Abstract
The nonreceptor, protein-tyrosine kinase Syk is a suppressor of breast cancer progression whose expression is inversely correlated with the invasive behavior of cancer cells. In contrast, Syk plays a positive role in murine mammary tumor virus-mediated tumorigenesis. A yeast two-hybrid screen using a library from human mammary gland identified TRAF-interacting protein (TRIP) as a Syk-binding partner. This interaction is mediated by the C-terminal region of TRIP and is enhanced by the treatment of cells with tumor necrosis factor (TNF) and the tyrosine-phosphorylation of Syk. Syk and TRIP play opposing roles in TNF-signaling pathways. Syk enhances the activation of NF-κB by TNF and this is antagonized by TRIP. The overexpression of TRIP sensitizes cells to TNF-induced apoptosis, an effect that can be reversed by the co-expression of Syk.
Keywords: Syk, tyrosine kinase, breast cancer, tumor necrosis factor, TRIP, NF-κB
Introduction
Syk is a non-receptor protein-tyrosine kinase that exhibits characteristics of a tumor suppressor to regulate malignant progression in breast cancer. Syk is expressed in normal breast epithelia and breast cancer cells with low tumorigenic potential, but is reduced or absent in more malignant and highly invasive tumor cells (Coopman et al., 2000; Moroni et al., 2004; Toyama et al., 2003). Decreased expression of Syk is correlated with poor prognosis and increased risk of distant metastases in patients (Toyama et al., 2003). On the other hand, the inappropriate activation of Syk in breast epithelial cells by the expression of proteins containing immunoreceptor tyrosine-based activation motifs (ITAMs) can be a positive event in the initiation of tumorigenesis (Katz et al., 2005). Thus, it will be important to understand the nature of the pathways in which Syk participates and the substrates and binding partners with which it interacts in epithelial cells to determine how it can both promote and inhibit the tumorigenic phenotype.
Syk has been best characterized as an element of the signaling apparatus that couples receptors bearing cytoplasmic ITAMs to the regulation of multiple intracellular pathways in hematopoietic cells (Kurosaki, 2000; Latour and Veillette, 2001). In addition to ITAM-bearing receptors, Syk also is coupled directly or indirectly to several different classes of receptors, but by mechanisms that are less well defined. One example is the receptor for tumor necrosis factor (TNF) (Takada and Aggarwal, 2004; Eliopoulos et al., 2006). In Jurkat T cells, TNF has stimulates the activity of Syk leading to the activation of NF-κB to promote cell survival (Takada and Aggarwal, 2004). The downregulation of Syk by siRNA inhibits TNF-signaling in Jurkat cells and airway epithelial cells (Takada and Aggarwal, 2004; Ulanova et al., 2005). We have found that Syk in breast epithelial cells also is a positive regulator of TNF-signaling.
Since the level at which Syk participates in TNF-signaling pathways has not been well defined, we conducted a yeast two-hybrid screen to search for binding partners known to participate in TNF-signaling. In this study, we identified TRAF-interacting protein (TRIP) as a novel Syk-binding partner. TRIP is a 53 kDa protein that contains an N-terminal RING finger, coiled-coil and leucine zipper regions that bind TRAF-family proteins (Lee et al., 1997; Besse et al., 2007) and a largely disordered C-terminal half reported to interact with CYLD, a ubiquitin hydrolase (Regamey et al., 2003). Here we demonstrate that in MCF7 breast cancer cells, Syk and TRIP interact with one another and have opposing effects on TNF-signaling. These studies provide an important clue as to one mechanism by which Syk participates in the regulation of cell growth and survival in breast epithelial cells.
Results
TRIP and Syk interact in a yeast two-hybrid assay
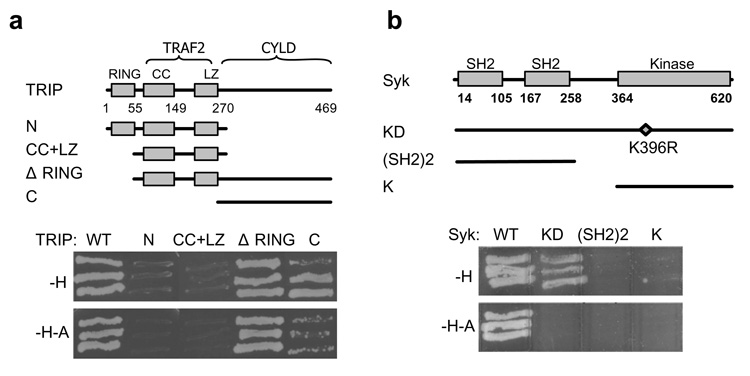
To search for Syk-interacting proteins, we screened a cDNA library from normal human mammary gland using a yeast two-hybrid approach with Syk as bait. Three independent cDNA clones were identified that encoded full length TRIP. Neither the N-terminal half (aa1–271) nor the coiled-coil plus leucine zipper domains of TRIP were capable of interacting with Syk (Fig. 1A). The N-terminal RING finger (aa1–51) could be eliminated without disrupting the interaction. The C-terminal half of TRIP alone (aa271–469) was sufficient for binding. However, under the most stringent screening conditions, a region (aa51–271) within the N-terminal half also was needed for an optimal interaction. TRIP interacted with both wild-type Syk and catalytically inactive Syk(K396R) under low stringency selection conditions (Fig. 1B). However, under highly stringent conditions, TRIP interacted only with active Syk. Neither the tandem pair of SH2 domains nor the kinase domain bound to TRIP. The preferential binding of TRIP to active Syk suggests a role for tyrosine-phosphorylation in promoting an optimal interaction.
Figure 1. TRIP and Syk interact in a yeast two-hybrid assay.
(a) Schematic diagram of TRIP indicating the positions of the RING finger motif (RING), coiled-coil region (CC), leucine-zipper (LZ) and binding sites for TRAF2 and CYLD. Wild-type TRIP and various truncation mutants were fused to the Gal4 activation domain and tested for binding to wild-type Syk under conditions of low (-H, histidine-deficient) or high (-H-A, histidine- and adenine-deficient) stringency. (b) Gal4 DNA binding domain-fusion proteins containing wild-type Syk (WT), the kinase dead mutant (KD), the tandem SH2 domains ((SH2)2) and the kinase domain (K) were examined in a yeast two-hybrid assay for binding to full-length TRIP under conditions of low (-H) or high (-H-A) stringency.
Syk and TRIP interact in co-immunoprecipitation and pull-down assays
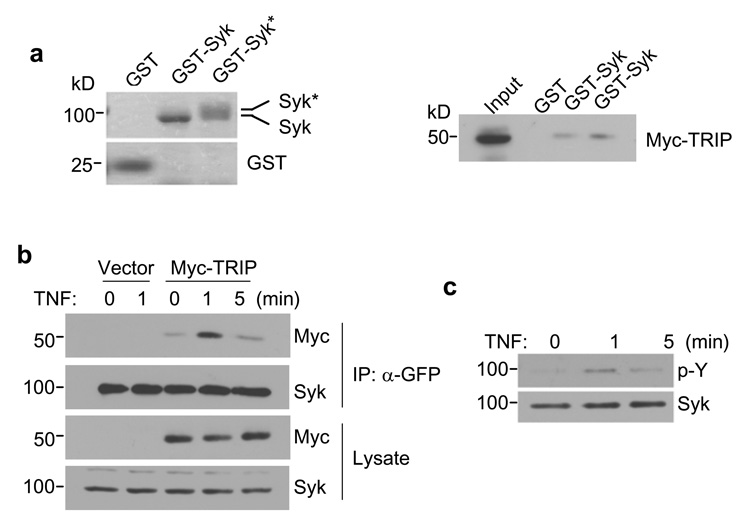
Immobilized GST-Syk was able to bind and recover transiently expressed Myc-TRIP from MCF7(BD) cell lysates (Fig. 2A). MCF7(BD) are MCF7 cells that lack endogenous Syk (see below). This interaction was moderately enhanced by the autophosphorylation of GST-Syk. Similarly, Myc-TRIP and Syk-EGFP could be co-immunoprecipitated by anti-GFP antibodies from lysates of MCF7(BD) cells transiently expressing both proteins (Fig. 2B). Syk is phosphorylated rapidly following the treatment of Jurkat cells and macrophages with TNF (Takada and Aggarwal, 2004; Eliopoulos et al., 2006). The phosphorylation of Syk-EGFP also was transiently enhanced in MCF7(BD) cells treated with TNF (Fig. 2C). This correlated with an increase in the interaction of Myc-TRIP with Syk-GFP as measured by co-immunoprecipitation (Fig. 2B). This is consistent with a role for tyrosine-phosphorylation in enhancing the interaction between Syk and TRIP.
Figure 2. TRIP and Syk interact in vitro and in cells.
(a) Purified GST, GST-Syk (without autophosphorylation) and GST-Syk* (with autophosphorylation) were separated by SDS-PAGE and stained with Coomassie blue (left panel). Autophosphorylated Syk showed a slower mobility following SDS-PAGE. Lysates prepared from MCF7(BD) cells expressing Myc-TRIP were incubated with equal amounts of immobilized GST, GST-Syk or GST-Syk* (right panel). The adsorbed proteins were analyzed by Western blotting with an anti-Myc antibody. (b) Syk-deficient MCF7(BD) cells stably expressing Syk-EGFP were transfected with a vector expressing Myc-TRIP or an empty vector. Cells were then treated with 40 ng/ml TNF-α for the indicated periods of time. Syk-EGFP was immunoprecipitated with an anti-GFP antibody (α-GFP) and the immune complexes were analyzed by Western blotting with anti-Myc (Myc) or anti-Syk (Syk) antibodies. The level of expression of each protein in cell lysates was determined by Western blotting. (c) Syk-EGFP-expressing MCF7(BD) cells were treated with TNF-α (40 ng/ml) for 0, 1 or 5 min. Syk-EGFP was immunoprecipitated with anti-GFP antibodies and subjected to Western blotting with antiphosphotyrosine (p–Y) or anti-Syk (Syk) antibodies.
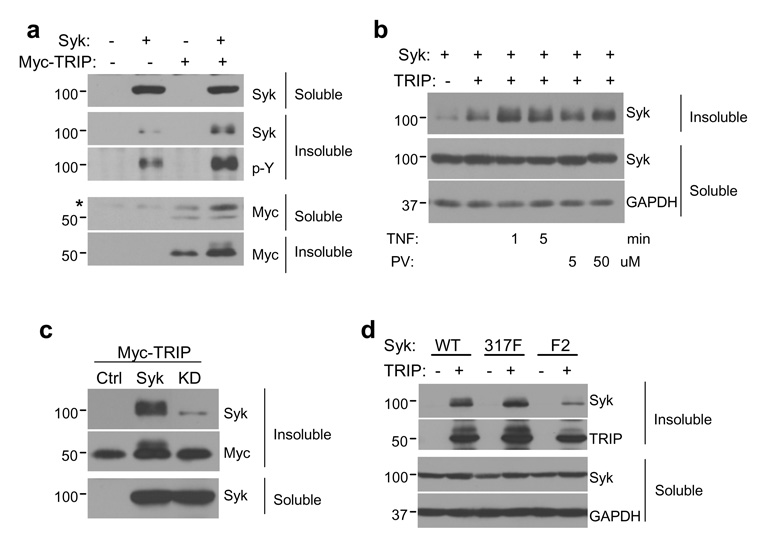
Figure 3. Syk and TRIP interact in the detergent-insoluble fraction.
(a) MCF7(BD) cells were transiently transfected with an empty vector, a vector coding for Myc-TRIP alone, Syk-EGFP alone or a combination of both constructs. Both soluble and insoluble fractions were analyzed by Western blotting with anti-Syk, anti-Myc and antiphosphotyrosine (p–Y) antibodies. (b) MCF7(BD) cells transfected to express TRIP and Syk-EGFP were treated with TNF-α (100 ng/ml) for 1 or 5 min, or with pervanadate (PV) at 5 or 50 µM for 20 min. Both soluble and insoluble fractions were analyzed by Western blotting with anti-Syk antibodies. (c) The Myc-TRIP expression plasmid was co-transfected into MCF7(BD) cells with an empty vector (Ctrl) or vectors expressing Syk-EGFP (Syk) or Syk-EGFP(K396R) (KD). Detergent-soluble and -insoluble fractions were analyzed by Western blotting with anti-Syk and anti-Myc antibodies. (d) Vectors coding for wild-type Syk-EGFP (WT), Syk-EGFP(Y317F) (317F), or Syk-EGFP(Y342F/Y346F) (F2) were co-transfected with an empty vector (−) or a TRIP-expression vector (+) into MCF7(BD) cells. Detergent-soluble and -insoluble fractions were analyzed by Western blotting with anti-Syk and anti-TRIP antibodies. GAPDH was probed to show equal loading in each lane.
Syk and TRIP interact in the detergent insoluble fraction
When TRIP or Myc-TRIP was expressed in MCF7(BD) cells, a major portion of each was insoluble in detergent lysis buffer while the majority of expressed Syk-EGFP was soluble (Fig. 3A). Considerably more Syk-EGFP was present in the detergent insoluble fraction of cells that also expressed exogenous TRIP. A portion of Syk-EGFP in this fraction exhibited a slower electrophoretic mobility characteristic of Syk when it is phosphorylated on tyrosine (Keshvara et al., 1998). This was confirmed by Western blotting with antiphosphotyrosine antibodies (Fig. 3A). Treatment of cells with TNF induced a further increase in the amount of Syk within the insoluble fraction (although the majority of the kinase remained in the soluble fraction). A similar effect was observed when cells were treated with the protein-tyrosine phosphatase inhibitor, pervanadate (Fig. 3B). Unlike Syk-EGFP, catalytically inactive Syk-EGFP(K396R) (Supplemental Fig. 1A) remained primarily in the soluble fraction of lysates from cells also expressing TRIP (Fig. 3C). Three linker B tyrosine residues on Syk (Y317, Y342 and Y346) serve as docking sites for proteins containing phosphotyrosine-binding domains. Mutation of Y317 did not affect the relocation of the kinase to the detergent-insoluble fraction (Fig. 3D). However, replacement of both Y342 and Y346 by phenylalanine resulted in a much reduced TRIP-dependent relocalization. These results suggest that phosphotyrosines at 342 and/or 346 promote the interaction of Syk with TRIP.
Syk induces phosphorylation of TRIP on tyrosine
When co-expressed with Syk-EGFP, a fraction of TRIP also migrated on SDS-PAGE with a reduced mobility. This altered mobility was dependent on its co-expression with a catalytically active kinase (Fig. 3C). Similarly, this shift was reduced in cells expressing Syk-EGFP(Y342F/Y346F), which interacts poorly with TRIP (Fig. 3D). This result suggested that Syk could catalyze the phosphorylation of TRIP. Consistent with this, a Western blot of TRIP immunoprecipitated from MCF7(BD) cells indicated that wild-type SykEGFP and SykEGFP(Y317F) were able to induce the phosphorylation of TRIP while the kinase dead version and Syk-EGFP(Y342F/Y346F) failed to do so (Supplemental Fig. 1B). Thus, the ability of Syk mutants to phosphorylate TRIP correlated with their ability to interact with the protein. Furthermore, GFP-TRIP isolated from transiently transfected MCF7(BD) cells could be phosphorylated by GST-Syk in an in vitro kinase assay (Supplemental Fig, 1C).
TRIP is a nucleolar protein that co-localizes with Syk in punctate cytoplasmic structures in response to TNF
We examined by immunofluorescence TRIP’s location in breast epithelial cells using affinity purified antibodies. The endogenous protein was predominantly co-localized with nucleolin in nucleoli (Fig. 4A). Overexpression of TRIP greatly enhanced this nucleolar staining (Fig. 4A) while suppression of endogenous TRIP by siRNA led to the disappearance of such staining (Fig. 4B). Similarly, TRIP tagged with EGFP also localized to nucleoli (Supplemental Fig. 2A). Subcellular fractionation of MCF7(BD) cells confirmed a nuclear localization for TRIP (Supplemental Fig. 2B). TRIP also localized to nucleoli in MDA-MB-231 cells and in human DG75 B cells (Supplemental Fig. 2C) as well as MCF10A and MCF7(ATCC) cells (data not shown). In contrast, Syk-EGFP was distributed throughout the cell. However, when Syk-EGFP and TRIP or Myc-TRIP were co-expressed in MCF-7(BD) cells, punctate complexes containing both proteins were observed in the cytoplasm of a small percentage of cells (Fig. 4C and D). Following treatment with TNF, more cells exhibited these punctate structures containing both Syk and TRIP (Fig. 4D and Supplemental Fig. 2D).
Figure 4. Localization of TRIP and Syk in epithelial cells.
(a) Endogenous or transiently expressed TRIP in MCF7(BD) cells was detected by immunofluorescence using an anti-TRIP antibody. Nucleolin was stained with a monoclonal anti-nucleolin antibody. The nuclei were stained with DAPI. (b) MCF7(BD) cells transfected with 50 nM of TRIP siRNA or a control siRNA (Ctrl) were analyzed by immunofluorescence using anti-TRIP antibodies or by treatment with DAPI. (c) MCF7(BD) cells expressing Syk-EGFP and TRIP were treated with TNF-α (100 ng/ml) for 5 min (+TNF) or left untreated (−TNF). TRIP was detected by immunofluorescence with the anti-TRIP antibody. An example of the colocalization of TRIP and Syk is indicated. E, MCF7(BD) cells expressing Syk-EGFP and Myc-TRIP were subjected to TNF-treatment. Myc-TRIP was immunostained with an anti-Myc antibody. An example of the colocalization of Syk-EGFP and Myc-TRIP is indicated. Bars represent 10 µm.
Syk enhances TNF-signaling to NF-κB in breast epithelial cells
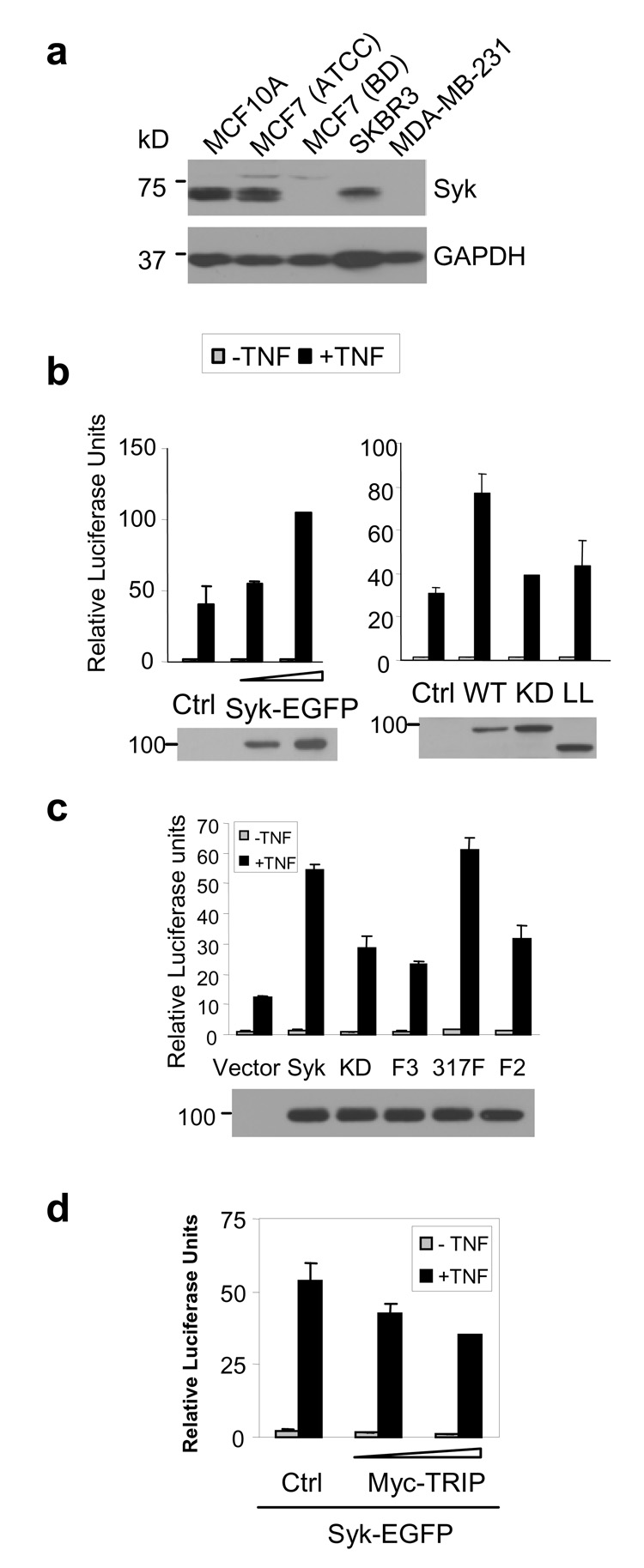
Consistent with previous reports (1), endogenous Syk was present in MCF10A, MCF7 (purchased from ATCC) and SKBR3 cells, but not in highly invasive MDA-MB-231 cells (Fig. 5A). Interestingly, one clone of MCF7 cells purchased from BD Biosciences (MCF7(BD)) lacked Syk. These originated from ATCC, retained a normal epithelioid morphology, expressed cell surface TNFR1, and lacked endogenous caspase 3, which is a characteristic of MCF7 cells (data not shown). We used these cells to explore a role for Syk in TNF-signaling. The expression of Syk-EGFP, but not Syk-EGFP(K396R), enhanced the ability of TNF to activate NF-κB (Fig. 5 B). Expression of a form of Syk-EGFP lacking the linker B region also failed to enhance TNF-signaling (Fig. 5B). Syk-EGFP(Y317F) was active in enhancing the activation of NF-κB while Syk-EGFP(Y342F/Y346F) and Syk-EGFP(Y317F/Y342F/Y346F) exhibited only a marginal effect on TNF-signaling. Thus, the ability of Syk to promote NF-κB activation required Y342 and Y346. This enhanced activation of NF-κB by Syk-EGFP was abrogated by the co-expression of exogenous TRIP (Fig. 5D). Similarly, the TNF-stimulated activation of NF-κB in MCF7(ATCC) cells that express endogenous Syk was inhibited by the expression of TRIP (Supplemental Fig. 3A). Thus Syk and TRIP had opposing effects on the TNF-stimulated activation of NF-κB.
Figure 5. Syk enhances TNF-mediated activation of NF-κB.
(a) The detergent-soluble lysates from various breast cancer cell lines were subjected to Western blotting with an anti-Syk antibody. GAPDH was blotted as a loading control. (b) Syk-deficient MCF7(BD) cells were transfected with increasing amounts of Syk-EGFP expression vector (0.1 or 0.3 µg, left panel), or with vectors expressing different versions of Syk-EGFP (right panel). WT, wild type; KD, kinase dead (i.e., Syk-EGFP(K396R)); LL, linkerless. Relative luciferase activities were measured 18 h after TNF-treatment. The expression levels of Syk were determined by a Western blot. (c) The abilities of wild-type Syk-EGFP (Syk), Syk-EGFP(K396R) (KD), Syk-EGFP(Y317F/Y342F/Y346F) (F3), Syk-EGFP(Y317F) (317F), or Syk-EGFP(Y342F/Y346F) (F2) to enhance the TNF-mediated activation of NF-κB were tested by a reporter assay in MCF7(BD) cells. The data represent the means and standard deviations of results from two independent experiments performed in duplicate. The expression levels of Syk-EGFP and the mutant proteins are shown by a Western blot. (d) Relative luciferase units of the NF-κB reporter assay were measured in MCF7(BD) cells that were transfected to express Syk-EGFP and an empty vector (Ctrl) or increasing amounts of Myc-TRIP, and treated with or without TNF. These data represent the means and standard deviations of four trials.
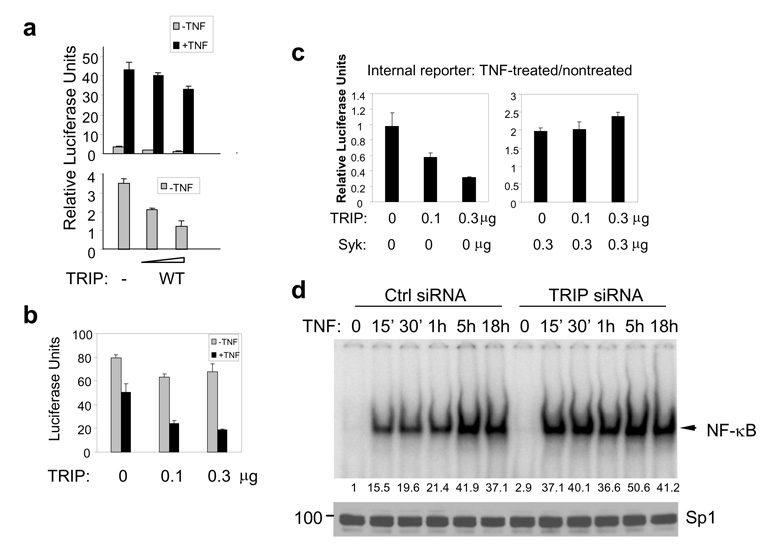
TRIP negatively regulates the TNF-mediated activation of NF-κB
To determine if TRIP also affected TNF-signaling in the absence of Syk, we expressed TRIP in the Syk-deficient cells and examined the activity of NF-κB. TRIP significantly reduced the basal activity of NF-κB and moderately inhibited the TNF-stimulated activation of NF-κB (Fig. 6A). However, luciferase expression from both the NF-κB and internal control reporter plasmids was reduced significantly by TNF. In TRIP-transfected cells, treatment with TNF led to a dramatic decline in luciferase expressed from a constitutively active promoter (Fig. 6B). This inhibition was independent of the nature of the promoter and was seen with HSV TK, CMV or even a promoterless construct (data not shown). Thus, the expression of exogenous TRIP sensitized cells to a TNF-dependent decrease in gene expression, most likely as a result of its ability to decrease the level of active NF-κB—an important survival factor—and the subsequent loss of transfected cells. Consistent with this hypothesis, overexpression of TRIP correlated with a loss of viable, adherent cells in response to treatment with TNF (Supplemental Fig. 3B). Furthermore, transfection of cells with siRNA to reduce the level of endogenous TRIP resulted in increased DNA-binding of NF-κB in response to TNF (Fig. 6D) and decreased TNF-induced activation of caspase 8 (Supplemental Fig. 3C). The expression of exogenous Syk protected cells from the inhibitory effects of TRIP on cell survival following treatment with TNF (Fig. 6C).
Figure 6. TRIP inhibits NF-κB activity.
(a) MCF7(BD) cells were transfected with NF-κB-luciferase reporter plasmids and increasing amounts (0.1 or 0.3 µg) of expression plasmids for wild-type TRIP (WT). Following treatment with or without TNF-α (20 ng/ml) for 18 h, relative luciferase units were measured. Basal NF-κB activities (−TNF) are expanded and shown in the lower panel. (b) MCF7(BD) cells were transiently transfected with a constitutively active luciferase reporter construct along with increasing amounts of the TRIP-expression vector. Cells were treated with or without TNF for 18 h and the amount of luciferase in the cell lysates was quantified. (c) Increasing amounts of the TRIP-expression plasmid were transfected into MCF7(BD) cells with 0.3 µg g of empty vector (left panel) or with 0.3 µg of the Syk-EGFP-expression vector (right panel). The luciferase activities of the constitutively expressed control reporter (pRL-TK) are shown. The Relative Luciferase Units represent the luciferase activities of TNF-treated samples divided by those of nontreated samples within the same group. (d) Nuclear extracts from control (Ctrl) and TRIP siRNA-transfected MCF7(BD) cells were prepared for the gel-shift assay. The shifted bands representing NF-κB complexes are indicated by the arrowhead. Their relative intensities were quantified and results are indicated below the panel. The nuclear extracts were analyzed by Western blotting with an anti-Sp1 antibody to show equal loading.
Discussion
Since the initial characterization of Syk as a potential tumor suppressor, several mechanisms have been proposed to explain how the kinase regulates the growth and proliferation of breast epithelial cells including maintenance of centrosomal function and suppression of transcription (Zyss et al., 2005; Wang et al., 2005). How Syk participates in signal transduction pathways triggered by extracellular modulators of cell growth, however, remains poorly understood. TNF is a particularly interesting cytokine in that it has pleiotropic effects on epithelial cells due to its ability to couple to both cell death and cell survival pathways. For example, TNF is a proliferation and survival factor for primary mammary epithelial cells and an inducer of apoptosis for many mammary tumor cells (Wilson and Balkwill, 2002). We observed a 2- to 3-fold increase in the activity of a NF-κB-driven reporter by the re-expression of Syk in Syk-deficient MCF7(BD) cells, suggesting that Syk plays a positive role in TNF-signaling. This result is consistent with other reports of a role for Syk in enhancing the TNF-dependent activation of NF-κB (Takada and Aggarwal, 2004; Ulanova et al., 2005).
Despite descriptions of connections between Syk and TNF-signaling, little is yet known regarding the molecular mechanism or mechanisms by which these occur. We initiated a search for Syk-associated proteins using a yeast two-hybrid screening strategy and identified TRIP as a novel Syk-interacting protein. TRIP is an interesting candidate since it was reported originally as a binding partner of TRAF2, which associates with the liganded TNFR1 as part of a signaling assembly required for the activation of NF-κB and initiation of the survival pathway. Consequently, the overexpression of TRIP through transfection has been reported to inhibit the TNF-mediated activation of NF-κB in HEK293 cells and HeLa cells (Lee et al., 1997; Regamey et al., 2003). However, in a subsequent study, no effect of TRIP on proximal signaling events initiated by TNF was observed (Besse et al., 2007). In this study, we did detect an inhibitory effect of exogenously expressed TRIP on the basal activity of NF-κB and on the Syk plus TNF-stimulated activation of NF-κB in breast epithelial cells. However, the level within the signaling pathway where this inhibition occurs has yet to be completely determined. Since activated NF-κB transcription factors rapidly turn on genes that antagonize the initiation of apoptosis, inhibition of the NF-κB branch of the pathway is known to sensitize cells to TNF-induced cell death, which likely explains the ability of overexpressed TRIP to sensitize cells to the inhibitory effects of TNF. However, this inhibitory activity TRIP can be reversed by the co-expression of Syk, which might inhibit the activity of cytoplasmic TRIP, sequester TRIP from important nucleolar targets or even counter TRIP’s inhibitory effects on TRAF and TNF-signaling by regulating the activity of alternative components of the pathway.
The opposing roles of Syk and TRIP in the same pathway prompted us to determine if and where they interact within cells. A previous report identified the localization of overexpressed Myc-TRIP as predominantly perinuclear in COS-7 cells (Regamey et al., 2003). To our surprise, we found that both endogenous TRIP and exogenously overexpressed TRIP are predominantly found in nucleoli in MCF7(BD) and multiple other cell types. Although the majority of TRIP protein is localized to the nucleus, a small portion of TRIP colocalizes with Syk in punctate cytoplasmic structures and this association is increased significantly in response to TNF. The translocation of proteins out of the nucleus to participate in cytoplasmic signaling events is not without precedent. Daxx, for example, is a RING-finger containing protein that exhibits a largely nuclear localization within PML bodies, but translocates to the cytoplasm to bind and activate apoptosis signal-regulating kinase 1 and promote apoptotic pathways initiated by cell surface receptors including Fas and TGF-β(Salomoni and Khelifi, 2006).
TNF-treatment enhances the co-immunoprecipitation of Syk and TRIP and the translocation of Syk into a detergent insoluble fraction in TRIP-expressing cells. This increased association correlates with the transient induction of the phosphorylation of Syk on tyrosine following the treatment of cells with TNF. Our yeast two-hybrid and biochemical interaction experiments indicate that the interaction between Syk and TRIP is enhanced by the autophosphorylation of Syk on tyrosine residues. Also, the movement of Syk to the detergent insoluble fraction of TRIP-expressing cells is dependent on its catalytic activity and phosphorylation on Y342 and Y346. It is interesting to note that kinase activity and phosphorylation on Y342 and Y346 are also necessary for Syk’s ability to enhance TNF-signaling. The correlation of Syk’s effect on NF-κB with its ability to interact and phosphorylate TRIP suggests a functional interplay between these two proteins.
Our results suggest a model in which a receptor-approximate event rapidly induces the tyrosine-phosphorylation of Syk following TNF-stimulation in MCF7 cells. A portion of active Syk with phosphorylation on Y342 and/or Y346 interacts with the C-terminal regions of TRIP. The interaction between Syk and TRIP results in the tyrosine-phosphorylation of TRIP and also shifts a portion of Syk to a detergent insoluble fraction, which may represent the Syk associated with TRIP in punctuate protein complexes observed in the cytoplasm. It is possible that Syk, which can shuttle in and out of the nucleus (Zhou et al., 2006), is responsible the export of the Syk-TRIP complex to the cytoplasm. However, it is equally possible that it is TRIP that modulates the intracellular localization of Syk. It is not yet known if the Syk-TRIP complex also is associated with TRAF2. Since it has been reported that the N-terminal region of TRIP, but not the C-terminal region where Syk binds, mediates the interaction with TRAF2, it is possible that TRIP may interact with both Syk and TRAF2 simultaneously. Thus it will be of considerable interest to examine the composition of the punctate complexes observed in the cytoplasm to determine if they also contain TRAF2 and other components of TNF-signaling pathways. Future investigations also will be directed toward understanding how Syk and TRIP modulate the function of one another and how this interaction contributes to the signaling events leading to the activation of NF-κB.
Although an anti-apoptotic role of Syk appears to be contradictory with its proposed function as a tumor suppressor, Syk appears to have more complicated effects on breast epithelial cells than what was perceived originally. Although the enforced expression of Syk in malignant breast cancer cells leads to defective mitosis and cell growth (Coopman et al., 2000), Syk plays a positive role in virus-mediated cellular transformation when it is activated by ITAM-containing viral proteins (Katz et al., 2005; Lu et al., 2006). While TRIP is expressed in multiple breast epithelial cell lines ranging from non-malignant MCF10A to highly invasive MDA-MB-231, Syk is reduced in level or is lost from more malignant cells. Since many transformed cells exhibit an enhanced sensitivity toward the pro-apoptotic effects of TNF, it is possible that the absence of Syk contributes to this phenotype by leaving the function of TRIP unchecked.
Materials and methods
Cells and plasmids
MCF7 cell lines were cultured in DMEM containing 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin. For the generation of stable Syk-EGFP-expressing cells, Syk-deficient MCF7(BD) cells were transfected with a plasmid expressing Syk-EGFP and a puromycin resistance gene and selected by treatment with puromycin (1 µg/ml). The series of Syk-EGFP-fusion constructs were described previously (Ma et al., 2001). The plasmids expressing non-tagged TRIP or its truncation mutants were generated by inserting TRIP cDNA or cDNA fragments into the pcDNA4/TO vector (Invitrogen). Similarly, full-length TRIP cDNA was cloned into the pCMV-Myc vector (BD Biosciences) for expressing Myc-tagged TRIP. The NF-κB-specific firefly luciferase reporter, pNF-κB-luc, was obtained from Stratagene. The internal control plasmid, pRL-TK, was from Promega.
Reagents
Primary antibodies against Syk (N19), Myc (9E10), p38, Sp1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotechnology. The caspase 8 antibody (1C12) was from Cell Signaling Technology. Anti-phosphotyrosine (4G10) was from Millipore/Upstate Biotechnology. Anti-GFP was from BD Biosciences. Rodamine-conjugated goat anti-rabbit and Alexa Flour 488-conjugated goat anti-mouse secondary antibodies were obtained from Jackson ImmunoResearch Laboratories and Molecular Probes, respectively. TNF-α was purchased from R&D Systems. The N-terminal (aa1–275) and C-terminal (aa278–469) halves of TRIP were expressed in bacteria as GST-fusion proteins and used as antigens for the immunization of rabbits (Lampire Biological Laboratories). Anti-TRIP antibodies were affinity-purified using each fusion protein immobilized on glutathione-agarose.
Yeast two-hybrid analyses
Plasmids expressing the Gal4 DNA-binding domain fused to Syk, Syk(K396R), the tandem SH2 domains, or the catalytic domain were constructed using the pGBKT7 vector (Moon et al., 2004). The human mammary gland cDNA library (Clontech) was transformed into AH109 cells pre-transformed with pGBKT7-Syk. Plasmids were eluted from colonies that grew on stringent selection media, amplified and sequenced. TRIP cDNA or its fragments were cloned into pACT2 for mapping analyses. For interaction assays, AH109 cells were transformed simultaneously with two plasmids expressing wild-type or mutated versions of TRIP and Syk and then tested on selection media with low (Leu−, Trp− and His−) or high (Leu−, Trp−, His− and Ade−) stringency.
GST pull-down and co-immunoprecipitation assays
GST-Syk was isolated from Sf9 cell lysates as described (Peters et al., 1996). Immobilized GST-Syk was autophosphorylated by incubation at 37 °C for 20 min in 50 mM Hepes, pH 7.5, 1 mM ATP, 10 mM MnCl2, 1 mM Na3VO4 and 10 µg/ml each of aprotinin and leupeptin. Immobilized proteins were incubated with lysates of MCF7(BD) cells expressing Myc-TRIP and then washed extensively with buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol and1% NP-40. Bound proteins were eluted in SDS-sample buffer and detected by Western blotting. For co-immunoprecipitation experiments, MCF7(BD) cells stably expressing Syk-EGFP were transfected with the Myc-TRIP expression vector and treated with TNF-α (40 ng/ml) for the indicated time at 37°C. Cells (5 × 106) were lysed in buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.2% NP-40, and 10 µg/ml each of aprotinin and leupeptin. Anti-GFP immune complexes were separated by SDS-PAGE.
Fluorescence microscopy
Cells transfected with Syk-EGFP- or the various TRIP-expression constructs were fixed in 3.7% formaldehyde and then permeablized with 1% NP-40 for 5 min at room temperature. After washing with PBS, cells were incubated with a blocking buffer (10% goat serum in PBS) for 1 h and then with primary (anti-TRIP or anti-Myc) and secondary (Rhodamine-labeled goat anti-rabbit IgG or Alexa Fluor 488-labeled goat anti-mouse IgG) antibodies. Cells were washed, stained with 4',6'-diamidino-2-phenylindole (DAPI; Sigma) and viewed by fluorescence microscopy.
Cellular fractionation
For fractionations based on detergent solubility, MCF7(BD) cells were lysed in 0.2% NP-40, 20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonylfluoride (PMSF), 10 µg/ml aprotinin, 10 µg/ml leupeptin and 1 mM Na3VO4. The detergent-soluble fraction was separated from the insoluble fraction by centrifugation for 5 min at 18,000 × g.
Reporter and gel-shift assays for the measurement of NF-κB activity
For luciferase-based reporter assays, 3 × 104 MCF7(BD or ATCC) cells were co-transfected using FuGENE 6 (Roche) with the indicated amounts of expression plasmids, 0.2 µg of the pNF-κB-luc reporter plasmid and 0.02 µg of the pRL-TK internal control plasmid. Transfected cells were treated with or without TNF-α for the indicated times. The activities of both luciferases were measured by the Dual Reporter Assay System (Promega). In general, the relative luciferase units represent the NF-κB reporter activities normalized against those of the internal control unless indicated otherwise. For gel shift assays, nuclear extracts (5 µg) from TNF-treated cells were incubated with a 32P-labeled NF-κB specific DNA probe (35 fmol) at room temperature for 30 min in a 10 µl reaction containing 10 mM Tris-HCl, pH 8.0, 250 mM KCl, 0.5 mM EDTA, 0.2 mM DTT, 0.1% Triton-X 100, 12% glycerol and 1 µg of poly-dI-dC. The 22-mer double-stranded probe was prepared from a sense strand (5’-AGT TGA GGG GAC TTT CCC AGG C-3’) and its complementary strand and labeled by T4 kinase. The DNA-protein complexes were separated by 5% native polyacrylamide gel electrophoresis. The radioactive bands were visualized and quantified by a phosphoimager using Optiquant software. The specificity of NF-κB complexes was confirmed by competition assays using the wild-type probe or a probe containing a single nucleotide mutation.
siRNA transfection
SmartPool siRNA duplexes against TRIP were obtained from Dharmacon. siRNA (50 nM) was transfected using Lipofectamine 2000. Suppression of TRIP was monitored by immunofluorescence and Western blotting.
Supplementary Material
Supplementary Information accompanies the paper on the Oncogene website.
Acknowledgments
This work was supported by United States Public Health Services grant CA115465 awarded by the National Cancer Institute and U.S. Army Breast Cancer Research Program award DAMD17-02-1-0554.
References
- Besse A, Campos AD, Webster WK, Darnay BG. TRAF-interacting protein (TRIP) is a RING-dependent ubiquitin ligase. Biochem Biophys Res Commun. 2007;359:660–664. doi: 10.1016/j.bbrc.2007.05.149. [DOI] [PubMed] [Google Scholar]
- Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- Eliopoulos AG, Das S, Tsichlis PN. The tyrosine kinase Syk regulates TPL2 activation signals. J Biol Chem. 2006;281:1371–1380. doi: 10.1074/jbc.M506790200. [DOI] [PubMed] [Google Scholar]
- Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, et al. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201:431–439. doi: 10.1084/jem.20041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshvara LM, Isaacson CC, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–281. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee SY, Choi Y. TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation. J Exp Med. 1997;185:1275–1285. doi: 10.1084/jem.185.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lin W-H, Chen S-Y, Longnecker R, Tsai S-C, Chen C-L, Tsai C-H. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281:8806–8814. doi: 10.1074/jbc.M507305200. [DOI] [PubMed] [Google Scholar]
- Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- Moon KD, Post CB, Durden DL, Zhou Q, De P, Harrison ML, et al. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J Biol Chem. 2004;280:1543–1551. doi: 10.1074/jbc.M407805200. [DOI] [PubMed] [Google Scholar]
- Moroni M, Soldatenkov V, Zhang L, Zhang Y, Stoica G, Gehan E, et al. Progressive loss of Syk and abnormal proliferation in breast cancer cells. Cancer Res. 2004;64:7346–7354. doi: 10.1158/0008-5472.CAN-03-3520. [DOI] [PubMed] [Google Scholar]
- Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates alpha-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- Regamey A, Hohl D, Liu JW, Roger T, Kogerman P, Toftgard R, et al. The tumor suppressor CYLD interacts with TRIP and regulates negatively nuclear factor kappaB activation by tumor necrosis factor. J Exp Med. 2003;198:1959–1964. doi: 10.1084/jem.20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. TNF activates Syk protein tyrosine kinase leading to TNF-induced MAPK activation, NF-kappaB activation, and apoptosis. J Immunol. 2004;173:1066–1077. doi: 10.4049/jimmunol.173.2.1066. [DOI] [PubMed] [Google Scholar]
- Toyama T, Iwase H, Yamashita H, Hara Y, Omoto Y, Sugiura H, et al. Reduced expression of the Syk gene is correlated with poor prognosis in human breast cancer. Cancer Lett. 2003;189:97–102. doi: 10.1016/s0304-3835(02)00463-9. [DOI] [PubMed] [Google Scholar]
- Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, et al. Syk tyrosine kinase participates in beta1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 2005;65:10289–10297. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol. 2002;12:113–120. doi: 10.1006/scbi.2001.0419. [DOI] [PubMed] [Google Scholar]
- Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Nucleocytoplasmic trafficking of the Syk protein-tyrosine kinase. Mol Cell Biol. 2006;26:3478–3491. doi: 10.1128/MCB.26.9.3478-3491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyss D, Montcourrier P, Vidal B, Anguille C, Mérezègue F, Sahuquet A, et al. The Syk tyrosine kinase localizes to the centrosomes and negatively affects mitotic progression. Cancer Res. 2005;65:10872–10880. doi: 10.1158/0008-5472.CAN-05-1270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on the Oncogene website.