Abstract
The evolutionary diversification of spiders is attributed to spectacular innovations in silk. Spiders are unique in synthesizing many different kinds of silk, and using silk for a variety of ecological functions throughout their lives, particularly to make prey-catching webs. Here, we construct a broad higher-level phylogeny of spiders combining molecular data with traditional morphological and behavioral characters. We use this phylogeny to test the hypothesis that the spider orb web evolved only once. We then examine spider diversification in relation to different web architectures and silk use. We find strong support for a single origin of orb webs, implying a major shift in the spinning of capture silk and repeated loss or transformation of orb webs. We show that abandonment of costly cribellate capture silk correlates with the 2 major diversification events in spiders (1). Replacement of cribellate silk by aqueous silk glue may explain the greater diversity of modern orb-weaving spiders (Araneoidea) compared with cribellate orb-weaving spiders (Deinopoidea) (2). Within the “RTA clade,” which is the sister group to orb-weaving spiders and contains half of all spider diversity, >90% of species richness is associated with repeated loss of cribellate silk and abandonment of prey capture webs. Accompanying cribellum loss in both groups is a release from substrate-constrained webs, whether by aerially suspended webs, or by abandoning webs altogether. These behavioral shifts in silk and web production by spiders thus likely played a key role in the dramatic evolutionary success and ecological dominance of spiders as predators of insects.
Keywords: Araneidae, behavioral evolution, cribellate silk, orb web, speciation
Spiders are exceptionally diverse and abundant in terrestrial ecosystems. In contrast to megadiverse orders of insects, evolutionary diversification of spiders is not coupled with major trophic shifts. All spiders are predators of arthropods, and spiders are dominant consumers at intermediate trophic levels (1, 2). Spider diversification is instead linked to key innovations in silk use (3–7). For instance, the araneoid orb web (Fig. 1) with stretchy capture spirals, coated by adhesive viscid silk secretions, provides access to abundant flying insects (3, 8). However, many spiders produce cribellate silk, a radically different dry adhesive that adheres to prey, using van der Waals interactions and hygroscopic forces (9). Some cribellate spiders also construct aerial orb webs, whereas most spin sheet-like webs on the substrate (Fig. S1) or have abandoned capture webs altogether. Furthermore, the most diverse families within “orb-weavers” (Orbiculariae) no longer build orb webs, but instead spin aerial sheet webs (Linyphiidae) or cobwebs (Theridiidae) (Fig. S2). Thus, discovering the pattern of evolution of web spinning behaviors is essential for understanding spider diversification.
Fig. 1.
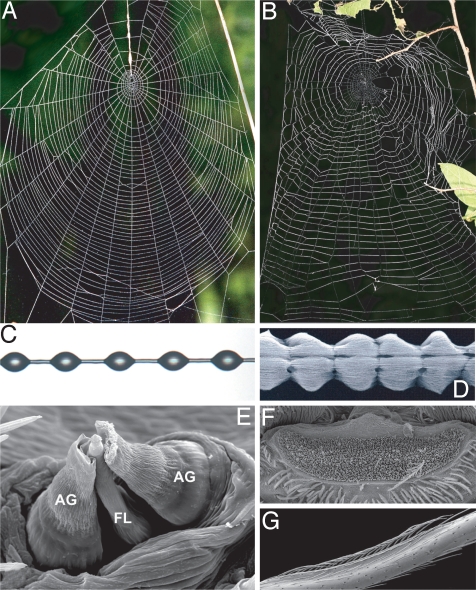
Comparison of modern gluey orb webs spun by araneoid spiders to cribellate orb webs. (A and B) The web architectures are strikingly similar. (C and D) However, they use radically different adhesive silks. (C) Capture threads in araneoid orbs are coated by droplets of aqueous glue that are chemically adhesive. (D) Cribellate spiders coat capture threads with puffs of tiny, dry silk fibrils. (E) Araneoid spiders quickly spin the central capture fiber and its surrounding glue simultaneously, using a triad of silk spigots on their posterior lateral spinnerets (the outer pair of aggregate spigots produces the glue, whereas the central flagelliform spigot produces the core fiber). The droplet morphology arises spontaneously soon after the glue is applied to the silk. (F) Cribellate spiders also produce the core fibers of their capture threads from spigots on the posterior lateral spinneret. However, they use hundreds of tiny spigots on the cribellum, anterior of the spinnerets, to produce the adhesive fibrils. (G) The puffed morphology results from the spiders behaviorally combing the silk, using a calamistrum located on their hind legs. D is courtesy of B. Opell.
Orb webs possessing dry cribellate capture spirals are architecturally similar to those spun from aqueous viscid silk (Fig. 1 A and B). Cribellate capture silk is produced by spiders first spinning a core axial fiber and then physically combing fine fibrils onto it to make functional capture spiral. This multistep process is metabolically expensive and time consuming (Fig. 1 D and F) (10, 11). In contrast, aqueous viscid silk is spun by simultaneously laying down a layer of glue while spinning the core axial fiber. The viscid coating then spontaneously forms glue droplets (Fig. 1 C and E). This streamlined process allows modern (ecribellate) spiders to complete orb webs in a fraction of the time required for cribellate orb webs. Given the radical differences in how dry cribellate and gluey wet capture silks are produced and function, the 2 types of orbs have been considered a classic example of evolutionary convergence (12–14). However, both types of webs are spun using many of the same behaviors, such as the pattern of leg movements used to manipulate silk (8, 14–18) and the spiders share many morphological similarities (19, 20). Despite similar architectures, the 2 types of orbs differ fundamentally in function. A major distinction is that viscid threads depend on water absorbed by the chemical glue coating them to maintain stickiness (21). In contrast, cribellate threads lose stickiness when water mats together their puffy fibrils. Overall, most characters supporting orb web monophyly relate to the spinning of the orb itself and, if the orb architecture is strongly adaptive, they may easily be convergent.
Inferring the evolutionary origin of orb web weaving is also necessary to understand the subsequent transformation and loss of the orb and associated web spinning behaviors. Although the orb weaver (Orbiculariae) clade constitutes ≈1/4 of the world's spider diversity, most do not spin orb webs. Cobweb spiders (Theridiidae) and sheet web spiders (Linyphiidae) encompass almost half of all extant species in the orb weaver clade. Moreover, many speciose families of spiders do not spin prey capture webs at all. Thus, a robust phylogenetic hypothesis is needed to determine how spider diversification relates to transformations in web architectures and silk specializations.
Higher level systematics of spiders currently relies heavily on morphological and behavioral data (19, 22, 23). Molecular data are used almost exclusively at the species/genus level (24–27) or within families (28–32). However, DNA has proven useful for groups of orbicularian spiders, including the biogeography of Hawaiian tetragnathid and linyphiid species (33, 34), relationships among cobweb weaving genera (29, 35), and relationships among micropholcommatids (36). These studies did include more distantly related taxa as outgroups, but the relationships among them varied greatly. The few DNA-based higher level analyses of spiders focus on clades outside orb weavers, such as the infraorder Mygalomorphae (tarantulas and relatives) (28), micropholcommatids and a few ecribellate orb weavers (36), or the RTA clade spiders (including wolf spiders, crab spiders, and their relatives) (37). Ayoub et al. (38) included a single ecribellate and 2 cribellate orb weavers in their analysis of the utility of EF-1γ for mygalomorph phylogeny. Vink et al. (39) used actin 5C to analyze higher level spider relations, but concentrated on jumping spiders. Hausdorf (40) published a single-gene study of 9 distantly related families, which included 1 cribellate and 2 ecribellate orb weavers, and found weak evidence for convergent orb architectures. Finally, Garb et al. (41) used the expression of viscid silk genes in cribellate spiders to argue for monophyly. All of these studies are too limited in taxon and character sampling to test strongly orb-weaver monophyly.
Here, we present the first combined evidence phylogeny to reconstruct higher level relationship of orb weaving spiders and relatives. We use this phylogeny to test the single origin of orb webs, and to understand the implications of major transformations in silk production and web architectures for spider diversification.
Results
General Description.
We performed a broad range of analyses, for 2 different molecular alignments, using diverse methods of phylogenetic reconstruction. Most analyses that included the complete molecular dataset yielded concordant results about fundamental relationships among spiders, regardless of the inclusion of morphological data, alignment parameters, or phylogenetic method. We summarize the results in Fig. 2, showing our preferred total evidence topology (MP, 8/4 alignment, implied weighting with concavity K = 3), and in Figs. S3–S5. Despite being culled from existing literature, morphology on its own recovered only a portion of traditionally hypothesized clades (Fig. S3).
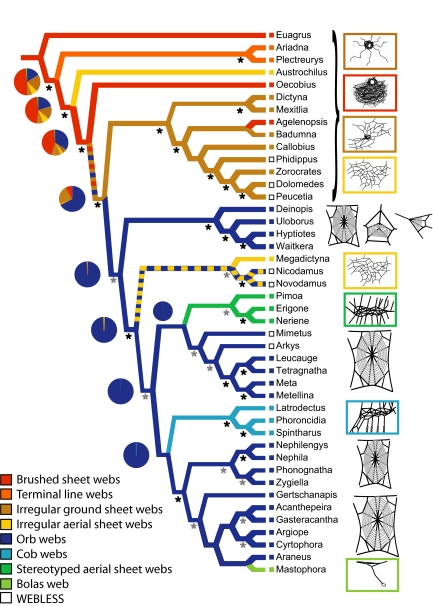
Fig. 2.
Optimization of web architecture on the preferred topology. Black stars indicate strong support for a node from both MP (jackknife > 75%) and Bayesian (posterior probabilities > 90%) analyses, and gray stars indicate nodes strongly supported only by one methodology or with jackknife 50–74%. Branch colors represent MP reconstruction of webs, and pie charts represent the relative probabilities from ML reconstructions. Colors of boxes to the left of taxon names represent their webs, and open boxes indicate that taxa do not spin prey capture webs.
Preferred Topology.
Our preferred tree includes the greatest number of most frequently supported groups across all analyses (8/4 alignment of full dataset, implied weights, K = 3; see Fig. 2 and Dataset S1). Our preference is merely heuristic in that it best summarizes results of all analyses, not because we particularly advocate implied weights or an 8/4 alignment. Most full dataset analyses resulted in very similar tree topologies (Dataset S1). Furthermore, the results of virtually all 65 analyses are consistent with our main conclusions (see below). Fig. S5 demonstrates the general similarity of results from a different phylogenetic method (Bayesian analysis of molecular data only, 24/6 alignment). Comparison of Fig. 2 and Fig. S5 illustrates the two common points of incongruity across analyses—placement of RTA clade relative to deinopoids and the tendency for Megadictyna to sometimes group with theridiids rather than nicodamids. The correspondence between our different analyses and traditional taxonomic groups is summarized in Fig. S3.
Web Evolution.
Orb webs unambiguously optimized as monophyletic under maximum parsimony (MP) for 10 of 14 different total evidence analyses of both the complete and pruned taxon sets, whereas monophyly was one of several ambiguous reconstructions for the remaining 4 analyses. Only 1 of 65 analyses (POY analysis of mitochondrial data alone) refuted the hypothesis of orb monophyly under MP ancestral character state reconstruction. Similarly, maximum likelihood (ML) ancestral character reconstruction also supported orb web monophyly as the most likely character reconstruction for all topologies, with the exceptions of 2 analyses of the 8/4 alignment of mitochondrial data alone.
Our results unambiguously refute the hypothesis that all orbicularian “sheet webs” are homologous [“araneoid sheet web weaver clade” (42)]. Instead, sheets evolved at least twice from ancestral orbs, resulting in linyphioid stereotyped aerial sheet webs and sticky gumfooted cobwebs of theridioids. The Megadictyna sheet is quite similar to linyphiid webs but its phylogenetic placement demonstrates the autapomorphic origin of its architecture. Moreover, Megadictyna utilizes cribellate silk in contrast to linyphiids' viscid adhesive.
Our analysis shows that orb webs arose a single time. The orb appears to be derived from a substrate-bound web, likely an irregular ground web or brushed sheet web (Fig. 2). The evolution of orb webs was marked by a dramatic increase in geometric regularity, resulting from increased behavioral stereotypy of spinning. This shift, coupled with suspension of orbs in midair, via structural support threads, likely released webs from constraints on shape imposed by the substrate in basal taxa. Subsequent to the orb's origin, aqueous viscid silk replaced cribellate silk and the orb was transformed at least 3 times into less regular appearing aerial sheet webs. Moreover, the orb web has been highly reduced independently in tetragnathoids and Araneidae (among “bolas spiders,” here represented by Mastophora) (Fig. 2). Furthermore, although the orb has been lost independently at least 3 times within the context of our current taxon sampling, it has likely been lost even more frequently among all orb-weavers (e.g., refs. 43–47).
Discussion
Monophyletic Origin of the Orb Web.
The monophyletic origin of orb webs is strongly supported, despite conspicuous differences in the silk used to spin different types of orbs (Fig. 1). This has important implications for understanding both web evolution and spider diversification. Instead of cribellate and ecribellate orb webs evolving in parallel, orb monophyly explicitly implies that dry cribellate capture spirals were replaced by ecribellate gluey spirals. This involves 2 major changes. First, a shift in the silk used to produce the core fibers of capture threads, resulting in novel tensile properties. The core fibers of modern (ecribellate) orb weavers are composed of flagelliform silk, which is much more elastic than the pseudoflagelliform silk core fibers of cribellate spiders (48). Mechanically, flagelliform silk functions like rubber, relying on entropy to resist motion of silk molecules and absorb kinetic energy during prey capture, allowing the capture spiral to expand and contract repeatedly (49). In contrast, cribellate silk relies on permanent rupturing of molecular bonds to absorb kinetic energy and deforms irreversibly during prey capture (48). The second major shift involves the mechanism of adhesion, from dry cribellate fibrils that adhere through van der Waals forces and hygroscopic interactions to chemically adhesive viscid glue in ecribellate spiders. This results in webs with greater adhesion per surface area (50) and may have facilitated the transition from horizontal to vertical web orientation in modern orb spiders, which is associated with increased prey interception rates (51).
An evolutionary shift in capture silk has been considered improbable because it necessitates the origin of both novel silk production apparatuses (e.g., glands and spigots) and spinning behaviors (refs. 13, 14, and 52; see summary in ref. 53). Modification in production of axial core fibers is relatively easy to understand because these threads are produced from spigots on the same spinneret in both types of spiders (54, 55). Moreover, Garb et al. (41) recently discovered the expression of flagelliform silk genes, once thought confined only to modern orb weavers, in the silk glands of cribellate orb weavers. Thus, a simple increase in the expression of flagelliform silk genes could explain the development of modern flagelliform core fibers. The transition between glue types is more challenging because cribellate fibrils function in a dry state whereas viscid glue functions in an aqueous state. Currently, the chemical composition of cribellate adhesive silk is unknown whereas aggregate silk has only been described in detail from one species (56), thus precluding meaningful insight into the transition.
Orb monophyly clearly implies multiple secondary derivations of alternative web types, and web losses. Far from being a highly stable and optimal behavior, orb spinning is instead a stepping stone to further behavioral innovation and evolutionary diversification. The orb web may be a preadaptation that freed spiders from constraints imposed by spinning sheet webs with substrate determined architectures.
Web Evolution and Spider Diversification.
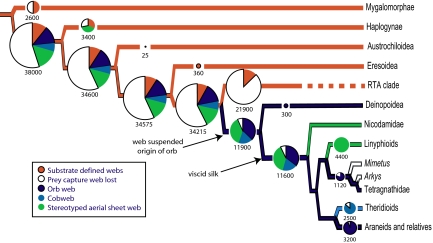
Most of the current diversity of spiders results from 2 major radiations −RTA clade (>21,000 spp.) and Orbiculariae (>11,000 spp.), Fig. 3. Our analysis indicates that these clades evolved from ancestors that spun capture webs whose overall architectures were largely defined by the substrates on which webs were placed. These spiders spin an array of different sheet-like webs distinguishable by the silk used to construct them or the details of how threads are interconnected, but which all share relatively variable shapes constrained by the locations in which they are spun. In contrast, diversification in Orbiculariae and RTA clade is associated with transformations away from these ancestral, terrestrial sheet webs. Strikingly, the Orbiculariae and RTA clade encompass >2/3 of all known spider species, making them extremely diverse compared with any of their likely sister groups [certainly compared with Eresoidea (e.g., Oecobiidae), their sister group in this study]. In both groups, diversity is dominated by species that no longer spin substrate-bound webs nor rely on expensive cribellate sticky silk for prey capture.
Fig. 3.
Association between web-spinning behaviors and species diversity of spider clades. The phylogeny is a summary of the results presented in Fig. 2. Size of circles is proportional to total species diversity of each clade, as also indicated by numbers. The branch colors indicate inferred ancestral state of web spinning behaviors for each clade, and the colored pie charts represent estimated proportion of each behavior within the clade. Diversity data are summarized from the World Spider Catalog (http://research.amnh.org/entomology/spiders/catalog/INTRO1.html).
The monophyletic origin of orbs implies 2 major concurrent transformations in spinning behaviors. First, extreme behavioral stereotypy allowed spinning of the highly regularly spaced radial lines and adhesive capture spirals of orb webs. Second, the suspension of webs on frames of discrete structural threads of major ampullate silk freed webs from constraints of the substrate and allowed occupation of novel niches. In particular, vertical orbs may have allowed spiders access to abundant flying insects concomitant with the ability to spin the viscid sticky threads needed to snare those prey.
The putative sister group of Orbiculariae in our hypothesis, the RTA clade, is even more diverse (>21,000). Hence, we cannot, by sister group comparison alone, claim that the invention of the orb web per se dramatically affected diversification rates. Notably, the most diverse families of spiders within the RTA clade are largely, or entirely webless, and have secondarily lost cribellate silk [e.g., ecribellate lycosoids and Dionycha: jumping spiders (Salticidae, ≈5,200 spp.), crab spiders (Thomisidae, ≈2,100 spp.), wolf spiders (Lycosidae, ≈2,300 spp.), and ground spiders (Gnaphosidae, ≈2,100 spp.)]. The key similarity is that spiders again shifted ecologies away from the constraints of substrate-bound sheet webs, this time by abandoning capture webs altogether rather than suspending webs in the air. Although we currently lack the detailed phylogenetic hypothesis of RTA clade relationships necessary to conduct sister group comparisons of diversification, it is clear from existing phylogenies that many of these families lost capture webs independently of one another (19, 22, 37), supporting a strong selective advantage to web abandonment.
Perhaps more important, many RTA clade spiders lost the cribellum, an event occurring repeatedly within this group (37). More than 90% of RTA clade species, even in families that still use prey capture webs, are ecribellate—a proportion comparable to Orbiculariae (57). Cribellate capture threads appear very costly for spiders (11, 58). Viscid capture silk of the Araneoidea can be laid down rapidly, allowing webs to be spun in as little as 30 min, rather than the 3 h that cribellate orb weaving spiders require to physically comb out their adhesive capture silk (10). This results in metabolic savings, increased foraging time, and potentially reduced exposure to predators. The lower cost of viscid capture silk also likely facilitates web renewal and relocation during conditions of low prey densities. In comparison, many cribellate orbweavers exhibit higher site tenacity, presumably because of the high cost of web production (59).
Evolution Beyond the Orb Web.
The most species-rich families within Orbiculariae are the linyphiids and the theridiids. Each has independently transformed the orb into different aerial sheets. In both cases, these webs are distinguished from ancestral sheets not only in being aerial but also in the overall stereotypy of their architectures, a characteristic inherited from orb weaving ancestors. This suggests that it was not the spinning of webs with sheet-like architectures per se that limited diversification of basal spiders, but rather constraints imposed from having web shape defined by microhabitat location and the costliness of producing the cribellate silk.
We speculate that the success, both in terms of species richness and abundance, of linyphiid and theridiid spiders may be due in part to further reductions in the cost of web spinning from sparse use of glue. Linyphiid webs contain few glue droplets and often lack sticky silk altogether whereas theridiid cobwebs restrict glue to the termini of sticky gumfooted threads. This continues the trend in economization of glue production during the transition from cribellate to ecribellate orb-weavers. Interestingly, ecribellate orb spiders are the only taxa known to regularly recycle webs (58, 60, 61). Sheet and cobweb weavers do not consume their own webs, whereas spider kleptoparasites (Argyrodinae) consume silk from their host orb webs, suggesting it is a valuable resource. Although often interpreted as a mechanism to economize on the protein in silk (58, 61), we suggest that web recycling may have to do with recovery of materials in the viscid glue itself (see also ref. 60), explaining why derived orbicularian taxa lost this behavior.
We suspect that other factors also played important roles in diversification of derived orb-weaving spiders. Many theridiids specialize on ants, an ecologically dominant resource avoided by other spiders. Moreover, both theridiids and linyphiids spin highly 3-dimensional webs that allow escape from common predators of orb spiders, especially parasitic wasps (4). Finally, although the evolution of extreme stereotypy in spinning behaviors appears to have been a crucial prerequisite for the transition from substrate defined sheet webs to architecturally defined aerial orbs, subsequent loss of that stereotypy may then allow continued diversification of web shape and thus occupation of novel niches (62, 63). In other words, like the orb architecture itself, stereotypy of spinning behaviors is not an evolutionary end point but rather a stepping stone that allows for new web architectures to evolve.
Summary.
Silk use is central to spider ecologies and our analyses reveal how evolutionary shifts in web production likely relate to the diversification of major spider radiations. Both molecular and morphological data support single origins for both orb and RTA clade spiders. We argue that the evolution of aerial orb webs and the evolution of webless hunting strategies were crucial for these 2 groups to escape the constraints of ancestral, substrate-defined cribellate prey capture webs. In the case of the orb, its symmetrical design is distinguished from ancestral webs by an overall architecture defined by the spinning behaviors of the spider, rather than the shape of its microhabitat location. However, the orb is certainly not the final apex of web design. Our analyses also demonstrate subsequent rampant transformation of the orb into other architectures, suggesting that the origin of stereotypical orb webs provides a critical gateway for the evolution of novel web types and the diversification associated with them.
Methods
Taxon, Gene, and Morphology Sampling.
We selected 44 species from 24 families (Table S1) to broadly represent modern orb spiders, hypothesized outgroups, and distantly related spiders. To test monophyly of orbicularian spiders we included 4 genera from 2 families of cribellate orb weavers, 14 genera from 4 families of ecribellate orb weavers, and 9 genera from 5 families that morphological evidence place as descendants of orb-weaving ancestors but who now spin nonorb architectures or no prey-catching webs at all. We sampled 17 genera from 13 families as potential outgroups, including the nicodamids Megadictyna, Nicodamus and Novodamus, several representatives of the diverse RTA clade, Oecobiidae, and Austrochilidae, and the more distantly related Haplogynae and Mygalomorphae.
We used routine DNA extraction, amplification, and sequencing methods for partial fragments of 2 mitochondrial (16S rRNA) and 4 nuclear (18S rRNA, 28S rRNA, H3, wingless) loci, providing ≈4,600 bp of data (Table S2). Five loci were used in previous studies of spider phylogeny (29, 64, 65). However, our study includes a new marker for spider systematics, wingless gene (wnt1), that we obtained by modifying lepidopteran primers (66) to match spider cDNA sequences (Cupiennius (67) and Achaearanea GenBank accession no. AB167808).
We assembled a morphological matrix from the literature (8, 19, 42, 62, 68–74), extracting 143 characters (SI Appendix, section 5). Ten genera lacked morphological data, which led to the construction of 2 matrices: The full 44 taxon dataset missing some morphological/ethological data, and a reduced dataset of 34 taxa for which both molecular and morphological/ethological data were available for all taxa. In 5 cases, we used morphological data from closely related genera to complete the 34 taxon matrix [Thaida for Austrochilus (Austrochilidae), Tricholathys for Mexitlia (Dictynidae), Steatoda for Latrodectus (Theridiidae), Linyphia for Neriene (Linyphiidae) and Neoramia for Agelenopsis (Agelenidae)]. Missing data were scored as question marks.
Phylogenetic Analysis.
We conducted 64 different analyses, using 4 phylogenetic approaches—a model-based approach (Bayesian), equal weights MP, implied weights MP, all for 2 different alignments of ribosomal data, and an implied alignment approach (POY). We analyzed molecular data in isolation and combined with morphological data for both the full and reduced datasets. Finally, we analyzed the morphological data alone as a 65th analysis. Details are in SI Appendix.
Ancestral Character Reconstruction.
We reconstructed ancestral characters in Mesquite 2.5 (75) to examine trait evolution and test the single origin of the orb web, using both equal-weights MP and ML [one parameter MK1 model (76)].
Web Homology.
The extreme regularity of orbs makes their coding straightforward. Indeed, similarity in architecture and spinning behaviors leads to the a priori hypothesis of orb homology that we test herein. Most other webs appear, at least superficially, less regular such that recognizing potential homologies among distantly related taxa is difficult.
We delimit diagnostic characteristics (see SI Appendix) to differentiate potentially homologous categories of sheet webs. Eight major web categories emerge: simple terminal line webs (Ariadna, Plectreurys); brushed sheet webs (Euagrus, Oecobius, and, secondarily, Agelenopsis), irregular ground sheet webs (Callobius, Megadictyna, Dictyna, Mexitlia, Badumna), irregular aerial sheet webs (Austrochilus, Megadictyna), stereotyped aerial sheet webs (Erigone, Linyphia, Pimoa), cobwebs, or sticky gumfooted thread webs (Steatoda, Spintharus, Phoroncidia), bolas webs (Mastophora), and orb webs (Acanthepeira, Araneus, Argiope, Cyrtophora, Gasteracantha, Gertschanapis, Leucauge, Meta, Metellina, Nephila, Nephilengys, Phonognatha, Tetragnatha, Zygiella, Deinopis, Hyptiotes, Uloborus, Waitkera). This categorization ignores the type of sticky silk in webs because it seems to be far less conservative evolutionarily than web architecture. For instance, cribellate silk is often lost in derived lineages (37).
Supplementary Material
Acknowledgments.
We thank Charles Griswold (California Academy of Sciences, San Francisco), Marshal Hedin (San Diego State University, San Diego), Brent Opell (Virginia Polytechnic Institute, Blacksburg, VA), and Meghan Rector (Ohio State University, Columbus, OH) for providing specimens for this study; Mark Stowe for assisting in specimen collection; Mariam Lekveishvili and Alpana Chaudhuri for assisting with some sequencing; Ward Wheeler, Gonzalo Giribet, and Dimitar Dimitrov for advising us on POY4. This work was supported by National Science Foundation Awards DEB-0516038 (to T.A.B.), DEB-0516028 (to J.W.W.), and EAR-0228699 (to J.C.); Slovenian Research Agency Research Fellowship ARRS Z1-9799-0618-07 (to I.A.), Danish Natural Science Research Council Award 21020502 (to N.S.); and Marie Curie Intra European Fellowship Award 025850 (to T.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901377106/DCSupplemental.
References
- 1.Wise DH. Spiders in Ecological Webs. New York: Cambridge Univ Press; 1993. p. 328. [Google Scholar]
- 2.Foelix RF. Biology of Spiders. 2nd Ed. New York: Oxford Univ Press; 1996. p. 330. [Google Scholar]
- 3.Bond JE, Opell BD. Testing adaptive radiation and key innovation hypotheses in spiders. Evolution. 1998;52:403–414. doi: 10.1111/j.1558-5646.1998.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 4.Blackledge TA, Coddington JA, Gillespie RG. Are three-dimensional spider webs defensive adaptations? Ecol Lett. 2003;6:13–18. [Google Scholar]
- 5.Shear WA, editor. Spiders: Webs, Behavior, and Evolution. Stanford, CA: Stanford Univ Press; 1986. p. 492. [Google Scholar]
- 6.Craig CL. Spider Webs and Silk: Tracing Evolution from Molecules to Genes to Phenotypes. New York: Oxford Univ Press; 2003. p. 256. [Google Scholar]
- 7.Vollrath F, Selden P. The role of behavior in the evolution of spiders, silks, and webs. Ann Rev Ecol Evol Systematics. 2007;38:819–846. [Google Scholar]
- 8.Coddington JA. Cladistics and spider classification Araneomorph phylogeny and the monophyly of orb-weavers (Araneae: Araneomorphae: Orbiculariae) Acta Zoologica Fennica. 1990;190:75–88. [Google Scholar]
- 9.Hawthorn AC, Opell BD. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J Exp Biol. 2003;206:3905–3911. doi: 10.1242/jeb.00618. [DOI] [PubMed] [Google Scholar]
- 10.Zschokke S, Vollrath F. Unfreezing the behaviour of two orb spiders. Physiol Behav. 1995;58:1167–1173. doi: 10.1016/0031-9384(95)02062-4. [DOI] [PubMed] [Google Scholar]
- 11.Opell BD. The material cost and stickiness of capture threads and the evolution of orb-weaving spiders. Biol J Linnean Soc. 1997;62:443–458. [Google Scholar]
- 12.Kaston BJ. The evolution of spider webs. Am Zool. 1964;4:191–207. [Google Scholar]
- 13.Kullmann EJ. Convergent development of orb webs in cribellate and ecribellate spiders. Am Zool. 1972;12:395–405. [Google Scholar]
- 14.Eberhard WG. Behavioral characters for the higher classification of orb-weaving spiders. Evolution. 1982;36:1067–1095. doi: 10.1111/j.1558-5646.1982.tb05475.x. [DOI] [PubMed] [Google Scholar]
- 15.Coddington J. Monophyletic origin of orb webs. Am Zool. 1982;22:886–886. [Google Scholar]
- 16.Eberhard WG. Function and phylogeny of spider webs. Annu Rev Ecol Syst. 1990;21:341–372. [Google Scholar]
- 17.Coddington J. In: Spiders: Webs, Behavior and Evolution. Shear WA, editor. Stanford, CA: Stanford Univ Press; 1986. pp. 319–363. [Google Scholar]
- 18.Coddington JA. Orb webs in “non-orb weaving” ogre-faced spiders (Araneae: Dinopidae): A question of genealogy. Cladistics. 1986;2:53–67. doi: 10.1111/j.1096-0031.1986.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 19.Griswold CE, Ramírez MG, Coddington JA, Platnick NI. Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proc CA Acad Sci. 2005;56(Suppl II):1–324. [Google Scholar]
- 20.Griswold CE, Coddington JA, Platnick NI, Forster RR. Towards a phylogeny of entelegyne spiders (Araneae, Araneomorphae, Entelegynae) J Arachnol. 1999;27:53–63. [Google Scholar]
- 21.Opell BD, Schwend HS. Persistent stickiness of viscous capture threads produced by araneoid orb-weaving spiders. J Exp Zool A. 2008;309A:11–16. doi: 10.1002/jez.426. [DOI] [PubMed] [Google Scholar]
- 22.Coddington JA, Levi HW. Systematics and evolution of spiders (Araneae) Annu Rev Ecol Syst. 1991;22:565–592. [Google Scholar]
- 23.Lopardo L, Hormiga G. Phylogenetic placement of the Tasmanian spider Acrobleps hygrophilus (Araneae, Anapidae) with comments on the evolution of the capture web in Araneoidea. Cladistics. 2008;24:1–33. [Google Scholar]
- 24.Agnarsson I, Maddison WP, Avilés L. The phylogeny of the social Anelosimus spiders (Araneae : Theridiidae) inferred from six molecular loci and morphology. Mol Phylogenet Evol. 2007;43:833–851. doi: 10.1016/j.ympev.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Arnedo MA, Gillespie RG. Species diversification patterns in the Polynesian jumping spider genus Havaika Proszynski, 2001 (Araneae, Salticidae) Mol Phylogenet Evol. 2006;41:472–495. doi: 10.1016/j.ympev.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Arnedo MA, Agnarsson I, Gillespie RG. Molecular insights into the phylogenetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna. Zoologica Scripta. 2007;36:337–352. [Google Scholar]
- 27.Bond JE, Stockman AK. An integrative method for delimiting cohesion species: Finding the population-species interface in a group of Californian trapdoor spiders with extreme genetic divergence and geographic structuring. Syst Biol. 2008;57:628–646. doi: 10.1080/10635150802302443. [DOI] [PubMed] [Google Scholar]
- 28.Hedin M, Bond JE. Molecular phylogenetics of the spider infraorder Mygalomorphae using nuclear rRNA genes (18S and 28S): Conflict and agreement with the current system of classification. Mol Phylogenet Evol. 2006;41:454–471. doi: 10.1016/j.ympev.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Arnedo MA, Coddington J, Agnarsson I, Gillespie RG. From a comb to a tree: Phylogenetic relationships of the comb-footed spiders (Araneae, Theridiidae) inferred from nuclear and mitochondrial genes. Mol Phylogenet Evol. 2004;31:225–245. doi: 10.1016/S1055-7903(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 30.Maddison WP, Hedin MC. Jumping spider phylogeny (Araneae : Salticidae) Invertebr Syst. 2003;17:529–549. [Google Scholar]
- 31.Benjamin SP, Dimitrov D, Gillespie RG, Hormiga G. Family ties: Molecular phylogeny of crab spiders (Araneae: Thomisidae) Cladistics. 2008;24:708–722. [Google Scholar]
- 32.Arnedo M, Hormiga G, Scharff N. Higher level phylogenetics of linyphiid spiders (Araneae, Linyphiidae) based on morphological and molecular evidence. Cladistics. 2009 doi: 10.1111/j.1096-0031.2009.00249.x. in press. [DOI] [PubMed] [Google Scholar]
- 33.Gillespie RG. Geographical context of speciation in a radiation of Hawaiian Tetragnatha spiders (Araneae, Tetragnathidae) J Arachnol. 2005;33:313–322. [Google Scholar]
- 34.Hormiga G, Arnedo M, Gillespie RG. Speciation on a conveyor belt: Sequential colonization of the Hawaiian islands by Orsonwelles spiders (Araneae, Linyphiidae) Syst Biol. 2003;52:70–88. doi: 10.1080/10635150390132786. [DOI] [PubMed] [Google Scholar]
- 35.Agnarsson I. Phylogenetic placement of Echinotheridion (Araneae : Theridiidae)—do male sexual organ removal, emasculation, and sexual cannibalism in Echinotheridion and Tidarren represent evolutionary replicas? Invertebr Syst. 2006;20:415–429. [Google Scholar]
- 36.Rix MG, Harvey MS, Roberts JD. Molecular phylogenetics of the spider family Micropholcommatidae (Arachnida : Araneae) using nuclear rRNA genes (18S and 28S) Mol Phylogenet Evol. 2008;46:1031–1048. doi: 10.1016/j.ympev.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Spagna JC, Gillespie RG. More data, fewer shifts: Molecular insights into the evolution of the spinning apparatus in non-orb-weaving spiders. Mol Phylogenet Evol. 2008;46:347–368. doi: 10.1016/j.ympev.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Ayoub NA, Garb JE, Hedin M, Hayashi CY. Utility of the nuclear protein-coding gene, elongation factor-1 gamma (EF-1 gamma), for spider systematics, emphasizing family level relationships of tarantulas and their kin (Araneae : Mygalomorphae) Mol Phylogenet Evol. 2007;42:394–409. doi: 10.1016/j.ympev.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Vink CJ, et al. Actin 5C, a promising nuclear gene for spider phylogenetics. Mol Phylogenet Evol. 2008;48:377–382. doi: 10.1016/j.ympev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Hausdorf B. Molecular phylogeny of araneomorph spiders. J Evol Biol. 1999;12:980–985. [Google Scholar]
- 41.Garb JE, DiMauro T, Vo V, Hayashi CY. Silk genes support the single origin of orb webs. Science. 2006;312:1762. doi: 10.1126/science.1127946. [DOI] [PubMed] [Google Scholar]
- 42.Griswold CE, Coddington JA, Hormiga G, Scharff N. Phylogeny of the orb-web building spiders (Araneae, Orbiculariae : Deinopoidea, Araneoidea) Zool J Linn Soc. 1998;123:1–99. [Google Scholar]
- 43.Stowe MK. In: Spiders: Webs, Behavior, and Evolution. Shear WA, editor. Stanford: Stanford Univ Press; 1986. pp. 101–131. [Google Scholar]
- 44.Gillespie RG. Predation through impalement of prey: The foraging behavior of Doryonychus raptor (Araneae, Tetragnathidae) Psyche. 1991;98:337–350. [Google Scholar]
- 45.Gillespie RG, Croom HB, Palumbi SR. Multiple origins of a spider radiation in Hawaii. Proc Natl Acad Sci USA. 1994;91:2290–2294. doi: 10.1073/pnas.91.6.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levi HW. The orb-weaver genera Metepeira, Kaira and Aculepeira in America north of Mexico (Araneae, Araneidae) Bull Mus Compar Zool. 1977;148:185–238. [Google Scholar]
- 47.Opell BD. Increased stickiness of prey capture threads accompanying web reduction in the spider family Uloboridae. Funct Ecol. 1994;8:85–90. [Google Scholar]
- 48.Blackledge TA, Hayashi CY. Unraveling the mechanical properties of composite silk threads spun by cribellate orb-weaving spiders. J Exp Biol. 2006;209:3131–3140. doi: 10.1242/jeb.02327. [DOI] [PubMed] [Google Scholar]
- 49.Gosline JM, Denny MW, Demont ME. Spider silk as rubber. Nature. 1984;309:551–552. [Google Scholar]
- 50.Opell BD. Redesigning spider webs: Stickiness, capture area and the evolution of modern orb-webs. Evol Ecol Res. 1999;1:503–516. [Google Scholar]
- 51.Opell BD, Bond JE, Warner DA. The effects of capture spiral composition and orb-web orientation on prey interception. Zoology. 2006;109:339–345. doi: 10.1016/j.zool.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Heimer S, Nentwig W. Thoughts on the phylogeny of the Araneoidea Latreille, 1806 (Arachnida, Araneae) Zeitschrift Fur Zoologische Systematik Und Evolutionsforschung. 1982;20:284–295. [Google Scholar]
- 53.Shear WA. The Evolution of Web-Building Behavior in Spiders: A Third Generation of Hypotheses. Stanford, CA: University Press; 1986. [Google Scholar]
- 54.Coddington JA. Spinneret silk spigot morphology: Evidence for the monophyly of orb-weaving spiders, Cyrtophorinae (Araneidae), and the group Theridiidae plus Nesticidae. J Arachnol. 1989;17:71–96. [Google Scholar]
- 55.Kovoor J. L'appareil séricigène dans le genre Uloborus Latr. (Araneae: Uloboridae) Revue Arachnologique. 1977;1:89–102. [Google Scholar]
- 56.Hu XY, et al. Analysis of aqueous glue coating proteins on the silk fibers of the cob weaver, Latrodectus hesperus. Biochemistry. 2007;46:3294–3303. doi: 10.1021/bi602507e. [DOI] [PubMed] [Google Scholar]
- 57.Platnick NI. The world spider catalog, version 9.0. New York: American Museum of Natural History; 2008. Available at http://research.amnh.org/entomology/spiders/catalog/index.html. [Google Scholar]
- 58.Opell BD. Economics of spider orb-webs: The benefits of producing adhesive capture thread and of recycling silk. Funct Ecol. 1998;12:613–624. [Google Scholar]
- 59.Kawamoto TH, Japyassú HF. Tenacity and silk investment of two orb weavers: Considerations about the Araneoidea diversification. J. Arachnol. 2008;36:418–424. [Google Scholar]
- 60.Townley MA, Tillinghast EK, Neefus CD. Changes in composition of spider orb web sticky droplets with starvation and web removal, and synthesis of sticky droplet compounds. J Exp Biol. 2006;209:1463–1486. doi: 10.1242/jeb.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carico JE. In: Spiders: Webs, Behavior, and Evolution. Shear WA, editor. Stanford, CA: Stanford Univ Press; 1986. [Google Scholar]
- 62.Agnarsson I. Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae) Zool J Linn Soc. 2004;141:447–626. [Google Scholar]
- 63.Eberhard WG, Agnarrson I, Levi HW. Web forms and phylogeny of theridiid spiders (Araneae: Theridiidae) Systematics and Biodiversity. 2008;6:415–475. [Google Scholar]
- 64.Hormiga G. Orsonwelles, a new genus of giant linyphiid spiders (Araneae) from the Hawaiian Islands. Invertebr Syst. 2002;16:369–448. [Google Scholar]
- 65.Hedin MC, Maddison WP. A combined molecular approach to phylogeny of the lumping spider subfamily Dendryphantinae (Araneae : Salticidae) Mol Phylogenet Evol. 2001;18:386–403. doi: 10.1006/mpev.2000.0883. [DOI] [PubMed] [Google Scholar]
- 66.Brower AVZ, DeSalle R. Patterns of mitochondrial versus nuclear DNA sequence divergence among nymphalid butterflies: The utility of wingless as a source of characters for phylogenetic inference. Insect Mol Biol. 1998;7:73–82. doi: 10.1046/j.1365-2583.1998.71052.x. [DOI] [PubMed] [Google Scholar]
- 67.Damen WGM. Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development. 2002;129:1239–1250. doi: 10.1242/dev.129.5.1239. [DOI] [PubMed] [Google Scholar]
- 68.Coddington JA. Ontogeny and homology in the male palpus of orb-weaving spiders and their relatives with comments on phylogeny Araneoclada Araneoidea Deinopoidea. Smithsonian Contr Zool. 1990;(496):1–49. [Google Scholar]
- 69.Platnick NI, Coddington JA, Forster RR, Griswold CE. Spinneret morphology and the phylogeny of haplogyne spiders (Araneae, Araneomorphae) Am Mus Novitates. 1991:1–73. [Google Scholar]
- 70.Hormiga G, Eberhard WG, Coddington JA. Web-construction behaviour in Australian Phonognatha and the phylogeny of nephiline and tetragnathid spiders (Araneae: Tetragnathidae) Aust J Zool. 1995;43:313–364. [Google Scholar]
- 71.Scharff N, Coddington JA. A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae) Zool J Linn Soc. 1997;120:355–434. [Google Scholar]
- 72.Álvarez-Padilla F. Systematics of the spider genus Metabus O. P.-Cambridge, 1899 (Araneoidea : Tetragnathidae) with additions to the tetragnathid fauna of Chile and comments on the phylogeny of Tetragnathidae. Zool J Linn Soc. 2007;151:285–335. [Google Scholar]
- 73.Kuntner M, Coddington JA, Hormiga G. Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): Testing morphological and ethological homologies. Cladistics. 2008;24:147–217. [Google Scholar]
- 74.Schütt K. The limits of the Araneoidea (Arachnida : Araneae) Aust J Zool. 2000;48:135–153. [Google Scholar]
- 75.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis, Version 2.5. 2008. Available at http://mesquiteproject.org.
- 76.Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.