Abstract
Abdominal obesity is associated with metabolic risk factors for coronary heart disease (CHD). Although we previously found that using liposuction surgery to remove abdominal subcutaneous adipose tissue (SAT) did not result in metabolic benefits, it is possible that postoperative inflammation masked the beneficial effects. Therefore, this study provides a long-term evaluation of a cohort of subjects from our original study. Body composition and metabolic risk factors for CHD, including oral glucose tolerance, insulin resistance, plasma lipid profile, and blood pressure were evaluated in seven obese (39 ± 2 kg/m2) women before and at 10, 27, and 84–208 weeks after large-volume liposuction. Liposuction surgery removed 9.4 ± 1.8 kg of body fat (16 ± 2% of total fat mass; 6.1 ± 1.4 kg decrease in body weight), primarily from abdominal SAT; body composition and weight remained the same from 10 through 84–208 weeks. Metabolic endpoints (oral glucose tolerance, homeostasis model assessment of insulin resistance, blood pressure and plasma triglyceride (TG), high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol concentrations) obtained at 10 through 208 weeks were not different from baseline and did not change over time. These data demonstrate that removal of a large amount of abdominal SAT by using liposuction does not improve CHD metabolic risk factors associated with abdominal obesity, despite a long-term reduction in body fat.
INTRODUCTION
Abdominal obesity is associated with metabolic risk factors for coronary heart disease (CHD), including insulin resistance, impaired oral glucose tolerance, dyslipidemia, and increased blood pressure (1). Diet-induced fat loss is recommended for obese patients who have these cardiometabolic risk factors, because even moderate (e.g., 10%) weight loss improves all risk factors simultaneously (1–3). Unfortunately, successful long-term weight management is difficult to achieve with lifestyle therapy alone (4), which has stimulated considerable interest in developing new, safe and effective obesity treatment options.
We previously evaluated the potential use of liposuction surgery as a therapeutic tool for treating persons who have abdominal obesity (5). Our data demonstrated that removal of large amounts of abdominal subcutaneous adipose tissue (SAT) (~10 kg) did not improve CHD risk factors or insulin sensitivity when subjects were evaluated ~10 weeks after the liposuction procedure was performed. However, it was suggested that liposuction-induced–adipose tissue inflammation could have obscured the detection of metabolic benefits in our subjects and that a longer time is needed to allow postprocedure inflammation to fully subside (6–9).
The purpose of the present study was to evaluate the hypothesis that large-volume liposuction has long-term beneficial effects on CHD risk that were missed at our 10–12 week postprocedure evaluation. Therefore, we conducted long-term longitudinal assessments of body composition and metabolic CHD risk in a subset of subjects who participated in our original study.
METHODS AND PROCEDURES
Subjects
Of the 15 women (47%) who participated in an earlier study that evaluated the short-term effects (~10 weeks) of large-volume liposuction on cardiovascular disease risk factors (5) seven women agreed to participate in this long-term follow-up evaluation. All subjects in the original study had abdominal obesity and a waist circumference > 100 cm. Subjects completed a comprehensive medical evaluation, which included a history and physical examination and standard blood and urine tests. All subjects provided written informed consent before participating in this the study, which was approved by the Human Studies Committee and the General Clinical Research Center Advisory Committee (GCRC) of Washington University School of Medicine in St. Louis.
Study design
Baseline assessments before liposuction
Subjects were admitted to the GCRC after they fasted overnight (12 h). A 2-h oral glucose- tolerance test was performed. A catheter was placed into an antecubital hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized blood samples. After baseline blood samples were obtained (time = 0) to determine plasma glucose, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride (TG) concentrations, subjects ingested 75 g of glucose. Blood samples were then taken at 30, 60, 90, and 120 min after glucose ingestion to determine plasma glucose concentrations.
Total body fat and fat-free mass were determined by using dual-energy X-ray absorptiometry (Delphi-W densitometer; Hologic, Waltham, MA). Abdominal SAT, visceral adipose tissue, and thigh adipose tissue masses were quantified by using magnetic resonance imaging (Siemens, Iselin, NJ). Eight, 10-mm thick, slice images were obtained at, and proximal to, the L4–L5 intervertebral space and the superior border of the medial condoyle of the tibia, and analyzed for subcutaneous and intracompartmental (abdomen or muscle) adipose tissue content (10).
Liposuction procedure
After all baseline evaluations were obtained, each subject underwent large-volume tumescent liposuction. Superficial and deep subcutaneous abdominal fat was primarily removed, but smaller amounts of fat were removed from the arms, flanks, hips, and thighs, to achieve additional cosmetic benefits. An average of 18 ± 2 l of Ringers Lactate plus epinephrine-infiltrated adipose tissue, containing ~10 kg of abdominal subcutaneous fat, was aspirated from each subject.
Assessments after liposuction
Subjects were instructed to resume their normal lifestyle after the initial recovery period and to weigh themselves weekly at home. Each subject was contacted via phone by one of the investigators, at least once every week until week 27 after liposuction, to reinforce the maintenance of their usual food intake and physical activity and to maintain a stable body weight. Body composition analyses, blood tests, and the oral glucose-tolerance test performed before liposuction were repeated at weeks 10 and 27 after liposuction and at a final evaluation between 84 and 208 weeks after liposuction.
sample analyses
Plasma glucose concentrations were determined by using a glucose analyzer (Yellow Springs Instrument, Yellow Springs, OH). Plasma total cholesterol, HDL-cholesterol, and TGs were determined enzymatically (Roche/Hitachi 747 Analyzer; Roche Diagnostics Corporation, Indianapolis, IN) by using commercially available kits; LDL-cholesterol was calculated by using the Friedewald equation (11).
Statistical analyses
A one-way analysis of variance with repeated measures, followed by Tukey’s least significant difference post hoc testing where indicated, was used to compare body composition and glucose and lipid concentrations between baseline and after liposuction. A P value ≤0.05 was considered statistically significant. All values are expressed as means ± s.e.m.
RESULTS
Body weight and composition
Body composition analyses at 10 weeks after the liposuction procedure demonstrated that liposuction caused a 9.4 ± 1.8 kg decrease in body fat (16 ± 2% of total fat mass), which resulted in decreases in body weight and BMI, without a significant change in fat-free mass (Table 1). In addition, abdominal SAT volume decreased by 23 ± 7% 10 weeks after liposuction, whereas visceral adipose tissue and thigh SAT volumes did not change. No subsequent changes in body weight, BMI, or any component of body composition occurred at subsequent analyses performed at 27 weeks and between 84 and 208 weeks after liposuction.
Table 1.
Body composition and metabolic characteristics of study participants before and after liposuction
| After liposuction |
||||
|---|---|---|---|---|
| Before liposuction | 10 Weeks | 27 Weeks | 84–208 Weeks | |
| BMI (kg/m2) | 39 ± 2 | 36 ± 2a | 36 ± 2a | 36 ± 2a |
| Weight (kg) | 108 ± 5 | 101 ± 5a | 102 ± 4a | 101 ± 4a |
| Fat mass (kg) | 59 ± 4 | 49 ± 3a | 51 ± 3a | 52 ± 3a |
| Abdominal SAT (cm3) | 3,793 ± 281 | 2,875 ± 223a | 2,844 ± 339a | 3,178 ± 266a |
| VAT (cm3) | 1,736 ± 389 | 1,604 ± 268 | 1,608 ± 234 | 1,716 ± 245 |
| Thigh SAT (cm3) | 1,846 ± 234 | 1,797 ± 229 | 1,888 ± 200 | 1,813 ± 188 |
| Systolic blood pressure (mm Hg) | 124 ± 5 | 132 ± 4 | 122 ± 5 | 133 ± 10 |
| Diastolic blood pressure (mm Hg) | 70 ± 3 | 67 ± 5 | 65 ± 2 | 72 ± 5 |
| LDL-cholesterol (mg/dl) | 109 ± 14 | 110 ± 13 | 117 ± 12 | 95 ± 13 |
| Triglyceride (mg/dl) | 120 ± 29 | 94 ± 11 | 116 ± 10 | 108 ± 6 |
| HDL-cholesterol (mg/dl) | 48 ± 4 | 50 ± 6 | 44 ± 5 | 42 ± 5 |
| Glucose (mg/dl) | 117 ± 16 | 105 ± 11 | 117 ± 18 | 106 ± 10 |
| Glucose AUC during 2-h OGTT (mg/dl × 120 min) | 22,390 ± 3,079 | 20,509 ± 2,177 | 22,448 ± 3,484 | 20,857 ± 2,361 |
| HOMA-IR | 2.6 ± 0.5 | 3.0 ± 0.7 | 3.1 ± 1.0b | 2.7 ± 0.7c |
Values are means ± s.e.m.
AUC, area under the curve; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; OGTT, oral glucose-tolerance test; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Value significantly different from corresponding before liposuction value, P < 0.05.
Data available for only five subjects.
Data available for only six subjects.
CHD risk factors
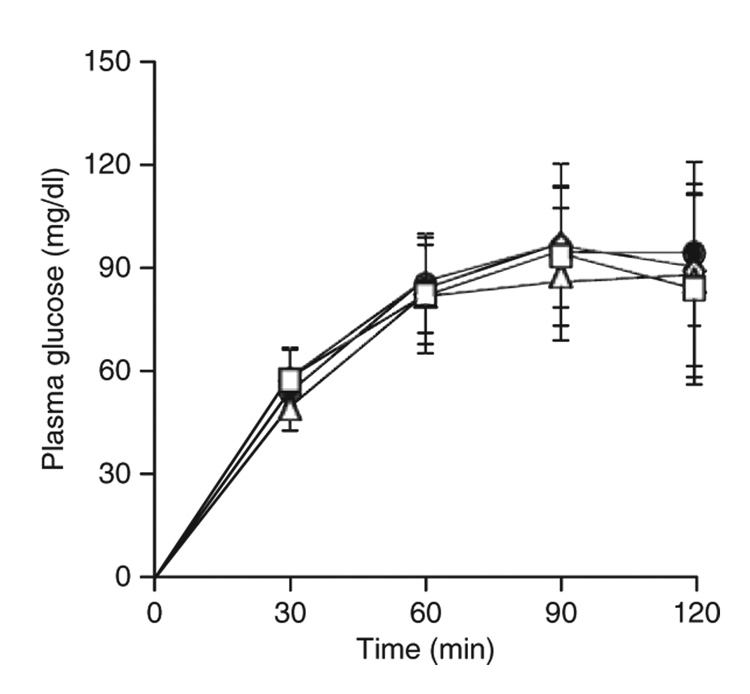
At baseline, four subjects had normal oral glucose tolerance and three had type 2 diabetes that was being treated with oral hypoglycemic agents only. Liposuction did not alter blood pressure, or plasma LDL-cholesterol, TG, HDL-cholesterol, and fasting glucose concentrations throughout the duration of the study (Table 1). In addition, plasma glucose concentrations during the oral glucose-tolerance test at 10, 27, and 84–208 weeks after liposuction were similar to values obtained before liposuction (Figure 1).
Figure 1.
Plasma glucose concentrations during a 2-h oral glucose-tolerance test obtained before (filled circle) and 10 (open triangle), 27 (open diamond), and 84–208 (open square) weeks after large-volume liposuction. Plasma glucose concentrations are absolute values above baseline (time 0).
Changes in medications
During the period between week 84 and week 208 of this study, treatment with medications that affect plasma glucose or lipoproteins was changed in some subjects as directed by their primary physician. Three subjects who had diabetes experienced changes in medications: One began treatment with glimepiride and glargine insulin; one began treatment with glargine insulin and concomitantly decreased pioglitazone from 45 to 30 mg/day; and one decreased glucovance treatment from t.i.d. to b.i.d. Two subjects started statin therapy (pravastatin and rosuvastatin), and one required an increased dose of pravastatin (40–80 mg). Among subjects, who did not have diabetes, one started treatment with pravastatin and olmesartan.
DISCUSSION
In this study, we evaluated the long-term effect of removing large amounts of SAT on body composition and CHD risk factors. Our data demonstrate that even though more than a 10% reduction in total body fat mass (~7% reduction in body weight) was maintained for 1.5–4 years after liposuction, oral glucose tolerance, blood pressure, plasma TG, and HDL- and LDL-cholesterol concentrations did not change. These long-term results are consistent with our findings obtained 10 weeks after large-volume liposuction (5) and make it unlikely that persistent postsurgical inflammation masked the metabolic benefits of liposuction that we reported previously. The data from the present study and our earlier study demonstrate that liposuction does not result in short-term or long-term improvement in most of the key metabolic risk factors for CHD.
The absence of a metabolic therapeutic effect in our subjects is strikingly different than the outcome expected from similar or even smaller decreases in body fat induced by diet therapy. Small long-term reductions in body weight (fat) improve glucose tolerance and the other metabolic CHD risk factors evaluated in the present study (2,3,12–14). Our results suggest that decreasing body fat by generating a negative energy balance, which decreases fat-cell size, intra-abdominal fat, and ectopic fat (e.g., intrahepatic and intramyocellular TG content), is necessary to achieve the metabolic benefits of weight loss. Surgical aspiration of fat can improve physical appearance and function, but not the CHD metabolic risk factors associated with obesity, even after a long-term reduction in body fat. However, the results from our study cannot exclude the possibility that removing larger amounts of body fat or fat from different locations would have resulted in metabolic improvements.
Weight regain is common after diet-induced weight loss (15). In contrast, our subjects maintained their body weight and body composition for years after liposuction-induced fat and weight loss. Several factors could have been responsible for weight and body fat maintenance in our study subjects. First, obese subjects who lose weight by dieting experience a decrease in body mass of multiple tissues and an obligate decrease in energy expenditure (16,17). It is difficult for many people to sustain the lifestyle changes in energy intake and physical activity needed to prevent an increase in body size back toward their baseline. Although we did not measure energy expenditure, it is unlikely that weight loss in our subjects had an important effect on energy metabolism because the loss in weight was completely due to aspiration of body fat, which has low energy requirements. Therefore, our subjects did not need to change their lifestyle habits to either achieve or maintain their fat loss. Second, removal of the large panniculus of subcutaneous abdominal fat in our subjects likely improved their ability to ambulate and be more physically active. Third, our subjects experienced considerable cosmetic benefits after liposuction, which likely improved their self-esteem and reinforced their desire not to gain weight. This observation raises the possibility that body image should be used as an incentive within the framework of behavioral therapy for obesity. Fourth, it is possible that the subjects who failed to achieve long-term weight and fat loss refused to participate in the scheduled follow-up study visits. Therefore, our study population would have been biased by only including those who had a successful weight outcome.
Liposuction surgery removes billions of adipocytes from selected adipose tissue depots and disrupts the connective tissue framework that supports adipocytes and other adipose tissue cells. Therefore, it is possible that weight (fat) gain after liposuction could have adverse cosmetic and metabolic effects if newly formed TGs are unable to accumulate in the aspirated areas and are redirected to other sites. However, the effect of liposuction on fat distribution and metabolic outcomes after subsequent weight gain in humans is not clear. Data from studies conducted in lipectomized animal models demonstrate that regeneration of the removed fat pad is rare, but compensatory fat accumulation at other sites commonly occurs (18). Information obtained from case reports in patients is consistent with the findings in animals, and suggest that weight gain after liposuction results in an increase in subcutaneous fat in areas that were not aspirated, such the back and breasts (19–21). In addition, it is possible that the removal of subcutaneous fat by liposuction will enhance ectopic fat deposition in other organs, such as the liver and skeletal muscle, which is associated with insulin resistance and inflammation (22,23). Additional studies are needed to determine the long-term effects of large-volume liposuction in patients who gain weight.
In summary, surgical aspiration of a large amount of abdominal SAT does not improve CHD metabolic risk factors associated with abdominal obesity, despite a long-term reduction in body fat. The absence of a therapeutic effect suggests that losing weight by inducing a negative energy balance, not by decreasing adipose tissue mass alone, is needed to achieve the metabolic benefits of weight loss.
ACKNOWLEDGMENTS
We thank the nursing staff of the General Clinical Research Center for their help in performing the studies, Freida Custodio for her technical assistance, and the study subjects for their participation. This study was supported by National Institutes of Health Grants DK 37948, RR-00036 (General Clinical Research Center), and DK 56341 (Clinical Nutrition Research Unit).
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:S51–S209. [PubMed]
- 2.Wing RR, Koeske R, Epstein LH, et al. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med. 1987;147:1749–1753. [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Arch Intern Med. 2001;161:218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- 5.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano G, Nicoletti G, Grella E, et al. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg. 2004;57:190–194. doi: 10.1016/j.bjps.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea F, Grella R, Rizzo MR, et al. Changing the metabolic profile by large-volume liposuction: a clinical study conducted with 123 obese women. Aesthetic Plast Surg. 2005;29:472–478. doi: 10.1007/s00266-005-0089-x. discussion 479–481. [DOI] [PubMed] [Google Scholar]
- 9.Esposito K, Giugliano G, Giugliano D. Metabolic effects of liposuction—yes or no? N Engl J Med. 2004;351:1354–1357. doi: 10.1056/NEJM200409233511320. author reply 1354–1357. [DOI] [PubMed] [Google Scholar]
- 10.Yarasheski KE, Tebas P, Claxton S, et al. Visceral adiposity, C-peptide levels, and low lipase activities predict HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285:E899–E905. doi: 10.1152/ajpendo.00036.2003. [DOI] [PubMed] [Google Scholar]
- 11.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 13.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 15.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13 Suppl 2:39–46. [PubMed] [Google Scholar]
- 16.Nelson KM, Weinsier RL, James LD, et al. Effect of weight reduction on resting energy expenditure, substrate utilization, and the thermic effect of food in moderately obese women. Am J Clin Nutr. 1992;55:924–933. doi: 10.1093/ajcn/55.5.924. [DOI] [PubMed] [Google Scholar]
- 17.Amatruda JM, Statt MC, Welle SL. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993;92:1236–1242. doi: 10.1172/JCI116695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 19.Yun PL, Bruck M, Felsenfeld L, Katz BE. Breast enlargement observed after power liposuction: a retrospective review. Dermatol Surg. 2003;29:165–167. doi: 10.1046/j.1524-4725.2003.29041.x. discussion 167. [DOI] [PubMed] [Google Scholar]
- 20.van der Lei B, Halbesma GJ, van Nieuwenhoven CA, van Wingerden JJ. Spontaneous breast enlargement following liposuction of the abdominal wall: does a link exist? Plast Reconstr Surg. 2007;119:1584–1589. doi: 10.1097/01.prs.0000256069.54596.0b. [DOI] [PubMed] [Google Scholar]
- 21.Yost TJ, Rodgers CM, Eckel RH. Suction lipectomy: outcome relates to region-specific lipoprotein lipase activity and interval weight change. Plast Reconstr Surg. 1993;92:1101–1108. discussion 1109–1111. [PubMed] [Google Scholar]
- 22.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]