Abstract
Background
Despite surveillance efforts, unexpected and serious adverse drug reactions (ADRs) repeatedly occur after marketing. The aim of this article is to analyse ADRs reported by available ADR signal detection approaches and to explore which information about new and unexpected ADRs these approaches have detected.
Methods
We selected three therapeutic cases for the review: antibiotics for systemic use, non-steroidal anti-inflammatory medicines (NSAID) and selective serotonin re-uptake inhibitors (SSRI). These groups are widely used and represent different therapeutic classes of medicines. The ADR studies were identified through literature search in Medline and Embase. The search was conducted in July 2007. For each therapeutic case, we analysed the time of publication, the strengths of the evidence of safety in the different approaches, reported ADRs and whether the studies have produced new information about ADRs compared to the information available at the time of marketing.
Results
79 studies were eligible for inclusion in the analysis: 23 antibiotics studies, 35 NSAID studies, 20 SSRI studies. Studies were mainly published from the end of the 1990s and onwards. Although the drugs were launched in different decades, both analytical and observational approaches to ADR studies were similar for all three therapeutic cases: antibiotics, NSAIDs and SSRIs. The studies primarily dealt with analyses of ADRs of the type A and B and to a lesser extent C and D, cf. Rawlins' classification system. The therapeutic cases provided similar results with regard to detecting information about new ADRs despite different time periods and organs attacked. Approaches ranging higher in the evidence hierarchy provided information about risks of already known or expected ADRs, while information about new and previously unknown ADRs was only detected by case reports, the lowest ranking approach in the evidence hierarchy.
Conclusion
Although the medicines were launched in different decades, approaches to the ADR studies were similar for all three therapeutic cases: antibiotics, NSAIDs and SSRIs. Both descriptive and analytical designs were applied. Despite the fact that analytical studies rank higher in the evidence hierarchy, only the lower ranking descriptive case reports/spontaneous reports provided information about new and previously undetected ADRs. This review underscores the importance of systems for spontaneous reporting of ADRs. Therefore, spontaneous reporting should be encouraged further and the information in ADR databases should continuously be subjected to systematic analysis.
Background
The thalidomide catastrophe around 1960 and additional experiences such as serious adverse drug reactions to high oestrogen oral contraceptives in the 1960s were probably the main reasons for the increasingly stringent requirements set to document development safety and the establishment of spontaneous reporting systems [1,2]. Over the years, the repeated occurrence of unexpected, serious adverse drug reactions (ADRs) has attracted wide professional and public attention, with the result that doubt has been cast on the effectiveness and quality of drug safety surveillance systems. The COX-2 scandal resulting in worldwide withdrawal of Vioxx® (rofecoxib) from the market in 2004 is a recent example of an ADR case that emerged unexpectedly and took the world by surprise [3]. Several other ADR cases have been discovered after marketing; well known are fenfluramine and the risk of pulmonal hypertension, vigabatrine and visual field defects and tolcapone and the risk of liver toxicity [4-6]. The repeated occurrence of serious ADR cases after medicines have been released on the market questions the extent to which existing systems and methods for predicting ADRs are effective [7]. Information about the ADR profile of a new medicine appears from observations made during the clinical development process [8,9]. The gold standard for the design of these clinical trials is the randomised controlled clinical trial (RCCT) [8,9]. The RCCT was designed to measure efficacy rather than ADRs as outcome. The design of the RCCT as hypothesis testing in itself sets narrow limits for the detection of information about serious and unexpected ADRs due to the short treatment period, the relatively small number of carefully selected participants in the trial, fixed drug doses, and hospital settings that do not reflect the conditions under which the medicines are used after marketing [8,9]. Data on well-recognised, easily detectable ADRs may potentially be observed in RCCTs, but unknown, rare or long-term adverse effects are seldom detected in these trials due to the limitations of the RCCT. Detection of unknown or rare ADRs may include other pharmacovigilance designs, e.g. the spontaneous reporting systems, cohort or case-control studies [1,10-12]. This article aims to review ADRs reported by available ADR signal detection approaches and to explore which information about new and unexpected ADRs these approaches have detected.
Methods
We selected three different therapeutic groups of medicines for review. The groups were characterised by different:
a. Therapeutic groups
• Antibiotics for systemic use
• Non-steroidal anti-inflammatory drugs (NSAIDs)
• Selective serotonin re-uptake inhibitors (SSRIs)
b. Market launch
Antibiotics were first marketed in the 1940s and NSAIDs in the 1960s, while SSRIs were not launched until the middle of the 1980s (internal documents, The Danish Medicines Agency).
c. ADR profiles
The therapeutic categories present different ADR profiles due to their specific pharmacological characteristics and functions.
Literature search
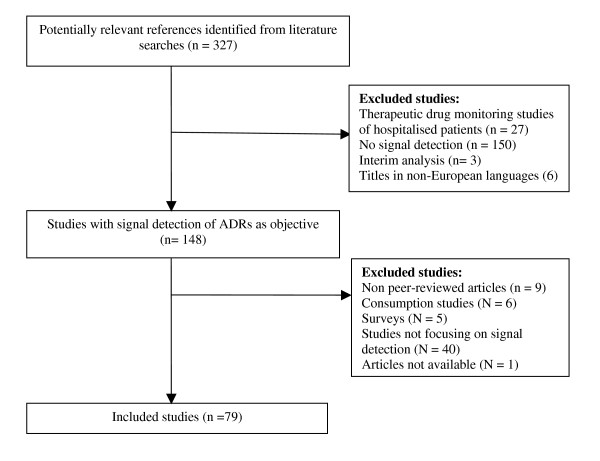
Studies were identified through Medline (from 1966) and Embase (from 1989) using the following MESH terms: serotonin re-uptake inhibitors, anti-inflammatory agents, non-steroidal, anti-bacterial agents, adverse drug reaction reporting systems, pharmacoepidemiology and the key words: adverse drug reactions and information in combination. The literature search was conducted in July 2007 without language restriction. Studies written in non-European languages were later excluded. To be considered relevant for this review, articles had to be empirical in origin and focus on signal detection. Titles and abstracts of the search results were screened and relevant articles identified. The reference lists of included publications were hand-searched for possible additional relevant studies. Non peer-reviewed articles or unpublished observations were not considered. A flow chart of the study selection process for the therapeutic cases is illustrated in figure 1.
Figure 1.
Flow chart of the study selection process for the cases.
Characteristics of the included studies
We developed a taxonomy inspired by general guidelines for pharmacoepidemiological research to analyse the studies systematically [13]. The taxonomy covers the following characteristics: publication year, design, method, explored medicine and adverse drug reactions, geographic setting, sampling period, sample size, outcome measures and results. We extracted and compared the results of published empirical studies in which various signal detection methods were used. Extracted information was entered into data sheets, one for each article. Data were extracted and handled by the first author and checked by the second author.
Analyses
For each of the three selected therapeutic groups, we analysed the time of publication, the strengths of the evidence in the different approaches, reported ADRs and whether the studies had produced new information about ADRs compared to the information available at the time of marketing.
Classification of the tested/detected ADRs
For each included literature reference, the ADRs tested or detected via the various signal detection approaches were classified according to Rawlins' classification system [14]. An overview of the classification system is shown in table 1. The reported/detected ADRs were also classified according to System Organ Classes in keeping with MedDRA terminology [13].
Table 1.
Rawlins' classification system of ADRs
| Type | Definition |
| A | Dose-dependent ADRs related to the pharmacological effect of the drug: |
| • Increased pharmacological effect | |
| • ADRs that occur secondarily to the desired pharmacological effect | |
| • ADRs due to other well known pharmacological effects | |
| B | Sensitivity reactions – not dose-dependent |
| • Allergic reactions | |
| • Idiosyncratic reaction | |
| C | Long-term ADRs |
| • Carcinogines | |
| • Teratogenes | |
| • Chronic organ damage | |
| D | Drug-drug interactions |
| • Pharmacodynamic | |
| • Pharmacokinetic | |
| • Non-classifiable | |
Classification of applied approaches
The explored approaches were classified into analytical or observational approaches according to Strom's definitions [13]. Case-control and cohort studies are classified as analytical methods, while spontaneous reporting, case series/case reports and PEM studies are observational [13].
Time of publication
For each therapeutic group, we analysed whether there was a connection between time of publication and the applied study design.
Strength of evidence
Evidence-based medicine operates with an evidence hierarchy for evaluating the quality of the various study designs used for therapeutic studies [13]. At the top of this hierarchy are the meta-analyses (level 1), followed by RCCTs at the second level and other controlled trials at the third level. Cohort studies are placed at the fourth level, followed by case-control studies at the fifth level. At the bottom of the evidence hierarchy are cross-sectional surveys (level 6) and anecdotal case reports (level 7) [13].
Results
The literature search identified 327 potentially relevant references for all three therapeutic groups, 149 of which were selected from the titles and abstracts and further screened for relevance. Eventually 79 references were included in this analysis. A flow chart of the selection and exclusion process is illustrated in figure 1. The included studies were distributed on the three therapeutic cases as follows: antibiotics: 23 studies; NSAID: 35 studies; SSRI: 20 studies. One reference was not accessible.
ADR detection approaches applied
Table 2 provides an overview of the categorisation of the designs used in the included studies and their rank in the evidence hierarchy [13]. As the table indicates, the majority of the included studies dealt with analyses of data reported in Prescription Event Monitoring (PEM) programs and ADRs reported to national ADR databases, approaches ranking at levels six and seven in the evidence hierarchy.
Table 2.
The analysed studies categorised by study design
| Study design | Rank in evidence hierarchy |
Therapeutic cases |
|||
| Antibiotics | NSAIDs | SSRIs | Total | ||
| Cohort | 4 | 5 | 4 | 1 | 10 |
| Case control | 5 | 2 | 3 | 2 | 8 |
| PEM* | 6 | 2 | 9 | 4 | 15 |
| National ADR databases | 7 | 7 | 14 | 11 | 32 |
| Case series | 7 | 2 | 3 | 1 | 6 |
| Case reports | 7 | 5 | 2 | 1 | 8 |
| Total number of studies | 23 | 36 | 20 | 79 | |
*Prescription Event Monitoring Studies
Study characteristics
Tables 3, 4 and 5 display the characteristics and descriptions of the analysed studies for each therapeutic case [15-92]. The tables show that the studies primarily dealt with analyses of ADRs of the type A and B, and to a lesser extent C and D. The evidence level of ADRs varied widely; some of the ADRs were documented in both the analytical and observational studies, others in only one of the designs.
Table 3.
Characteristics of studies of the occurrence of ADRs related to antibiotics use
| Reference | Setting | Medicines | ADRs | Sampling period | Sample size |
Outcome measures |
Results (95% CI) |
Type of ADRs |
| Case control studies | ||||||||
| Czeizel 1999 [15] | HU | Erythromycin | Teratology | 1980–1996 | 113 cases/38,151 controls | OR | 1.1; 0.5–2.3 | C |
| Seeger 2006 [16] | Fluoroquinolones | Achilles tendon rupture | 1997–2001 | 947 cases/ 18,940 controls |
OR | 1.2; 0.9–1.7 | B | |
| Cohort studies | ||||||||
| Chysky 1991[17] | DE | Ciprofloxacin | Not specified | 44 days | 634 patients | % ADRs | Different categories reported | A/B |
| Derby 1993 [18] | AU | Flucloxacillin | Cholestatic hepatitis | 45 days | 132,087 patients | PRR/100,000 users | 7.6; 3.5–13.9 | B |
| Jick 1994 [19] | AU | Flucloxacillin | Cholestatic hepatitis | 1991–1992 | 77,552 patients | PRR/ 100,000 users |
6.5; 2.7–15.1 | B |
| Derby 1993b [20] | AU | Erythromycin | Cholestatic hepatitis | - | 366,064 patients | PRR/100,000 users | 3,6; 1.9–6.1 | B |
| Heymann 2007 [21] | Israel | Penicillins | Pemphigus | 1997–2001 | 150,000 patients | OR | 2.03; 1.56–2.64 | B |
| PEM | ||||||||
| Clark 2001 [22] | UK | Fluoroquinolones | Cardiovascular events | 1988–1991 | 36,410 patients | CRR (crude relative risk) | Atrial fibrillation: 1.0; 0.02 – 8.92 | B |
| Inman1994 [23] | UK | Fluconazole | All | 1988–1989 | 15,015 patients | Frequencies | Different categories reported | A |
| National ADR databases | ||||||||
| Polimeni 2006 [49] | Sicilian | Antibacterials | All | 1998–2002 | 1585 cases | ADRs | Different categories reported | A |
| Sachs 2006 [24] | DE | Fluoroquinolones | Anaphylaxis | 1993–2004 | 204 cases | PRR > 2 | Moxifloxacin: 2.1; Ofloxacin: 2.3 Ciprofloxacin: 2.3 Levofloxacin: 2.0 |
B |
| Fleisch 2000 [25] | CH | Levofloxacin | Tendinopathy | 1986–1999 | 19 cases/460 non-cases | Reporting rate | Different categories reported | B |
| Leone 2003 [26] | IT | Fluroquinolones | Not specified | 1999–2001 | 432 cases/ 10,011 non cases |
Reporting rate | Different categories reported | A |
| Pierfitte 2000 [27] | FR | Sparfloxacin | Phototoxicity | 1994–1996 | 371 cases | RtR/1000 patients | 0.4 | B |
| Frothingham 2005 [28] | US | Gatifloxacin | Glucose homeostatis abnormalities | 1997–2003 | 453 cases/ 1427 non cases |
Reporting rate/107 prescriptions | 477 | A |
| Hedenmalm 1996 [29] | SE | Fluorquinolones | Sensory disturbances | 1965–1993 | 37 cases | ADRs | Different categories reported | A |
| Case series | ||||||||
| Abouesh 2002 [30] | - | Fluorquinolones Macrolides |
Mania | - | 102 cases | Case review | Case review | B |
| Smith 2005 [31] | - | Doxycycline Minocycline |
ADRs | 1966–2003 | 130 cases | Incidences | Doxycycline: 0–61% Minocycline: 11.7 – 83.3% |
A |
| Case reports | ||||||||
| Hällgren 2003 [32] | - | Ciprofloxacin | Steven-Johnson syndrome | 1988–2000 | 8 cases | IC pr. 100,000 patients | 0.045 | B |
| Warner 2000 [33] | - | Clarithromycin | Acute Psychotic Stress | - | 1 case | Causality assessment | Possible | A |
| ADRAC 1992 [34] | - | Flucloxacillin | Cholestatic hepatitis | - | 1 case | Case review | Case review | B |
| Greco 1997 [35] | - | Clarithromycin | Glossitis, stomatitis, black tongue | - | 1 case | Case review | Case review | B |
| Björnsson 1996 [36] | - | Doxycycline | Liver reactions | 1966–1995 | 23 cases | Causality assessment | Likely (n = 3) Possible (n = 8) |
B |
Table 4.
Characteristics of studies of the occurrence of ADRs related to NSAID use
| Reference | Setting | Medicines | ADRs | Sampling period | Sample size | Outcome measures |
Results (95%CI) |
Type of ADRs |
|
Case control studies |
||||||||
| Hernandez-Diaz 2001[37] | UK | NSAIDs | Gastrointestinal events | 1993–1998 | 2,105 cases/ 11,500 controls |
OR | 1.8; 1.3 – 2.4. | A |
| Mockenhaupt 2003 [38] | DE/US | NSAIDs | Steven-Johnson syndrome | 1989–1995 | 245 cases/ 1147 controls |
PRR | 34, 95; 11–105 | B |
| Lacroix 2004 [39] | FR | NSAIDs | Liver injury | 1998–2000 | 88 cases/ 178 controls |
OR | Women: 6.49; 1.67–25.16 Men: 1.06; 0.36–3.12 |
B |
| Cohort studies | ||||||||
| Lipworth 2004 [40] | DK | Ibuprofen | Mortality | 1989–1995 | 113,538 patients |
SMR (standard mortality rate) | 1.21; 1.19–1.24 | A/B |
| Ashworth 2004 [41] | CA | Diclofenac Naproxen Arthrotec |
Mortality | 1991–1994 | 18,424 patients | OR | Arthrotec: 1.4; 0.9–2.1. Diclofenac: 2.0; 1.3–3.1. Naproxen: 3.0; 1.9–4.6 |
A/B |
| Morant 2004 [42] | UK | NSAIDs | Gastrointestinal haemorrhage | 1987–2001 | 628000 patient year | PRR | 0.84; 0.60 – 1.17 | A |
| Martin 2000 [43] | UK | Meloxicam | Gastrointestinal events | 1996–1997 | 19,087 patients | Events/ 1000 patient-months of exposure |
Dyspepsia: 28.3 Gastrointestinal haemorrhage: 0.4 |
A + B |
| National ADR databases | ||||||||
| Lugardon 2004 [44] | FR | COX-2 inhibitors | Oeso-gastro-duodenal events: | 2000–2002 | 505 cases/ 2,525 non-cases |
OR | 14.9; 9.3–23.7 | A |
| Durrieu 2005 [45] | FR | COX-2 inhibitors | Arterial hypertension | 2000–2003 | 34 cases | OR | 3.3; 1.6–6.9. | A |
| Clinard 2004 [46] | FR | NSAIDs | Excess risk of adverse drug reactions | 1995–1999 | 3983 cases/ 54,583 non- cases |
OR | Different categories reported | B |
| Brinker 2004 [47] | US | COX-2 inhibitors | Hypertension | < 2002 | 34 cases | Reporting rate/106person years | Rofecoxib: 5.0 Celecoxib: 1.3 |
A |
| La Grenade 2005 [48] | US | COX-2 inhibitors Meloxicam |
Steven-Johnson syndrome Toxic Epidermal Necrolysis |
< 2004 | 123 cases | Reporting rate/106person years | Valdecoxib: 49 Celecoxib: 6 Rofecoxib: 3 |
B |
| Polimeni 2006 [49] | Sicilian | NSAIDs | All | 1998–2002 | 1585 cases | PRR | Hepatitis: 14.20 Vasculitis: 7.72 Hypertension: 15.40 |
B |
| Conforti 2001 [50] | IT | NSAIDs | Gastrointestinal events | 1996–1999 | 705 cases/ 10,608 non cases |
% ADRs | Nimesulid: 10.4 Diclofenac: 21.2 Ketoprofen: 1.7 Piroxicam: 18.6 |
A |
| Ahmad 2002 [51] | US | COX-2 inhibitors | Renal failure | 1969–2000 | Celecoxib: 122 cases Rofecoxib: 142 cases |
Case review | Case review | A |
| Puijenbroek 2000 [52] | NL | NSAIDs Diuretics |
Drug interactions | 1990–1999 | 305 cases/ 9517 non cases |
OR | OR: 2.0, 1.1–3.7 | D |
| Lapeyre-Mestre 2004 [53] | FR/ES | NSAIDs | Hepatic events | 1982–2001 | 29,486 cases | OR | Different OR calculated for NSAIDs. | B |
| Leone 1999 [54] | IT | Nimesulide | Renal impairment | 1988–1997 | 11cases/ 7438 non cases |
Causality assessment | Possible (n = 6) Probable (n = 4) Certain (n = 1) |
A |
| Brown 1998 [55] | UK | Tiaprofenic acid | Cystitis | 1981–1996 | 221 cases/ 1327 non cases |
ADRs/105 prescriptions | 1991: 4.2 1992: 5.9 1993: 4.2 1994: 34.4 1995: 18.5 1996: 6.5 |
B |
| Verrico 2003 [56] | US | COX2-inhibitors | Not specified | 1999–2002 | 24 cases | Causality assessment | Possible (n = 29) Probable (n = 16) |
A |
| Kahn 1997 [57] | US | NSAIDs | Necrotizing soft tissue infections | 1969–1995 | 33 cases | Case review | N = 26 | C |
| PEM | ||||||||
| Layton 2004a [58] | UK | Celecoxib | Not specified | 2000 | 17,458 patients | IDs (event incidence densitites) | Dyspepsia = 25.4 Abdominal pain = 10.6 |
A + B |
| Layton 2003b [59] | UK | Celecoxib Meloxicam |
Not specified | 1996–1997 | 34,355 patients | PRR | Different categories reported | A |
| Layton 2003c [60] | UK | Rofecoxib | Not specified | 2000 | 15,268 patients | Event rate pr. 1000 patient months exposure | 76 upper GI bleedings and 101 thromboembolic events | A + B |
| Layton 2004d [61] | UK | Rofecoxib | Exacerbation of colitis | 1999 | 15,268 patients | IRR | 5.8; 2.7–11.3 | A |
| Kasliwal 2005 [62] | UK | COX-2 inhibitors | Gastrointestinal + thromboembolic events |
1999–2000 | 32,726 patients | PRR | GI: 1.21; 1.09 – 1.36. Thromboembolic: 1.04; 0.50 – 2.17. |
A + B |
| Layton 2003e [63] | UK | Rofecoxib Meloxicam |
Thromboembolic events | 1996–1997 | 34,355 patients | PRR | 1.68; 1.15 – 2.46. | A |
| Layton 2003f [64] | UK | Rofecoxib Meloxicam |
Upper GI events | 1996–1997 | 34,355 patients | IR | 0.71; 0.65 – 0.79. | A |
| Layton 2006g [65] | UK | COX-2 inhibitors | Serious skin reactions | 1999–2000/ | 52,644 patients | IR/1000 patient-months | IR: 0.019 | B |
| Layton 2003h [66] | UK | Celecoxib Meloxicam |
Gastrointestinal events | 1996–1997 | 36,545 patients | PRR | 0.77; 0.69 – 0.85. | A |
| Case series | ||||||||
| Onder 2004 [67] | - | NSAIDs | Psychiatric ADRs | 1965–2003 | 27 reports with data on 453 cases | Risk factors | Age, psychiatric disorders, parturients | B |
| Fraunfelder 2006 [68] | - | NSAIDs | Ocular ADRs | - | 569 cases | Reported ADRs | Blurred vision, conjunctivitis, visual hallucinations | B |
| Zimer 2007 [69] | DE | Valdecoxib | Cutaneous adverse reactions | 2002–2005 | 5 cases | Case review | Erythematous, facial edema, dyspnea | B |
| Case reports | ||||||||
| Hunter 1999 [70] | - | Bromfenac | Hepatic Failure | - | 1 case | Causality assessment | Related | B |
| ADRAC 1998 [71] | - | Diclofenac Indomethacin Mefenamic acid |
Closure of fetal ductus arterious | - | 3 cases | Case review | Case review | C |
Table 5.
Studies of the occurrence of ADRs related to SSRI use
| Reference | Setting | Medicines | ADRs | Sampling period | Sample size | Outcome measures |
Results (95% CI) |
Type of ADRs |
| Case-control studies | ||||||||
| Schillevoort 2002 [72] | NL | SSRIs | Extrapyramidal Syndromes (EPS) |
1985–1999 | 41cases/1,264 controls | OR | 2.2; 1.2–3.9 | A |
| Movig 2002 [73] | NL | SSRIs | Hyponatraemia | 1990–1998 | 203 cases/608 controls | OR | 3.96; 1.33 – 11.83 | A |
| Cohort study | ||||||||
| Bell 2006 [74] | US | Fluoxetine | Testosterone levels | - | 14 patients | Testosterone level | No changes | B |
| National ADR databases | ||||||||
| Trenque 2002 [75] | FR | SSRIs | Withdrawal syndrome | < 2000 | 60 cases/166,327 non cases | OR | 5.05, 3.81–6.68. | A |
| Gony 2003 76] | FR | SSRIs | Extrapyramidal Symptoms |
1995–2000 | 9 cases | OR | 2.18; 0.47–11.35 | A |
| Hedenmalm 2006 [77] | SE | SSRIs | Alopecia | < 2004 | 27 cases | IC | Sertraline = 1.63, 0.85–2.41 Citalopram = 1.22, 0.97–1.47 |
B |
| Goldstein 1997 [78] | - | Fluoxetine | First-trimester exposure on newborns | < 1996 | 796 cases | Rate % | 5.0 | C |
| Spigset 2003 [79] | SE | Nefazodone | Hepatic injury | < 2002 | 27,542 cases/ 2830764 non cases |
IC | 0.42, 0.12–0.72 | B |
| Khan 2003 [80] | US | SSRIs | Suicide | 1985–2000 | 77 cases/48,277 non cases |
Suicide rate | 0.59, 0.31 – 0.87 | A |
| Egberts 1997 [81] | NL | SSRIs | Non-puerperal lactation | 1986–1996 | 38cases/14,439 non cases | OR | 2.7; 6.4–25.4 | A |
| Kvande 2001 [82] | NO | SSRIs | Pancreatitis | < 2000 | 160 cases | No. of cases | 160 cases | B |
| Stahl 1997 [83] | SE | SSRIs | Withdrawal reactions | < 1995 | 49, 393 cases | Number of reports/106/ DDD |
Paroxetine = 1.9 Sertraline = 2.1 Fluoxetine = 0.48 |
A |
| Spigset 1999 [84] | SE | SSRIs | Not specified | 1965–1997 | 1202 cases | ADRs | Different categories reported | A + B |
| Sanz 2005 [85] | SE | SSRIs | Neonatal withdrawal syndrome | 1968–2002 | 102 cases | IC | Paroxetine = 4.07 Sertraline = 1.20 Citalopram = 1.92 Fluoxetin = 1.07 |
C |
| PEM | ||||||||
| Price 1996 [86] | UK | SSRIs | Withdrawal reactions | 1987–1992 | 50,150 patients | Reports/ 1000 prescriptions |
Paroxetine = 0.3 Sertraline = 0.03 Fluvoxamine = 0.03 Fluoxetine = 0.002 |
A |
| Layton 2001 [87] | UK | SSRIs | Abnormal bleeding | 1986–1998 | 135,754 patients | PRR | Day 1–30 = 1.38 Month 2–6 = 1.17 |
A |
| Edwards 1994 [88] | UK | Fluvoxamine | All | 1987–1988 | 10,401 patients | Incidences | A | |
| MacKay 1997 [89] | UK | SSRIs | All | 1988–1991 | 56,145 patients | Nausea, vomiting, withdrawal symptoms | ||
| Case series | ||||||||
| de Abajo 2006 [90] | - | SSRIs Venlafaxine |
Bleeding Disorders | 1988–2003 | 1,651 cases/ 10,000 controls |
PRR | 3.0, 2.1–4.4 | A |
| Gram 1999 [91] | DK | SSRIs | Bleeding Thrombocytopenia |
- | 8 cases | - | Case review | A + B |
| Case report | ||||||||
| Demers 2001 [92] | - | Fluvoxamine | Serotonin syndrome | - | 1 case | - | Case review | A |
Data sources
Case-control studies were carried out on data from various national registers and/or data from spontaneous ADR databases, physicians' databases such as the General Practitioners' database in the UK and Health Insurance Databases [15,16,37,38,72,73]. The studies were reported in the literature from the mid-1980s to the end of the 1990s. Cohort studies analysed ADR data collected from the mid-1980s to the end of the 1990s. The cohort studies varied in size from less than 20,000 patients to between 20,000–50,000 and more than 100,000 patients [17,19,21,40,41]. The PEM studies were conducted in the UK at the Drug Safety Unit in Southampton, and were based on data collected from the mid-1980s to the end of the 1990s [22,23,58-66,86-89]. Studies analysing spontaneously reported ADRs were conducted on large spontaneous reporting databases such as the French, American, British and the Uppsala Monitoring Centre WHO database [44-48,51,55-57,62,74-76,79,82,83,85].
Design and historical perspective
The antibiotic studies were published from 1990 and onwards, most of them from 1995. Cohort studies were published during 1990–1994, while the PEM studies, spontaneous reporting, case reports/case series primarily were published after 1995. The majority of the NSAID studies were published after year 2000. The SSRI studies were published from 1990 to present, most of them from 1995 to 2005. Table 6 shows the distribution of the analysed studies by type of approach, therapeutic case, and time of publication. For all therapeutic cases, data were collected and the studies published a long time after the drugs were first marketed. Despite the decades of difference in market launches for the therapeutic cases, the studies are mainly published from the end of the 1990s and on. Data were collected earlier.
Table 6.
Number of studies categorised by number, design and time of publication
| Year of publication | < 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005- |
| Study design | ||||||||||||||||
| Antibiotics | ||||||||||||||||
| Case control studies | 1 | 1 | ||||||||||||||
| Cohort studies | 1 | 3 | 1 | |||||||||||||
| National ADR databases | 1 | 2 | 1 | 2 | ||||||||||||
| PEM* | 1 | 1 | ||||||||||||||
| Case series | 1 | 1 | ||||||||||||||
| Case reports | 1 | 1 | 1 | 1 | 1 | |||||||||||
| NSAIDs | ||||||||||||||||
| Case control studies | 1 | 1 | 1 | 1 | ||||||||||||
| Cohort studies | 1 | 3 | ||||||||||||||
| National ADR databases | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 3 | |||||||
| PEM* | 5 | 2 | 2 | |||||||||||||
| Case series | 1 | 2 | ||||||||||||||
| Case reports | 1 | 1 | ||||||||||||||
| SSRIs | ||||||||||||||||
| Case control studies | 2 | |||||||||||||||
| Cohort studies | 1 | |||||||||||||||
| National ADR databases | 3 | 1 | 1 | 1 | 3 | 2 | ||||||||||
| PEM* | 1 | 1 | 1 | 1 | ||||||||||||
| Case series | 1 | 1 | ||||||||||||||
| Case reports | 1 | |||||||||||||||
*Prescription Event Monitoring Studies
Explored and detected ADRs
Antibiotics
ADRs from newer types of antibiotics, such as fluoroquinolones, have been reported much more frequently in the literature than ADRs from the older antibiotics, such as penicillins and macrolides [15-17,19,20,22-24,26,29,33,34,36]. The studies explore a possible risk between the use of antibiotics and the risk of liver, cardiovascular, CNS and dermatological ADRs [18-20,22,24,27,29,30,32,33,36]. Three cohort studies documented a correlation between cholestatic hepatitis and the use of flucloxacillin [18-20]. Increased risk of palpitation from the use of norfloxacin compared to ciprofloxacin/ofloxacin was demonstrated [22]. Cohort studies further demonstrated a risk of pemphigus related to penicillins, liver injury related to flucloxacillin and erythromycin [18-21]. CNS and dermatological ADRs from treatment with antibiotics have been reported rarely and on the case report level [30,32,33]. New information about ADRs was only produced by case reports: acute psychotic stress and glossitis/black tongue [34,35].
NSAIDs
Studies explored the risk of gastrointestinal [37-44,50] and dermatological ADRs as well as the development of liver and kidney toxicity which are well known ADRs associated with NSAIDs and their pharmacological characteristics[38,39,48,51,53,54,57,65,69,70]. The studies were generated after the launch of COX-2 inhibitors in the mid-1990s. A case-control study documented increased risk of developing dermatological ADRs of the type Steven-Johnson Syndrome and toxic epidermal necrolysis as did spontaneously reported ADRs [38,48]. A case-control study documented hepatic injury related to the use of NSAIDs, as did spontaneously reported ADRs, while renal injury and hypertension was documented in spontaneous reports and thromboembolic events in a PEM study [39,45,47,51,53,54,63,70]. With the exception of case reports, the approaches used did not produce information about ADRs that had not been reported previously.
SSRIs
Studies explored the risk of extrapyramidal symptoms, withdrawal syndromes and serotonin syndrome with the use of SSRIs, other ADRs investigated were: changes in testosterone and natrium level, alopecia, liver injury and bleeding. ADRs reported only via spontaneous reports are first-trimester exposure on newborns and neonatal withdrawal syndrome, hepatic injury and pancreatitis, suicide, non-puerperal lactation and serotonin syndrome [72-81,79,83,85,86,90-92]. With the exception of case reports, the approaches used did not detect new ADR signals that had not been reported previously [90-92].
Information about ADRs reported across approaches
Analytical
The approaches produced information about ADR risks compared to placebo or similar drugs as either odds ratios (OR), proportional reporting ratio (PRR) estimates, incidences (IC) and frequencies of ADRs. These parameters are built into the design and based on previous information or hypothesis. The studies were conducted on various patient populations, various medicines within the individual sub-groups, and different types of ADRs, different outcome measures, data sources and time periods. The purpose of the approaches made it possible to adjust the ADR estimate for known confounders and risk factors.
Observational
The approaches produced information about ADRs as estimates (OR, PRR, IC) or as single observations compared to placebo/similar medicines. Case reporting was the only approach that contributed new information about new ADRs in all three therapeutic cases.
Discussion
This review has several main findings:
First, analytical approaches ranging higher in the evidence hierarchy provided information about risks of already known or expected ADRs, while information about new and unknown ADRs was detected by case reports only, which range at the lowest level in the evidence hierarchy. Second, the studies primarily dealt with analyses of ADRs of type A and B, and only a few studies analysed type C and D. Third, similar approaches, both analytical and observational, were applied to all therapeutic cases. Fourth, the ADR cases provided similar results with regard to detecting new ADRs despite their connection to different time periods and organs attacked.
Methodological quality and capability of approaches
There is a general lack of standards in the field of ADRs, particularly because many ADRs are not detected until after marketing and the studies are based on selected patient groups, which makes it difficult to generalise the results to other patient groups. As previously argued in the literature, testing specific hypotheses in the analytical approaches makes it difficult to capture information about new and unknown ADRs [13]. Despite the fact that these types of studies rank high in the evidence hierarchy, the weaker design of the observational studies makes them more suitable for discovering previously undetected ADRs. Healthcare professionals have conventionally considered cohort and case-control studies to be well suited for post-marketing surveillance of ADRs, despite their lack of randomisation and lower position in the evidence hierarchy, level 4 and 5 respectively [14]. These studies primarily detected/analysed ADRs of type A and B and less frequently type C and D [14]. Thus, the approaches are not designed and therefore are not suitable for predicting new information about other ADRs that have not previously been detected or ADRs of the type C or D [14]. Case reports have provided data about patients, suspected ADRs, medicines involved and so on, but this information is often anecdotal in nature and collected retrospectively. However, it is interesting that despite their low rank in the evidence hierarchy, these reports provide new information about rare and previous undetected ADRs. Case reports may serve as whistleblowers, thereby initiating larger systematic analyses of patient populations or registering data to quantify the risk. A large majority of spontaneously reported ADRs are stored in databases hosted by regulatory agencies. Information about these observations is typically only released to the public in the form of press releases, insertions in product information or messages in national bulletins. If all these signals were published in the scientific literature or made public on the web pages of regulatory agencies, the number of spontaneous reports/case series would probably have been larger and added to the relative dominance of this design [93,94]. The results confirm that spontaneous post-marketing reporting of ADRs is of great importance and that regulatory agencies must continue to encourage spontaneous reporting of ADRs [93,94].
Alternative signal detection approaches
New ADR signals are often documented by only a small number of case reports, and systematic inclusion of data mining procedures in assessment of new ADR signals would probably contribute to earlier detection and quantification of serious ADR signals [95,96]. However, data mining was not applied in the three therapeutic cases studied here. Examples of data mining are cumulative techniques, time scans and Poisson methods, proportional reporting ratios (PRRs) and Bayesian data mining [97]. These methods assess how much the observed reporting frequency of a given drug-event combination deviates from that expected, given statistical independence between drug and event. Methodological and practical experiences with data mining in signal detection are limited [97,98].
Strengths and limitations of the study
The objective of this review was to analyse which information signal detection approaches have produced about new ADRs in selected and published therapeutic cases, rather than to perform a systematic review of the entire body of ADR literature covering all therapeutic groups. The choice of widely different therapeutic cases and the similar results obtained across therapeutic cases make us believe that the results qualitatively reflect the general, published experience on ADRs based on signal detection approaches. Findings across therapeutic cases were similar with respect to methodological approaches and time of publication, despite the fact that ADRs differed in nature and affected different organs. Although antibiotics have been marketed since the 1940s, it was not possible to search for literature before the mid-1960s due to the limitations of current databases. Lack of consistency in reporting ADRs, different methodologies used in the studies and their impact on the results are difficult to evaluate in this review.
Conclusion
Although the medicines were launched in different decades, approaches to the ADR studies were similar for all three therapeutic cases: antibiotics, NSAIDs and SSRIs. Descriptive as well as analytical designs were applied. Despite the fact that the analytical studies rank higher in the evidence hierarchy, only the descriptive case reports/spontaneous reports provided information about new and previously undetected ADRs. This review underscores the importance of systems for spontaneous reporting of ADRs. Therefore, spontaneous reporting should be encouraged further and the information in ADR databases should continuously be subjected to systematic analysis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LA and EHH designed the study, analysed the data and wrote the various drafts of the manuscript. LA did the sampling and literature search. Both authors read and approved the final version of the manuscript.
Acknowledgements
We thank The Danish Medicines Agency and the Hørslev Foundation for their financial support of the study.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Lise Aagaard, Email: laa@farma.ku.dk.
Ebba Holme Hansen, Email: ehh@farma.ku.dk.
References
- Inman WHW, Vessey MP, Westerholm B, Engelund A. Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. BMJ. 1970;25:203–9. doi: 10.1136/bmj.2.5703.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes MNG. The effects of drug regulation. Lancaster: MTP Press Limited; 1985. [Google Scholar]
- Horton R. Vioxx, the implosion of Merck, and the after shocks at the FDA. Lancet. 2004;364:1995–6. doi: 10.1016/S0140-6736(04)17523-5. [DOI] [PubMed] [Google Scholar]
- Tscholl D, Langer F, Wendler O, Wilkens H, Georg T, Schäfers HJ. Pulmonary thromboendarterectomy- risk factors for early survival and hemodynamic improvement. Eur J Cardiothorac Surg. 2001;19:771–6. doi: 10.1016/S1010-7940(01)00686-8. [DOI] [PubMed] [Google Scholar]
- Stefan H, Bernatik J, Knorr J. Visual field defects due to antiepileptic drugs. Nervenartz. 1999;70:552–5. doi: 10.1007/s001150050479. [DOI] [PubMed] [Google Scholar]
- Watkins P. COMT inhibitors and liver toxicity. Neurology. 2000;55:S51–2. [PubMed] [Google Scholar]
- Aagaard L, Soendergaard B, Andersen E, Kampmann JP, Hansen EH. Creating knowledge about adverse drug reactions: a critical analysis of the Danish reporting system from 1968 to 2005. Soc Sci Med. 2007;65:1296–309. doi: 10.1016/j.socscimed.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Hansen EH. Technology assessment of pharmaceuticals – the necessity of user perspective. Cah Sociol Demogr Med. 1990;30:313–27. [PubMed] [Google Scholar]
- Hansen EH. Technology assessment in a user perspective – experiences with drug technology. Int J Technol Assess Health Care. 1992;8:150–65. doi: 10.1017/s0266462300008011. [DOI] [PubMed] [Google Scholar]
- Dukes MNG. The law and ethics of the pharmaceutical industry. Amsterdam, Elsevier; 2006. [Google Scholar]
- Loke YK, Derry S, Aronson JK. A comparison of three different sources of data in assessing the frequencies of adverse reactions to amiodarone. Br J Clin Pharmacol. 2004;57:616–621. doi: 10.1111/j.0306-5251.2003.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke YK, Price D, Herxheimer A. Systematic reviews of adverse effects: framework for a structured approach. BMC Medical Research Methodology. 2007;7:32. doi: 10.1186/1471-2288-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom BL. Pharmacoepidemiology. West Sussex, John Wiley & Sons Ltd; 2005. [Google Scholar]
- Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies DM, editor. Textbook of Adverse Drug Reactions. 1977. pp. 10–31. [Google Scholar]
- Czeizel AE, Rockenbauer M, Sørensen HT, Olsen J. A population-based case-control teratologic study of oral erythromycin treatment during pregnancy. Reprod Toxicol. 1999;13:531–6. doi: 10.1016/S0890-6238(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15:784–92. doi: 10.1002/pds.1214. [DOI] [PubMed] [Google Scholar]
- Chyský V, Kapila K, Hullmann R, Arcieri G, Schacht P, Echols R. Safety of ciprofloxacin in children: worldwide clinical experience based on compassionate use. Emphasis on joint evaluation. Infection. 1991;19:289–96. doi: 10.1007/BF01644970. [DOI] [PubMed] [Google Scholar]
- Derby LE, Jick H, Henry DA, Dean AD. Cholestatic hepatitis associated with flucloxacillin. Med J Aust. 1993;158:596–00. doi: 10.5694/j.1326-5377.1993.tb137624.x. [DOI] [PubMed] [Google Scholar]
- Jick H, Derby LE, Dean AD, Henry DA. Flucloxacillin and cholestatic hepatitis. Med J Aust. 1994;18:160–525. [PubMed] [Google Scholar]
- Derby LE, Jick H, Henry DA, Dean AD. Erythromycin-associated cholestatic hepatitis. Med J Aust. 1993;158:600–2. doi: 10.5694/j.1326-5377.1993.tb137625.x. [DOI] [PubMed] [Google Scholar]
- Heymann AD, Chodick G, Kramer E, Green M, Shalev V. Pemphigus variant associated with penicillin use: a case-cohort study of 363 patients from Israel. Arch Dermatol. 2007;143:704–7. doi: 10.1001/archderm.143.6.704. [DOI] [PubMed] [Google Scholar]
- Clark DW, Layton D, Wilton LV, Pearce GL, Shakir SA. Profiles of hepatic and dysrhythmic cardiovascular events following use of fluoroquinolone antibacterials: experience from large cohorts from the Drug Safety Research Unit Prescription-Event Monitoring database. Drug Saf. 2001;24:1143–54. doi: 10.2165/00002018-200124150-00005. [DOI] [PubMed] [Google Scholar]
- Inmann W, Pearce G, Wilton L. Safety of fluconazole in the treatment of vaginal candidiasis. A prescription-event monitoring study, with special reference to the outcome of pregnancy. Eur J Clin Pharmacol. 1994;46:115–8. doi: 10.1007/BF00199872. [DOI] [PubMed] [Google Scholar]
- Sachs B, Riegel S, Seebeck J, Beier R, Schichler D, Barger A, Merk HF, Erdmann S. Fluoroquinolone-associated anaphylaxis in spontaneous adverse drug reaction reports in Germany: differences in reporting rates between individual fluoroquinolones and occurrence after first-ever use. Drug Saf. 2006;29:1087–00. doi: 10.2165/00002018-200629110-00008. [DOI] [PubMed] [Google Scholar]
- Fleisch F, Hartmann K, Kuhn M. Fluoroquinolone-induced tendinopathy: also occurring with levofloxacin. Infection. 2000;28:256–7. doi: 10.1007/s150100070050. [DOI] [PubMed] [Google Scholar]
- Leone R, Venegoni M, Motola D, Moretti U, Piazzetta V, Cocci A, Resi D, Mozzo F, Velo G, Burzilleri L, Montanaro N, Conforti A. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluoroquinolone consumption data from three Italian regions. Drug Saf. 2003;26:109–20. doi: 10.2165/00002018-200326020-00004. [DOI] [PubMed] [Google Scholar]
- Pierfitte C, Royer RJ, Moore N, Bégaud B. The link between sunshine and phototoxicity of sparfloxacin. Br J Clin Pharmacol. 2000;49:609–12. doi: 10.1046/j.1365-2125.2000.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frothingham R. Glucose homeostasis abnormalities associated with use of gatifloxacin. Clin Infect Dis. 2005;41:1269–76. doi: 10.1086/496929. [DOI] [PubMed] [Google Scholar]
- Hedenmalm K, Spigset O. Peripheral sensory disturbances related to treatment with fluoroquinolones. J Antimicrob Chemother. 1996;37:831–7. doi: 10.1093/jac/37.4.831. [DOI] [PubMed] [Google Scholar]
- Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22:71–81. doi: 10.1097/00004714-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther. 2005;27:1329–42. doi: 10.1016/j.clinthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hällgren J, Tengvall-Linder M, Persson M, Wahlgren CF. Stevens-Johnson syndrome associated with ciprofloxacin: a review of adverse cutaneous events reported in Sweden as associated with this drug. J Am Acad Dermatol. 2003;49:S267–9. doi: 10.1016/S0190-9622(03)00478-X. [DOI] [PubMed] [Google Scholar]
- Warner A. Clarithromycin–a precipitant for acute psychotic stress. Psychosomatics. 2000;41:539. doi: 10.1176/appi.psy.41.6.539. [DOI] [PubMed] [Google Scholar]
- ADRAC Drug-induced cholestatic hepatitis from common antibiotics. MJA. 1992;157:531. [PubMed] [Google Scholar]
- Greco S, Mazzaglia G, Caputi AP, Pagliaro L. Glossitis, stomatitis, and black tongue with lansoprazole plus clarithromycin and other antibiotics. Ann Pharmacother. 1997;31:1548. doi: 10.1177/106002809703101220. [DOI] [PubMed] [Google Scholar]
- Björnsson E, Lindberg J, Olsson R. Liver reactions to oral low-dose tetracyclines. Scand J Gastroenterol. 1997;32:390–5. doi: 10.3109/00365529709007690. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz S, Rodríguez LA. Steroids and risk of upper gastrointestinal complications. Am J Epidemiol. 2001;153:1089–93. doi: 10.1093/aje/153.11.1089. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt M, Kelly JP, Kaufman D, Stern RS, SCAR Study Group The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with nonsteroidal antiinflammatory drugs: a multinational perspective. J Rheumatol. 2003;30:2234–40. [PubMed] [Google Scholar]
- Lacroix I, Lapeyre-Mestre M, Bagheri H, Pathak A, Montastruc JL. Nonsteroidal anti-inflammatory drug-induced liver injury: a case-control study in primary care. Fundam Clin Pharmacol. 2004;18:201–6. doi: 10.1111/j.1472-8206.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- Lipworth L, Friis S, Blot WJ, McLaughlin JK, Mellemkjaer L, Johnsen SP, Nørgaard B, Olsen JH. A population-based cohort study of mortality among users of ibuprofen in Denmark. Am J Ther. 2004;11:156–63. doi: 10.1097/00045391-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Ashworth NL, Peloso PM, Muhajarine N, Stang M. A population based historical cohort study of the mortality associated with nabumetone, Arthrotec, diclofenac, and naproxen. J Rheumatol. 2004;31:951–6. [PubMed] [Google Scholar]
- Morant SV, Pettitt D, MacDonald TM, Burke TA, Goldstein JL. Application of a propensity score to adjust for channelling bias with NSAIDs. Pharmacoepidemiol Drug Saf. 2004;13:345–53. doi: 10.1002/pds.946. [DOI] [PubMed] [Google Scholar]
- Martin RM, Biswas P, Mann RD. The incidence of adverse events and risk factors for upper gastrointestinal disorders associated with meloxicam use amongst 19,087 patients in general practice in England: cohort study. Br J Clin Pharmacol. 2000;50:35–42. doi: 10.1046/j.1365-2125.2000.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugardon S, Lapeyre-Mestre M, Montastruc JL. Upper gastrointestinal adverse drug reactions and cyclo-oxygenase-2 inhibitors (celecoxib and rofecoxib): a case/non-case study from the French Pharmacovigilance Database. Eur J Clin Pharmacol. 2004;60:673–7. doi: 10.1007/s00228-004-0813-5. [DOI] [PubMed] [Google Scholar]
- Durrieu G, Olivier P, Montastruc JL. COX-2 inhibitors and arterial hypertension: an analysis of spontaneous case reports in the Pharmacovigilance database. Eur J Clin Pharmacol. 2005;61:611–4. doi: 10.1007/s00228-005-0964-z. [DOI] [PubMed] [Google Scholar]
- Clinard F, Sgro C, Bardou M, Hillon P, Dumas M, Kreft-Jais C, Escousse A, Bonithon-Kopp C. Association between concomitant use of several systemic NSAIDs and an excess risk of adverse drug reaction. A case/non-case study from the French Pharmacovigilance system database. Eur J Clin Pharmacol. 2004;60:279–83. doi: 10.1007/s00228-004-0761-0. [DOI] [PubMed] [Google Scholar]
- Brinker A, Goldkind L, Bonnel R, Beitz J. Spontaneous reports of hypertension leading to hospitalisation in association with rofecoxib, celecoxib, nabumetone and oxaprozin. Drugs Aging. 2004;21:479–84. doi: 10.2165/00002512-200421070-00005. [DOI] [PubMed] [Google Scholar]
- La Grenade L, Lee L, Weaver J, Bonnel R, Karwoski C, Governale L, Brinker A. Comparison of reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis in association with selective COX-2 inhibitors. Drug Saf. 2005;28:917–24. doi: 10.2165/00002018-200528100-00008. [DOI] [PubMed] [Google Scholar]
- Polimeni G, Salvo F, Cutroneo P, Morreale I, Patrizio Caputi A. Adverse reactions induced by NSAIDs and antibacterials: analysis of spontaneous reports from the Sicilian regional database. Drug Saf. 2006;29:449–59. doi: 10.2165/00002018-200629050-00006. [DOI] [PubMed] [Google Scholar]
- Conforti A, Leone R, Moretti U, Mozzo F, Velo G. Adverse drug reactions related to the use of NSAIDs with a focus on nimesulide: results of spontaneous reporting from a Northern Italian area. Drug Saf. 2001;24:1081–90. doi: 10.2165/00002018-200124140-00006. [DOI] [PubMed] [Google Scholar]
- Ahmad SR, Kortepeter C, Brinker A, Chen M, Beitz J. Renal failure associated with the use of celecoxib and rofecoxib. Drug Saf. 2002;25:537–44. doi: 10.2165/00002018-200225070-00007. [DOI] [PubMed] [Google Scholar]
- van Puijenbroek EP, Egberts AC, Heerdink ER, Leufkens HG. Detecting drug-drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2000;56:733–8. doi: 10.1007/s002280000215. [DOI] [PubMed] [Google Scholar]
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Del Pozo JG, Requejo AA, Arias LM, Montastruc JL, Carvajal A. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. doi: 10.1111/j.1472-8206.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Leone R, Conforti A, Ghiotto E, Moretti U, Valvo E, Velo GP. Nimesulide and renal impairment. Eur J Clin Pharmacol. 1999;55:151–4. doi: 10.1007/s002280050610. [DOI] [PubMed] [Google Scholar]
- Brown EG, Waller PC, Sallie BA. Tiaprofenic acid and severe cystitis. Postgrad Med J. 1998;74:443–4. doi: 10.1136/pgmj.74.873.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico MM, Weber RJ, McKaveney TP, Ansani NT, Towers AL. Adverse Drug Events Involving COX-2 Inhibitors. Ann Pharmacother. 2003;37:1203–13. doi: 10.1345/aph.1A212. [DOI] [PubMed] [Google Scholar]
- Kahn LH, Styrt BA. Necrotizing soft tissue infections reported with nonsteroidal antiinflammatory drugs. Ann Pharmacother. 1997;31:1034–9. doi: 10.1177/106002809703100914. [DOI] [PubMed] [Google Scholar]
- Layton D, Wilton LV, Shakir SA. Safety profile of celecoxib as used in general practice in England: results of a prescription-event monitoring study. Eur J Clin Pharmacol. 2004;60:489–01. doi: 10.1007/s00228-004-0788-2. [DOI] [PubMed] [Google Scholar]
- Layton D, Hughes K, Harris S, Shakir SA. Comparison of the incidence rates of selected gastrointestinal events reported for patients prescribed celecoxib and meloxicam in general practice in England using prescription-event monitoring (PEM) data. Rheumatology (Oxford) 2003;42:1332–41. doi: 10.1093/rheumatology/keg376. [DOI] [PubMed] [Google Scholar]
- Layton D, Riley J, Wilton LV, Shakir SA. Safety profile of rofecoxib as used in general practice in England: results of a prescription-event monitoring study. Br J Clin Pharmacol. 2003;55:166–74. doi: 10.1046/j.1365-2125.2003.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton D, Heeley E, Shakir SA. Identification and evaluation of a possible signal of exacerbation of colitis during rofecoxib treatment, using Prescription-Event Monitoring data. J Clin Pharm Ther. 2004;29:171–81. doi: 10.1111/j.1365-2710.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- Kasliwal R, Layton D, Harris S, Wilton L, Shakir SA. A comparison of reported gastrointestinal and thromboembolic events between rofecoxib and celecoxib using observational data. Drug Saf. 2005;28:803–16. doi: 10.2165/00002018-200528090-00005. [DOI] [PubMed] [Google Scholar]
- Layton D, Heeley E, Hughes K, Shakir SA. Comparison of the incidence rates of thromboembolic events reported for patients prescribed rofecoxib and Meloxicam in general practice in England using Prescription-event monitoring (PEM) data. Rheumatology. 2003;42:1342–53. doi: 10.1093/rheumatology/keg379. [DOI] [PubMed] [Google Scholar]
- Layton D, Heeley E, Hughes K, SAW Shakir. Comparison of the incidence rates of selected gastrointestinal events reported for patients prescribed rofecoxib and meloxicam in general practice in England using prescription-event monitoring data. Rheumatology. 2003;42:622–31. doi: 10.1093/rheumatology/keg141. [DOI] [PubMed] [Google Scholar]
- Layton D, Marshall V, Boshier A, Friedmann P, Shakir SA. Serious Skin Reactions and Selective COX-2 Inhibitors. A Case Series from Prescription-Event Monitoring In England. Drug Saf. 2006;29:687–96. doi: 10.2165/00002018-200629080-00005. [DOI] [PubMed] [Google Scholar]
- Layton D, Hughes K, Harris S, Shakir SA. Comparison of the incidence rates of thromboembolic events reported for patients prescribed celecoxib and meloxicam in general practice in England using Prescription-Event Monitoring (PEM) data. Rheumatology. 2003;42:1354–64. doi: 10.1093/rheumatology/keg401. [DOI] [PubMed] [Google Scholar]
- Onder G, Pellicciotti F, Gambassi G, Bernabei R. NSAID-Related Psychiatric Adverse Events. Who is at risk? Drugs. 2004;64:2619–27. doi: 10.2165/00003495-200464230-00001. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FW, Solomon J, Mechelas TJ. Ocular Adverse Effects Associated With Cyclooxygenase-2 Inhibitors. Arch Ophthalmol. 2006;124:277–79. doi: 10.1001/archopht.124.2.277. [DOI] [PubMed] [Google Scholar]
- Ziemer M, Wiesend CL, Vetter R, Weiss J, Blaschke S, Norgauer J, Mockenhaupt M. Cutaneous Adverse Reactions to Valdecoxib Distinct From Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Arch Dermatol. 2007;143:711–16. doi: 10.1001/archderm.143.6.711. [DOI] [PubMed] [Google Scholar]
- Hunter EB, Johnston PE, Tanner G, Pinson CW, Awad JA. Bromfenac (Duract)- Associated Hepatic Failure Requiring Liver Transplantation. AJG. 1999;94:2299–01. doi: 10.1111/j.1572-0241.1999.01321.x. [DOI] [PubMed] [Google Scholar]
- ADRAC Premature closure of the fetal ductus arteriosus after maternal use of non-steroidal anti-inflammatory drugs. MJA. 1998;169:270–71. [PubMed] [Google Scholar]
- Schillevoort I, van Puijenbroek EP, de Boer A, Ross RA, Jansen PA, Leufkens HG. Extrapyramidal syndromes associated with selective serotonin reuptake inhibitors: a case-control study using spontaneous reports. Int Clin Psychopharmacol. 2002;17:75–9. doi: 10.1097/00004850-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Movig KLL, Leufkens HGM, Lenderink AW, Eberts ACG. Serotonergic antidepressants associated with an increased risk for hyponatraemia in the elderly. Eur J Clin Pharmacol. 2002;58:143–48. doi: 10.1007/s00228-002-0438-5. [DOI] [PubMed] [Google Scholar]
- Bell S, Shipman M, Bystritsky A, Haifley T. Fluoxetine Treatment and Testosterone Levels. Annals of Clinical Psychiatry. 2006;18:19–22. doi: 10.1080/10401230500464612. [DOI] [PubMed] [Google Scholar]
- Trenque T, Piednoir D, Frances C, Millart H, Germain ML. Reports of withdrawal syndrome with the use of SSRIs: a case/non-case study in the French Pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2002;11:281–3. doi: 10.1002/pds.704. [DOI] [PubMed] [Google Scholar]
- Gony M, Lapeyre-Mestre M, Montastruc JL. Risk of serious extrapyramidal symptoms in patients with Parkinson's disease receiving antidepressant drugs: a pharmacoepidemiologic study comparing serotonin reuptake inhibitors and other antidepressant drugs. Clin Neuropharmacol. 2003;26:142–5. doi: 10.1097/00002826-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Hedenmalm K, Sundström A, Spigset O. Alopecia associated with treatment with selective serotonin reuptake inhibitors (SSRIs) Pharmacoepidemiol Drug Saf. 2006;15:719–25. doi: 10.1002/pds.1270. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Corbin LA, Sundell KL. Effects of First-Trimester Fluoxetine Exposure on the Newborn. Obstet Gynecol. 1997;89:713–8. doi: 10.1016/S0029-7844(97)00070-7. [DOI] [PubMed] [Google Scholar]
- Spigset O, Hägg S, Bate A. Hepatic injury and pancreatitis during treatment with serotonin reuptake inhibitors: data from the World Health Organization database of adverse drug reactions. Int Clin Psychopharmacol. 2003;18:157–61. doi: 10.1097/00004850-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Khan A, Khan S, Kolts R, Brown WA. Suicide Rates in Clinical Trials of SSRIs, other Antidepressants and placebo: Analysis of FDA Reports. Am J Psychiatry. 2003;160:790–92. doi: 10.1176/appi.ajp.160.4.790. [DOI] [PubMed] [Google Scholar]
- Egberts ACG, Meyboom RHB, De Koning FHP, Bakker A, Leufkens HGM. Non-puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol. 1997;44:277–81. doi: 10.1046/j.1365-2125.1997.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvande KT, Madsen S. Selective serotonin reuptake inhibitors and pancreatitis. Tidsskr Nor Laegeforen. 2001;121:177–8. [PubMed] [Google Scholar]
- Stahl MMS, Lindquist M, Pettersson M, Edwards IR, Sanderson JH, Taylor NFA, Fletcher AP, Schou JS. Withdrawal reactions with selective serotonin re-uptake inhibitors as reported to the WHO system. Eur J Clin Pharmacol. 1997;53:163–69. doi: 10.1007/s002280050357. [DOI] [PubMed] [Google Scholar]
- Spigset O. Adverse Reactions of Selective Serotonin Reuptake Inhibitors. Reports from a Spontaneous Reporting System. Drug Saf. 1999;20:277–87. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482–87. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- Price JS, Waller PC, Wood SM, MacKay AV. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42:757–63. doi: 10.1046/j.1365-2125.1996.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton D, Clark DW, Pearce GL, Shakir SA. Is there an association between selective serotonin reuptake inhibitors and risk of abnormal bleeding? Results from a cohort study based on prescription event monitoring in England. Eur J Clin Pharmacol. 2001;57:167–76. doi: 10.1007/s002280100263. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Inman WH, Wilton L, Pearce G. Prescription-event monitoring of 10,401 patients treated with fluvoxamine. Br J Psychiatry. 1994;164:387–95. doi: 10.1192/bjp.164.3.387. [DOI] [PubMed] [Google Scholar]
- MacKay FJ, Dunn NR, Wilton LV, Pearce GL, Freemantle SN, Mann RD. A comparison of fluvoxamine, fluoxetine, sertraline and paroxetine examined by observational cohort studies. Pharmacoepidemiol Drug Saf. 1997;6:235–46. doi: 10.1002/(SICI)1099-1557(199707)6:4<235::AID-PDS293>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- De Abajo FJ, Montero D, Garcia Rodriguez LA, Madurga M. Antidepressants and Risk of Upper Gastrointestinal Bleeding. Pharmacology & Tocicology. 2006;98:304–10. doi: 10.1111/j.1742-7843.2006.pto_303.x. [DOI] [PubMed] [Google Scholar]
- Gram LF. Blødning og trombocytopeni ved behandling med selektive SSRI [In Danish] Ugeskr Laeger. 1999;161:2697–98. [PubMed] [Google Scholar]
- Demers JC, Malone M. Serotonin Syndrome Induced by Fluvoxamine and Mirtazapine. Ann Pharmacother. 2001;35:1217–20. doi: 10.1345/aph.10418. [DOI] [PubMed] [Google Scholar]
- Aagaard L, Soendergaard B, Andersen E, Kampmann JP, Hansen EH. Creating knowledge about adverse drug reactions: a critical analysis of the Danish reporting system from 1968 to 2005. Soc Sci Med. 2007;65:1296–309. doi: 10.1016/j.socscimed.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Aagaard L, Stenver DI, Hansen EH. Structures and processes in spontaneous ADR reporting systems: a comparative study of Australia and Denmark. Pharm World Sci. 2008;30:563–70. doi: 10.1007/s11096-008-9210-y. [DOI] [PubMed] [Google Scholar]
- Mann RD. Drug safety alerts – a review of "current problems". Pharmacoepidemiol Drug Saf. 1992;1:269–79. doi: 10.1002/pds.2630010509. [DOI] [Google Scholar]
- Meyboom RHB, Gribnau FWJ, Hekster YA, de Koning GHP, Egberts ACG. Characteristics of Topics in Pharmacovigilance in the Netherlands. Clin Drug Invest. 1996;12:207–19. doi: 10.2165/00044011-199612040-00006. [DOI] [Google Scholar]
- Hauben M, Zhou X. Quantitative methods in pharmacovigilance: focus on signal detection. Drug Saf. 2003;26:159–86. doi: 10.2165/00002018-200326030-00003. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Kuroda Y, Niwa S, Sonehara S, Hamada C, Yoshimura I. Criteria Revision and Performance Comparison of Three Methods of Signal Detection Applied to the Spontaneous Reporting Database of a Pharmaceutical Manufacturer. Drug Saf. 2007;30:715–26. doi: 10.2165/00002018-200730080-00008. [DOI] [PubMed] [Google Scholar]