Abstract
Although the core region of the nucleus accumbens (NAcc) has been implicated in motor control and the acquisition of appetitive learning, these processes are altered by environmental experience. To assess how environment influences neuronal processing in NAcc core, we recorded single-unit activity during acquisition of an appetitive learning task in which rats reared in an environmentally enriched condition (EC) learned the operant response (nosepoke into a lit hole) for sucrose reinforcement faster than rats reared in an isolated condition (IC). In the first training session, even before the emergence of learning differences, core neurons were more likely to respond (increase or decrease activity) during the operant and consummatory responses in EC than IC rats. By the third training session, when learning differences emerged, EC neurons continued to be more responsive than IC neurons, but in very different ways: the response shifted to the cues that signaled trial onset (1900 Hz tone and green LED) and reward availability (4500 Hz tone and yellow LED). Cue-related responding, moreover, was dominated by neuronal excitations. In contrast, post-acquisition recordings revealed no EC-IC differences. Collectively, these results suggest that core neurons are initially more responsive to discrete, goal-directed movements in EC rats, but as learning materializes, the neuronal response shifts to the cues that predict these movements. Thus, environmental experience alters core neuronal processing of both motor- and sensory-related events but at different stages over the course of learning.
Keywords: nucleus accumbens, environmental enrichment, social isolation, single-unit activity, appetitive conditioning, goal-directed behavior
1. INTRODUCTION
Anatomical, pharmacological, and electrophysiological investigations have long established that the nucleus accumbens (NAcc), a brain region found in ventral striatum, is involved in the regulation of adaptive, goal-directed activity, such as feeding (Heffner et al., 1980), drinking (Young et al., 1992), and sex (Fiorino et al., 1997; Mas et al., 1990). Accumulating data suggest that the NAcc, in particular, plays an important role in flexible approach of appetitive stimuli (Ikemoto and Panksepp, 1999; Mogenson and Nielsen, 1984; Wood et al., 2004; Wood and Rebec, 2004). Examination of the anatomical underpinnings of these behaviors reveals functional differences between the core and shell subregions. For example, shell is more sensitive to novel circumstances and more involved in visceral and motivational mechanisms (Carlezon and Wise, 1996; Maldonado-Irizarry et al., 1995; Rebec et al., 1997a; Rebec et al., 1997b), while core is involved specifically in motor control and learning of appetitive behavioral responses (Kelley et al., 1997; Maldonado-Irizarry and Kelley, 1995b; Smith-Roe and Kelley, 2000).
Other research shows that rats reared in an environmentally enriched (EC) or isolated condition (IC) exhibit clear behavioral and physiological differences. For example, in addition to enhanced learning capacity (for review, see (Renner and Rosenzweig, 1987), EC rats exhibit reduced reward-seeking behavior (Bardo et al., 2001; Lamden and Rose, 1979; Rose and Lamden, 1983; Rose et al., 1985; Rose et al., 1986; van der Harst et al., 2003) and altered structure and function of the NAcc (Bowling et al., 1993; Wood et al., 2005) relative to IC littermates. To assess the impact of differential environmental experience on learning and reward-seeking behavior, we recently analyzed the behavioral performance of EC and IC rats during acquisition of a discriminative learning task for sucrose reinforcement (Wood et al., 2006). Because of reduced discriminative responding to the operant stimulus, IC rats acquired an appetitive learning task more slowly than EC rats. Our working hypothesis is that this learning difference occurs in conjunction with differential responsiveness of NAcc core neurons to individual task events as learning progresses. To test this prediction, we recorded single-unit activity in EC and IC rats during and after task acquisition.
2. RESULTS
Operant Performance
Training in our discriminative learning task was provided in three successive approximations or phases (see Methods). Briefly, in phase one, animals learned that sucrose was available from a spout; this availability temporally corresponded with a feeder cue. In phase two, nosepokes were required to elicit this feeder cue (sucrose availability). In the third and final phase of training, a discriminative nosepoke to one of these two nosepoke holes (correct response) was required for the activation of the feeder cue.
As we observed in previous work (Wood et al., 2006), EC rats (n = 21) acquired this discriminative behavioral response (phase-three training) more rapidly than IC rats (n = 17). Because EC-IC behavioral differences were evident by the third session in this training phase, our analyses during acquisition of this discriminative learning response focused on the first and third sessions.
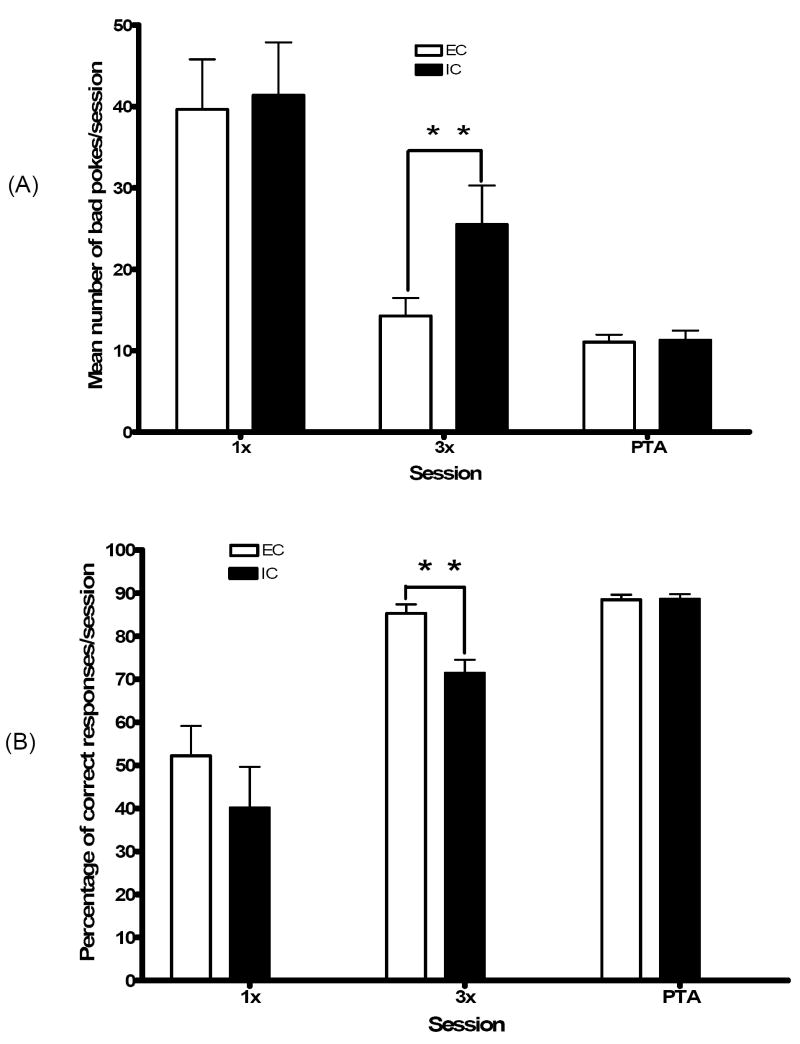
Although in the first session, both groups performed comparably in the first session, by the third session EC relative to IC rats made significantly fewer bad pokes (t = 3.30; p < 0.01; see Fig. 1A) and had a significantly higher percentage of correct responses (t = 3.60; p < 0.01; see Fig. 1B). Thus, as we observed in previous work (Wood et al., 2006), third-session performance revealed a critical EC-IC behavioral difference. This difference was no longer evident in the post-training acquisition (PTA) session, indicating that with additional training IC rats can match EC performance.
Fig. 1.
(A) Mean number of bad pokes in EC and IC rats during the first training session (1x), the third training session (3x), and post-training acquisition (PTA) recording sessions. As indicated (*), IC rats performed significantly more bad pokes than EC rats during 3X (p<.01), although no differences were observed during 1X or PTA. (B) Percentage of correct responses in EC and IC rats during 1X, 3X, and PTA. As indicated (*), EC rats exhibited a significantly higher percentage of correct responses than IC rats during 3X (p<.05) although no differences were observed during 1X or PTA.
Electrophysiology
During task acquisition (first and third training sessions) and PTA, we discriminated and recorded a total of 271 neurons in NAcc core in EC and IC rats (see Fig. 2). In both groups, similar proportions of neurons were identified during each session (see Table 1), and spike waveforms ranged between 0.6 and 1.2 ms in duration and 300 and 500 μV in amplitude. Signal-to-noise ratios of discriminated waveforms typically ranged between 3 and 4:1. Pre-trial baselines were similar in EC and IC rats, typically averaging between 2-3 spikes/s in each case.
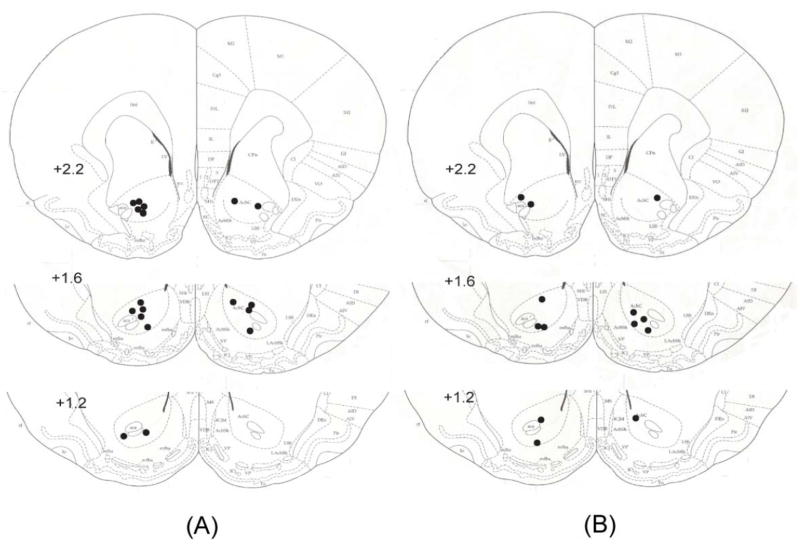
Fig. 2.
Placement of microwire electrode bundles lowered to the NAcc core in EC rats (A) and IC rats (B). As indicated by black dots, the locations of the tip of these bundles were distributed along the rostral-caudal axis (2.7-1.2 mm anterior to bregma) in the left and right of NAcc core.
Table 1.
Number of single units recorded from EC and IC rats during the 1st training session, the 3rd training session, and PTA recordings
| Recording | EE core | SI core | Total |
|---|---|---|---|
| 1st session | 49 | 32 | 81 |
| 3rd session | 48 | 43 | 91 |
| PTA sessions | 57 | 42 | 99 |
Unit Responses during Task Acquisition
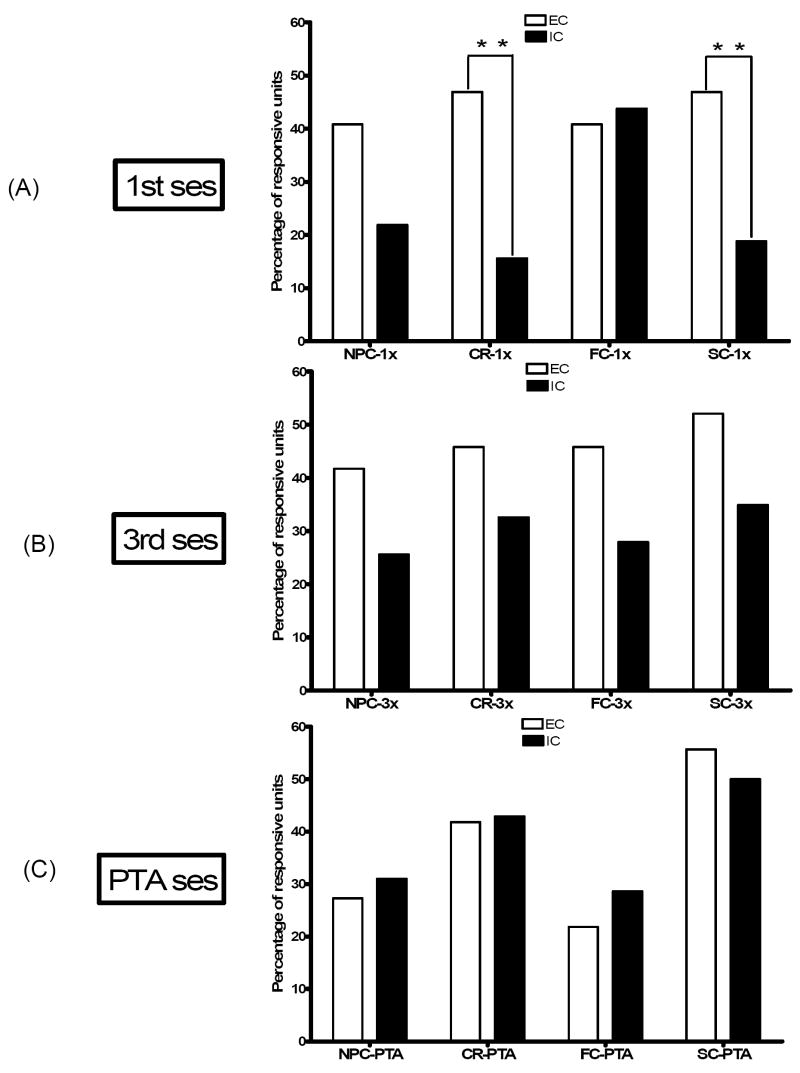
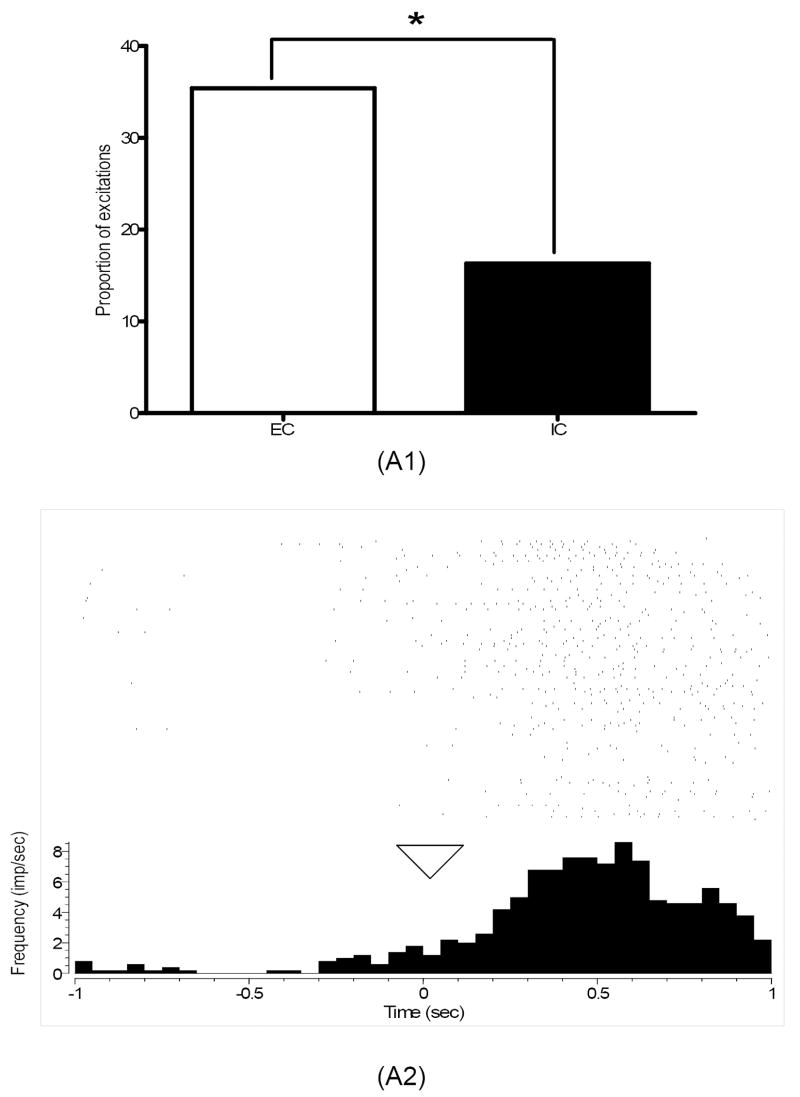
During the first training session, roughly half of core neurons in EC rats exhibited phasic firing changes (excitations or inhibitions) during the nosepoke response to the lit hole (47%) and during sucrose consumption (47%), while significantly fewer neurons responded to these events (16%, χ2 = 8.39; p< 0.01; 22%, χ2 = 6.69; p < 0.01, respectively) in IC rats (see Fig. 3). In the third training session, when there were clear learning differences, EC, but not IC, rats shifted neuronal responsiveness to the predictive cues. The shift, however, was evident not in overall responsiveness (see Fig. 3) but in excitations alone. As indicated, the proportion of core excitations in EC relative to IC rats was roughly three times greater during nosepoke cue (see Fig. 4; χ2 = 5.64; p < 0.05) and more than twice as great during feeder cue (see Fig. 5; χ2 = 4.28; p < 0.05). The magnitude of the neuronal response, however, was comparable in both groups, ranging between 220 and 390% and 60 and 90% of pre-event baseline.
Fig. 3.
A summary of the percentage of responsive units overall in NAcc core during nosepoke cue (NPC), correct responses (CR), feeder cue (FC) and sucrose consumption (SC) in EC and IC rats organized by recording session. In the first training session, a significantly higher proportion of core neurons in EC relative to IC rats responded during CR and SC in the first training session (** indicates p<.01). No significant differences were evident during the any of one of these task events during the third training session or during the PTA recording sessions. Note the similarity of overall task-related responsiveness in core neurons in EC and IC rats in the PTA recording sessions.
Fig. 4.
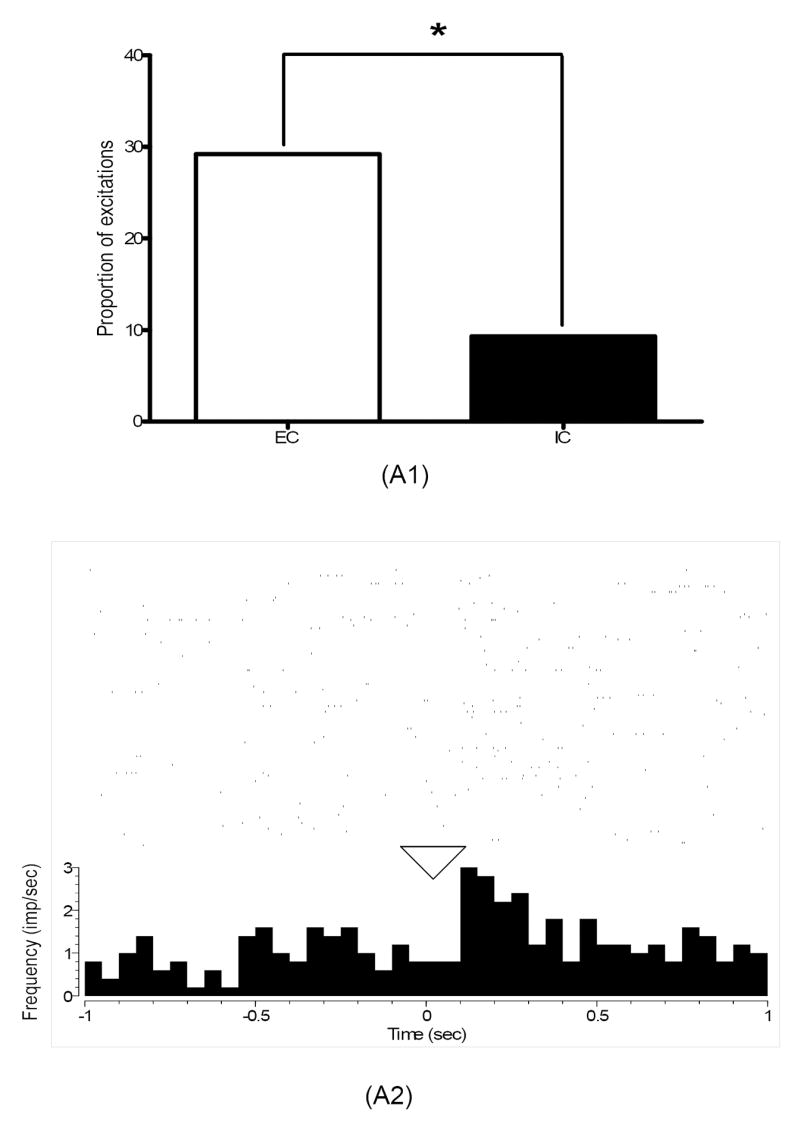
(A1) Percentage of excitations in core neurons recorded in EC and IC rats during nosepoke cue in the third training session. As indicated (*), a significantly larger proportion of excitations were present in EC relative to IC rats during this task event (p<0.05). (A2) A perievent histogram raster plot of a single-unit excitation recorded from an EC rat during nosepoke cue in the third training session. As indicated in the histogram, neuronal firing in this single unit increased right after time 0 (marked by triangle), the onset of this task event. The rasters above the histogram plot neuronal firing 1 sec before and after nosepoke cue during the 100 trials performed by this animal in this training session.
Fig. 5.
(A1) Percentage of excitations in core neurons recorded in EC and IC rats during feeder cue in the third training session. As indicated (*), a significantly larger proportion of excitations were present in EC relative to IC rats during this task event (p<.05). (A2) A perievent histogram raster plot of a single-unit excitation recorded from an EC rat during nosepoke cue in the third training session. As indicated in the histogram, neuronal firing in this single unit increased right after time 0 (marked by triangle), the onset of this task event. The rasters above the histogram plot neuronal firing 1 sec before and after nosepoke cue during the 100 trials performed by this animal in this training session.
Unit Responses during PTA
After the operant task was acquired, group differences vanished: neuronal responses during all four assessed task-related events during PTA were similar in EC and IC rats. In both groups, as shown in Fig. 3, the proportion of units responding during nosepoke to the lit hole and sucrose consumption appeared to be elevated relative to the proportion of responsive units during nosepoke cue and feeder cue. In fact, the combined proportion of responsive units during nosepoke to the lit hole and sucrose consumption (48%) was significantly greater (χ2 = 3.84; p < 0.05) than the combined proportion during nosepoke and feeder cues (27%). Moreover, as observed during task acquisition, the magnitude of PTA neuronal responses was similar in EC and IC rats.
3. DISCUSSION
Environmental experience alters neuronal processing in NAcc core during the acquisition of our discriminative learning task. Core neurons were more responsive in EC rats during goal-directed movements (correct responses and sucrose consumption) in the initial trial of acquisition. As learning materialized, this enhanced neuronal response shifted to the cues (nosepoke cue and feeder cue) predicting these movements. Thus, environmental experience alters core neuronal processing of both motor- and sensory-related events but at different stages over the course of learning. To ensure that neuronal responses were temporally associated with these respective task events, firing rate changes during each event were assessed in relation to pre-event baseline periods that did not overlap in time with the pre-event baseline period or response period of other task events.
In contrast to this work, most electrophysiological investigations of NAcc in drug or natural reward paradigms have limited their analysis to neuronal activity changes expressed during operant responses only after task acquisition. Our decision to attend to task-related neuronal responses during acquisition was influenced by previous pharmacological reports (Kelley et al., 1997; Smith-Roe and Kelley, 2000) indicating that the function of the NAcc core is critical for the acquisition of appetitive behavioral responses. We recognize that the categorization of task events as sensory or motor-related is not without its limitations; nevertheless, this analysis utilizes the separation in time that exists between discrete task events, which are temporally tied to either specific sensory input or motor output.
Despite the greater responsiveness of core neurons in EC rats during goal-directed behaviors in the initial training session, no group differences in nosepoke accuracy were observed at this point in training; however, this neuronal response difference was likely activity-dependent. IC rats are reliably more active than EC rats when placed in novel environments (Bowling et al., 1993; Larsson et al., 2002; Smith et al., 1997), and by definition, novel circumstances were experienced by all animals in this training session. Consistent with this prospect, NAcc core is involved in motor control, and neurons in this brain area are known to respond during forward locomotion (Wood and Rebec, 2004).
In the third training session, EC rats performed fewer bad pokes and exhibited a higher percentage of correct responses than IC rats. Thus, IC rats required significantly more training to hone their response to the active operant stimulus. Consistent with the less selective response pattern, IC rats extinguish operant responding for various rewards (e.g. sucrose, cocaine and amphetamine) more slowly than EC rats after reward is no longer available (Green, 2003). Since IC animals more readily engage operant stimuli for access to natural and drug rewards (Bardo et al., 2001; Green et al., 2002; Lamden and Rose, 1979; Rose and Lamden, 1983; Rose et al., 1985; Rose et al., 1986; Smith et al., 1997), their inclination towards augmented approach may interfere with the capacity to alter behavioral responses when contingencies change in appetitive contexts.
One might predict that the profile presented by IC rats during the third training session, increased proportion of bad pokes and decreased proportion of correct responses, was a consequence of a disinhibitory phenotype. If IC animals learned as rapidly as EC rats, but were simply expressing more intrusive responses, then IC rats should have exhibited more inter-trial interval (ITI) pokes (nosepokes between trials) during this training session. The number of ITI pokes, however, was not significantly different between the two groups indicating that this explanation is unlikely. Instead, our data suggests that EC rats learned the contingencies of the phase-three procedure more rapidly than IC rats.
Along with the accelerated learning rate of EC rats during the third training session, core neurons in these animals were more likely to increase firing during sensory-related cues (nosepoke cue and feeder cue). Because EC rats generally acquire complex learning associations more rapidly than IC rats (Doty, 1972; Nyman, 1967), the increased responsiveness of core neurons in EC rats to these cues may represent an important mechanism underlying an enhanced capacity to detect and respond effectively to changes in environmental conditions. Consistent with the learning-related neuronal response differences in this study, previous research has implicated NAcc core in various reinforcement learning tasks. For example, local infusions of AP-5, a NMDA antagonist, into NAcc core disrupted path learning in a spatial food-gathering task (Maldonado-Irizarry and Kelley, 1995a). Likewise, intra-core AP-5 injections disturbed discriminative approach to a conditioned stimulus before task learning but not after post-acquisition (Kelley et al., 1997). Collectively, these results suggest that glutamatergic transmission in NAcc core is critical for reinforcement learning, including learning in those paradigms involving discriminative appetitive behavioral responses.
Glutamate is primarily responsible for activating NAcc neurons in behaving rats (Kiyatkin and Rebec, 1996; Sandstrom and Rebec, 2003), and thus is presumably responsible for the preferential excitation of core neurons in EC rats during nosepoke cue and feeder cue in the third training session, when task performance in these animals was superior. Core neurons receive extensive glutamatergic input from variety of cortical, limbic, and thalamic sources (Zahm, 2000), including dorsal prefrontal cortex (dPFC), which selectively innervates the NAcc core (Berendse et al., 1992). The dPFC, which includes the anterior cingulate and prelimbic cortices, has been implicated in the control of executive function (Fuster, 2001). In fact, reduced activation of dPFC is associated with reduced discriminative behavioral responding (Jentsch and Taylor, 1999). Thus, if dPFC in IC rats is less responsive to these cued events during acquisition of our nosepoke operant task, this difference in neural function may have contributed to observed behavioral differences in EC and IC rats in this discriminative learning task.
A leading hypothesis of NAcc function advocates that NAcc is implicated in flexible, approach behavior leading to the formation of association with incentive stimuli (Ikemoto and Panksepp, 1999). As animals obtained phase-three training in our discriminative learning task, their goal-directed behavior generally became less variable and more discriminative as indicated by an accelerated trial completion rate and a decreased number of incorrect responses/session. Thus, based upon the flexible approach hypothesis, task-related neuronal responding should decrease as animals learned the phase-three protocol. Our task-related neuronal responses in NAcc core during acquisition oppose this hypothesis. The proportion of task-related responses of core neurons in IC rats was, in most cases, initially low at the beginning of phase-three training but generally increased with task acquisition. In contrast, the responsiveness of core neurons in EC rats was usually high during each assessed task event in the initial training session; however, as these animals improved their performance in the third training session of acquisition, the proportion of task-related responses generally remained high and in some cases (e.g. during feeder cue) exceeded the proportion of responses in the first training session. These observations clearly indicate that core neuronal responding is not reduced as EC and IC animals learn the phase-three protocol, but our data are consistent with the notion that activation of core neurons is necessary for the acquisition of a learned appetitive behavioral response.
Interestingly, once the operant task was acquired, group differences were no longer apparent: neuronal responses during all four assessed task-related events in PTA recordings were similar in EC and IC rats. Nevertheless, core neurons were preferentially responsive during specific goal-directed events (correct responses and sucrose consumption) compared to sensory stimulating events (nosepoke cue and feeder cue). This PTA effect may indicate that NAcc core is preferentially involved in motor control after learning has been acquired.
In sum, our results suggest that the functional role of core neurons in appetitive behavior not only changes in the context of learning and previous experience but may be particularly apparent prior to the learning of a reinforced behavioral response. In a learning context, core neurons are initially more responsive during the acquisition of goal-directed movements in EC rats, but as learning materializes, the neuronal response shifts to the cues that predict these movements. Thus, environmental experience alters core neuronal processing of both motor- and sensory-related events but at different stages over the course of learning.
4. EXPERIMENTAL PROCEDURE
Animal Care
Male Sprague-Dawley rat pups (bred in our colony from animals originating from Harlan Industries, Indianapolis, IN, USA) were cross-fostered to a single mother on post-natal day (p) 2, weaned on p28, and placed into either IC (20 × 24 × 18 cm) or EC (43 × 90 × 46 cm) housing. IC rats were housed individually; EC rats were housed in small groups of 3-5 rats each. The EC cage contained toys, tunnels, and other interactive objects, all of which were repositioned within the cage 3 times/week. To eliminate handling differences, neither group was handled during the experimental housing period. Animals were maintained on a 12 hr light/dark cycle (07:00 lights on) with ad libitum access to food and water. From p45 to p55, food (but not water) was restricted to maintain animals at 85% of their free feeding weight. Operant training started 5 days later. After phase-one and phase-two training (see below), animals were put on free feeding for at least 48 hr before undergoing surgery. Following recovery from surgery, animals were returned to food restriction for the duration of the study. Recordings were typically collected within ~ 7-10 days from the time animals reached 85% of their feeding weight. All animal protocols were approved by the Indiana University Institutional Animal Care and Use Committee.
Training Protocol
With the house lights off, rats were trained in a three-phase shaping procedure for sucrose (10% w/v) reinforcement In phase one, rats were prompted to lick a spout for sucrose in association with the feeder cue consisting of a tone (4500 Hz, 70 dB) and a yellow LED located above the spout. The yellow LED remained illuminated until the rat broke the photo beam located in front of the spout triggering a 1 s release of sucrose (sucrose consumption) followed by a 5 s intertrial interval (ITI). To facilitate association of the feeder cue and sucrose availability, the ITI was increased by 5 s for every 5 trials thereafter. Following 5 trials with a 30 s ITI, phase-one training was complete.
In phase two, a partition that blocked access to two nosepoke holes on the opposing wall of the chamber was removed, and animals were required to poke one of those two lit holes (nosepoke) to elicit the feeder cue. The first session of phase two began with an initial, free reinforcement (signaled by a feeder cue). After a 3–8 s ITI, each subsequent trial began with a lit green LED in both nosepoke holes coincident with a 900 ms nosepoke cue tone (1900 Hz, 70 dB). The green LED remained lit until a nosepoke was performed, which was followed by the feeder cue 700 ms later. When the rat broke the photo beam in front of the spout, the feeder cue was deactivated, and sucrose consumption occurred. To ensure sucrose consumption during initial trials of the first session of phase two, sucrose was available for 60 s following a nosepoke. After the first 10 reinforced responses, this period was reduced to 15 s. When animals performed 30 trials in 30 min, phase two was complete.
In phase three, only one nosepoke hole was lit (pseudorandomly selected) at trial onset to stimulate selective responding. After an initial, free reinforcement, the feeder cue was activated 700 ms after a nosepoke to the lit hole (correct response). As was the case in phase two, the feeder cue was deactivated with sucrose consumption, which was followed by an ITI of 3–8 s. After feeder cue onset, sucrose was available for 10 s. Following the first 70 trials, sucrose availability was reduced from 10 to 5 s. Each training session lasted 45 min or 100 trials, whichever occurred first. Phase-three training was considered complete after three training sessions. Subsequent sessions were known as post-training acquisition (PTA) sessions in which rats expressed at least 80% accuracy (typically 20 or fewer bad pokes in a 100-trial session). Bad pokes were defined as nosepokes to the incorrect hole after nosepoke cue onset prior to a correct response.
Surgical Procedure
To record neuronal responses from NAcc core, rats were implanted with microwire electrode bundles. In preparation for surgery, all animals were anesthetized (90 mg/kg ketamine HCl; 10 mg/kg xylazine HCl; im), and secured in a stereotaxic frame. After the skull was exposed, holes were drilled bilaterally over NAcc core (1.5 mm lateral and 1.7 mm anterior to bregma) according to standard coordinates (Paxinos and Watson, 1998). Electrode assemblies, which were composed of a cylindrical bundle of eight microwire electrodes (Teflon-coated, 50 μ diameter, stainless-steel) and one ground wire soldered to a 10-pin connector (NB Labs, Denison, Texas), were lowered to the proper depth (6.5-7.5 mm ventral from brain surface). Six stainless-steel support screws were attached to the skull along with dental acrylic to stabilize neuronal recordings. At the conclusion of surgery, each animal was injected with Ringer’s solution (5-10 ml, sc) to counter dehydration.
Electrophysiology
After animals recovered from surgery and returned to 85% of their free-feeding weight, a flexible, shielded cable harness was attached to the connector of each implanted electrode bundle and plugged into the commutator located above the operant chamber. Partitions were placed in the operant chamber to impede access to operant stimuli while neuronal activity was assessed. Neuronal signals from the rat were amplified, filtered (bandpass, 0.3 – 10 kHz), and routed to a Multi-channel Neuronal Acquisition Processor (MNAP, Plexon, Inc., Dallas, TX) to isolate and discriminate spike activity. Signals also were routed to an oscilloscope and audio monitor to facilitate single-unit discrimination. Time stamps for each unit were confined to those waveforms that selectively passed through a closely matched, digitized waveform template.
Different waveforms of units recorded on the same microwire were separated on the basis of their distinct characteristics including amplitude, duration, and voltages at specific points on the ascending and descending limbs of each respective waveform. Waveforms with a consistent amplitude and signal-to-noise ratio (at least 2.5 to 1) were identified as single units; this characterization was later confirmed by ad hoc analysis of autocorrelogram data.
After spike activity was discriminated, the partitions within the operant chamber were removed, and the first session of phase-three training or the PTA session began. Behavioral protocols were controlled and monitored by a computer that interacted with the operant chamber and MNAP via a software interface (Q-UNIT) that sent digital pulses to the MNAP system corresponding to behavioral output. MNAP simultaneously tracked operant events along with the collection of electrophysiological data.
Tissue Preparation
After the last recording session, rats were overdosed with a mixture of pentobarbital and chloral hydrate (chloropent; 0.33 ml/kg; i.p.) and current (30 μA, 5 s) was passed through the electrodes to mark recording sites. Rats were perfused transcardially with an isotonic saline followed by a solution of 4% paraformaldehyde and 5% potassium ferrocyanide. Metal deposits left at each lesion site reacted with this solution to produce a localized blue-green stain (Prussian Blue reaction). Brains were removed, fixed in 10% formalin, and placed in a 20% sucrose solution until they were sectioned. Tissue was cut in 40 μm coronal sections, stained with Cresyl Violet, and examined for the location of each electrode bundle.
Data Analyses
Peri-event histograms (PEHs) were used to assess changes in spike activity in NAcc core neurons during four task events (nosepoke cue, correct operant response, feeder cue, and sucrose consumption) during the first and third sessions of phase-three training and the PTA session. To assess firing rate changes in relation to each event, we established a pre-event baseline, defined as mean firing during the 300 ms period immediately before event onset. Neuronal excitations and inhibitions were said to occur if the firing rate in three, consecutive 50-ms bins during the 300 ms immediately after event onset increased or decreased from the pre-event baseline by at least 30%. These criteria are designed to detect changes beyond normal fluctuations in baseline activity, and are consistent with established standards used to identify responses in NAcc neurons during appetitive behaviors (Carelli et al., 2000; Chang et al., 2000). Analysis of variance was used to compare pre-event baseline activity preceding each of the four task events. Response magnitudes were defined as the ratio of the mean response firing rate divided by the pre-event baseline. Pearson’s chi-square tests were used to compare the proportion of task–related responsive units for the phase-one, phase-three and PTA recording sessions. These tests also were used to compare the combined proportion of neuronal responses during either motor-related task events (correct responses and sucrose consumption) or sensory-related task events (nosepoke cue and feeder cue). Student’s t-tests were used to assess baseline rates, task-related neuronal responses, neuronal response magnitudes, and behavioral performance, including bad pokes/session and percentage of correct operant responses/session [number of correct responses/total responses (correct responses + bad pokes)/session].
Acknowledgments
This research was supported by U.S. Public Health Service grants from the National Institute on Drug Abuse (DA 02451, DA 12964, DA 05312, DA 19300). We thank Jason Pope for his surgical assistance. We also thank Faye Caylor for administrative support and Paul Langley for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–66. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–22. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;99:433–43. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Doty BA. The effects of cage environment upon avoidance responding of aged rats. J Gerontol. 1972;27:358–60. doi: 10.1093/geronj/27.3.358. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Coury A, Phillips AG. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. J Neurosci. 1997;17:4849–55. doi: 10.1523/JNEUROSCI.17-12-04849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–33. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Green TA. Environmental and incentive salience factors affecting operant responding for drug and non-drug reinforcers. Diss Abstr Int Sect B Sci Eng. 2003;63:3970. [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. Feeding increases dopamine metabolism in the rat brain. Science. 1980;208:1168–70. doi: 10.1126/science.7375926. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci U S A. 1997;94:12174–9. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–53. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Lamden PJ, Rose FD. Sensorily reinforcing learning in rats reared in enriched and impoverished environments. IRCS Medical Sci. 1979;7:139. [Google Scholar]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Kelley AE. Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in the rat. Behav Pharmacol. 1995a;6:527–539. [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Kelley AE. Excitotoxic lesions of the core and shell subregions of the nucleus accumbens differentially disrupt body weight regulation and motor activity in rat. Brain Res Bull. 1995b;38:551–9. doi: 10.1016/0361-9230(95)02030-2. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas M, Gonzalez-Mora JL, Louilot A, Sole C, Guadalupe T. Increased dopamine release in the nucleus accumbens of copulating male rats as evidenced by in vivo voltammetry. Neurosci Lett. 1990;110:303–8. doi: 10.1016/0304-3940(90)90864-6. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen M. Neuropharmacological evidence to suggest that the nucleus accumbens and subpallidal region contribute to exploratory locomotion. Behav Neural Biol. 1984;42:52–60. doi: 10.1016/s0163-1047(84)90424-2. [DOI] [PubMed] [Google Scholar]
- Nyman AJ. Problem solving in rats as a function of experience at different ages. J Genet Psychol. 1967;110:31–9. doi: 10.1080/00221325.1967.10533713. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain and Stereotaxis Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997a;776:61–7. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997b;76:707–14. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Rosenzweig MR. Enriched and impoverished environments: effects on brain and behavior. Springer-Verlag; New York: 1987. [Google Scholar]
- Rose FD, Lamden PJ. Go no-go learning in rats reared in enriched and impoverished environments. IRCS Medical Sci. 1983;11:433–434. [Google Scholar]
- Rose FD, Dell PA, Love S. Behavioural consequences of different types of environmental enrichment in the rat. IRCS Medical Sci. 1985;13:748–749. [Google Scholar]
- Rose FD, Love S, Dell PA. Differential reinforcement effects in rats reared in enriched and impoverished environments. Physiol Behav. 1986;36:1139–45. doi: 10.1016/0031-9384(86)90491-9. [DOI] [PubMed] [Google Scholar]
- Sandstrom MI, Rebec GV. Characterization of striatal activity in conscious rats: contribution of NMDA and AMPA/kainate receptors to both spontaneous and glutamate-driven firing. Synapse. 2003;47:91–100. doi: 10.1002/syn.10142. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–42. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- van der Harst JE, Baars AM, Spruijt BM. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res. 2003;142:151–6. doi: 10.1016/s0166-4328(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Wood DA, Kosobud AE, Rebec GV. Nucleus accumbens single-unit activity in freely behaving male rats during approach to novel and non-novel estrus. Neurosci Lett. 2004;368:29–32. doi: 10.1016/j.neulet.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Wood DA, Rebec GV. Dissociation of core and shell single-unit activity in the nucleus accumbens in free-choice novelty. Behav Brain Res. 2004;152:59–66. doi: 10.1016/j.bbr.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Wood DA, Buse JE, Wellman CL, Rebec GV. Differential environmental exposure alters NMDA but not AMPA receptor subunit expression in nucleus accumbens core and shell. Brain Res. 2005;1042:176–83. doi: 10.1016/j.brainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–7. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Young AM, Joseph MH, Gray JA. Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience. 1992;48:871–6. doi: 10.1016/0306-4522(92)90275-7. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]