in the current cell line isolates that was not present when the original karyotype of the Bge cell line was reported (Hansen, 1976; Bayne et al., 1978). Also, the resolution of previously published ex vivo karyotypes by Raghunathan (1976) and Goldman (1984) (both karyotypes were produced from snail tissue) were not of a quality we could use as a comparison. However, we were able to relate some other designated groups for these Bge cells back to that of Raghunathan’s karyotype. Indeed, certain chromosomes from Raghunathan’s published karyotype were easily identifiable, such as the largest chromosomes in groups A and C, which are chromosomes 1 and 8 in Raghunathan’s (1976) publication.
3.3. Gene mapping via FISH
The main reason for investigating the genomic content of these Bge cell line isolates was to assess how relevant and important they would be as a resource for the molluscan genome sequencing project. They would be a useful tool to perform physical mapping upon, and develop protocols required for, performing FISH on snail cells and chromosomes. Thus, we used the Bge cell line to develop a methodology for physical mapping of BACs by FISH for Biomphalaria. Figs. 3A and B demonstrate the mapping of biotinylated probes of BACs containing BgPrx4 and piwi, respectively. Interestingly, both probes hybridised onto a pair of homologous chromosomes. BgPrx4 was located proximally from the centromere of the largest metacentric chromosome in group A (Fig. 3A) while piwi was located distally from the centromere of the smallest metacentric chromosome in group A (Fig. 3B).
Fig. 3.
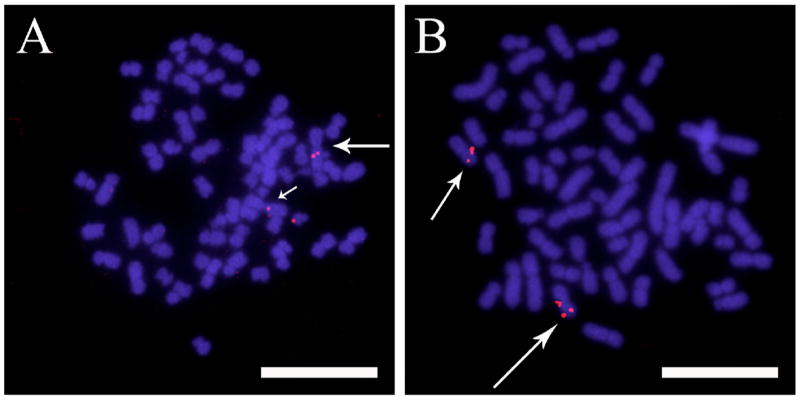
Representative 2-dimensional fluorescence in situ hybridization (FISH) images of hybridization of non-repetitive (single copy) genes of Biomphalaria glabrata, onto two homologous chromosomes. (A) Chromosomes were fixed, hybridized with DNA from Bacterial Artificial Chromosome (BAC) clones corresponding to genes for B. glabrata peroxiredoxin, BgPrx4 (seen as red fluorescence) and, (B) P-element induced wimpy testis, piwi (seen as red fluorescence) and counterstained with DAPI (seen as blue fluorescence). The arrows indicate the location of the genes on the chromosome arms. Scale bar = 10 μm.
4. Discussion
Interest in the mollusc B. glabrata has increased greatly during the past few years with it being chosen as a model organism for whole genome sequencing (Raghavan and Knight, 2006). Thus, it is not only parasitologists who will champion this era of modern genomics biology of this lochotrophozoan but other investigators as well, especially those interested in comparative genomics and genome evolution. The existence of a cell line from B. glabrata, indeed the only established molluscan cell line (Hansen, 1976; Bayne et al., 1978, 1998), has been a useful tool for parasitologists and has been used for many in vitro experiments. Because the original Bge cells that we purchased commercially from ATCC® could not be propagated in our laboratories, we obtained Bge cells from two different sources as mentioned earlier. As differences were observed between the cell line isolates, we analyzed the karyotype of these cells to determine if any differences occurred at the chromosomal level compared with the original Bge cell line (Hansen 1976; Bayne et al., 1978).
Based on our analysis, we discovered that the Bge cell line has undergone significant cytological changes since Hansen first established it in the 1970s. Therefore, those designing experiments assuming normal gene dosage and expression may be misled without accounting for these changes. The natural diploid number of chromosomes for B. glabrata is 36. The changes in two Bge cell line isolates are such that there is considerable aneuploidy amongst the cells, to the effect that a chromosome count of 100 metaphase plates produces modal chromosome complements of 63 and 67. It is intriguing that two supposedly identical cell lines, which have been cultured separately in different laboratories, have such disparate numbers of chromosomes. These findings will need to be taken into account when designing in vitro experiments that hope to extrapolate to the in vivo situation, as there may well be extra copies of genes and alterations to transcriptional profiles.
To achieve construction of the karyotypes from these cell line isolates with such divergent modal counts, it has been difficult arranging these chromosomes into groups as previously performed from the snail itself by Raghunathan (1976) and Goldman et al. (1984). We had difficulties identifying these chromosomes from those of the aforementioned previously published karyotypes. However, certain chromosomes, such as the largest chromosomes in groups A and C, were very distinctive and were easily identifiable in Raghunathan’s (1976) and Goldman et al., (1984) karyotypes (Raghunathan designated these chromosomes as 1 and 8, respectively).
We initially hypothesised a simple case of triploidy as observed in the human cervical cancer cell line, HeLa (Ghosh and Ghosh, 1975). However, such theories were negated not only by the modal numbers of 64 and 67 chromosomes in the Bge 1 and 2 cell line isolates but also by the presence of ‘diploid’ pairs of homologous chromosomes (see Fig. 1). Hence the results we present here indicate an uneven duplication of chromosomes. Such duplications exist most prominently in the chromosomes we have deemed medium metacentric and medium submetacentric.
As mentioned previously, aneuploidy is common in many cancer cell lines such as that of HeLa cells (Ghosh and Ghosh; 1975). In mammalian cells it can arise via irregular mitotic division or indeed by the knockout of DNA repair genes such as BRCA1 and BRCA2 (Tutt et al., 1999; Weaver et al., 2002). The Bge cell line’s “immortality” was apparently due to a spontaneous transformation. One could postulate that the spontaneous transformation of the Bge cells as observed and noted by Hansen in 1976, may have given certain aberrant cells a selective advantage over other cells within the culture. Such a transformation may have been a mutation of a gene controlling mitotic checkpoint signalling and silencing. Or it may have occurred through chromosome missegregation via the presence of multipolar spindles (Kops et al., 2005). We can only speculate that the changes that took place in these cells favoured the duplication of the medium metacentric and submetacentric chromosomes. Thus, researchers using the Bge cell line will have to consider their experiments carefully if genes of interest are found to be located on these medium-sized grouped chromosomes. While one can also speculate that the extra chromosomes could be derived from another organism (the laboratory in which the original Bge cell line was established also cultured cells from the mosquito Aedes albopictus), it was unequivocally shown, however, by serology and antigen-based analysis that the Bge cell line was not contaminated (Bayne et al., 1978).
It has been discussed by interested parties in the B. glabrata genome sequencing consortium that the Bge cell line would be a useful tool for mapping genes since it is easier to prepare DNA and chromosomes from a cultured cell line than from whole organisms. Thus, it was imperative to re-evaluate its karyotype and develop protocols for physical mapping of genes by FISH that can be implemented for the future mapping of ex vivo chromosomes.
Further, we previously mentioned the monomorphism of B. glabrata chromosomes, with only the larger chromosomes being very distinctive. Hence, mapping of genes onto homologues allows these chromosomes to be characterized more thoroughly and provides an identifying probe.
The B. glabrata genome is highly repetitive and we found that robust FISH methods, where no suppression of repetitive DNA is required, is not suitable for this organism (Odoemelam and Bridger, data not shown); although they have been successful when assessing the distribution of repetitive elements such as Nimbus by FISH (Knight et al., 2008). These problems were overcome via the implementation of unlabelled B. glabrata genomic DNA, to prevent the probe from hybridizing onto repetitive sequences in the genome. We found 40 μg was an optimum amount of DNA to suppress the repetitive sequences in the B. glabrata genome and to give clear signals on two chromatids of both homologues. Despite having surmised that there is aneuploidy and possibly unrecognisable translocations in these cells, this study shows from FISH mapping experiments with the first non-repetitive (single-copy) BACs that, as expected, hybridisation was seen to two homologous chromosomes.
This is an important step forward because it shows that despite its imperfections, the Bge cell line is still a useful first line reagent for developing standardized protocols that can eventually be used to perform similar physical mapping experiments by FISH with non-repetitive probes on ex vivo chromosomes.
Acknowledgments
We would like to thank Dr. Julio Masabanda for helpful suggestions concerning the FISH methodology and Drs. E. Sam Loker and Christopher Bayne for the Bge cell line isolates 1 and 2, respectively. We also thank Dr. Fred Lewis for his support and help with the writing of this manuscript. This work was funded by a grant from NIH-NIAID AI63480.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adema CM, Luo M, Hanelt B, Hertel LA, Marshall JJ, Zhang S, DeJong RJ, Kim H, Kudrna D, Wing RA, Soderlund C, Knight M, Lewis FA, Caldeira RL, Jannotti-Passos LK, Carvalho O, Loker ES. A bacterial artificial chromosome library for Biomphalaria glabrata, intermediate snail host of Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2006;101(Suppl I):167–177. doi: 10.1590/s0074-02762006000900027. [DOI] [PubMed] [Google Scholar]
- Bayne CJ, Owczarzak A, Allen JR. Molluscan (Biomphalaria) cell line: Serology, karyotype, behavioural and enzyme elecrophoretic characterization. J Inv Path. 1978;32:35–39. [Google Scholar]
- Bayne CJ. Invertebrate cell culture considerations: insects, ticks, shellfish, and worms. Methods Cell Biol. 1998;57:187–201. doi: 10.1016/s0091-679x(08)61578-2. [DOI] [PubMed] [Google Scholar]
- Benex J. Survival of explants of planorbidae (Australorbis glabrata) in synthetic, and nutritive medium. Comptes rendus hebdomadaires des seances de l’Academie des sciences. 1961;253:734–736. [PubMed] [Google Scholar]
- Benex J. Attempts at infestation, by Schistosoma mansoni miracidia, of planorbid tentacles maintained in organ-type culture in renewed liquid medium. Comptes rendus hebdomadaires des seances de l’Academie des sciences. 1965;260:4080–4082. [PubMed] [Google Scholar]
- Chen SN, Wen CM. Establishment of cell lines derived from the oyster, Craasostrea gigas Thunberg and hard clam, Meretrix lusoria Röding. Meth Cell Sci. 1999;21:183–192. doi: 10.1023/a:1009829807954. [DOI] [PubMed] [Google Scholar]
- Coustau C, Yoshino TP. Flukes without snails: advances in the in vitro cultivation of intramolluscan stages of trematodes. Exp Parasitol. 2000;94:62–66. doi: 10.1006/expr.1999.4462. [DOI] [PubMed] [Google Scholar]
- DeJong RJ, Emery AM, Adema CM. The mitochondrial genome of Biomphalaria glabrata (Gastropoda: Basommatophora), intermediate host of Schistosoma mansoni. J Parasitol. 2004;90:991–997. doi: 10.1645/GE-284R. [DOI] [PubMed] [Google Scholar]
- El-Sayed NM, Bartholomeu D, Ivens A, Johnston DA, LoVerde PT. Advances in Schistosome genomics. Trends in Parasitol. 2004;20:154–157. doi: 10.1016/j.pt.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anaemia: the relationship and potential mechanisms. Trends in Parasitol. 2005;21:382–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Ghosh I. Variation of stemline karyotype in a HeLa cell line. J Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975;84:129–133. doi: 10.1007/BF00304038. [DOI] [PubMed] [Google Scholar]
- Goldman MA, LoVerde PT, Chrisman L, Franklin DA. Chromosomal evolution in planorbid snails of the genera Bulinus and Biomphalaria. Malacologia. 1984;25:427–446. [Google Scholar]
- Gregory TR. Genome size estimates for two important freshwater molluscs, the zebra mussel (Dreissena polymorpha) and the schistosomiasis vector snail (Biomphalaria glabrata) Genome. 2003;46:841–844. doi: 10.1139/g03-069. [DOI] [PubMed] [Google Scholar]
- Hansen EL. A cell line from embryos of Biomphalaria glabrata (Pulmonata): Establishment and characteristics. In: Maramorosch K, editor. In Invertebrate Tissue Culture: Research Applications. New York: Academic Press; 1976. pp. 75–97. [Google Scholar]
- Humphries JE, Yoshino TP. Schistosoma mansoni excretory-secretory products stimulate a P38 signalling pathway in Biomphalaria glabrata embryonic cells. Intl J Parasitol. 2006;36:37–46. doi: 10.1016/j.ijpara.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Lerner JP, McCormick RS, Toumadje A, Allen B, Fischer K, Hedstrom O, Helmrich A, Barnes DW, Bayne CJ. Continuous in vitro propagation and differentiation of cultures of the intramolluscan stages of the human parasite Schistosoma mansoni. Proc Natl Natl Acad Sci USA. 1999;96:4965–4970. doi: 10.1073/pnas.96.9.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Miller AN, Patterson CN, Rowe CG, Michaels G, Carr D, Richards CS, Lewis FA. The identification of markers segregating with resistance to Schistosoma mansoni infection in the snail Biomphalaria glabrata. Proc Natl Acad Sci USA. 1999;96:1510–1515. doi: 10.1073/pnas.96.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Bridger JM, Ittiprasert W, Odoemelam EC, Masabanda J, Miller A, Raghavan N. Endogenous retrotransposon sequences of the Schistosoma mansoni intermediate snail host, Biomphalaria glabrata. In: Brindley PJ, editor. Mobile Genetic Elements in Metazoan Parasites. Austin: Landes Bioscience; 2008. In press. Epub ahead of print. http://www.eurekah.com/chapter/3457. [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Langer PR, Waldrop AA, Ward DC. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probe. Proc Natl Acad Sci USA. 1981;78:6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:201–220. [Google Scholar]
- Lockyer AE, Spinks JN, Walker AJ, Kane RA, Noble LR, Rollinson D, Dias-Neto E, Jones CS. Biomphalaria glabrata transcriptome: identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES) Dev Comp Immunol. 2007;31:763–82. doi: 10.1016/j.dci.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerde PT, Hirai H, Merrick JM, Lee NH, El-Sayed NMA. Schistosoma mansoni genome project: an update. Parasitol Int. 2004;53:183–192. doi: 10.1016/j.parint.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Burch JB. Chromosomes of pulmonate molluscs. In: Fretters V, Peake J, editors. Pulmonates: systematics, evolution and ecology. Academic Press; New York: 1978. pp. 171–217. [Google Scholar]
- Raghavan N, Miller AN, Gardner M, FitzGerald PC, Kerlavage AR, Johnston DA, Lewis FA, Knight M. Comparative gene analysis of Biomphalaria glabrata hemocytes pre- and post-exposure to miracidia of Schistosoma mansoni. Mol Biochem Parasitol. 2003;126:181–191. doi: 10.1016/s0166-6851(02)00272-4. [DOI] [PubMed] [Google Scholar]
- Raghavan N, Knight M. The snail (Biomphalaria glabrata) genome project. Trends in Parasitol. 2006;22:148–151. doi: 10.1016/j.pt.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Raghavan N, Tettelin H, Miller A, Hostetler J, Tallon L, Knight M. Nimbus (BgI): An active non-LTR retrotransposon of the Schistosoma mansoni snail host Biomphalaria glabrata. Int J Parasitol. 2007;37:1307–1318. doi: 10.1016/j.ijpara.2007.04.002. Epub 2007 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan L. The karyotype of Biomphalaria glabrata, the snail vector of Schistosoma mansoni. Malacologia. 1976;15:447–450. [PubMed] [Google Scholar]
- Sano Y, Akimaru H, Okamura T, Nagao T, Okada M, Ishii S. Drosophila Activating Transcription Factor-2 Is Involved in Stress Response via Activation by p38, but Not c-Jun NH2-Terminal Kinase. Mol Biol Cell. 2005;16:2934–2946. doi: 10.1091/mbc.E04-11-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A, Gabriel A, Bertwhistle D, Connor F, Patterson H, Peacock J, Ross G, Ashworth A. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr Biol. 1999;9:1107–1110. doi: 10.1016/s0960-9822(99)80479-5. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;297:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Weaver Z, Montagna C, Xu X, Howard T, Gadina M, Brodie SG, Deng C, Ried T. Mammary tumors in mice conditionally mutant for Brca1 exhibit gross genomic instability and centrosome amplification yet display a recurring distribution of genomic imbalances that is similar to human breast cancer. Oncogene. 2002;21:5097–5107. doi: 10.1038/sj.onc.1205636. [DOI] [PubMed] [Google Scholar]