Abstract
Deficiencies in brain serotonergic neurotransmission, which is in part associated with the alteration of brain serotonin (5-HT) receptors, have been proposed as part of a neurochemical imbalance in affective disorders, including depression. The drugs used for the treatment of these disorders generally act through and/or on the serotonergic system. Different animal models of depression have provided researchers with tools to obtain a better understanding of drug actions and possibilities to obtain insight into the neurochemical bases of these disorders. The measurements of the 5-HT1A and 5-HT1B receptor densities in a rat model of depression, Flinders Sensitive Line (FSL) rats, and comparisons with Sprague-Dawley (SPD) and Flinders Resistant Line (FRL) rats, are reported here. The receptor sites were quantified by autoradiography in more than twenty-five distinct brain regions known to have relatively large densities of respective sites. Some brain regions (e.g., dental gyrus, septal nucleus) were divided into several parts, according to previously known subdivisions, because of a substantial heterogeneity of these receptors. The densities in the FSL rats (“depressed” rats) were compared statistically to those in the SPD rats. In addition, comparisons were made to the densities in the FRL rats (rats not showing depressive symptoms). Comparisons were performed with the SPD and FRL rats because both of these strains have been used as control animals in studies of FSL rats. The results show that the densities of 5-HT1A receptors are not significantly different between the FSL and SPD rats, but they are significantly different from the FRL rats. 5-HT1A receptor density is significantly higher in the FRL rats than the SPD rats. The 5-HT1B receptors were significantly greater in the FSL rats than in either the SPD or FRL rats. In addition, the FRL rats have 5-HT1B receptor densities significantly lower in many brain regions than the SPD rats. The data presented here, in addition to previously reported differences in regional synthesis between these strains and the effect of acute citalopram on synthesis, suggest that SPD rats are likely a more appropriate control than FRL rats, when studies of FSL rats are performed with drugs acting directly or indirectly on, or through, the brain serotonergic system. However, comparisons, particularly of neurochemical and/or biological parameters in FRL rats, may reveal new insight into the alterations of 5-HT neurotransmission in this animal model of depression and possibly human depression, as well as the elevation of symptoms with treatments. The data also suggest that there could be a different fraction of 5-HT1A receptors in high and low affinity states in these strains, as well as the possibility of different intracellular signalling.
Keywords: Depression model, autoradiography, brain receptors, FRL rats, Sprague-Dawley rats, animal models, depression, serotonin receptors, autoradiography
Introduction
Decreased activity of the brain serotonergic system, both in humans with depression and different animal models, have been described (Lesch and Heils, 2000). There are many animal models of depression (Kelly et al. 1997; Nestler et al. 2002; O’Neil and Moore, 2003; Willner and Mitchell, 2002). These models should have three distinct values: (1) face validity (how closely the model resembles the psychiatric condition); (2) construct validity (consistency of the model with the theoretical rationale); and (3) predictive validity (how closely the action of the drugs in the model resembles the actions of these drugs in the human disease) (Willner and Mitchell, 2002). The Flinders Sensitive Line (FSL) model of depression possesses all of these three values, with a relatively large number of similarities to the human disease (Yadid et al. 2000; Overstreet et al, 2005). The FSL rats have been proposed as a rat model of human depression with psychomotor retardation (Overstreet at al. 2005), a type of depression described to be present in humans (Lapierre and Butter, 1980; clinically described as retarded depression - a state of clinical depression in which the individual is lethargic and slow to initiate action). One very important aspect of an animal model of depression is the alleviation of depressive symptoms following chronic, but not acute, treatment with antidepressants. This aspect is true for the FSL rat model, but is not the case with the majority of other rat models of depression (O’Neil and Moore, 2003). In addition, it has been proposed that the FSL model could also model a predisposition to depression as well as depression per se (Overstreet et al. 2005). In a recent review (Nestler et al. 2002), it was clearly stated that the models that may be good predictors of an antidepressant effect (e.g., learned helplessness) are nonetheless unrealistic models of depression, as they show behavioural changes following acute treatment. In the FSL rats, neurochemical alterations are present in both presynaptic (e.g., autoreceptors, neurotransmitter synthesis) and postsynaptic functions (e.g., receptors densities/affinities, postsynaptic loops) of the brain serotonergic neurotransmission. In addition, there is also the possibility that intracellular processes are altered, which could influence the efficiency of the signal transduction (Shayit et al. 2003).
The FSL and FRL lines of rats were generated by selective breeding from SPD rats by selecting the animals which showed low (FRL) and high (FSL) sensitivity to the cholinesterase inhibitor, diisopropyl fluorophosphate (Overstreet, 2002). This supersensitivity of the FSL rats accords with the cholinergic hypothesis of depression (Janowsky and Risch, 1984) and the hypothesis of serotonergic and cholinergic actions of antidepressants (Dilsaver, 1986; Janowsky and Risch, 1984; Plaznik et al. 1989). In addition, FSL rats show neurochemical deficiencies in other brain systems (cholonergic, gabaergic, neurosteroids, dopaminergic, Hypothalamic-Pituitary-Adrenal axis) and receptors (e.g., 5-HT2C, 5-HT3, GABAA) which have been associated with human depression (Overstreet et al. 2005 and 2008). The FSL rats possess many biochemical and behavioural differences when compared to FRL or SPD rats (Yadid et al. 2000; Overstreet et al. 2005 and 2008). A large majority of comparisons have been performed with FRL rats, and they should probably not be considered as controls, especially when serotonergic system is studied, because they differ in many respects from SPD rats (normal rats).
Drugs acting as 5-HT1A agonists exhibit antidepressant and/or anxiolytic activities in animal models (de Vry 1995) and humans (Amsterdam, 1992;Fulton and Brogden, 1997; Celada et al. 2004) and, as such, a quantitative evaluation of these receptor sites in the FSL rats, relative to the control rats, should help us understand the action of these drugs and possibly provide us with some clues into the development of depressive symptoms. Because an integral action of a neurotransmitter depends on both receptor densities and affinity, knowledge of only the densities may not represent a full picture of receptor functioning. Generally, receptor affinities for a ligand are very similar between different species (Palacios et al., 1987). It is reasonable to assume that the affinity will not differ much between these strains of rats, and, as such, comparisons of the densities (as reported here) should be informative. Therefore, one should be able to assume that differences in the densities in the first approximation reflect differences in their functionality. Additionally, it is very important to study the receptors in animal models of depression, rather than in normal animals, because it is well known that antidepressants have no or very little effect on the brain serotonergic system (e.g., 5-HT synthesis, some receptor densities) in normal animals (Alpers and Himwich, 1972; Caccia et al. 1993; Dewar et al. 1993; Hasegawa et al. 2005b).
Limbic brain regions have the highest density of 5-HT1A receptors, especially the Hip, lateral septum, cortical areas, as well as the mesencephalic raphe nuclei (both the DR and MR) (Barnes and Sharp, 1999). A down regulation of 5-HT1A receptors has been proposed as a general hypothesis of alteration in 5-HT receptor function in subjects with depressive symptoms (Blier and de Montigny, 1994; Srinivas et al. 2001), as well as the possible involvement of other 5-HT receptors (e.g., 5HT2, Stahl, 1994). Further, many clinical and preclinical studies have reported a down regulation of 5-HT1A and 5-HT2 sites with antidepressant treatments (e.g., Stahl, 1994, Celada et al., 2004). There is still no clear understanding of the mode of action of antidepressants and/or how the 5-HT receptors are altered (the 5-HT system in general) in affective disorders, as well as the changes that occur with antidepressants.
Similar to the neuronal positions of 5-HT1A receptors, 5-HT1B receptors are found pre- and post-synaptically where they serve as auto- and heteroceptors, respectively (Olivier and Mos, 1992; Pineyro et al. 1995). It has also been shown that 5-HT1B agonists, as is the case with 5-HT1A agonists and antagonists, can modulate 5-HT synthesis (Tohyama et al. 2002; Hasegawa et al. 2005a; Dobson et al. 2004), one of the most important presynaptic parameters of serotonergic neurotransmission (Nelson, 1993) and, as such, their study is very important. We have also found that acute citalopram treatment reduces regional 5-HT synthesis in FSL rats, without any significant effect in SPD rats, and a significant elevation in FRL rats (Kanemaru et al. 2008). This suggests that this SSRI and an indirect agonist, citalopram, modulate regional 5-HT synthesis rather differently in these three strains of rats. This highlights the importance of quantitatively mapping receptor sites through which 5-HT can be modulated.
Because of the important role played by both the 5-HT1A and 5-HT1B receptors in behaviour, the modulation of serotonergic neurotransmission, drug action, the antidepressant properties of 5-HT1A agonists, and because of the lack of regional information on the densities of these sites in either the FSL or FRL rats, a quantitative determination of these sites was deemed necessary. There have been no systematic studies of these receptor sites in the FSL and FRL rats, while the effects of many drugs on behaviour acting through the receptors have been studied. Here, the measurements were performed in the FSL rats (“depressed” rats), FRL rats (rats most often used as controls in studies of FSL rats; Overstreet et al. 1998 and 2005), and SPD rats (normal rats from which both FSL and FRL rats are derived). One of the important reasons for selecting both of these strains as controls was the fact that regional 5-HT synthesis is somewhat different between the FRL and SDP rats (Hasegawa et al. 2006), but there are fewer differences than between the FSL and SPD rats. This suggests that, biochemically, at least with respect to some aspects of brain serotonergic neurotransmission, the FRL rats are different from the SPD rats and probably possess specific biochemical differences from those found in the SPD rats.
The hypotheses tested in the present study were: 1) FSL rats have a higher density of 5-HT1B receptors in the terminal regions (they function as autoreceptors; upregulated because of low synthesis and extracellular levels of 5-HT) as compared to the control SPD rats; 2) the differences will be particularly pronounced in the brain limbic structures (one of the basis of behavioural differences); 3) the FRL rats will have different densities of 5-HT1B receptors in the terminal regions than those found in the FSL and SPD rats; and 4) the densities of the 5-HT1A receptors, serving as autoreceptors in the cell bodies and heteroreceptors (responsible for the creation of postsynaptic loops) in the terminal regions, will be lower in the FSL rats compared to the controls.
Material and Methods
Male SPD rats weighing between 180–230g were used for this experiment (Charles River Canada, St. Constant, Quebec, Canada), while the FSL and FRL rats (within the same weight range) were obtained from breeding colonies maintained at the Montreal Neurological Institute from virus free breeds kindly supplied by Dr. D. Overstreet (Center for Alcohol Studies, University of North Carolina, Chapel Hill, NC 27599-7178, U.S.A.). In the present study, 7 rats were used in the FSL and SPD groups, while 9 rats were used in the FRL group. The rats were housed in the animal facility (room temperature 22°C and on a 12h day-night cycle).
The rats were sacrificed by decapitation and the brains were quickly removed and frozen in isopentane (−25°C). The brains were stored at −84°C in a sealed, freezer bag until their use. The brains were sectioned in 20 μm thick slices in a microtome-cryostat at −25°C and placed on a gelatin coated microscopic glass as previously described (Palacios et al. 1988; Takeda et al. 1996). In short, the slices were mounted on the gelatin-coated microscope slides and stored at room temperature overnight and then stored in slide boxes with desiccant (at least 2 days) at −20°C until use. The brain slices were preincubated for 30 min in a 0.17 M TRIS buffer (170 nM TRIS.HCl, 4 mM CaCl2, 0.01% ascorbic acid, and 10 μM pargylin for 5-HT1A receptors, which was replaced by 0.1 μM 8-OH-DPAT and 1 μM mesulgerine for 5-HT1B receptors; pH=7.6) at room temperature. The density of 5-HT1A was determined from the binding with [3H]8-OH-DPAT (KD=1.2 nM (Palacios et al. 1987); concentration=1.35 nM; SA=170 Ci/mmol; non specific binding was measured in the presence of 1 μM of non-radioactive 5-HT; Marcinkiewicz et al. 1984), while the densities of 5-HT1B were determined from the binding with [3H]5-HT (KD=23.4 nM (Engel et al. 1986); concentration=1 nM; SA=30 Ci/mmol) in the presence of 100 nM (nonradioactive) of 8-OH-DPAT and 1μM of mesulgerine to block the 5-HT2 receptors (non-specific binding was measured in the presence of 1 μM of non-radioactive 5-HT; Pazos and Palacios, 1985). After 60 min of incubation at room temperature, the slices were washed for 5 min in an iced cold buffer and steam dried.
The dried slices were contacted with [3H]sensitive films (Amersham Bioscience Inc.) along with tissue equivalent calibrated tritium standards for up to three months. Following development, the binding in the brain regions selected a priori were quantified. The regions selected were those with relatively high densities of 5-HT1A and 5-HT1B receptors (Pazos and Palacios, 1985). The brain region identifications were performed by an aid of a rat atlas and, whenever possible, abbreviations from the atlas were used (Paxinos and Watson, 1997). The amount of radioactivity was quantified by an MCID imaging program (MCID-4; Imaging Research Inc. St. Catharines, Ont. Canada) using tritium standards calibrated to the tissue equivalent (Amarsham Biociences Inc.). The non-specific binding was subtracted from the total binding and the radioactivity was expressed in fmol/mg-brain. All animal use procedures were in strict accordance with the Canadian Council on Animal Care guidelines, and were approved by the Animal Care Committee of McGill University. All brain regions were quantified in at least three consecutive slices on the left and the right sides. The readings from the left and ride sides were averaged and the value used was representative of a particular region. The Bmax was calculated as: Bmax=B (1+KD/C) [pmol/mg], where B [pmol/mg] is the amount of the ligand bound, KD [pM] is the ligand affinity constant, and C [pM] is the concentration of the ligand in the bath.
Some brain structures, especially for the 5-HT1A receptor quantification, were divided into smaller distinct regions (e.g., DG): the DG was divided into other, inner and polymorphic layers (Fig. 1); the SepN was divided into lateral dorsal and lateral intermedial; the A was divided into posterior medial nucleus (AM) and the rest of A; and the layers were divided into the Fr and SM. The DG was divided into these specific regions because of the important projections from respective parts to different limbic and cortical regions (Patton and McNaughton, 1995), and the posterior medial nucleus of A was selected because of the dense serotonergic innervations (Knapska et al 2007). In addition, the PaS, the structure not previously quantified separately, was selected for 5-HT1A receptor quantification, as important connections between the CA1 layer of the Hip and Ent run through this structure (Amaral and Witter, 1989). The 5-HT1A receptors were quantified in the following regions: A; AM; Au; CA1 dorsal; CA1 vent; CA2; CA3; Cg; DGIBL; DGBL; DGPo; DR; Sd; Ent; Fr (layers I-III); Fr (layers IV-VI); Hyp; IC; LC; MPFr; MR; PaS; Pt; SC; SepNLD (lateral dorsal); SepNLM (lateral intermedial); SM (layers I-III); SM (layers IV-VI); and V.
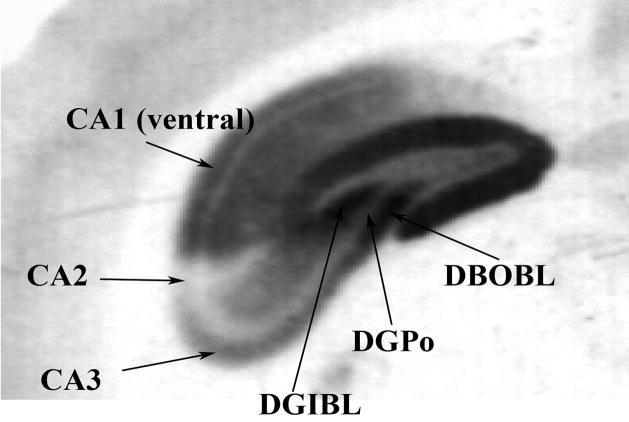
Figure 1.
An example of different parts of the dental gyrus in which the 5-HT1A receptors were quantified.
The 5-HT1B receptors were quantified in the following regions: A; Acx; Abc; AOM; Cg; CPuL; CPuM; DR; Ent; Fr; GP; HiD; HiV; Hyp; IC; LC; LG; MR; Pt; SC; Sd; SepN; SM; SN; Tha; V; and VP.
The receptor densities in twenty-nine brain regions in the above mentioned groups of rats were first evaluated by two-factor ANOVA (factor-1=strain; factor-2=brain region) to determine if there were significant differences between the groups and regions. Next, a Newman-Keuls post hoc correction was performed on the region specific ANOVA because we were only interested in the comparison between the groups in any individual brain region. We were not interested in comparing the densities among the different brain regions. p<0.05 was used as a level of significance. Comparisons between FRL and SPD rats were evaluated because, as noted in the Introduction section, it was important to establish if the FRL and SPD rats differed in these neurochemical parameters, given that both strains have been used as controls in many past studies.
Results
A set of representative cross sections to depict the diversity of bindings in the FSL, FRL and SPD rats are provided in Fig. 2 for the 5-HT1A receptor and in Fig. 3 for the 5-HT1B receptor. Great heterogeneities are obvious in the densities of both receptors. The laminal organization of the 5-HT1A receptors is also obvious in the cortical region, as well as the hippocampus (Fig 1). The quantitative densities (fmol/mg-brain) of the 5-HT1A and 5-HT1B receptors are provided in Tables 1 and 2, respectively, for all three groups of rats. The highest density of 5-HT1A receptors was observed in the PaS, followed by the SepNLM and DR. Other parts of the SepNLD and parts of the DG (DGOBL, DGIBL, and DGPo) and hippocampus had relatively higher densities as well (Table 1). Among the cortical regions, the greatest densities were observed in the MPFr and Cg, two very important limbic structures. The highest density of 5-HT1B receptors was found in the SN, followed by the VP, while the GP had a density of approximately half of that in the SN. The smallest density, from the brain regions measured here, was found in the V in the FSL rats, the Fr in the FRL rats, and the Tha in the SPD rats, suggesting a somewhat different, relative distribution of 5-HT1B sites in these three strains.
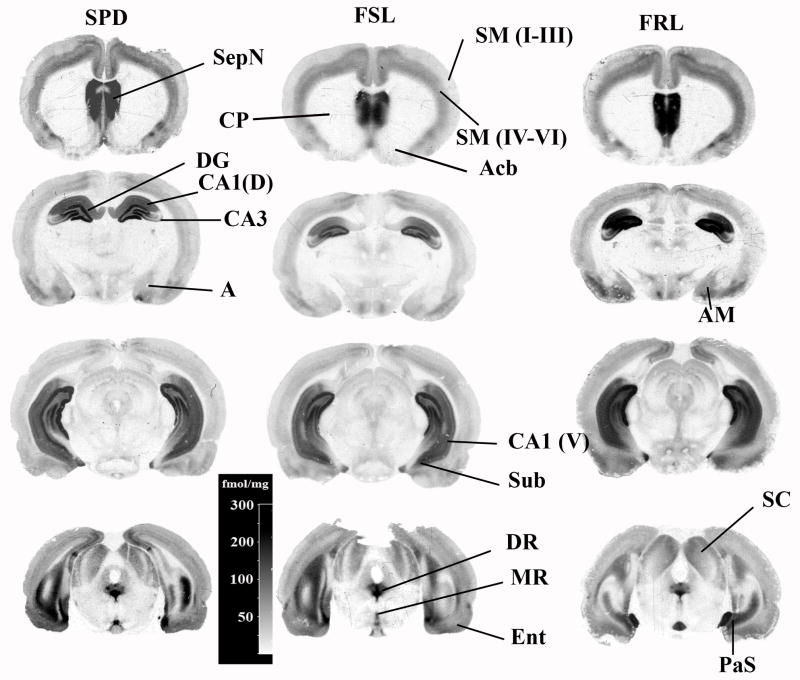
Figure 2.
A set of autoradiograms depicting the distribution of 5-HT1A receptors visualized by [3H]8-OH-DPAT in the FSL, FRL and SPD rats. The abbreviations of the structures are: Acb-accumbens; SeN-septal nucleus; DG-dental gyrus; DR-dorsal raphe; Ent-enthorinal cortex; SM-somatosensory cortex (layers I to VI are separately identified in parenthesis); A-amygdala; Hyp-hypothalamus; CA1 (V) ventral part of the hippocampal layer CA1; CP-caudate-putamen; Sub-subiculum; and PaS-parasubiculum.
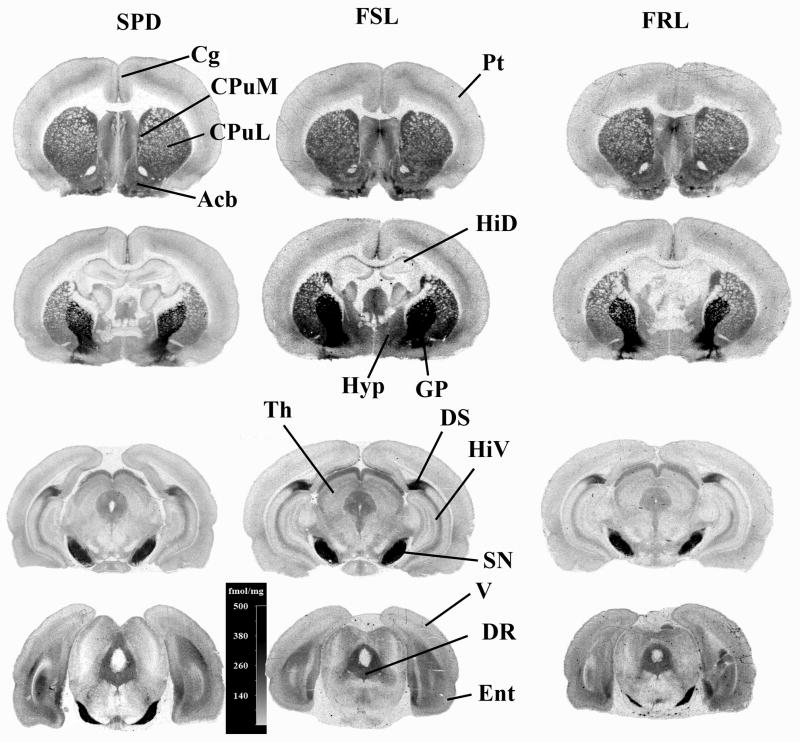
Figure 3.
A set of autoradiograms depicting the distribution of 5-HT1B receptors visualized by [3H]serotonin in the FSL, FRL and SPD rats. The abbreviations of the structures are: Acb-accumbens; SeN-septal nucleus; GP-globus pallidus; DR-dorsal raphe; SN-substantia nigra; Cg-cingulate cortex; Ent-enthorinal cortex; SM-somatosensory cortex (layers I to VI are separately identified in parenthesis); V-visual cortex; A-amygdala; Hyp-hypothalamus; HiD-dorsal hippocampus; HiV-ventral hippocampus; CPuL-lateral part of caudate-putamen; and CPuM-median part of caudate-putamen.
Table 1.
5-HT1A receptor densities. The values are given as means ± standard errors of the means in fmol/mg with the number of rats provided in the parentheses after the group name.
| REGIONS1 | FSL (N=7) | FRL (N=9) | SPD (N=7) |
|---|---|---|---|
| A | 4.0±0.4 | 29.1±3.6 | 4.6±0.6 |
| AM (medial nucleus) | 14.0±0.8 | 17.3±0.9 | 16.4±2.2 |
| CA1 dorsal | 19.7±1.4* | 27.5±1.5 | 23.9±2.3 |
| CA1 vent | 26.7±2.7 | 30.7±2.0 | 27.9±4.0 |
| CA2 | 3.3±0.4 | 5.2±0.7 | 3.6±0.4 |
| CA3 | 16.0±1.4 | 19.0±1.2 | 18.1±1.9 |
| DGIBL | 35.6±2.4 | 41.1±2.3 | 39.6±4.6 |
| DGOBL | 44.0±4.5 | 49.4±3.2 | 49.5±6.7 |
| DGPo | 21.9±1.5 | 25.5±1.7 | 23.2±2.5 |
| DR | 31.1±2.9 | 34.1±1.9 | 31.9±3.7 |
| MR | 13.7±1.4 | 18.2±1.5 | 14.2±2.4 |
| Au | 3.7±0.6* | 7.5±1.0# | 4.2±0.5 |
| Cg | 10.5±1.1* | 15.5±0.0# | 11.0±1.6 |
| Ent | 10.0±1.0* | 15.1±1.4 | 12.2±1.5 |
| Fr (layers IV-VI) | 6.9±0.7* | 11.1±0.9# | 8.2±1.1 |
| Fr (layers I-III) | 1.8±0.2* | 4.5±0.5 | 3.3±0.4† |
| MPFr | 19.9±2.1 | 21.1±1.3 | 15.1±2.8 |
| Pt | 3.7±0.4* | 6.1±0.8# | 3.9±0.5 |
| SM (layers IV-VI) | 7.8±0.8* | 11.7±1.0# | 8.3±1.1 |
| SM (layers I=III) | 2.1±0.3* | 5.0±0.5# | 2.9±0.4 |
| V | 4.1±0.5* | 8.3±1.0# | 4.8±0.7 |
| Hyp | 2.9±0.3 | 4.7±0.7 | 3.7±0.4 |
| IC | 4.9±0.4 | 6.5±0.7 | 5.8±0.5 |
| SC | 4.4±0.6 | 6.3±0.9 | 4.7±0.4 |
| LC | 7.9±0.9 | 11.3±1.5# | 6.4±0.8 |
| Sd | 5.6±1.7 | 8.3±2.4 | 3.1±0.5 |
| PaS | 55.6±6.1 | 58.1±3.2 | 53.0±7.1 |
| SepNLD | 28.4±2.8* | 40.0±2.3 | 44.3±6.5† |
| SepNLIM | 33.2±3.3 | 40.0±2.6 | 36.0±4.4 |
| Tha | |||
Abbreviations are listed at the beginning.
Difference is significant when the 5-HT1A receptor densities between the FSL and FRL rats were compared; p<0.05 with post-hoc Newman-Keuls correction
Difference is significant when the 5-HT1A receptor densities between the FRL and SPD rats were compared; p<0.05 with post-hoc Newman-Keuls correction
Difference is significant when the 5-HT1A receptor densities between the FSL and SPD rats were compared; p<0.05 with post-hoc Newman-Keuls correction
Table 2.
5-HT1B receptor densities in three groups of rats: FSL, FRL and SPD. The values are given as means ±standard errors of the means in fmol/mg-tissue.
| REGIONS1 | FSL (N=5) | FRL (N=7) | SPD (N=7) |
|---|---|---|---|
| A | 63.4±5.3* | 26.5±4.5# | 43.5±4.5† |
| Acb | 129.3±7.6* | 60.7±6.4# | 87.5±6.4† |
| AOM | 45.3±7.3 | 26.1±6.2 | 30.4±6.2 |
| Cg | 45.7±5.4* | 21.3±4.6# | 38.4±4.6 |
| Ent | 53.9±5.3* | 21.7±4.5# | 40.1±4.5 |
| HiD | 35.7±3.2* | 11.3±2.7 | 19.8±2.7† |
| HiV | 59.0±4.3* | 24.9±3.7# | 52.8±3.7 |
| Hyp | 111.7±6.8* | 44.7±5.7# | 92.3±5.7† |
| CPuL | 104.0±4.3* | 41.6±3.6# | 80.2±3.6† |
| CPuM | 115.1±3.9* | 54.4±3.3# | 94.6±3.3† |
| DR | 126.8±4.6* | 49.0±3.9# | 113.9±3.9† |
| MR | 65.5±4.3* | 24.5±3.6# | 51.0±3.6† |
| Au | 37.5±3.9* | 17.5±3.3 | 27.7±3.3 |
| Fr | 40.0±6.4 | 20.5±5.4 | 31.3±5.4 |
| Pt | 26.6±2.3* | 11.5±2.0 | 15.1±2.0† |
| SM | 35.8±3.4* | 16.5±2.9# | 27.3±2.9 |
| V | 24.1±3.0* | 12.0±2.5 | 16.4±2.5 |
| GP | 274.6±13.2* | 127.±11.# | 237.±11. ( |
| VP | 403.(31.* | 199.(24.# | 349.(26. |
| IC | 40.7(3.5* | 10.7(3.0# | 22.9(3.0( |
| SC | 148.9(2.9* | 61.7(2.5# | 137.5(2.5.( |
| LC | 148.0±8.8* | 46.3±7.5# | 110.8±7.5† |
| LG | 69.1±3.0* | 22.2±2.6# | 52.5±2.6† |
| Sd | 380.7±23.8* | 179.±20.# | 326.±20. |
| SepN | 140.4±6.1* | 57.5±5.1# | 119.5±5.1† |
| SN | 537.±47.* | 298.±39. | 376.±39.† |
| Tha | 24.1±2.6* | 9.4±2.2 | 15.0±2.2† |
Abbreviations are listed at the beginning.
Difference is significant when 5-HT1B receptor densities between the FSL and FRL rats were compared; p<0.05 with post-hoc Newman-Keuls correction
Difference is significant when 5-HT1B receptor densities between the FRL and SPD rats were compared; p<0.05 with post-hoc Newman-Keuls correction
Difference is significant when 5-HT1B receptor densities between the FSL and SPD rats were compared; p<0.05 with post-hoc Newman-Keuls correction
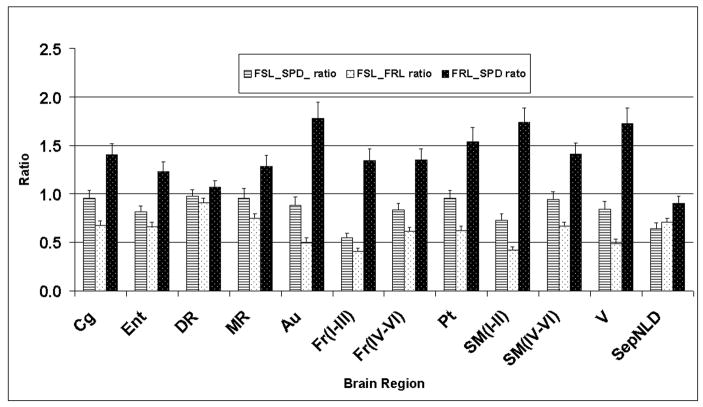
The evaluation of the 5-HT1A receptors by two-factor main effect ANOVA showed significant differences between groups (F(2,600)=24.4; p<0.005; partial Eta2=0.08; power=1.0). A post hoc analysis using Newman-Keuls correction for three groups revealed that there is an overall significant difference (p<0.001) between the FSL and FRL, FRL and SPD, and FSL and SPD (p<0.02) rats. The post-hoc analysis using the Newman-Keuls correction of the region specific ANOVA revealed significant differences in the FSL rats relative to the FRL rats in eleven regions. In the FSL rats, when compared to the SPD rats, there were significant differences (p<0.05 after correction for multiple tests) in two brain regions out of thirty regions investigated (Table 1). The FRL rats had significantly greater densities of 5-HT1A receptors than the FSL rats in the following brain regions: Fr (layers IV-VI), Fr (layers I-III), Cg, SepNLD, SM (layers IV-VI), SM (layers I-III), CA1 dorsal, Pt, Au, Ent, and V. When the FSL rats were compared to the SPD rats, the post hoc significant differences were found in the Fr (layers I-III) and SepNLD regions. The comparisons of the FRL and SPD rats showed significant differences in eight out of thirty regions evaluated [Fr (layers IV-VI), Cg, SM (layers IV-VI), SM (layers I-III), Pt, Au, V, and LC) (Table 1). A graphical presentation of the brain regions in which the FSL and FRL rats showed significant differences, as well as the DR and MR, is provided in Fig. 4. From this, one can note that the 5-HT1A receptors in the FSL and SPD rats have similar densities, but the densities seem to be somewhat lower in the FSL rats. The greatest density of the 5-HT1A receptors is in the FRL rats (Fig. 4). Also, similarities of the densities in the DR and MR among different strains can be easily appreciated.
Figure 4.
A graphical presentation of the ratios of the 5-HT1A receptor densities (y-axis) in different brain regions (x-axis). The ratios are presented with the standard error of the ratio. The relative error of the ratios was calculated as the square root of the sum of squares of individual errors of the nominator and denominator. The meaning of these abbreviations is provided at the beginning, in the list of abbreviations.
The 5-HT1B receptor density evaluated by two-factor ANOVA showed a highly significant difference between three groups (group; F(2,459)=242.0; p<0.001; partial Eta2=0.51; power=1.0 ). The Newman-Keuls corrected post hoc analysis revealed significant differences between three groups (strain of rats) (p<0.001). A post-hoc Newman-Keuls corrected comparison of the region specific ANOVA revealed significant differences in all brain regions except for the FSL rats, relative to the FRL rats (Table 2). For the FSL rats, relative to the SPD rats, there were significant differences in seventeen brain regions out of twenty seven regions investigated (Table 2). No significant differences between the FSL and SPD rats were found in the following regions: AOM, Au, Cg, Ent, Fr, GP, HiV, Pt, Sd, SM, V, and VP (Table 2). A post hoc evaluation between the FRL and SPD rats also showed differences in all brain regions except the following five; AOM, Au, Fr, HiD, Pt, SN, Tha, and V (Table 2). This also suggests that with respect to the 5-HT1B receptors, another important serotonergic parameter, the density of these sites in the FRL and SPD rats are not the same (i.e., similar or equivalent). A graphical presentation of selected brain regions representing some limbic structures, Fr, DR, and MR, clearly indicate that the density of 5-HT1B receptors is the greatest in the FSL rats and the smallest in the FRL rats (Fig. 5). The densities in the normal SPD rats is in between the FSL and FRL rats.
Figure 5.
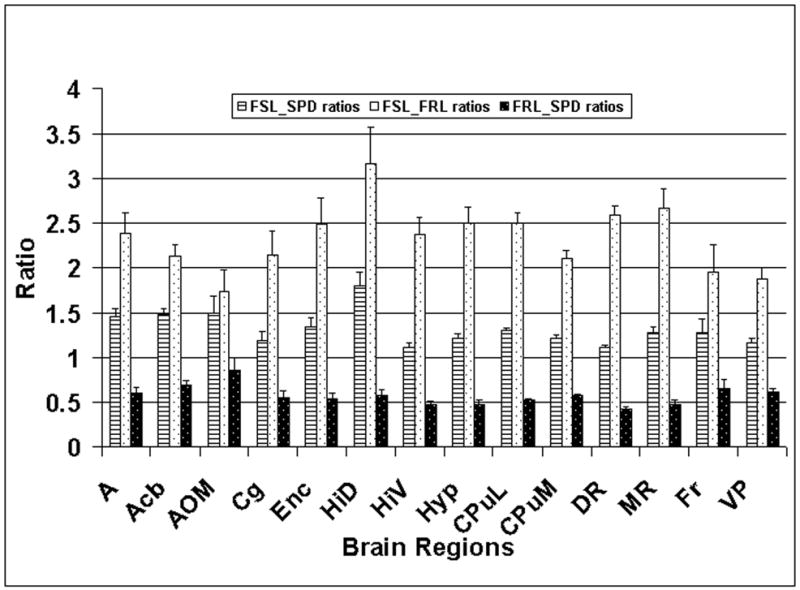
A graphical presentation of the ratios of the 5-HT1B receptor densities (y-axis) in different brain regions (x-axis). The standard errors were calculated as described in the caption to Fig. 4. The meaning of these abbreviations is provided at the beginning, in the list of abbreviations.
Discussion
The main finding of this study is the fundamental neurochemical/biochemical difference between the FSL rats (“depressed” rats) and both the SPD and FRL rats, as well as between the FRL and SPD rats. There were only a few regions in which significant differences were found between the FSL and SPD rats, when the 5-HT1A receptors were compared (Table 1; Fig 4), but very large differences were found when the 5-HT1B receptors were compared (Table 2; Fig. 5). This, combined with previous findings on the differences of 5-HT synthesis between these strains (Hasegawa et al. 2006), tissue 5-HT levels (Zangen et al. 1997), and a large array of other differences related to the receptors and the brain systems (Markon et al., 1994; Overstreet et al. 2005 and 2008; Yadid et al. 2000), suggest that SPD rats should likely be used as the control of choice in studies of the serotonergic system in FSL rats. However, the comparison of both the FSL and FRL rats with the SPD rats may help in understanding the behavioural and/or neurochemical differences between the “depressed” FSL rats and the normal SPD rats, as the FSL and FRL rats represent two genetic variants of the SPD rats with different behaviours and sensitivities to antidepressants (Yadid et al. 2000; Overstreet et al. 1998 and 2005). Further, comparisons between all three of these strains may also shed some light on human depression.
The relative regional distribution of both the 5-HT1A (Fig. 2) and 5-HT1B (Fig. 3) receptors observed in the SPD rats (Tables 1 and 2, respectively) accords, in general, with the distributions of these sites in normal rats (Pazos and Palacios, 1985; Verge et al. 1986). The two receptor sites studied here cannot provide a full explanation of the FSL rat model of depression, because the FSL rat model of depression is very complex (Yadid et al., 2000; Overstreet et al. 2005 and 2008). Rather, the presented results represent a novel contribution to a better understanding of the neurochemistry/biology and possible behavioural differences between these strains of rats. It is interesting to note that the genetic alteration accomplished by selective breeding likely produced a compensatory mechanism by which the reduction in 5-HT1A receptors in the FSL rats produced an over expression of the 5-HT1B sites (Tables 1 and 2). This could be related to the 5-HT1A knockout mice which has an upregulation of 5-HT1B receptors (Ramboz et al. 1998).
Significant differences in the densities of 5-HT1A receptors between the FSL and SPD rats were expected, but only differences in two regions were significant (Table 1 and Fig. 4). However, it is important to note that in many brain regions the receptors have lower densities in the FSL than in the SPD rats, but following correction for multiple comparisons, the significance is lost (Fig. 4). These differences were expected because of the reported supersensitivity of the 5-HT1A receptors (Shayit et al. 2003) and a greater hypothermic response to 5-HT1A agonists, which are drugs known to lower body temperature (Overstreet et al. 1998 and 2005). Also, the FSL rats have, throughout the brain, lower 5-HT synthesis than the SPD rats (Hasegawa et al. 2006), which at least in part relates to an inhibition (Hamon et al. 1972), as they have elevated intracellular 5-HT (Zangen et al. 1997). A relatively small number of the brain terminal regions in which 5-HT1A receptor density is significantly lower in the FSL than the SPD rats (Table 1) could be related to the observation that, at baseline, there is no difference in the extracellular levels of 5-HT between these strains (Zangen et al. 2001). This conclusion follows from an assumption that lower extracellular levels of 5-HT would upregulate receptor sites, assuming no change in the receptor affinity. The above mentioned results suggest differences in intracellular signalling (Shayit et al. 2003) between these strains. The fact that 5-HT1A receptors exist in high and low affinity states (Assie et al. 1999) could also contribute towards an apparent discrepancy between 5-HT synthesis (Hasegawa et al. 2006), extracellular levels of 5-HT (Zangen et al. 1997), and receptor density (present report).
Significant differences in the density of 5-HT1A receptors between the FSL and FRL rats (Table 1; Fig. 4) are in accordance with the reported effects of the 5-HT1A agonist, 8-OH-DPAT, which has a rather different effect on lowering body temperature, primarily modulated through the 5-HT1A receptors, in the FSL rats than in the FRL rats (Overstreet et al. 1998). The lowering of the body temperature by the 5-HT1A agonist in the FRL rats (Overstreet et al. 1998) is approximately the same as that produced in the SPD rats with buspirone, another 5-HT1A acting drug modulating 5-HT synthesis (Okazawa et al. 1999). A small difference in 5-HT synthesis between the FRL and SPD rats (Hasegawa et al. 2006) could not explain the significant differences in the 5-HT1A receptor densities, unless there is an inefficient intracellular signalling in the FRL rats and/or a greater proportion of 5-HT1A receptors in a high affinity state in the FRL rats in comparison to the SPD rats. From this, it is obvious that information on the differences in extracellular and/or intracellular levels of 5-HT between the FRL and SDP rats would be very helpful.
The 5-HT1A receptor densities between the FSL and FRL rats varies with the data reported in an abstract suggesting a 20% increase in 5-HT1A receptors in adult FSL rats relative to the FRL rats (Schiller, 1991). This apparent discrepancy in 5-HT1A receptor density is somewhat surprising, but the difference in finding could be, in part, explained by the fact that the measurements were performed on the tissue membranes in the previous study, as opposed to on brain slices, as was the case in the present study. It is also possible that the rats used in the previous study (Schiller, 1991) were younger (no age is provided), as it has been reported that younger rats have a larger density of 5-HT1A receptors (Yamaguchi and Yamagata, 1991). The age effect on the density of 5-HT1A receptors has also been reported in a human positron emission tomography study (Meltzer et al. 2004; Møller et al. 2007), as well as in studies on post-mortem specimens (Marcusson et al. 1984).
Lower 5-HT1A receptor densities in the FSL rats (“depressed”), relative to the FRL rats (not depressed) could be related to the observation and designation of FSL rats as “depressed” in many behavioural studies (Overstreet et al. 1998 and 2005). The reduction in 5-HT1A receptors has been reported in depressed patients (Sargent et al. 2000; Drevets et al. 1999; Parsey et al. 2002), and that accords with the differences between the FSL and FRL rats reported here. Similarly, mice lacking 5-HT1A receptors show increased anxiety (Parks et al. 1998), which is in line with a general behavioural pattern of FSL rats relative to either the SPD or FRL rats (Overstreet et al. 1998 and 2005), and a significant reduction in 5-HT1A sites in many cortical and limbic regions (present report).
In another rat model of depression, the olfactory bulbectomized (OBX) rat model, it has been found that the densities of 5-HT1A receptors are significantly lower in the OBX than in the sham operated rats (Sato et al. 2008). However, there is a large difference in the extracellular 5-HT between the FSL rats (about the same in the FSL and SPD rats; Zangen et al. 2001) and the OBX rats (larger in the OBX rats than in the sham operated rats; Lumia et al. 1992; van der Stelt et al. 2005), relative to their respective controls. These differences in 5-HT tissue levels (Lumia et al. 1992; van der Stelt et al. 2005; Zangen et al. 2001) and regional 5-HT synthesis rates (Watanabe et al. 2003 and 2006; Hasegawa et al. 2006) between these strains and their respective controls could be, in part, responsible for differences in the regional receptor densities presented here and those reported for OBX rats (Sato et al. 2008).
The increase in densities of the 5-HT1B receptors (Table 2; Fig. 5) in the FSL rats relative to the SPD rats accords with the lower 5-HT synthesis in the terminal regions in FSL rats (Hasegawa et al. 2006). This elevation in 5-HT1B receptors would be a consequence of the upregulation of receptors. The findings reported here for 5-HT1B receptors do not accord with the reported observation of no difference between the extracellular 5-HT in FSL and SPD rats (Zangen et al. 2001). One would not expect relatively large differences in 5-HT1B receptor densities between the FRL and SPD rats, as those observed here (Table 2; Fig. 5), because at baseline in the terminal regions, 5-HT synthesis in these strains is not significantly different (Hasegawa et al. 2006). Of course, this would only be correct if 5-HT synthesis was the only factor controlling the expression of these receptor sites, which is likely not the case. Unfortunately, there are no measurements of extracellular 5-HT in the FRL rats. One can therefore hypothesize a difference in intracellular signalling, as previously proposed, for 5-HT1A receptors (Shayit et al. 2003). From this, it is clear that the reason(s) for the differences in 5-HT1B receptor densities and the differences and/or similarities in 5-HT synthesis are substantially more complex and require further study before a clear understanding is obtained. As mentioned above, no differences in the affinities are expected between these strains.
Taken together, one can hypothesize that the reduction in 5-HT1A receptors coupled by an elevation of 5-HT1B sites could be part of the pathophysiology in the development of depressive symptoms. This hypothesis is derived from the results of this present study in FSL rats, when compared to both the FRL and SPD rats, as well as from previous work performed on human depression and other animal models, as discussed above.
In conclusion, it can be stated that both the 5-HT1A and 5-HT1B receptor sites are significantly different when comparing the FSL, FRL and SPD rats. The relative differences in 5-HT1A sites between the strains indicate that the FRL rats have the largest, while FSL rats have the lowest density of these sites. On the contrary, with respect to the densities of 5-HT1B receptors, the greatest density is in the FSL rats and the smallest is in the FRL rats. This suggests a genetic influence on the receptor densities and possibly a compensatory mechanism between these two strains produced through genetic alteration. It should be emphasized that these two sites form only a part of these very complex receptors and system deficiencies found in FSL rats. As such, they are only a component of complex mechanisms responsible for the manifestation of depressive symptoms in FSL rats. While in many brain terminal regions, the FSL rats have a significantly lower density of 5-HT1A receptors than the FRL rats, there was a significant difference in the three terminal regions (Sd, SepNLD and Fr (layers I-III)), when the FSL and SPD rats were compared. With respect to the densities of the 5-HT1B receptors in the terminal and cell body areas, all three strains are very different. This could suggest that the 5-HT1B receptors play an important role in the manifestation of depressive symptoms found in FSL rats and possibly in humans (there is no systematic study available), and the changes in these sites could be important in antidepressant therapy. Taking this together, there is no overall mechanism that can explain all of the differences reported here and those reported previously, which indicates the involvement of other neurotransmitter systems in the pathophysiology of depressive symptoms in FSL rats. These, as well as other neurochemical data reported previously, suggest that the SPD rats (normal rats) are likely the controls of choice in the study of serotonergic drugs in FSL rats.
Acknowledgments
The research reported here was supported in part by a grant from the Canadian Institute for Health Research (MOP-42438) and a grant from the US National Institute of Health (R01-NS29629). We would also like to thank Ms. Valerie-Ann Cherneski for the editorial help.
List of abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tertraline
- A
amygdala
- Acb
nucleus accumbens
- AM
posterior medial nucleus of amygdala
- ANOVA
analysis of variance
- AOM
medial anterior olfactory nucleus
- Au
auditory cortex
- Bmax
maximum binding capacity
- Cg
cingulate cortex
- CPuL
caudate putamen (lateral)
- CPuM
caudate putamen (medial)
- DG
dentate gyrus
- DGIB
dental gyrus inner blade layer
- DGBL
dental gyrus other blade layer
- DR
dorsal raphe nucleus
- Ent
enthorinal cortex
- Fr
frontal cortex
- FRL
Flinders Resistant Line
- FSL
Flinders Sensitive Line
- GABA
γ-iso-butyric acid
- GP
globus pallidus
- HiD
dorsal hippocampus
- HiV
ventral hippocampus
- Hyp
hypothalamus
- IC
inferior colliculus
- LC
locus coeruleus
- LG
lateral geniculate
- MPFr
medial prefrontal cortex
- MR
median raphe
- PaS
parasubiculum
- Pcx
parietal cortex
- SA
specific activity
- SC
superior colliculus
- Sd
subiculum, dorsal part
- SepN
septal nucleus
- SERT
serotonin transporter
- SM
sensory-motor cortex
- SN
substantia nigra
- SPD
Sprague-Dawley
- Tha
thalamus
- RIS-2
Amino-2-hydroxymethyl-propane-1,3-diol
- V
visual cortex
- VP
ventral pallidus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpers HS, Himwich HE. The effects of chronic imipramine administration on rat brain levels of serotonin, 5-hydroxyindoleacetic acid, norepinephrine and dopamine. Journal of Pharmacology and Experimental Therapeutics. 1972;180:531–8. [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–91. doi: 10.1016/0306-4522(89)90424-7. Review. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD. Gepirone, a selective serotonin (5HT1A) partial agonist in the treatment of major depression. Progress in Neuropshychopharmacology and Biological Psychiatry. 1992;16:271–80. doi: 10.1016/0278-5846(92)90079-t. [DOI] [PubMed] [Google Scholar]
- Assié MB, Cosi C, Koek W. Correlation between low/high affinity ratios for 5-HT(1A) receptors and intrinsic activity. European Journal of Pharmacology. 1999;386(1):97–103. doi: 10.1016/s0014-2999(99)00738-4. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barton CL, Hutson PH. Inhibition of hippocampal 5-HT synthesis by fluoxetine and paroxetine: Evidence for the involvement of both 5-HT1A and 5-HT1B/D autoreceptors. Synapse. 1999;31:13–19. doi: 10.1002/(SICI)1098-2396(199901)31:1<13::AID-SYN3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends in Pharmacological Sciences. 1994;15(7):220–6. doi: 10.1016/0165-6147(94)90315-8. Review. [DOI] [PubMed] [Google Scholar]
- Caccia S, Anelli M, Codegoni AM, Fracasso C, Garattini S. The effects of single and repeated anorectic doses of 5-hydroxytryptamine uptake inhibitors on indole levels in rat brain. British Journal of Pharmacology. 1993;110:355–9. doi: 10.1111/j.1476-5381.1993.tb13817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. Journal of Psychiatry and Neuroscience. 2004;29(4):252–65. [PMC free article] [PubMed] [Google Scholar]
- DeVry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl) 1995;121(1):1–26. doi: 10.1007/BF02245588. Review. [DOI] [PubMed] [Google Scholar]
- Dewar KM, Grondin L, Nénonéné EK, Ohayon M, Reader TA. [3H] paroxetine binding and serotonin content of rat brain: absence of changes following antidepressant treatments. European Journal of Pharmacology. 1993;235:137–42. doi: 10.1016/0014-2999(93)90833-4. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC. Cholinergic mechanisms in affective disorders. Future directions for investigation. Acta Psychiatrica Scandinavica. 1986;74(4):312–34. doi: 10.1111/j.1600-0447.1986.tb06250.x. [DOI] [PubMed] [Google Scholar]
- Dobson CF, Tohyama Y, Diksic M, Hamel E. Effects of acute or chronic administration of anti-migraine drugs sumatriptan and zolmitriptan on serotonin synthesis in the rat brain. Cephalalgia. 2004;24(1):2–11. doi: 10.1111/j.1468-2982.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986;332(1):1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Hamon M, Bourgoih S, Morot-Gaudry Y, Glowinski J. End product inhibition of serotonin synthesis in the rat striatum. Nature: New Biology. 1972;237:184–187. doi: 10.1038/newbio237184a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Watanabe A, Nishi K, Nguyen KQ, Diksic M. Selective 5-HT(1B) receptor agonist reduces serotonin synthesis following acute, and not chronic, drug administration: results of an autoradiographic study. Neurochemistry International. 2005a;46:261–72. doi: 10.1016/j.neuint.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Watanabe A, Nguyen KQ, Debonnel G, Diksic M. Chronic administration of citalopram in olfactory bulbectomy rats restores brain 5-HT synthesis rates: an autoradiographic study. Psychopharmacology. 2005b;179:781–790. doi: 10.1007/s00213-004-2122-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Nishi K, Watanabe A, Overstreet DH, Diksic M. Brain 5-HT synthesis in the Flinders Sensitive Line rat model of depression: an autoradiographic study. Neurochemistry International. 2006;48(5):358–66. doi: 10.1016/j.neuint.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Risch SC. Cholinomimetics and anticholinergic drugs used to investigate an acetylcholine hypothesis of affective disorders and stress. Drug Development Research. 1984;4:125–142. [Google Scholar]
- Kanemaru K, Hasegawa S, Nishi K, Diksic M. Acute citalopram has different effects on regional 5-HT synthesis in FSL, FRL, and SDP rats; an autoradiographic evaluation. Brain Research Bulletin. 2008 doi: 10.1016/j.brainresbull.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: An update. Pharmacology & Therapeutics. 1997;174:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiological Reviews. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Lapierre YD, Butter HJ. Agitated and retarded depression. A clinical psychophysiological evaluation. Neuropsychobiology. 1980;6:217–23. doi: 10.1159/000117755. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Research. 1992;587(2):181–5. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Vergé D, Gozlan H, Pichat L, Hamon M. Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Research. 1984;291:159–63. doi: 10.1016/0006-8993(84)90664-4. [DOI] [PubMed] [Google Scholar]
- Marcusson J, Oreland L, Winblad B. Effect of age on human brain serotonin (S-1) binding sites. Journal of Neurochemistry. 1984;43:1699–705. doi: 10.1111/j.1471-4159.1984.tb06098.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Matthews K, Overstreet DH, Koob GF, Geyer MA. Flinders resistant hypocholinergic rats exhibit startle sensitization and reduced startle thresholds. Biological Psychiatry. 1994;36:680–8. doi: 10.1016/0006-3223(94)91177-0. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29(12):2258–65. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Hutson PH. The 5-HT1B receptors. Annals of the New York Academy of Sciences. 1990;600:132–47. 347–48. doi: 10.1111/j.1749-6632.1990.tb16878.x. Review. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Fulton M, Kramer G, Petty F. A preliminary neuroendocrine study with buspirone in major depression. Neuropsychopharmacology. 1994;10:75–83. doi: 10.1038/npp.1994.9. [DOI] [PubMed] [Google Scholar]
- Møller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacology. 2007;32(8):1707–14. doi: 10.1038/sj.npp.1301310. [DOI] [PubMed] [Google Scholar]
- Nelson N. Presynaptic events involved in neurotransmission. Journal of Physiology (Paris) 1993;87(3):171–8. doi: 10.1016/0928-4257(93)90028-r. Review. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- O’Neil MF, Moore NA. Animal models of depression: Are there Any? Human Psychopharmacology: Clinical and Experimental. 2003;18:239–254. doi: 10.1002/hup.496. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Yamane F, Blier P, Diksic M. Effects of acute and chronic administration of the serotonin1A agonist buspirone on serotonin synthesis in the rat brain. Journal of Neurochemistry. 1999;72:2022–31. doi: 10.1046/j.1471-4159.1999.0722022.x. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J. Rodent models of aggressive behavior and serotonergic drugs. Progress in Neuropsychopharmacology and Biological Psychiatry. 1992;16(6):847–70. doi: 10.1016/0278-5846(92)90104-m. Review. [DOI] [PubMed] [Google Scholar]
- Österlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Brain Research Molecular Brain Research. 1999;74:158–66. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behavior Genetics. 2002;32(5):335–48. doi: 10.1023/a:1020262205227. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Daws LC, Schiller GD, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: implications for rat models of depression. Pharmacology Biochemistry and Behaviour. 1998;59:777–85. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathé AA, Yadid G. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neuroscience and Biobehavioural Reviews. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Stemmelin J, Griebel G. Confirmation of antidepressant potential of the selective beta3 adrenoceptor agonist amibegron in an animal model of depression. Pharmacology Biochemistry and Behaviour. 2008;89:623–6. doi: 10.1016/j.pbb.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Cortes R, Dietl MM. A laboratory guide for the in vivo labeling of receptors in tissue sections for radiography. In: Van Leeuwen FW, et al., editors. Molecular Neuroanatomy. Elsevier; Amsterdam: 1988. pp. 95–109. [Google Scholar]
- Palacios JM, Pazos A, Hoyer D. Characterization and mapping of 5-HT1A sites in the brain of animals and man. In: Dourish CT, Ahlenius S, Hutson PH, editors. Brain 5-HT1A Receptors. VCH; Chichester Horwood: 1987. pp. 65–81. [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proceedings of the National Academy of Sciences U S A. 1998;95:10734–9. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Research. 2002;954:173–82. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Patton PE, McNaughton B. Connection matrix of the hippocampal formation: I. The dentate gyrus. Hippocampus. 1995;5:245–86. doi: 10.1002/hipo.450050402. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Atlas in Stereotaxic Coordinates. 3. Academic Press; New York: 1997. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Research. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Deveault L, de Montigny C, Blier P. Effect of prolonged administration of tianeptine on 5-HT neurotransmission: an electrophysiological study in the rat hippocampus and dorsal raphe. Naunyn Schmiedebergs Archives of Pharmacology. 1995;351:119–25. doi: 10.1007/BF00169325. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Kostowski W, Archer T. Serotonin and depression: old problems and new data. Progress in Neuropsychopharmacology and Bioliogical Psychiatry. 1989;13(5):623–33. doi: 10.1016/0278-5846(89)90050-x. Review. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(24):14476–81. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Archives of General Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sato H, Skelin I, Debonnel G, Diksic M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: Assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Research Bulletin. 2008;75:545–555. doi: 10.1016/j.brainresbull.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Schiller GD. Altered behavioral sensitivity to serotonergic agonists in an animal model of depressive disorders: receptor binding correlates and cholineragic-serotonergic systems interaction. Journal of Neurochemistry. 1991;57:S138. [Google Scholar]
- Shayit M, Yadid G, Overstreet DH, Weller A. 5-HT(1A) receptor subsensitivity in infancy and supersensitivity in adulthood in an animal model of depression. Brain Research. 2003;980:100–8. doi: 10.1016/s0006-8993(03)02944-5. [DOI] [PubMed] [Google Scholar]
- Srinivas BN, Subhash MN, Vinod KY. Cortical 5-HT(1A) receptor downregulation by antidepressants in rat brain. Neurochemistry International. 2001;38(7):573–9. doi: 10.1016/s0197-0186(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacology Bulletin. 1994;30(1):39–43. Review. [PubMed] [Google Scholar]
- Takeda N, Diksic M, Yamamoto YL. The sequential changes in DNA synthesis, glucose utilization, protein synthesis, and peripheral benzodiazepine receptor density in C6 brain tumors after chemotherapy to predict the response of tumors to chemotherapy. Cancer. 1996;77:1167–79. doi: 10.1002/(sici)1097-0142(19960315)77:6<1167::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, Mück-Seler D, Diksic M. Acute flesinoxan treatment produces a different effect on rat brain serotonin synthesis than chronic treatment: an alpha-methyl-l-tryptophan autoradiographic study. Neurochemistry International. 2007;51(8):486–95. doi: 10.1016/j.neuint.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, Yamane F, Fikre Merid M, Blier P, Diksic M. Effects of serotonin receptors agonists, TFMPP and CGS12066B, on regional serotonin synthesis in the rat brain: an autoradiographic study. Journal of Neurochemistry. 2002;80(5):788–98. doi: 10.1046/j.0022-3042.2002.00757.x. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, Yamane F, Merid MF, Diksic M. Effects of selective 5-HT1A receptor antagonists on regional serotonin synthesis in the rat brain: an autoradiographic study with alpha-[14C]methyl-L-tryptophan. European Neuropsychopharmacology. 2001;11(3):193–202. doi: 10.1016/s0924-977x(01)00076-1. [DOI] [PubMed] [Google Scholar]
- van der Stelt HM, Breuer ME, Olivier B, Westenberg HGM. Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: An in vivo microdialysis study. Biological Psychiatry. 2005;57:1061–1067. doi: 10.1016/j.biopsych.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Marcinkiewicz M, Patey A, el Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. Journal of Neuroscience. 1986;6:3474–82. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Hasegawa S, Nishi K, Nguyen KQ, Diksic M. Chronic buspirone treatment normalizes regional serotonin synthesis in the olfactory bulbectomized rat brian: an autoradiographic study. Brain Research Bulletin. 2006;69:101–8. doi: 10.1016/j.brainresbull.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Tohyama Y, Nguyen KQ, Hasegawa S, Debonnel G, Diksic M. Regional brain serotonin synthesis is increased in the olfactory bulbectomy rat model of depression: an autoradiographic study. Journal of Neurochemistry. 2003;85:469–75. doi: 10.1046/j.1471-4159.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behavioral Pharmacology. 2002;13:169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Yadid G, Nakash R, Deri I, Tamar G, Kinor N, Gispan I, Zangen A. Elucidation of the neurobiology of depression: insights from a novel genetic animal model. Progress in Neurobiology. 2000;62:353–78. doi: 10.1016/s0301-0082(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamagata A. Serotonergic ligand binding in aging brain of experimental animals. Neurochemistry International. 1991;16(4):469–73. doi: 10.1007/BF00965568. [DOI] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155:434–9. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. Journal of Neurochemistry. 1997;69(6):2477–83. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]