Abstract
Rationale
Adenosine receptors are involved in cocaine and methamphetamine discrimination and exposure to caffeine can affect behavioral effects of nicotine in rats.
Objectives
Here we investigated the relative involvement of adenosine A1 and A2A receptors in nicotine, cocaine and methamphetamine discrimination, before and/or during chronic caffeine exposure.
Methods
The non-selective adenosine antagonist caffeine, the A1-receptor antagonist CPT and the A2A-receptor antagonist MSX-3 were evaluated in rats trained to discriminate 0.4 mg/kg nicotine from saline under a fixed-ratio schedule of food delivery. Effects of antagonists were then compared in rats discriminating nicotine, methamphetamine or cocaine during chronic caffeine exposure in their drinking water.
Results
Caffeine, CPT and MSX-3 partially generalized to nicotine and shifted nicotine dose-response curves leftwards. During chronic caffeine exposure, however, all three ligands failed to generalize to nicotine and failed to shift nicotine dose-response curves. In previous experiments, CPT and MSX-3 partially generalized to methamphetamine and cocaine and shifted dose-response curves leftwards. In the present experiments, CPT neither generalized nor shifted dose-response curves for methamphetamine or cocaine during chronic caffeine exposure. However, MSX-3 partially generalized to both psychostimulants and shifted their dose-response curves leftwards. Caffeine partially generalized to cocaine, but not methamphetamine, and shifted both dose-response curves leftwards.
Conclusions
Both adenosine A1 and A2A receptors are capable of modulating the discriminative-stimulus effects of nicotine. Chronic caffeine exposure produces complete tolerance to both A1- and A2A-mediated effects in nicotine-trained rats. In contrast, chronic caffeine exposure produces tolerance to adenosine A1-mediated, but not A2A-mediated, effects in methamphetamine- and cocaine-trained rats.
Keywords: Adenosine A1 receptor, adenosine A2A receptor, caffeine, cocaine, drug discrimination, methamphetamine, nicotine, rats
INTRODUCTION
Adenosine, by acting on adenosine A1 and A2A receptors, is known to antagonistically modulate dopaminergic neurotransmission by means of pre- and postsynaptic mechanisms that involve modulation of dopamine release and functional interactions between adenosine and dopamine receptors (Ferre et al. 1997; Ferre 2008). The non-selective adenosine receptor antagonist caffeine acts by blocking adenosine transmission in the brain and, notably, in the basal ganglia (Ferre 2008; Fisone et al. 2004). A critical aspect of the mechanisms underlying caffeine’s psychostimulant effects is a release of the pre- and postsynaptic brakes that adenosine imposes on striatal dopaminergic neurotransmission (Ferre 2008). Adenosine A1 and A2A receptors are involved in the motor effects of caffeine (Karcz-Kubicha et al. 2003) and there is evidence that tolerance develops to the motor-stimulant effects of caffeine after chronic oral caffeine exposure, a situation that may best mimic the habitual consumption of caffeine by humans. Caffeine also potentiates the behavioral responses to amphetamine and cocaine in rats responding for food reinforcement under a fixed-interval schedule (Jaszyna et al. 1998). In addition, it potentiates the discriminative-stimulus effects of amphetamine and cocaine (Gauvin et al. 1990; Harland et al. 1989; Schechter 1977; Young et al. 1998). We previously demonstrated that both subtypes of adenosine receptors are involved in potentiation of the discriminative-stimulus effects of methamphetamine and cocaine (Justinova et al. 2003; Munzar et al. 2002). In those experiments, we compared the involvement of adenosine A1 and A2A receptors in the modulation of the discriminative-stimulus effects of methamphetamine and cocaine by using the selective adenosine A1 and A2A receptor antagonists CPT and MSX-3, respectively (Justinova et al. 2003). Both antagonists produced high levels of drug-lever selection when substituted for either methamphetamine or cocaine and significantly shifted the dose-response curves of both psychostimulants to the left. Therefore, adenosine A1 and A2A receptors play important roles in the modulation of the discriminative-stimulus effects of methamphetamine and cocaine (Justinova et al. 2003). The relative involvement of adenosine A1 and A2A receptors in the modulation of the discriminative-stimulus effects of nicotine remains unknown.
Additive interactions between acutely administered nicotine and caffeine have been reported on locomotor activity (Cohen et al. 1991) and schedule-controlled behavior (White 1988) in rats. Also, behavioral effects of nicotine relevant to its addictive properties can be profoundly affected in rats chronically exposed to caffeine. For example, chronic exposure to caffeine in the drinking water facilitated the acquisition of both nicotine self-administration behavior (Shoaib et al. 1999) and nicotine-discrimination performance in rats (Gasior et al. 2000; Gasior et al. 2002). Caffeine also appeared to enhance the discriminative-stimulus effects of the threshold dose of nicotine in rats and no tolerance seemed to develop to this effect even after chronic oral exposure to caffeine (Gasior et al. 2002). These interactions between caffeine and nicotine were produced by low doses of nicotine that produced plasma levels of nicotine (10.4–13.3 ng/ml) in a range of plasma levels that would be produced by smoking one cigarette (Benowitz 1996).
The aim of the present study was threefold. We investigated the relative involvement of adenosine A1 and A2A receptors in the modulation of the discriminative-stimulus effects of nicotine by studying, whether selective adenosine A1 and A2A receptor agonists (CPA and CGS 21680, respectively) and adenosine A1 and A2A receptor antagonists (CPT and MSX-3, respectively) can mimic or modulate the discriminative-stimulus effects of nicotine in rats. In addition, we studied the involvement of adenosine receptors in the modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats chronically exposed to caffeine. We also tested the acute effects of caffeine in nicotine-, methamphetamine-, and cocaine-trained rats and its effects after chronic exposure to caffeine in the drinking water.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats (Charles River, Wilmington, MA) initially weighing 300–360g were housed individually. Before the start of the study, rats were diet restricted (3 NIH07 biscuits /day) for 10 days (weight was 330 – 370 g at the start of the study) and the diet restriction was maintained throughout the study. Enrichments (fresh fruits and vegetables) were provided on Saturdays. Water was available ad libitum. All rats were housed in a temperature- and humidity-controlled room and were maintained on a 12 hr light/dark cycle -the lights were on from 7 a.m. to 7 p.m. Experiments were conducted during the light phase.
Animals used in this study were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and all experimentation was approved by the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, NIH, and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Apparatus
Twelve standard operant-conditioning chambers (Coulbourn Instruments, Lehigh Valley, PA) were used. Each chamber contained a white house light and two levers, separated by a recessed tray into which a pellet dispenser could deliver 45 mg food pellets (F0021, Bioserv, Frenchtown, NJ). Each press of a lever with force of 0.4 N through 1 mm was recorded as a response and was accompanied by an audible click. The operant-conditioning chambers were controlled by microcomputers using the MED Associates MED-PC software package (MED Associates Inc., East Fairfield, VT).
Drug-discrimination procedure
Rats were trained as described previously (e.g., (Justinova et al. 2003; Le Foll et al. 2008) under a discrete-trial schedule of food-pellet delivery to respond on one lever after an injection of a training dose of 0.4 mg/kg nicotine, 1 mg/kg methamphetamine or 10 mg/kg cocaine and on the other lever after an injection of 1 ml/kg of saline vehicle. Injections of nicotine were given subcutaneously 10 min before the start of the session. Methamphetamine and cocaine were given intraperitoneally 15 min before the start of the session. At the start of the session, a white house light was turned on and in its presence the rats were required to make ten consecutive responses (fixed-ratio 10 schedule of food delivery) on the lever appropriate to the pre-session treatment in order to obtain food. The completion of ten consecutive responses on the correct lever produced delivery of a 45 mg food pellet and initiated a 45-s time-out during which lever-press responses had no programmed consequences and the chamber was dark. Responses on the incorrect lever had no programmed consequences other than to reset the fixed-ratio requirement on the correct lever. After each time-out, the white house light was again turned on and the next trial began. Each session ended after completion of 20 fixed-ratio trials or after 30 min elapsed, whichever occurred first. Discrimination-training sessions were conducted 5 days per week under a double alternation schedule (i.e. DDSSDDSS etc., D = drug; S = saline). Training continued until there were eight consecutive sessions during which rats completed at least 90% of their responses during the session on the correct lever and no more than four responses occurred on the incorrect lever during the first trial. Test sessions with other doses of training drug and other drugs were then initiated. Test sessions were identical to training sessions, with the exception that ten consecutive responses on either one of the two levers ended the trial. Switching responding from one lever to the other lever reset the ratio requirement. In a test phase, a single alternation schedule was introduced and test sessions were usually conducted on Tuesdays and Fridays. Thus, a 2-week sequence starting on Monday was: DTSDTSTDST (T = test). In this way, test sessions occurred with equal probability after saline and drug sessions. Test sessions were conducted only if the criterion of 90% accuracy and not more than 4 incorrect responses during the first trial was maintained in the two preceding training sessions.
Testing in nicotine-trained animals
The nicotine dose-response curve (0.01, 0.03, 0.1, 0.3, 0.4 mg/kg) was first determined after the discrimination was acquired, before testing other drugs (n = 27). In the nicotine-trained group, a range of doses of adenosine receptor antagonists CPT (1 – 30 mg/kg; n = 12), MSX-3 (1 – 30 mg/kg; n = 14), and caffeine (1 – 56 mg/kg; n = 15) and adenosine receptor agonists CPA (0.01 – 0.03 mg/kg; n = 6) and CGS 21680 (0.03 – 0.2 mg/kg; n = 7) was substituted for the training dose of nicotine. A range of doses of each drug was tested, and the dose of each drug was typically increased until there was either complete generalization to the nicotine-training stimulus or until the test drug produced a significant decrease in response rates. CPA and CGS 21680 were also administered together with the training dose of nicotine to assess possible alteration of its discriminative-stimulus effects. Subsequently, the effects of selected doses of all adenosinergic compounds on the nicotine dose-response curve were studied: CPT: 3 and 10 mg/kg (n = 10-11), MSX-3: 3 and 10 mg/kg (n = 8-9), caffeine: 10 and 30 mg/kg (n = 12), CPA: 0.01 and 0.02 mg/kg (n = 9-11), CGS 21680: 0.06 mg/kg (n = 12). Then rats were chronically exposed to caffeine in their drinking water (1 mg/ml). At least 10 sessions of training under caffeine exposure preceded the testing of adenosinergic ligands. A range of doses of CPT (3 – 30 mg/kg; n = 8-10), MSX-3 (3 – 30 mg/kg; n = 8-10) and caffeine (3 – 56 mg/kg; n = 11) was substituted for the training dose of nicotine. Then, the nicotine dose-response curve (0.01, 0.03, 0.1, 0.4 mg/kg) was re-determined (n = 12) and a selected dose of each adenosine antagonist was tested for its effects on the dose-response curve: CPT 10 mg/kg (n = 11-12), MSX-3 3 mg/kg (n = 11-12); caffeine 10 mg/kg (n = 11-12).
Testing in methamphetamine- and cocaine-trained animals
Methamphetamine (0.1, 0.18, 0.3, 0.56, 1 mg/kg), cocaine (1, 1.8, 3, 5.6, 10 mg/kg) and caffeine (3, 10, 30, 56 mg/kg) dose-response curves were established before chronic exposure to caffeine began. Then rats were chronically exposed to caffeine in their drinking water (1 mg/ml). After at least 10 days 14 days of training under the caffeine exposure condition, the testing of adenosinergic ligands began (methamphetamine group: n = 9; cocaine group n = 6). A range of doses of CPT (3 – 20 mg/kg), MSX-3 (1 – 20 mg/kg) and caffeine (3 – 56 mg/kg) was substituted for the training doses of methamphetamine or cocaine. Then, methamphetamine and cocaine dose-response curves were re-determined and a selected dose of each adenosine antagonist was tested for its effects on the dose-response curves. In the methamphetamine group, the following doses were tested (n = 9): CPT 3 mg/kg, MSX-3 3 mg/kg, caffeine 10 mg/kg. In cocaine group the following doses were tested: CPT 3 mg/kg (n = 5), MSX-3 3 mg/kg (n = 5), caffeine 3 mg/kg (n = 6).
Drugs
(−)-Cocaine HCl was obtained from NIDA, NIH (Rockville, MD). S(+)-Methylamphetamine HCl (methamphetamine), adenosine A1 receptor agonist CPA (N6-Cyclopentyladenosine), adenosine A1 receptor antagonist CPT (8-cyclopentyl-1,3-dimethylxanthine), adenosine A2A receptor agonist CGS 21680 (2-p-(2-Carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine hydrochloride hydrate), MSX-3 hydrate (7-Dihydro-8-[(1E)-2-(3-Methoxyphenyl)ethenyl]-7-methyl-3-[3-(phosphonooxy)propyl-1-(2-propynyl)-1H-purine-2,6-dione disodium salt hydrate), caffeine (caffeine anhydrous base), and nicotine [(−)-nicotine hydrogen tartrate] were purchased from Sigma Aldrich (St. Louis, MO).
Caffeine was administered chronically by giving the animals free access to bottles containing 1.0 mg/ml caffeine anhydrous base solution in tap water. Caffeine intake was monitored throughout the experiment. Daily caffeine intake (mg/kg per day) was estimated once every week, based on the subject’s fluid consumption over a 48-72 h period and its body weight.
Doses of methamphetamine, cocaine, MSX-3, and CGS 21680 refer to the weight of the salt, whereas doses of CPA, CPT, and nicotine refer to the weight of the base drug. One milligram of the salt form of MSX-3 is equivalent to 0.74 mg of base, and 1 mg of CGS 21680 is equivalent to 0.93 mg of base. All drugs were dissolved in saline (0.9% NaCl) with diluted NaOH for nicotine, MSX-3 and CPT (final pH 7.0) and sonicated if needed. The drugs were injected in a volume of 1 - 3 ml/kg. All drugs were administered intraperitoneally, except for nicotine, which was administered subcutaneously. CPA, CPT, CGS 21680, MSX-3, and caffeine were administered 10 min before the session in generalization tests. In combination tests, all tested compounds were administered 10 min before nicotine (i.e., 20 min before the session), methamphetamine or cocaine (i.e., 25 min before the session). The range of doses and pretreatment times for each compound were selected based on published studies showing behavioral effects (when possible discriminative effects) in rats, devoid of toxicity. For all combination tests, doses of CPA, CGS 21680, CPT, MSX-3 and caffeine were chosen based on the generalization results obtained in this study. For combination tests under chronic caffeine exposure, we considered generalization results during chronic caffeine exposure. Typically, the highest dose that did not produce significant depression of rates of responding or complete abolition of responding in any subject was used.
Data analysis
Discriminative-stimulus data were expressed as the percentage of the total responses on both levers that were made on the nicotine, methamphetamine- or cocaine-appropriate lever. Complete generalization to the training stimulus was defined as 80% or more of responses on the drug-appropriate lever. Partial generalization was defined as >20% to <80% of responding on the drug-appropriate lever. No generalization was defined as less than 20% of responses on the drug-appropriate lever. Response-rate data were expressed as responses per second averaged over the session, with responding during time-out periods not included in calculations. The data from sessions during which rats did not complete at least one fixed ratio were excluded from analysis of drug-lever selection. All results are presented as group means ± S.E.M.
Statistical analysis of the generalization testing was done by using one-way ANOVA for repeated measures. Significant main effects were analyzed further by subsequent paired comparisons with vehicle treatment using the post-hoc Dunnett’s test. A probability value of p < 0.05 was considered significant. For pretreatment tests, ED50 values (doses required to evoke 50% of drug-appropriate responses) for each combination were obtained by nonlinear regression analysis with a sigmoidal dose-response (variable slope) equation, using GraphPad Prism 4 software (GraphPad Software, San Diego, CA). Dose-response curves were considered significantly different when 95% confidence intervals of ED50 values did not overlap. In addition, shifts in dose-response curves were evaluated by using two-way ANOVA for repeated measures. Statistical analysis of the effect of any treatment on rates of responding was done using one-way ANOVA for repeated measures in comparison with vehicle treatment followed, when appropriate, by the Dunnett’s post hoc test. A probability value of p < 0.05 was considered significant. SigmaStat program (http://www.systat.com) was used for ANOVA analysis.
RESULTS
When the dose of nicotine was varied, there was a dose-dependent increase in drug-lever selection with maximal selection of the drug-lever (97.32%) at the 0.4 mg/kg training dose of nicotine (F5,129 = 234.93, p < 0.001). When the dose of methamphetamine was varied, there was a dose-dependent increase in drug-lever selection with maximal selection of the drug-lever (100%) at the 1.0 mg/kg training dose of methamphetamine (F5,40 = 49.97, p < 0.001). Similarly, when the dose of cocaine was varied, there was a dose-dependent increase in drug-lever selection with maximal selection of the drug-lever (99.36%) at the 10.0 mg/kg training dose of cocaine (F5,25 = 27.70, p < 0.001).
During chronic caffeine exposure, rats demonstrated unchanged and reliable stimulus control under training conditions, i.e. responses occurred predominantly on the saline-associated lever following pretreatment with saline, whereas responses occurred predominantly on the drug-associated lever following pretreatment with the training dose of the drug. Nicotine, methamphetamine and cocaine increased drug-lever responding in a dose-dependent manner and comparably under both conditions (i.e., prior and during caffeine exposure) and there was no difference between dose-response curves established prior to and during caffeine exposure (Nicotine: Figures 1A and 4A, top panels; Methamphetamine: Figure 5A, top panel; Cocaine: Figure 5B, top panel). Furthermore, the potency of nicotine, methamphetamine and cocaine as a discriminative stimulus was comparable prior to and during caffeine exposure (Table 1: overlapping 95% CIs for ED50). This suggests that there was no shift in nicotine, methamphetamine or cocaine discrimination performance as a result of chronic caffeine exposure.
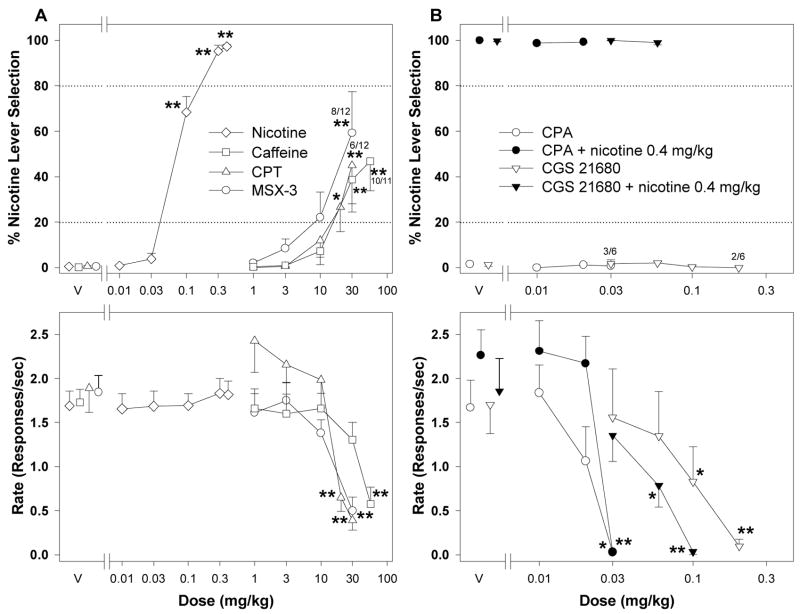
Figure 1.
Average percentage of responding on the nicotine-appropriate lever (top panels) and response rates (bottom panels) from generalization tests with adenosine antagonists and agonists in nicotine-trained rats. A) Effects of pretreatment with nicotine (n = 27), caffeine (n = 15), CPT (n = 12), or MSX-3 (n = 14) in rats trained to discriminate 0.4 mg/kg nicotine from vehicle. B) Effects of pretreatment with CPA (n = 6) or CGS 21680 (n = 7) alone and in combination with 0.4 mg/kg nicotine in rats trained to discriminate 0.4 mg/kg nicotine from saline. Data are means ± S.E.M. *p < 0.05, **p < 0.01, post hoc comparison with the vehicle pretreatment after significant ANOVA for repeated measures main effect, Dunnett’s test. Numbers with asterisks at higher doses indicate the number of rats that completed at least one fixed ratio (trial) during the session over the total number of rats in which the dose was tested.
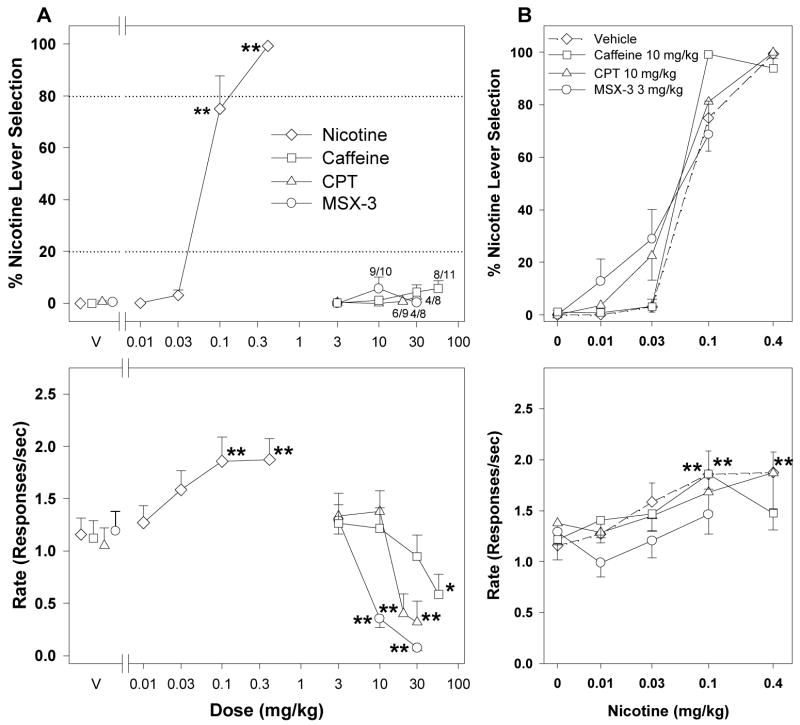
Figure 4.
Average percentage of responding on the nicotine-appropriate lever (top panels) and response rates (bottom panels) from generalization and combination tests with adenosine antagonists in nicotine-trained rats chronically exposed to caffeine in their drinking water. A) Effects of pretreatment with nicotine (n = 12), caffeine (n = 11), CPT (n = 8–10), or MSX-3 (n = 8–10) in nicotine-trained rats. B) Nicotine dose-response curves after pretreatment with vehicle, CPT, MSX-3, or caffeine (n = 11–12). Data are means ± S.E.M. *p < 0.05, **p < 0.01, post hoc comparison with the vehicle pretreatment after significant ANOVA for repeated measures main effect, Dunnett’s test. Numbers with asterisks at higher doses indicate the number of rats that completed at least one fixed ratio (trial) during the session over the total number of rats in which the dose was tested.
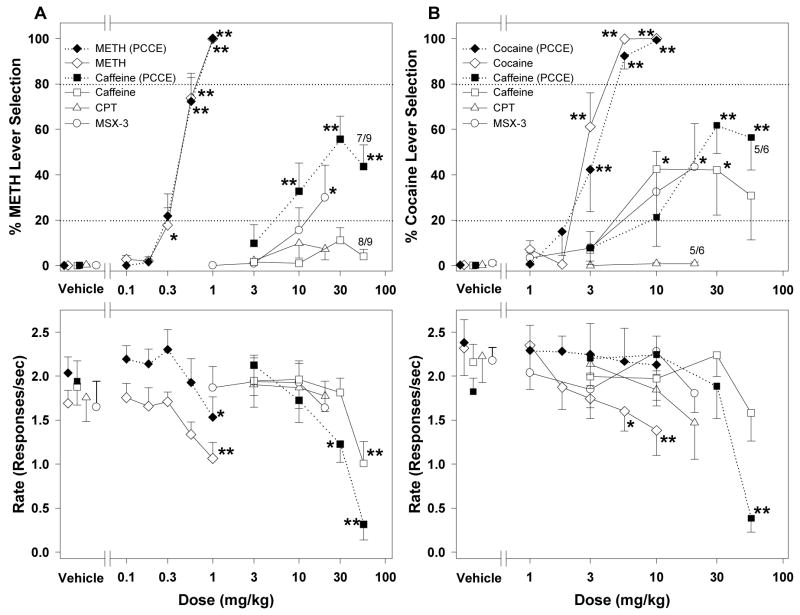
Figure 5.
Average percentage of responding on the drug-appropriate lever (top panels) and response rates (bottom panels) from generalization tests with adenosine antagonists in methamphetamine- or cocaine-trained rats chronically exposed to caffeine in their drinking water. A) Effects of methamphetamine (n = 9); caffeine (n = 9), CPT (n = 9), or MSX-3 (n = 9) in rats trained to discriminate 1.0 mg/kg methamphetamine from saline. Effects of pretreatment with methamphetamine or caffeine prior to chronic caffeine exposure (METH PCCE, caffeine PCCE; both n = 9). B) Effects of pretreatment with cocaine (n = 6), caffeine (n = 6), CPT (n = 6) or MSX-3 (n = 6) in rats trained to discriminate 10.0 mg/kg cocaine from saline. Effects of pretreatment with cocaine or caffeine prior to chronic caffeine exposure (Cocaine PCCE, caffeine PCCE; both n = 6). Data are means ± S.E.M. *p < 0.05, **p < 0.01, post hoc comparison with the vehicle pretreatment after significant ANOVA for repeated measures main effect, Dunnett’s test. Numbers with asterisks at higher doses indicate the number of rats that completed at least one fixed ratio (trial) during the session over the total number of rats in which the dose was tested. PCCE = prior to chronic caffeine exposure
Table 1.
ED50 values of selected treatments for drug-discrimination experiments. ED50 values were calculated by nonlinear regression analysis using a sigmoidal dose-response curve with variable slope.
| Drug | Dose Range Tested
(mg/kg) |
ED50 (95% CI)
(mg/kg) |
|---|---|---|
| No caffeine in drinking water | ||
| Nicotine + vehicle | 0.01 – 0.4 | 0.08 (0.07 – 0.09) |
| Nicotine + CPT 3 | 0.01 – 0.4 | 0.06 (0.04 – 0.09) |
| Nicotine + CPT 10a | 0.01 – 0.4 | 0.04 (0.02 – 0.07) |
| Nicotine + vehicle | 0.01 – 0.4 | 0.09 (0.05 – 0.14) |
| Nicotine + MSX 3 | 0.01 – 0.1 | 0.07 (0.03 – 0.12) |
| Nicotine + MSX 10a | 0.01 – 0.1 | 0.04 (0.01 – 0.05) |
| Nicotine + vehicle | 0.01 – 0.4 | 0.10 (0.08 – 0.11) |
| Nicotine + caffeine 10 | 0.01 – 0.4 | 0.06 (0.03 – 0.10) |
| Nicotine + caffeine 30a | 0.01 – 0.4 | 0.04 (0.03 – 0.06) |
| Nicotine + vehicle | 0.01 – 0.4 | 0.08 (0.06 – 0.09) |
| Nicotine + CPA 0.01 | 0.01 – 0.4 | 0.08 (0.07 – 0.10) |
| Nicotine + CPA 0.02 | 0.01 – 0.4 | 0.07 (0.06 – 0.07) |
| Nicotine + vehicle | 0.01 – 0.4 | 0.07 (0.06 – 0.08) |
| Nicotine + CGS 21680 0.06 | 0.01 – 0.4 | 0.06 (0.03 – 0.08) |
| METH + vehicle | 0.1 – 1.00 | 0.46 (0.40 – 0.51) |
| Cocaine + vehicle | 1.0 – 10.0 | 3.28 (2.63 – 3.93) |
| Caffeine in drinking water | ||
| Nicotine + vehicle | 0.01 – 0.4 | 0.08 (0.06 – 0.10) |
| Nicotine + CPT 10 | 0.01 – 0.4 | 0.06 (0.05 – 0.08) |
| Nicotine + MSX 3 | 0.01 – 0.1 | 0.07 (0.04 – 0.10) |
| Nicotine + caffeine 10 | 0.01 – 0.4 | 0.06 (0.03 – 0.08) |
| METH + vehicle | 0.1 – 1.00 | 0.46 (0.42 – 0.50) |
| METH + CPT 3 | 0.1 – 0.56 | 0.52 (0.44 – 0.59) |
| METH + MSX 3a | 0.1 – 0.56 | 0.25 (0.22 – 0.29) |
| METH + caffeine 10a | 0.1 – 0.56 | 0.30 (0.24 – 0.36) |
| Cocaine + vehicle | 1.0 – 10.0 | 2.89 (2.58 – 3.21) |
| Cocaine + CPT 3 | 1.0 – 3.0 | 2.51 (2.26 – 2.75) |
| Cocaine + MSX 3a | 1.0 – 3.0 | 0.76 (0.19 – 1.32) |
| Cocaine + caffeine 3a | 1.0 – 5.6 | 1.69 (1.03 – 2.36) |
Non-overlapping 95% CI compared with the dose-response curve after vehicle pretreatment.
Figure 1 shows the percentage of responses made on the drug lever (top panels) and overall rates of responding (bottom panels) during sessions when different doses of the three adenosine antagonists (Figure 1A) or two adenosine agonists (Figure 1B) were tested for generalization to the training dose of 0.4 mg/kg of nicotine. The non-selective adenosine receptor antagonist caffeine produced partial generalization to the nicotine-training stimulus (F5,62 = 10.10, p < 0.001), which was significant at doses of 30 and 56 mg/kg. The 56 mg/kg dose of caffeine significantly decreased rates of responding (F5,84 = 10.42, p < 0.001; 1 of 11 rats did not complete a single fixed ratio). The selective adenosine A1 receptor antagonist CPT (F5,43 = 5.18, p < 0.001) also partially generalized to the nicotine training stimulus at doses of 20 and 30 mg/kg, which also significantly decreased rates of responding (F5,49 = 14.49, p < 0.001). Six out of 12 rats did not complete a single fixed ratio after a dose of 30 mg/kg of CPT. MSX-3 also partially generalized to nicotine (F4,41 = 6.63, p < 0.001) at a dose of 30 mg/kg, which decreased rates of responding (F4,45 = 14.42, p < 0.001; four out of 12 rats did not complete a single fixed ratio).
Neither the adenosine A1 receptor agonist CPA (0.01–0.03 mg/kg) nor the adenosine A2A receptor agonist CGS 21680 (0.03–0.2 mg/kg) generalized to the nicotine-training stimulus when administered alone (Figure 1B, top panel). CPA, at a dose 0.03 mg/kg, markedly and significantly decreased response rates (Figure 1B, bottom panel; F3,15 = 6.86, p = 0.004; 3 of 6 rats did not complete a single fixed ratio). CGS 21680, at doses of 0.1 and 0.2 mg/kg, significantly decreased rates of responding (Figure 1B, bottom panel; F4,20 = 8.97, p < 0.001). Four of six rats did not complete a single fixed ratio after administration of 0.2 mg/kg of CGS 21680. Also, neither CPA nor CGS 21680 significantly attenuated the discriminative-stimulus effects of the training dose of nicotine (Figure 1B, top panel). When a dose 0.03 mg/kg of CPA was administered together with the training dose of nicotine, none of the six rats tested completed a single fixed ratio (Figure 1B, bottom panel). A combination of a low 0.06 mg/kg dose of CGS 21680 with 0.4 mg/kg nicotine caused a significant decrease in rates of responding (F3,12 = 8.01, p = 0.003). When a higher dose of 0.1 mg/kg CGS 21680 was administered together with the 0.4 mg/kg training dose of nicotine only five of six rats completed a single fixed ratio.
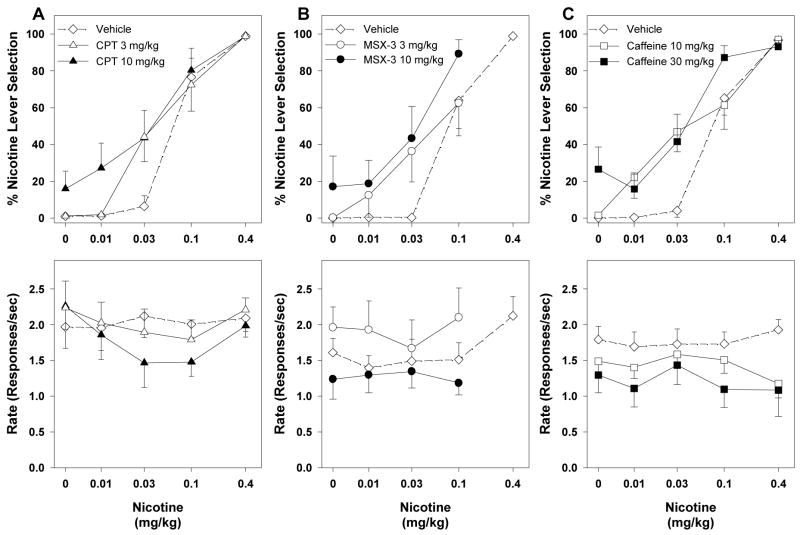
Figure 2 shows effects of selected doses of CPT, MSX-3 and caffeine on the nicotine dose-response curves. Doses chosen for combination tests did not produce significant generalization to the nicotine-training stimulus and did not significantly affect response rates when given alone. A 10 mg/kg dose of CPT produced a shift to the left of the nicotine dose-response curve (Figure 2A, top panel) without significantly altering rates of responding (Figure 2A, bottom panel). This leftward shift was significant, as indicated by non-overlapping 95% CIs of ED50 values for vehicle and CPT pretreatments (Table 1) as well as by two-way ANOVA for repeated measures (F1,19 = 6.29, p = 0.03). A 3 mg/kg dose of CPT did not significantly shift the nicotine dose-response curve (Table 1: overlapping 95% CIs of ED50 values). Pretreatment with a 10 mg/kg dose of MSX-3 shifted the nicotine dose-response curve markedly to the left (Figure 2B, top panel) without significantly altering rates of responding (Figure 2B, bottom panel), as revealed by non-overlapping 95% CIs of ED50 values for vehicle and MSX-3 pretreatments (Table 1) as well as by two-way ANOVA for repeated measures (F1,14 = 14.22, p = 0.007). A 3 mg/kg dose of MSX-3 did not significantly shift the nicotine dose-response curve (Table 1: overlapping 95% CIs of ED50 values). A 30 mg/kg dose of caffeine significantly shifted the nicotine dose-response curve to the left (Figure 2C, top panel) without significantly altering rates of responding (Figure 2C, bottom panel) (Table 1: non-overlapping 95% CIs of ED50 values; F1,17 = 18.87, p < 0.001). A 10 mg/kg dose of caffeine shifted the nicotine dose-response curve to the left, as revealed by significant two-way ANOVA for repeated measured (F1,21 = 5.40, p = 0.04), but 95% CIs for ED50 overlapped with values for vehicle pretreatment (Table 1).
Figure 2.
Average percentage of responding on the nicotine-appropriate lever (top panels) and response rates (bottom panels) from combination tests with adenosine antagonists in nicotine-trained rats. Nicotine dose-response curves after pretreatment with vehicle or CPT (A) or MSX-3 (B) or caffeine (C). Data are means (±S.E.M.) from 10–11 rats (A), 8–9 rats (B) or 12 rats (C).
Figure 3 shows the effects of selected doses of CPA (0.01 and 0.02 mg/kg) and CGS 21680 (0.06 mg/kg) on the nicotine dose-response curves. Doses of CPA and CGS 21680 chosen for combination tests did not produce significant generalization to the nicotine-training stimulus and did not significantly affect response rates when given alone. Both CPA (Figure 3A) and CGS 21680 (Figure 3B) failed to shift the nicotine dose-response curves at the selected doses (Table 1: overlapping 95% CIs of ED50 values) and did not significantly alter rates of responding.
Figure 3.
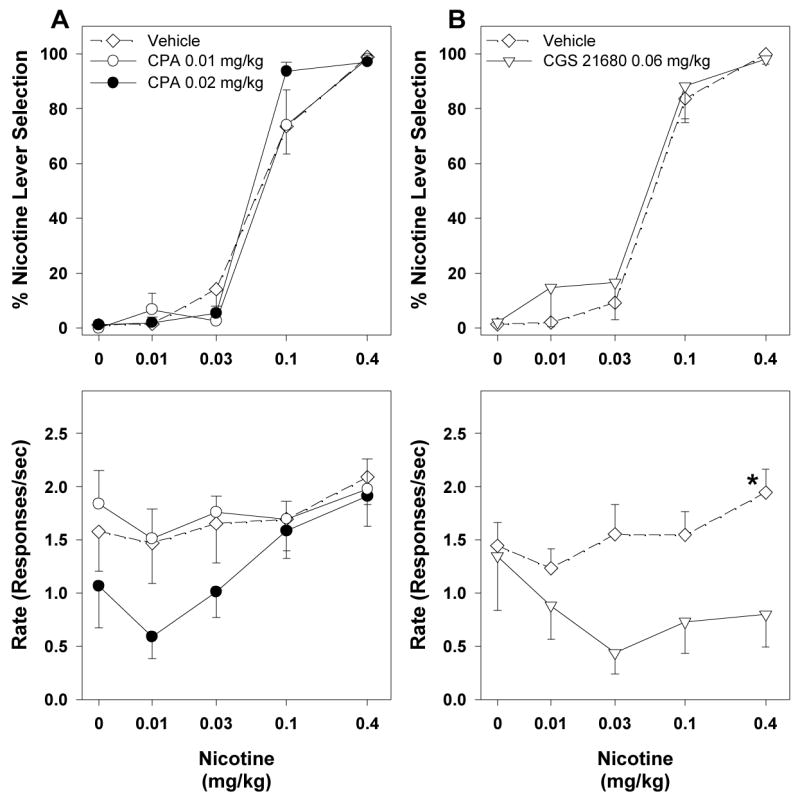
Average percentage of responding on the nicotine-appropriate lever (top panels) and response rates (bottom panels) from combination tests with adenosine agonists in nicotine-trained rats. Nicotine dose-response curves after pretreatment with vehicle or CPA (A) or CGS 21680 (B). Data are means (±S.E.M.) from 9–11 rats (A) or 12 rats (B). *p < 0.05, post hoc comparison with the vehicle pretreatment after significant ANOVA for repeated measures main effect, Dunnett’s test.
Figures 4, 5 and 6 show generalization and combination tests with different adenosine receptor antagonists during chronic caffeine exposure in nicotine-, methamphetamine-, and cocaine-trained rats. Rats were exposed to caffeine in their drinking water (1 mg/kg) for at least 14 days before the testing begin. Average daily caffeine intake was 104.4 ± 5.9 mg/kg. All three adenosine antagonists, caffeine, CPT and MSX-3, failed to generalize to the nicotine-training stimulus when rats were chronically exposed to caffeine (Figure 4A, top panel). The highest dose of caffeine (56 mg/kg) significantly reduced rates of responding (Figure 4A, bottom panel; F4,40 = 4.08, p = 0.007) and three out of 11 rats did not finish a single fixed ratio. The highest doses of CPT (20 and 30 mg/kg) also significantly decreased rates of responding (Figure 4A, bottom panel; F4,33 = 12.17, p < 0.001) and three of nine and four of eight rats, respectively, did not complete a single fixed ratio. Doses 10 and 30 mg/kg of MSX-3 significantly decreased rates of responding (Figure 4A, bottom panel; F3,25 = 23.62, p < 0.001) and one of 10 and four of eight rats, respectively, did not complete a single trial. Selected doses of caffeine (10 mg/kg), CPT (10 mg/kg), or MSX-3 (3 mg/kg) failed to shift the nicotine dose-response curve (Table 1: overlapping 95% CIs of ED50 values). CPT and MSX-3 showed a tendency to increase the discriminative effects of lower nicotine doses, but the effect was not statistically significant. These doses of adenosine antagonists did not significantly alter rates of responding, except for caffeine in combination with 0.1 mg/kg nicotine, which significantly increased rates of responding (Figure 4B, bottom panel; F3,33 = 4.58, p = 0.009).
Figure 6.
Average percentage of responding on the drug-appropriate lever (top panels) and response rates (bottom panels) from pretreatment tests with adenosine antagonists in methamphetamine- or cocaine-trained rats chronically exposed to caffeine in their drinking water. Methamphetamine (A) and cocaine (B) dose-response curves after intraperitoneal pretreatment with 1.0 ml/kg of vehicle or caffeine at doses 10 mg/kg (methamphetamine group) or 3 mg/kg (cocaine group), or 3 mg/kg CPT or 3 mg/kg MSX-3. Data are means (± S.E.M.) from 9 rats (methamphetamine-trained rats) and 5–6 rats (cocaine-trained rats). *p < 0.05, **p < 0.01, post hoc comparison with the vehicle pretreatment after significant ANOVA for repeated measures main effect, Dunnett’s test.
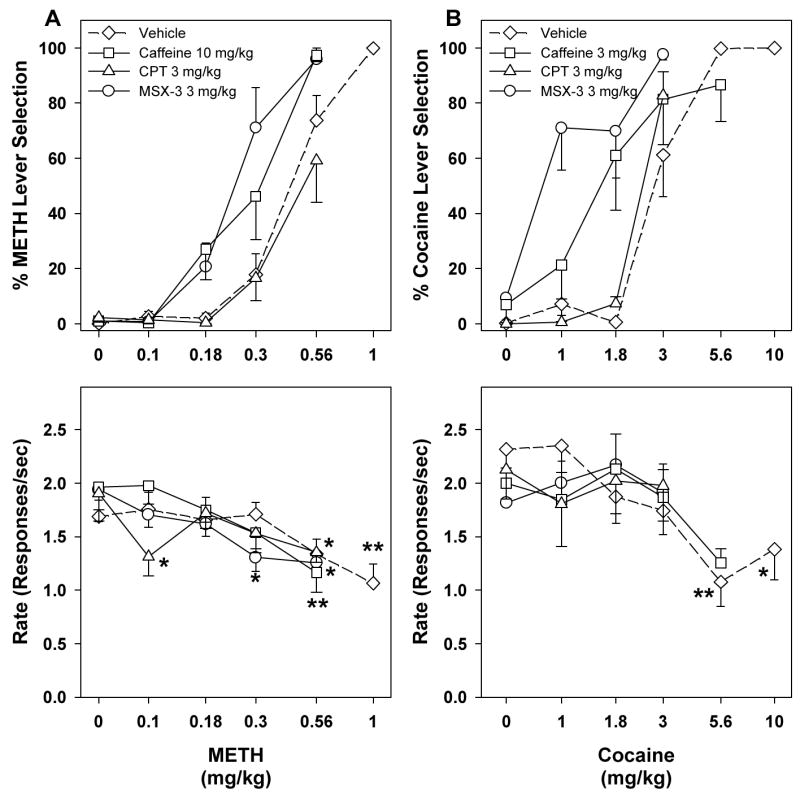
In methamphetamine-trained rats, caffeine doses of 10, 30 and 56 mg/kg produced partial generalization to the training stimulus before chronic exposure to caffeine in the drinking water (Figure 5A, top panel; F4,30 = 11.36, p < 0.001) and caffeine doses of 30 and 56 mg/kg significantly decreased rate of responding (Figure 5A, bottom panel; F4,32 = 19.79, p < 0.001; two of nine rats did not finish a single fixed-ratio at dose 56 mg/kg). In contrast, caffeine failed to produce significant generalization to the methamphetamine-training stimulus during chronic exposure to caffeine (Figure 5A, top panel). One-way ANOVA for repeated measures showed a significant increase in drug-lever selection after a dose 30 mg/kg caffeine (F4,31 = 3.76, p = 0.013). However, drug-lever selection reached only 11.10%, which is below our criterion (20%) for partial generalization. In methamphetamine-trained rats (Figure 5A, bottom panel), a caffeine dose of 56 mg/kg significantly decreased rates of responding (F4,32 = 4.86, p = 0.004; one of nine rats did not finish a single trial). In cocaine-trained animals, caffeine doses of 30 and 56 mg/kg partially generalized to the training stimulus before chronic caffeine exposure (Figure 5B, top panel; F4,19 = 12.81, p < 0.001) and the 56 mg/kg caffeine dose significantly decreased rates of responding (Figure 5B, bottom panel; F4,20 = 15.56, p < 0.001; one of six rats did not finish a single fixed-ratio). Caffeine also produced significant partial generalization to the cocaine-training stimulus at doses 10 and 30 mg/kg during chronic exposure to caffeine (Figure 5B, top panel; F4,20 = 3.55, p = 0.024) without significantly altering rates of responding.
When CPT was administered alone, neither methamphetamine- nor cocaine-lever selection was observed (Figure 5A). Increasing the dose of CPT to 20 mg/kg produced a decrease in rates of responding in the cocaine-trained group (one of six rats failed to complete at least one fixed-ratio; Figure 5B, bottom panel). MSX-3 produced partial generalization to both the methamphetamine- and cocaine-training stimuli without significantly altering rates of responding. In methamphetamine-trained rats, MSX-3 produced partial generalization (F4,32 = 3.51, p = 0.017) at a dose of 20 mg/kg (Figure 5A, top panel). In cocaine-trained rats, MSX-3 also produced partial generalization to the cocaine-training stimulus at a dose of 20 mg/kg (F4,20 = 3.97, p = 0.016).
Figure 6 shows effects of selected doses of adenosine antagonists, which did not produce significant generalization to the methamphetamine-training stimulus and did not significantly change response rates when given alone, on methamphetamine and cocaine dose-response curves. A 10 mg/kg dose of caffeine produced a leftward shift of the methamphetamine dose-response curve (Figure 6A, top panel). This leftward shift was significant, as indicated by non-overlapping 95% CIs of ED50 values for vehicle and caffeine pretreatments (Table 1) as well as by two-way ANOVA for repeated measures (F1,24 = 7.88, p = 0.02). In the cocaine-trained group (Figure 6B, top panel), a 3 mg/kg dose of caffeine produced a leftward shift of the cocaine dose-response curve (Table 1: non-overlapping 95% CIs of ED50 values; F1,15 = 9.30, p = 0.028). In the methamphetamine-trained group (Figure 6A, bottom panel), 10 mg/kg of caffeine in combination with 0.56 mg/kg of methamphetamine produced significant decreases in rates of responding (F4,32 = 8.20, p < 0.001). A 3 mg/kg dose of CPT failed to significantly shift either the methamphetamine or cocaine dose-response curves. In the methamphetamine-trained group (Figure 6A, bottom panel), CPT in combination with 0.1 or 0.56 mg/kg of methamphetamine significantly decreased rates of responding (F4,32 = 3.20, p = 0.026). Pretreatment with 3 mg/kg MSX-3 shifted both the methamphetamine and cocaine dose-response curves markedly to the left, as revealed by non-overlapping 95% CIs of ED50 values for vehicle and MSX-3 pretreatments (Table 1), as well as by two-way ANOVA for repeated measures (methamphetamine: F1,24 = 14.69, p = 0.005; cocaine: F1,8 = 44.20, p = 0.003). In the methamphetamine-trained group (Figure 6A, bottom panel), MSX-3 in combination with 0.3 or 0.56 mg/kg of methamphetamine produced significant decreases in rates of responding (F4,32 = 3.36, p = 0.021).
DISCUSSION
In the present study, effects of different adenosine receptor antagonists and agonists were investigated in rats trained to discriminate nicotine from saline in order to characterize the relative involvement of adenosine receptor subtypes in the discriminative-stimulus effects nicotine. The non-selective adenosine-receptor antagonist caffeine, the selective adenosine A1 antagonist CPT, as well as the selective adenosine A2A antagonist MSX-3, all produced nicotine-like discriminative-stimulus effects upon substitution and they all shifted the nicotine dose-response curve to the left. The discriminative-stimulus effects of nicotine are mostly mediated by nicotinic acetylcholine receptors (e.g., (Stolerman et al. 1984), but involvement of a dopaminergic component has been suggested (e.g., (Desai et al. 2003). Our results indicate that both adenosine A1 and A2A receptors are capable of modulating nicotine’s discriminative-stimulus effects and that their blockade can increase these actions of nicotine. The partial generalization levels found after CPT and MSX-3 administration were somewhat lower than was previously observed in methamphetamine- or cocaine-trained rats (Justinova et al. 2003), but the effects were observed over the same dose-range. Caffeine produced a level of partial generalization which was not observed previously in nicotine-trained rats (Gasior et al. 2002) but was very similar to what was previously observed in methamphetamine-trained rats over the same range of doses (Munzar et al. 2002). The leftward-shift of the nicotine dose-response curve produced by caffeine in this study is in agreement with the finding of Gasior and colleagues (Gasior et al. 2002) that caffeine acutely potentiates the discriminative-stimulus effects of a low threshold dose of nicotine under the same conditions.
Taking into consideration the effects of the adenosine antagonists described above, it could be expected that adenosine agonists would not produce nicotine-like discriminative-stimulus effects and that they would attenuate the discriminative-stimulus effects of nicotine. In accordance with this hypothesis, the adenosine A1- and A2A-receptor agonists (CPA and CGS 21680, respectively) did not mimic the nicotine discriminative-stimulus effects. However, they neither attenuated the discriminative effects of the nicotine-training stimulus nor produced any shift of the nicotine dose-response curve. Though unexpected, these results were similar to those we obtained in methamphetamine-trained rats, but were different from those in cocaine-trained animals in our previous study (Justinova et al. 2003). In cocaine-trained animals, CGS 21680 partially generalized to the training stimulus and both CPA and CGS 21680 shifted the cocaine dose-response curve to the left. We hypothesized that the observed differences in cocaine-trained animals were due to a cocaine-mediated increase in extracellular levels of adenosine in the ventral tegmental area (Fiorillo and Williams 2000), which is not observed after administration of amphetamines (Herrera-Marschitz et al. 1994).
Chronic caffeine exposure had a different effect on nicotine-trained rats compared to methamphetamine- or cocaine-trained rats. When nicotine-trained rats were chronically exposed to caffeine in their drinking water, we did not observe cross-tolerance developing to the effects of nicotine, but tolerance to the A1- as well as the A2A-mediated effects developed. After chronic exposure to caffeine in the drinking water, the adenosine antagonists, caffeine, CPT, and MSX-3, all failed to generalize to the nicotine-training stimulus and all failed to shift the nicotine dose-response curve. The lack of tolerance development to the rate-depressant effects of higher doses of adenosine-receptor antagonists is in accordance with studies showing that adenosine receptors are not involved in the motor-depressant effects of higher doses of caffeine (Halldner et al. 2004). Failure to mimic or alter the discriminative-stimulus effects of nicotine by selective A1 and A2A antagonists, as well as caffeine, might be related to the reported reduction of the dopaminergic component of the nicotine cue in caffeine-drinking rats (Gasior et al. 1999). A previous study by Gasior and colleagues (Gasior et al. 2002) reported that caffeine can potentiate discriminative-stimulus effects of low threshold doses of nicotine during chronic exposure to caffeine in the drinking water, but we could not replicate this finding under the same conditions in the present study. This discrepancy is apparent in human studies as well. For example, two studies showed that daily oral caffeine administration potentiates discriminative effects of nicotine administered intravenously (Jones and Griffiths 2003) or in the form of chewing gum (Duka et al. 1998), while another study (Perkins et al. 2005) showed that daily oral caffeine does not alter the discrimination of nicotine administered by nasal spray in chronic coffee drinkers. Different routes of nicotine administration as well as population samples studied might account for these discrepancies.
In methamphetamine- and cocaine-trained rats, there appeared to be complete tolerance to the A1-mediated effects developed during chronic exposure to caffeine in the drinking water, because CPT failed to generalize to either training stimulus and failed to produce any shift in the methamphetamine or cocaine dose-response curves. On the other hand, A2A-mediated effects were still present in both groups of rats chronically exposed to caffeine. Only MSX-3 produced partial generalization to the methamphetamine-training stimulus, but both caffeine and MSX-3 partially generalized to cocaine and both caffeine and MSX-3 shifted the methamphetamine and cocaine dose-response curves to the left. Also, we did not observe a cross-tolerance to methamphetamine or cocaine after chronic caffeine exposure. A comparative summary of the effects of adenosinergic compounds from our present and previous drug-discrimination studies (Justinova et al. 2003; Munzar et al. 2002) can be found in Table 2.
Table 2.
Summary of effects of different adenosinergic ligands on discriminative-stimulus effects of nicotine, methamphetamine, and cocaine (present study; Munzar et al. 2002; Justinova et al. 2003).
| Adenosine agonists | Adenosine antagonists | |||||
|---|---|---|---|---|---|---|
| Discriminated Drug | Effect | A1 CPA | A2A CGS 21680 | A1 CPT | A2A MSX-3 | Non-selective Caffeine |
| No Caffeine Exposure in Drinking water | ||||||
| Nicotine | Generalization | None | None | Partial | Partial | Partial |
| Shift of DRC | No shift | No shift | Left | Left | Left | |
| Methamphetamine | Generalization | None | None | Partial | Partial | Partial |
| Shift of DRC | No shift | No shift | Left | Left | Left | |
| Cocaine | Generalization | None | Partial | Partial | Partial | Partial |
| Shift of DRC | Left | Left | Left | Left | Left* | |
| Chronic Caffeine Exposure in Drinking Water | ||||||
| Nicotine | Generalization | None | None | None | ||
| Shift of DRC | No shift | No shift | No shift | |||
| Methamphetamine | Generalization | None | Partial | None | ||
| Shift of DRC | No shift | Left | Left | |||
| Cocaine | Generalization | None | Partial | Partial | ||
| Shift of DRC | No shift | Left | Left | |||
DRC = Dose-response curve
In conclusion, both adenosine A1 and A2A receptors appear to be capable of modulating discriminative-stimulus effects of nicotine in fashion similar to their involvement in the discriminative-stimulus effects of methamphetamine and cocaine. However, the capacity of adenosine receptors to modulate the discriminative-stimulus effects of nicotine substantially diminishes during chronic exposure to caffeine, but it persists during chronic caffeine exposure in methamphetamine- and cocaine-trained animals. We observed a complete tolerance to both the A1- and A2A-mediated effects in nicotine-trained rats during chronic caffeine exposure. In contrast, chronic caffeine exposure produced tolerance to adenosine A1-mediated, but not A2A-mediated, effects in methamphetamine- and cocaine-trained rats. Our study supports the evidence from previous preclinical studies that caffeine can acutely potentiate the effects of nicotine related to its abuse potential (Gasior et al. 1999; Gasior et al. 2002; Shoaib et al. 1999), but we show that tolerance can develop to this effect after chronic oral exposure to caffeine, which mimics the habitual consumption of caffeine by human smokers.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD. We thank Eric Thorndike for programming assistance and Dr. Leigh Panlilio for helpful comments on the manuscript.
All experimentation was approved by the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, NIH, and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
References
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Cohen C, Welzl H, Battig K. Effects of nicotine, caffeine, and their combination on locomotor activity in rats. Pharmacol Biochem Behav. 1991;40:121–123. doi: 10.1016/0091-3057(91)90331-u. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, Russell K, Stephens DN. Discriminative stimulus properties of nicotine at low doses: the effects of caffeine preload. Behav Pharmacol. 1998;9:219–229. [PubMed] [Google Scholar]
- Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends in Neurosciences. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Selective inhibition by adenosine of mGluR IPSPs in dopamine neurons after cocaine treatment. J Neurophysiol. 2000;83:1307–1314. doi: 10.1152/jn.2000.83.3.1307. [DOI] [PubMed] [Google Scholar]
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Munzar P, Witkin JM, Goldberg SR. Caffeine potentiates the discriminative-stimulus effects of nicotine in rats. Psychopharmacology (Berl) 2002;162:385–395. doi: 10.1007/s00213-002-1113-3. [DOI] [PubMed] [Google Scholar]
- Gasior M, Jaszyna M, Peters J, Goldberg SR. Changes in the ambulatory activity and discriminative stimulus effects of psychostimulant drugs in rats chronically exposed to caffeine: effect of caffeine dose. J Pharmacol Exp Ther. 2000;295:1101–1111. [PubMed] [Google Scholar]
- Gasior M, Shoaib M, Yasar S, Jaszyna M, Goldberg SR. Acquisition of nicotine discrimination and discriminative stimulus effects of nicotine in rats chronically exposed to caffeine. J Pharmacol Exp Ther. 1999;288:1053–1073. [PubMed] [Google Scholar]
- Gauvin DV, Criado JR, Moore KR, Holloway FA. Potentiation of cocaine’s discriminative effects by caffeine: a time-effect analysis. Pharmacol Biochem Behav. 1990;36:195–197. doi: 10.1016/0091-3057(90)90149-c. [DOI] [PubMed] [Google Scholar]
- Halldner L, Aden U, Dahlberg V, Johansson B, Ledent C, Fredholm BB. The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology. 2004;46:1008–1017. doi: 10.1016/j.neuropharm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Harland RD, Gauvin DV, Michaelis RC, Carney JM, Seale TW, Holloway FA. Behavioral interaction between cocaine and caffeine: a drug discrimination analysis in rats. Pharmacol Biochem Behav. 1989;32:1017–1023. doi: 10.1016/0091-3057(89)90075-0. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, Luthman J, Ferre S. Unilateral neonatal intracerebroventricular 6-hydroxydopamine administration in rats: II. Effects on extracellular monoamine, acetylcholine and adenosine levels monitored with in vivo microdialysis. Psychopharmacology (Berl) 1994;116:451–456. doi: 10.1007/BF02247477. [DOI] [PubMed] [Google Scholar]
- Jaszyna M, Gasior M, Shoaib M, Yasar S, Goldberg SR. Behavioral effects of nicotine, amphetamine and cocaine under a fixed-interval schedule of food reinforcement in rats chronically exposed to caffeine. Psychopharmacology (Berl) 1998;140:257–271. doi: 10.1007/s002130050766. [DOI] [PubMed] [Google Scholar]
- Jones HE, Griffiths RR. Oral caffeine maintenance potentiates the reinforcing and stimulant subjective effects of intravenous nicotine in cigarette smokers. Psychopharmacology (Berl) 2003;165:280–290. doi: 10.1007/s00213-002-1262-4. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Muller CE, Fuxe K, Goldberg SR, Popoli P, Ferre S. Involvement of Adenosine A(1) and A(2A) Receptors in the Motor Effects of Caffeine after its Acute and Chronic Administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Justinova Z, Wertheim CE, Barnes C, Goldberg SR. Topiramate does not alter nicotine or cocaine discrimination in rats. Behav Pharmacol. 2008;19:13–20. doi: 10.1097/FBP.0b013e3282f3cf84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzar P, Justinova Z, Kutkat SW, Ferre S, Goldberg SR. Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 2002;161:348–355. doi: 10.1007/s00213-002-1075-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Stolinski A, Blakesley-Ball R, Wilson AS. The influence of caffeine on nicotine’s discriminative stimulus, subjective, and reinforcing effects. Exp Clin Psychopharmacol. 2005;13:275–281. doi: 10.1037/1064-1297.13.4.275. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Caffeine potentiation of amphetamine: implications for hyperkinesis therapy. Pharmacol Biochem Behav. 1977;6:359–361. doi: 10.1016/0091-3057(77)90038-7. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Yasar S, Goldberg SR. Chronic caffeine exposure potentiates nicotine self-administration in rats. Psychopharmacology (Berl) 1999;142:327–333. doi: 10.1007/s002130050896. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- White JM. Behavioral interactions between nicotine and caffeine. Pharmacol Biochem Behav. 1988;29:63–66. doi: 10.1016/0091-3057(88)90274-2. [DOI] [PubMed] [Google Scholar]
- Young R, Gabryszuk M, Glennon RA. (−)Ephedrine and caffeine mutually potentiate one another’s amphetamine-like stimulus effects. Pharmacol Biochem Behav. 1998;61:169–173. doi: 10.1016/s0091-3057(98)00044-6. [DOI] [PubMed] [Google Scholar]