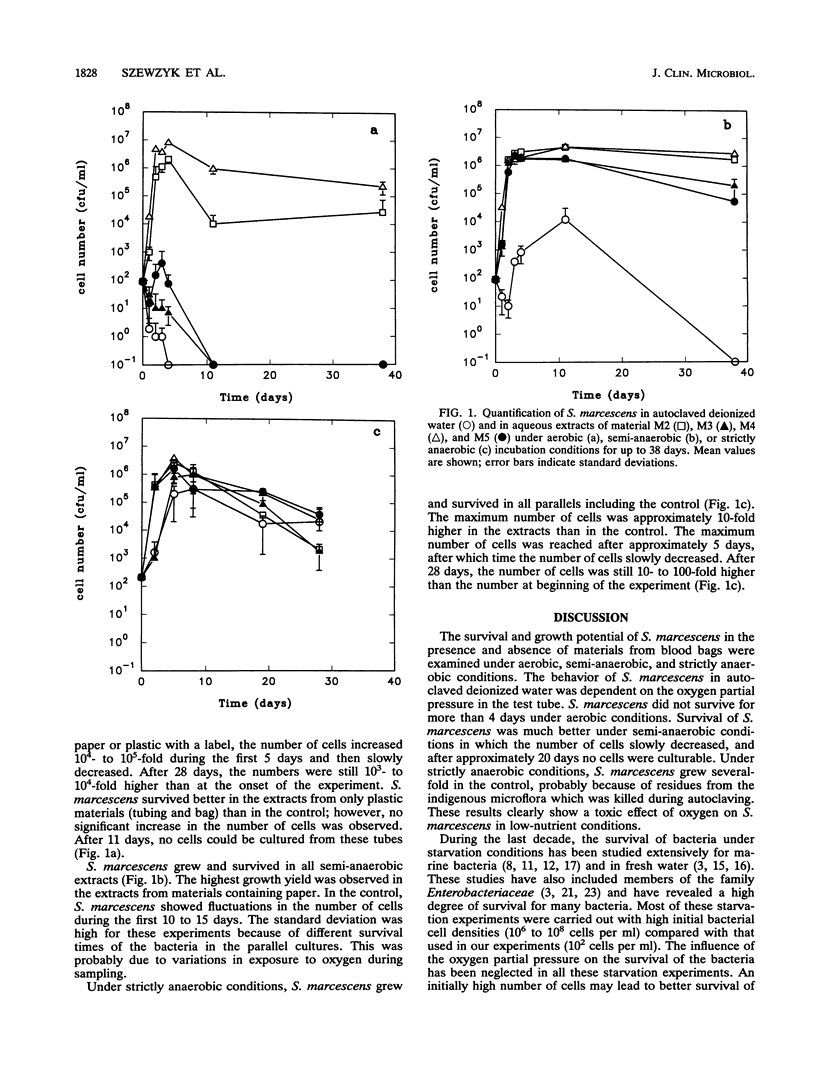
Abstract
Several patients receiving blood transfusions during the summer of 1991 developed bacteremia after the transfusion. In all cases, the infection was caused by Serratia marcescens. The same strain of Serratia marcescens was isolated from the patients and from the outer surface of unfilled blood bags. The transport containers for the blood bags were made anoxic by using a catalyst in order to prevent microbial growth. The survival and growth of S. marcescens K202, which was isolated from the blood bags, was studied at different oxygen concentrations in deionized water containing materials derived from the blood bags. The rate of survival and growth of S. marcescens was highest under anaerobic conditions, in which growth occurred with all materials and even in deionized water alone. In contrast, S. marcescens did not survive in control cultures under semi-anaerobic and aerobic conditions. Growth was observed, however, under both aerobic and semi-anaerobic conditions in the presence of each of the tested blood bag materials. These findings indicate that the conditions in the transport containers for the blood bags were favorable for the survival and growth of S. marcescens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botzenhart K., Kufferath R. Uber die Vermehrung verschiedener Enterobacteriaceae sowie Pseudomonas aeruginosa und Alkaligenes spec. in destilliertem Wasser, entioniertem Wasser, Leitungswasser und Mineralsalzlösung. Zentralbl Bakteriol Orig B. 1976 Dec;163(5-6):470–485. [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell B. A., Ye C., Griffiths R. P., Moyer C. L., Morita R. Y. Plasmid expression and maintenance during long-term starvation-survival of bacteria in well water. Appl Environ Microbiol. 1989 Aug;55(8):1860–1864. doi: 10.1128/aem.55.8.1860-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Davis B. R., Hickman F. W., Presley D. B., Bodey G. P., Negut M., Bobo R. A. Detection of Serratia outbreaks in hospital. Lancet. 1976 Aug 28;2(7983):455–459. doi: 10.1016/s0140-6736(76)92539-3. [DOI] [PubMed] [Google Scholar]
- Favero M. S., Carson L. A., Bond W. W., Petersen N. J. Pseudomonas aeruginosa: growth in distilled water from hospitals. Science. 1971 Aug 27;173(3999):836–838. doi: 10.1126/science.173.3999.836. [DOI] [PubMed] [Google Scholar]
- Geller A. Growth of bacteria in inorganic medium at different levels of airborne organic substances. Appl Environ Microbiol. 1983 Dec;46(6):1258–1262. doi: 10.1128/aem.46.6.1258-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Prolonged survival of Serratia marcescens in chlorhexidine. Appl Environ Microbiol. 1981 Dec;42(6):1093–1102. doi: 10.1128/aem.42.6.1093-1102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A. K., Highsmith A. K., Martone W. J. Survival of Serratia marcescens in benzalkonium chloride and in multiple-dose medication vials: relationship to epidemic septic arthritis. J Clin Microbiol. 1987 Jun;25(6):1019–1021. doi: 10.1128/jcm.25.6.1019-1021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parment P. A., Rönnerstam R., Walder M. Persistence of Serratia marcescens, Serratia liquefaciens and E. coli in solutions for contact lenses. Acta Ophthalmol (Copenh) 1986 Aug;64(4):456–462. doi: 10.1111/j.1755-3768.1986.tb06953.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Isolation of pigmented and nonpigmented mutants of Serratia marcescens with reduced cell surface hydrophobicity. J Bacteriol. 1984 Oct;160(1):480–482. doi: 10.1128/jb.160.1.480-482.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L. Serratia marcescens: historical perspective and clinical review. N Engl J Med. 1979 Apr 19;300(16):887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]


