Abstract
Alzheimer disease (AD) is the most common cause of dementia in adults. The current therapy for AD has only moderate efficacy in controlling symptoms, and it does not cure the disease. Recent studies have suggested that abnormal hyperphosphorylation of tau in the brain plays a vital role in the molecular pathogenesis of AD and in neurodegeneration. This article reviews the current advances in understanding of tau protein, regulation of tau phosphorylation, and the role of its abnormal hyperphosphorylation in neurofibrillary degeneration. Furthermore, several therapeutic strategies for treating AD on the basis of the important role of tau hyperphosphorylation in the pathogenesis of the disease are described. These strategies include (1) inhibition of glycogen synthase kinase-3β (GSK-3β), cyclin-dependent kinase 5 (cdk5), and other tau kinases; (2) restoration of PP2A activity; and (3) targeting tau O-GlcNAcylation. Development of drugs on the basis of these strategies is likely to lead to disease-modifying therapies for AD.
Keywords: Alzheimer disease, tau, neurodegeneration, GSK-3β, cdk5, protein phosphatases, O-GlcNAcylation, therapy
INTRODUCTION
Alzheimer disease (AD) is a chronic neurodegenerative disease that is characterized clinically by a progressive decline of cognitive function, leading to dementia. The disease eventually leads to the death of affected individuals an average of nine years after diagnosis [1]. Approximately 27 million individuals are suffering from AD worldwide, and it accounts for the majority of cases of dementia in adults. For the last three decades, the standard treatments for AD have been acetylcholinesterase inhibitors to improve cognitive function, and other drugs to manage the mood disturbance, agitation and psychosis that often occur in the later stages of the disease. In the recent years, memantine, an NMDA (Nmethyl-D-aspartate) receptor antagonist and a potentially neuroprotective agent, has been widely used. However, all of these treatments show only modest symptomatic effects. The major barrier to effective treatments is the lack of full understanding of the mechanism of AD.
The last two decades have marked a very significant era of AD research. During this period, the nature of amyloid plaques and neurofibrillary tangles (NTFs), the two histopathological hallmarks of AD, has been elucidated. Recent research efforts have led to several hypotheses to explain AD. As a result, numerous new therapeutic drugs for AD are at various stages of development. An internet search on “Alzheimer's disease” at the NIH's website “clinicalTrials.gov” (http://www.clinicaltrials.gov) yielded 180 ongoing trials of interventional studies for, or related to, AD at the time of this manuscript preparation. As some of the molecular pathways involved in the pathogenesis of AD have been revealed, it is time to develop a disease-modifying therapy for AD.
Amyloid β (Aβ) toxicity is believed to play a primary role in the development of AD [2]. Thus, anti-amyloid strategies have been the primary focus of AD drug development for the last 10 years. Recently, more and more evidence has demonstrated a crucial role of tau abnormalities in AD neurodegeneration, suggesting that tau could be a promising therapeutic target for developing disease-modifying drugs of AD. In this article, we first describe tau protein and the tau abnormalities involved in AD, followed by the molecular mechanism of neurofibrillary degeneration. Then, we discuss the therapeutic strategies that are based on reversal of abnormal hyperphosphorylation of tau.
TAU PROTEIN
Tau was first discovered as a microtubule-associated protein (MAP) that stimulates tubulin assembly into microtubules in the brain [3]. There was not much research interest in tau protein until a decade later, when it was found to make up the paired helical filaments (PHFs) of NFTs in AD brain [4, 5]. Human tau gene is located on the long arm of chromosome 17 (position 17q21) and was found to contain 16 exons [6]. This single tau gene encodes six tau isoforms in adult human brain as a result of alternative splicing of its mRNA [7]. The six tau isoforms differ from each other by the presence or absence of one or two inserts (29 or 58 amino acids) in the N-terminal part and by the presence of either three or four repeats in the C-terminal half. The N-terminal inserts are highly acidic. The repeats in the C-terminal half of tau are the domains by which tau binds to microtubules [8-10].
Each of the six tau isoforms possibly has its particular physiological role and differential biological activities, because they are differentially expressed during development and stimulate microtubule assembly with different efficiencies [11-13]. Only the shortest isoform of tau is expressed in fetal brain, whereas all six isoforms are seen in adult human brain [12, 13]. Altered proportions of various tau isoforms have been observed in several neurodegenerative diseases such as frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) and Pick disease (reviewed in [14]). Tau's ability to bind microtubules probably may have both beneficial and negative consequences. The beneficial effect of tau is to stabilize microtubules, permitting neurites' extension and stabilization. The negative effect is that tau might compete with the motor protein kinesin for microtubule binding, leading to decreased axonal transport [15-18]. This harmful effect of tau may explain the symptoms of amyotrophic lateral sclerosis with neurofilament accumulation in motor neurons in several transgenic mouse models of tau overexpression [17, 19-21]. However, a recent study, which showed no defects of axonal transport in mice that either over-express tau up to 4-folds over endogenous tau level or do not express tau at all [22], challenges this view.
ABNORMAL HYPERPHOSPHORYLATION OF TAU — THE KEY PLAYER OF NEUROFIBRILLARY DEGENERATION IN AD
Abnormal Hyperphosphorylation of Tau in AD
Tau is modified post-translationally by several ways in both normal and pathological conditions. These modifications include phosphorylation, glycosylation, ubiquitination, glycation, polyamination, nitration, truncation, and aggregation. Thorough reviews of the post-translational modifications of tau have been published elsewhere [23, 24]. Here, we focus our discussion only on alterations of tau phosphorylation because its role in AD neurodegeneration is most established.
Tau was found to be a phosphoprotein as early as 1977 [25]. Several years later, Lindwall and Cole [26] demonstrated that phosphorylation of tau negatively regulates its activity in promoting microtubule assembly. After the discovery that abnormally hyperphosphorylated tau is the major component of PHFs in AD, tau phosphorylation has been studied extensively. Normal brain tau contains 2–3 moles of phosphate per mole tau [27-29]. Studies on human brain biopsy tissue indicated that several serine and threonine residues of tau are normally phosphorylated at low substoichiometrical levels [30, 31]. The phosphorylation level of tau isolated from autopsied AD brain is 3- to 4-fold higher than that of normal human brains [27-29]. To date, more than 40 phosphorylation sites have been identified in tau protein isolated from AD brain. These hyperphosphorylation sites have been listed in a recent review [23] and updated further by a mass spectrometry study [32].
Tau phosphorylation at different sites has a different impact on its biological function and on its pathogenic role. A quantitative in vitro study demonstrated that phosphorylation of tau at Ser262, Thr231, and Ser235 inhibits its binding to microtubules by ∼35%, ∼25%, and ∼10%, respectively [33]. In vitro kinetic studies of the binding between hyperphosphorylated tau and normal tau suggest that Ser199/Ser202/Thr205, Thr212, Thr231/Ser235, Ser262/Ser356, and Ser422 are among the critical phosphorylation sites that convert tau to an inhibitory molecule that sequesters normal microtubule-associated proteins from microtubules [34]. Further phosphorylation at Thr231, Ser396, and Ser422 promotes self-aggregation of tau into filaments. Similarly, mutation of tau at Ser396 and Ser404 into Glu to mimic phosphoserine converts it to be more fibrillogenic [35], and a tau construct in which Ser422 is mutated to Glu shows a significantly increased propensity to aggregate [36]. It is obvious that tau phosphorylation at various sites impacts tau activity and aggregation collectively. Our recent study has demonstrated that tau phosphorylation at the proline-rich region, which is located upstream of the microtubule-binding domains, inhibits its microtubule assembly activity moderately and promotes its self-aggregation slightly. Tau phosphorylation at the C-terminal tail region increases its activity and promotes its self-aggregation markedly. Tau phosphorylation at both of these regions plus the microtubule-binding region nearly diminishes its activity and disrupts microtubules [37].
Although PHF-tau loses its ability to stimulate microtubules, the lack of overt phenotype of tau knockout transgenic mice [38-40] suggests that loss of normal function due to tau hyperphosphorylation may not be sufficient to lead to neurodegeneration. Instead, both the abnormally hyperphosphorylated tau isolated from AD brain and the in vitro hyperphosphorylated tau gain a toxic ability to sequester normal tau and other MAPs, such as MAP1 and MAP2, and cause microtubule disassembly [41-44]. Upon dephosphorylation, they lose this toxic ability. Thus, it is likely that the abnormal hyperphosphorylation of tau causes neurodegeneration by gain of toxic function rather than by loss of normal activity that can be compensated for by other MAPs.
The exact molecular basis of toxicity caused by abnormal tau remains to be an area of intense research. Earlier studies showing a strong correlation between the number of NFTs in the brain and the severity of dementia [45-47] suggested that the aggregated NFTs might cause neurodegeneration. This view was recently challenged by studies demonstrating that the unpolymerized abnormal tau or the oligomers, rather than the highly polymerized PHFs, are toxic. A correlation study of biopsied human brain tissue indicated no relationship between microtubule attenuation in AD/aging and tau filament formation [48]. In a conditional transgenic mouse model that expresses tau with the FTDP-17 P301L mutation and shows NFT pathology together with neuronal loss and behavioral deficits, when the mutant tau expression is turned off at the age of four months, the removal of mutant tau leads to reversal of the behavioral deficits, whereas NFT pathology continues to progress [49]. In vitro, polymerization of hyperphosphorylated tau into PHFs also abolishes its toxic activity to sequester other MAPs [50]. Thus, NFTs could be regarded as a marker of damage already done rather than a primary cause of neurodegeneration, in the same way that Aβ plaques have been viewed recently. Actually, polymerization of toxic abnormal tau into PHFs/NFTs could even be a defense mechanism by which neurons reduce the toxic activity of the abnormally hyperphosphorylated tau. This phenomenon is apparently common to other diseases characterized by abnormal protein aggregates, such as Huntington disease and cardiomyopathy, in which the abnormal non-fibrillar protein oligomers, rather then the protein aggregates themselves, appear to be pathogenic [51-53].
Potential Causes Leading to Abnormal Hyperphosphorylation of Tau in AD
The normal level of tau phosphorylation is a consequence of dynamic regulation of tau kinases and tau phosphatases. Numerous studies in the last decade have identified the major tau kinases and phosphatases in the brain. The major tau kinases include glycogen-synthase kinase-3β (GSK-3β), cyclin-dependent protein kinase 5 (cdk5), cAMP-dependent protein kinase (PKA), and stress-activated protein kinases (reviewed in [54, 55]). Unlike protein kinases, protein phosphatases (PP) usually have broad substrate specificities. However, we and others have found that PP2A is by far the most important and major tau phosphatase [56-59]. Therefore, targeting these enzymes is a logical consideration for reversing abnormal hyperphosphorylation of tau.
Despite extensive studies, the causes leading to abnormal hyperphosphorylation of tau are still not fully understood. Among tau kinases, cdk5 was reported to be upregulated in AD brain [60], but this observation has been challenged [61-64]. On the other hand, both the activity and the expression of PP2A are decreased in AD brain [59, 65-69]. Down-regulation of PP2A activity in AD brain might be partially attributed to the deregulation of two endogenous PP2A inhibitors, namely I1PP2A and I2PP2A [70, 71]. Consistent with the relatively broad substrate specificity of PP2A, several other neuronal proteins, such as neurofilaments, MAP1B, β-tubulin, and β-catenin, are also hyperphosphorylated in AD brain [72-74]. Thus, it appears that down-regulation of PP2A might, at least in part, underlie the abnormal hyperphosphorylation of tau in AD.
In addition to tau kinases and phosphatases, alterations of tau itself, the substrate of these enzymes, also plays a role in its hyperphosphorylation and aggregation. Besides phosphorylation, the serine/threonine residues of tau are modified by a monosaccharide called β-N-acetylglucosamine (GlcNAc) via a glycosidic bond, and this modification is called O-GlcNAcylation [75-77]. Most interestingly, O-GlcNAcylation regulates phosphorylation of tau inversely both in vitro and in vivo [76-79]. Tau O-GlcNAcylation was found to be indeed decreased in AD brain, and this decrease was correlated to tau hyperphosphorylation (Liu, et al. unpublished observations). A similar phenomenon has also been seen for neurofilaments [80]. Because tau O-GlcNAcylation is directly regulated by glucose metabolism that supplies Uridine diphosphate (UDP)–GlcNAc as a donor for protein O-GlcNAcylation, the above observations led us to propose a novel hypothesis that explains the molecular mechanism by which impaired glucose uptake/metabolism in AD brain, which has been well established for decades, contributes to neurodegeneration [81, 82]. In AD brain, impaired glucose metabolism may cause decreased tau O-GlcNAcylation which, in turn, facilitates hyperphosphorylation of tau that leads to neurofibrillary degeneration.
Mechanism of Neurofibrillary Degeneration
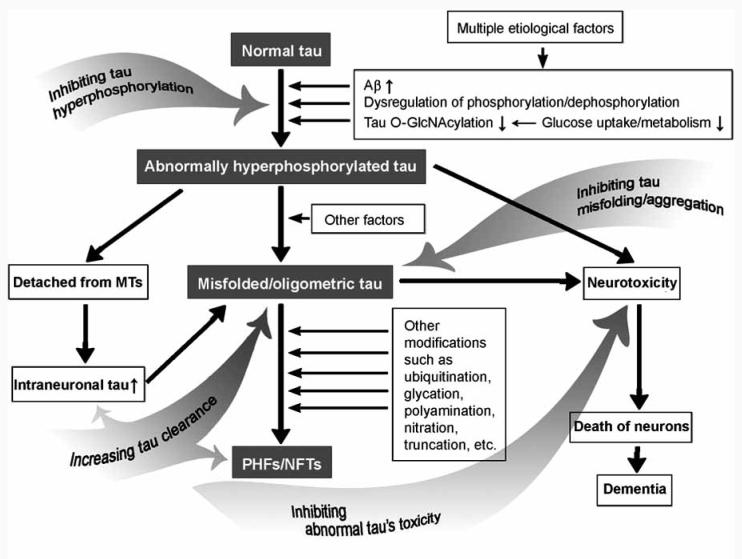
As discussed above, there is no doubt that abnormal hyperphosphorylation plays a central role in the neurofibrillary degeneration that is a leading cause of neuronal death in AD and other tauopathies. On the basis of recent studies, we propose the mechanism of neurofibrillary degeneration (Fig. 1).
Fig. (1).
Proposed mechanism of neurofibrillary degeneration and targets for therapeutic interventions. Multiple etiological factors cause abnormal hyperphosphorylation of tau via various pathways, including Aβ, dysregulation of phosphorylation/dephosphorylation, and impaired brain glucose metabolism. The abnormally hyperphosphorylated tau not only loses its biological activity and disassociates from microtubules (MTs), but also promotes its polymerization. The soluble abnormal tau and/or its oligomers are toxic to neurons and lead to neuronal death and dementia. Probably due to the defense mechanism of the neuron, abnormal tau further polymerizes into highly aggregated paired helical filaments (PHFs) and neurofibrillary tangles (NFTs) that might be inert but might finally choke the affected neurons and facilitate cell death. Thus, tau protein is obviously a promising target for developing disease-modifying therapy for AD. The gray arrows indicate several therapeutic strategies targeting neurofibrillary degeneration.
Multiple etiological factors cause abnormal hyperphosphorylation of tau via various pathways, including Aβ, dysregulation of phosphorylation/dephosphorylation, and impaired brain glucose metabolism. The abnormally hyperphosphorylated tau not only loses its biological activity and disassociates from MTs, but also promotes its polymerization. The soluble abnormal tau and/or its oligomers are toxic to neurons and lead to neuronal death and dementia. Probably due to the defense mechanism of the neuron, abnormal tau further polymerizes into highly aggregated PHFs/NFTs that might be inert but might finally choke the affected neurons and facilitate cell death.
The key role of tau abnormalities in neurodegeneration is further supported by recent in vivo studies. In a transgenic mouse model of amyloid pathology and cognitive deficits, the phenotype almost disappeared when tau was knocked out [83], indicating the essential role of tau in mediating neurodegeneration in this model. In another study, reduction of soluble Aβ and tau, but not Aβ alone, ameliorated cognitive decline in a 3×Tg-AD mouse model that develops amyloid plaques and NFTs [84].
TAU HYPERPHOSPHORYLATION — A THERAPEUTIC TARGET FOR AD
To develop disease-modifying therapy for AD, it is now obvious that tau protein is a promising target. On the basis of the key role tau abnormalities play in the neurodegeneration of AD, the therapeutic strategies may include (1) inhibiting and/or reversing tau hyperphosphorylation, (2) inhibiting tau misfolding/aggregation, (3) inhibiting abnormal tau's neurotoxicity, and (4) increasing tau's clearance (Fig. 1). Some of these strategies have been discussed recently [85-90]. We will limit our discussion now to inhibiting/reversing abnormal hyperphosphorylation of tau.
Targeting GSK-3β
Although there is no conclusive evidence showing an up-regulated tau kinase that could have caused hyperphosphorylation of tau in AD brain, the majority of drug developments in tau phosphorylation focus on kinase inhibitors rather than tau phosphatase activators. General pharmacologic experience indicates that enzyme inhibition by small molecules is much more easily realized than activation, especially if the target enzyme has a natural small-molecular binding site, such as kinases do for ATP. Most of the tau kinase–targeting drugs considered so far are GSK-3β inhibitors, because this kinase appears to play a critical role in AD pathogenesis [91, 92]. A recent study showed that GSK-3β activity may increase with aging [93], which is consistent with the fact that aging is the most important risk factor for AD. Both in vitro and in vivo studies have demonstrated that inhibition of GSK-3β, by either pharmacological or genetic means, can reverse hyperphosphorylation of tau and prevent behavioral impairments in mice [94-99]. These studies make GSK-3β inhibition very attractive as a therapeutic target for AD. Another important reason is that the human body tolerates GSK-3β inhibition well. Lithium, which inhibits GSK-3β both directly by competition with magnesium [100] and indirectly via the PI3K pathway [101-103], has been used for long-term treatment of bipolar disorders for decades. With the potentially pivotal role of GSK-3β in the pathogenesis of AD as well as in several other diseases such as type 2 diabetes and cancer, much effort directed at identifying selective GSK-3β inhibitors has been reported and recently reviewed [91, 92, 104-106]. Several GSK-3β inhibitors, including lithium, aloisines, flavopiridol, hymenialdisine, paullones, and staurosporine, are under active investigation and development, which have been reviewed in detail [55, 88]. Recent findings that GSK-3β inhibition may also inhibit Aβ production and help cell survival (reviewed in [91, 107]) make the development of GSK-3β inhibitors for treatment of AD even more attractive. However, critical questions regarding potential mechanism-based undesirable effects of the GSK-3β inhibitors linked to regulation of glucose metabolism and tumorigenic effects remain. Successful clinical development of a GSK-3β inhibitor for treating AD is both challenging and eagerly awaited.
Targeting cdk5
Cdk5 is another target kinase toward which pharmacological inhibitors are under development for treatment of AD. Many inhibitors of cdk5 have been described across several structural classes, although their selectivity for cdk5 over other cdk kinases important for cell cycle control is either poor or not disclosed (reviewed in [108, 109]). One of these inhibitors, purine roscovitine, has an IC50 of 0.16 μM for cdk5/p25 [110, 111] and useful selectivity for cdk5 over other kinases and can pass through the blood-brain barrier [112]. Administration of roscovitine showed a reduction of hyperphosphorylation of tau and other neuronal proteins in two different transgenic mouse models and in transient ischemic rats [113-115].
Other cdk5 inhibitors include purine olomoncine, flavopiridol, aloisines, and indirubins (reviewed in [88]). Many of these compounds are non-selective cdk inhibitors and have shown efficacy as anti-proliferatives. Their efficacy in inhibiting tau hyperphosphorylation has not been well studied, and their utility may be compromised by their cell cycle effects. Further truncation of p25, a potent cdk5 activator, was found to yield a cdk5 inhibitory peptide that specifically inhibits cdk5/p25 activity without affecting cdk5/p35 or mitotic cdk5 activities [116]. This inhibitory peptide was able to reduce tau hyperphosphorylation and neuronal death induced by cdk5/p25 and thus might be used for developing a specific cdk5 inhibition strategy in the treatment of neurodegeneration. A recent in vivo study demonstrated that inhibition of cdk5 could cause activation of GSK-3β that plays a more dominant role in overall tau phosphorylation than does cdk5 [93]. Thus, cdk5 inhibitors might be unable to reverse abnormal hyperphosphorylation of tau and treat neurofibrillary degeneration. The same study also demonstrated that cdk5 inhibitors can reduce Aβ level in the mouse brain. Therefore, cdk5 inhibitors deserve further investigation as potential therapeutic agents for AD.
Targeting Other Tau Kinases
In addition to GSK-3β and cdk5, other protein kinases that can catalyze tau phosphorylation have also been considered as targets for inhibiting tau hyperphosphorylation. These kinases include mitogen-activated protein kinases, casein kinases, Ca2+/calmodulin-dependent protein kinase II, microtubule affinity regulating kinase, and PKA, and the development of inhibitors of these kinases has been reviewed recently [88]. A common problem of the kinase inhibitors is their insufficient selectivity. However, a recent report using a less selective inhibitor, SRN-003-556, targeting GSK-3β, ERK2/cdc2, PKA, and PKC, demonstrated efficacy in reducing soluble aggregated hyperphosphorylated tau and delaying the motor deficits in the JNPL3 tau transgenic mice [97]. This study suggests that non-specific kinase inhibitors might be considered for developing AD drugs, as more than one kinase are probably involved in the abnormal hyperphosphorylation of tau [117]. Because many protein kinases are dynamically regulated and are critical to many vital signaling pathways, any short-term and long-term measurable side effects of using the kinase inhibitors will have to be a major focus for developing these therapies.
Targeting Tau Phosphatases
Up-regulation of tau phosphatases is certainly another approach to reverse abnormal hyperphosphorylation of tau. Because PP2A is the major tau phosphatase in the brain [56-59], it is a primary target to be considered. One important consideration of this issue is that all protein phosphatases, including PP2A, have much broader substrate specificities than protein kinases. Thus, more undesirable effects might be expected than when using kinase inhibitors. Because PP2A is down-regulated in AD brain [59, 65-69], these concerns may be eliminated if just the pathological PP2A down-regulation is corrected. The activity, substrate specificity, and subcellular localization of PP2A are regulated by various numbers of regulatory subunits. To date, the cellular and subcellular distributions of the PP2A down-regulation in AD remain elusive. Activating the right pool of PP2A to the right extent is certainly a challenge for drug development.
PP2A activity is regulated by two endogenous protein inhibitors, I1PP2A and I2PP2A [118, 119]. These two inhibitors may be deregulated in AD brain [70, 71], leading to PP2A inhibition. Targeting these inhibitors may serve as another approach to restore PP2A in AD.
Memantine, a low- to moderate-affinity antagonist of NMDA receptor, is clinically beneficial in treating moderate to severe AD. We found that it reverses okadaic acid–induced PP2A inhibition and prevents tau hyperphosphorylation in hippocampal slice cultures from adult rats [120]. The restoration of PP2A activity by memantine also leads to restoration of MAP2 expression in the neuropil and a reversal of hyperphosphorylation and accumulation of neurofilaments. As other NMDA antagonists failed to show similar effects to memantine, memantine's efficacy for treating AD might be involved in PP2A restoration. Similarly, melatonin has also been shown to restore PP2A activity and reverse tau hyperphosphorylation, both in vitro and in experimental animals [121-124].
Targeting Brain Glucose Metabolism and Tau O-GlcNAcylation
Glucose uptake and metabolism are impaired in AD brain, and this impairment appears to be a cause, rather than a consequence, of neurodegeneration [125]. However, what causes impaired brain glucose uptake/metabolism is not well understood. It could be a result of one or more of the followings: reduced cerebral blood flow [126], deficient brain insulin signaling [127, 128], deficient brain glucose transporters [129, 130], and oxidative stress [131]. Because impaired brain glucose uptake/metabolism can cause deregulation of tau phosphatases and decreased tau O-GlcNAcylation, which, in turn, facilitates abnormal hyperphosphorylation of tau [79, 81, 82, 132, 133], restoration of normal brain glucose metabolism could help reverse tau hyperphosphorylation in AD brain. Rosiglitazone, a well-known insulin sensitizer commonly used for treating type 2 diabetes, has been shown to reduce tau phosphorylation in cultured cells [134] and in obese rats [135], and to attenuate learning and memory deficits in Tg2567 transgenic APP mice [136]. A recent clinical trial has shown therapeutic efficacy of rosiglitazone in AD patients who did not carry the apoE4 allele [137]. It is possible that rosiglitazone achieves its efficacy via increasing brain insulin sensitivity, restoring brain glucose metabolism, increasing tau O-GlcNAcylation, and finally, inhibiting tau hyperphosphorylation.
Tau O-GlcNAcylation is also dynamically regulated by O-GlcNAc transferase and β-N-acetyl-glucosaminidase (O-GlcNAcase) [138]. This mechanism offers a possibility to alter tau phosphorylation via tau O-GlcNAcylation by targeting these two enzymes. Small inhibitory compounds that selectively inhibit O-GlcNAcase, the enzyme that removes O-GlcNAc from proteins, have been developed recently [139, 140]. These inhibitors and their future derivatives have the potential to be used to inhibit tau hyperphosphorylation via increasing tau O-GlcNAcylation.
CONCLUDING REMARKS
This article has discussed the rationale and the current status of therapeutic strategies targeting tau hyperphosphorylation for treating AD. AD is multifactorial and heterogeneous, and these causative factors appear to lead to the same pivotal downstream event of abnormal hyperphosphorylation of tau, leading to neurodegeneration. It is possible that inhibition of such a vital downstream event may be more effective than targeting an upstream event that might benefit only a subgroup of AD patients [86]. Alternatively, a “cocktail” strategy or multi-target therapy, targeting Aβ, tau, acetylcholinesterase, inflammation, oxidative stress, and cognitive symptoms, could be more efficacious than monotherapy. As pointed out by Zhu et al. [141], complete success in treating AD requires further understanding of the exact molecular mechanisms of this disease.
ACKNOWLEDGEMENTS
Our laboratories were supported in part by funds from the New York State Office of Mental Retardation and Developmental Disabilities; NIH grants (AG027429 and AG019158); and a U.S. Alzheimer's Association grant (IIRG-05-13095). We thank Ms. J. Murphy for secretarial assistance, and Ms. M. Marlow for editorial suggestions.
REFERENCES
- 1.Davis KL, Samuels SC. In: Pharmacological Management of Neurological and Psychiatric Disorders. Enna SJ, Coyle JT, editors. McGraw-Hill; New York: 1998. pp. 267–316. [Google Scholar]
- 2.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. Proc. Natl. Acad. Sci. USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. J. Biol. Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 5.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Proc. Natl. Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Brain Res. 1986;387:271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 8.Goode BL, Feinstein SC. J. Cell. Biol. 1994;124:769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirokawa N, Shiomura Y, Okabe S. J. Cell. Biol. 1988;107:1449–1459. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee G, Neve RL, Kosik KS. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- 11.Utton MA, Gibb GM, Burdett ID, Anderton BH, Vandecandelaere A. J. Biol. Chem. 2001;276:34288–34297. doi: 10.1074/jbc.M011384200. [DOI] [PubMed] [Google Scholar]
- 12.Kosik KS, Orecchio LD, Bakalis S, Neve RL. Neuron. 1989;2:1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Stanford PM, Shepherd CE, Halliday GM, Brooks WS, Schofield PW, Brodaty H, Martins RN, Kwok JB, Schofield PR. Brain. 2003;126:814–826. doi: 10.1093/brain/awg090. [DOI] [PubMed] [Google Scholar]
- 14.Brandt R, Hundelt M, Shahani N. Biochim. Biophys. Acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. J. Cell. Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. J. Cell. Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, Loos R, Van Leuven F. Am. J. Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunzi V, Glatzel M, Nakano MY, Greber UF, Van Leuven F, Aguzzi A. J. Neurosci. 2002;22:7471–7477. doi: 10.1523/JNEUROSCI.22-17-07471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 20.Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Acta Neuropathol. (Berl.) 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 22.Yuan A, Kumar A, Peterhoff C, Duff K, Nixon RA. J. Neurosci. 2008;28:1682–1687. doi: 10.1523/JNEUROSCI.5242-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. J. Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 24.Pevalova M, Filipcik P, Novak M, Avila J, Iqbal K. Bratisl. Lek. Listy. 2006;107:346–353. [PubMed] [Google Scholar]
- 25.Cleveland DW, Hwo SY, Kirschner MW. J. Mol. Biol. 1977;116:227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 26.Lindwall G, Cole RD. J. Biol. Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- 27.Ksiezak-Reding H, Liu WK, Yen SH. Brain Res. 1992;597:209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- 28.Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. J. Biol. Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 29.Kenessey A, Yen SH. Brain Res. 1993;629:40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo ES, Shin RW, Billingsley ML, Van deVoorde A, O'Connor M, Trojanowski JQ, Lee VM. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 31.Garver TD, Harris KA, Lehman RA, Lee VM, Trojanowski JQ, Billingsley ML. J. Neurochem. 1994;63:2279–2287. doi: 10.1046/j.1471-4159.1994.63062279.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. J. Biol. Chem. 2007;282:23645. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta A, Kabat J, Novak M, Wu Q, Grundke-Iqba I, Iqbal K. Neurobiol. Aging. 1998;19:S124–524. [Google Scholar]
- 34.Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. J. Biol. Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 35.Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. J. Cell Sci. 2000;113(Pt 21):3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- 36.Haase C, Stieler JT, Arendt T, Holzer M. J. Neurochem. 2004;88:1509–1520. doi: 10.1046/j.1471-4159.2003.02287.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Eur. J. Neurosci. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 39.Ikegami S, Harada A, Hirokawa N. Neurosci. Lett. 2000;279:129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- 40.Takei Y, Teng J, Harada A, Hirokawa N. J. Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Proc. Natl. Acad. Sci. USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso AD, Grundke-Iqbal I, Iqbal K. Nat. Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 43.Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Proc. Natl. Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. J. Biol. Chem. 2001;276:37967–37973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- 45.Tomlinson BE, Blessed G, Roth M. J. Neurol. Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 46.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Acta Neuropathol. (Berl.) 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 47.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 48.Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Am. J. Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso AD, Li B, Grundke-Iqbal I, Iqbal K. Proc. Natl. Acad. Sci. USA. 2006;23:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 52.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 53.Sanbe A, Osinska H, Villa C, Gulick J, Klevitsky R, Glabe CG, Kayed R, Robbins J. Proc. Natl. Acad. Sci. USA. 2005;102:13592–13597. doi: 10.1073/pnas.0503324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Curr. Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- 55.Mazanetz MP, Fischer PM. Nat. Rev. Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 56.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. J. Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 57.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 58.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. J. Biol. Chem. 2000;275:5535–5544. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 59.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Eur. J. Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 60.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen KC, Rosales JL, Barboza M, Lee KY. J. Alzheimers Dis. 2002;4:123–126. doi: 10.3233/jad-2002-4207. [DOI] [PubMed] [Google Scholar]
- 62.Tandon A, Yu H, Wang L, Rogaeva E, Sato C, Chishti MA, Kawarai T, Hasegawa H, Chen F, Davies P, Fraser PE, Westaway D, George-Hyslop PH. J. Neurochem. 2003;86:572–581. doi: 10.1046/j.1471-4159.2003.01865.x. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi S, Fujita Y, Hayashi S, Kakita A, Takahashi H, Murayama S, Saido TC, Hisanaga S, Iwatsubo T, Hasegawa M. FEBS Lett. 2001;489:46–50. doi: 10.1016/s0014-5793(00)02431-5. [DOI] [PubMed] [Google Scholar]
- 64.Yoo BC, Lubec G. Nature. 2001;411:763–764. doi: 10.1038/35081146. discussion - [DOI] [PubMed] [Google Scholar]
- 65.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. J. Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 66.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. J. Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 67.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. Exp. Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 68.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 69.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd J. Neuropathol. Exp. Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 70.Tsujio I, Zaidi T, Xu J, Kotula L, Grundke-Iqbal I, Iqbal K. FEBS Lett. 2005;579:363–372. doi: 10.1016/j.febslet.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 71.Tanimukai H, Grundke-Iqbal I, Iqbal K. Am. J. Pathol. 2005;166:1761–1771. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulloa L, Montejo de Garcini E, Gomez-Ramos P, Moran MA, Avila J. Brain Res. Mol. Brain Res. 1994;26:113–122. doi: 10.1016/0169-328x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 73.Vijayan S, El-Akkad E, Grundke-Iqbal I, Iqbal K. FEBS Lett. 2001;509:375–381. doi: 10.1016/s0014-5793(01)03201-x. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I. FEBS Lett. 2001;507:81–87. doi: 10.1016/s0014-5793(01)02944-1. [DOI] [PubMed] [Google Scholar]
- 75.Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. J. Biol. Chem. 1996;271:28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 76.Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire MJ, Delacourte A, Michalski JC, Caillet-Boudin ML. Biochim. Biophys. Acta. 2003;1619:167–176. doi: 10.1016/s0304-4165(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 77.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. Proc. Natl. Acad. Sci. USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson LA, Moya KL, Breen KC. J. Alzheimers Dis. 2004;6:489–495. doi: 10.3233/jad-2004-6505. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Lu F, Wang JZ, Gong CX. Eur. J. Neurosci. 2006;23:2078–2086. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 80.Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX. FASEB J. 2008;22:138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. J. Alzheimers Dis. 2006;9:1–12. doi: 10.3233/jad-2006-9101. [DOI] [PubMed] [Google Scholar]
- 82.Gong CX, Liu F, Grundke-Iqba I, Iqbal K. In: Alzheimer's Disease: New Advances. Iqbal K, Winblad B, Avila J, editors. Medimond International; Madrid, Spain: 2007. pp. 253–261. [Google Scholar]
- 83.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 84.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 85.Avila J. FEBS Lett. 2006;580:2922–2927. doi: 10.1016/j.febslet.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 86.Iqbal K, Grundke-Iqbal I. Cell. Mol. Life Sci. 2007;64:2234–2244. doi: 10.1007/s00018-007-7221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee VM, Trojanowski JQ. J. Alzheimers Dis. 2006;9:257–262. doi: 10.3233/jad-2006-9s328. [DOI] [PubMed] [Google Scholar]
- 88.Churcher I. Curr. Top. Med. Chem. 2006;6:579–595. doi: 10.2174/156802606776743057. [DOI] [PubMed] [Google Scholar]
- 89.Golde TE. J. Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 90.Roder HM, Hutton ML. Expert Opin. Ther. Targets. 2007;11:435–442. doi: 10.1517/14728222.11.4.435. [DOI] [PubMed] [Google Scholar]
- 91.Takashima A. J. Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 92.Avila J, Hernandez F. Expert Rev. Neurother. 2007;7:1527–1533. doi: 10.1586/14737175.7.11.1527. [DOI] [PubMed] [Google Scholar]
- 93.Wen Y, Planel E, Herman M, Figueroa HY, Wang L, Liu L, Lau LF, Yu WH, Duff KE. J. Neurosci. 2008;28:2624–2632. doi: 10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. J. Alzheimers Dis. 2003;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 95.Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. J. Neurochem. 2006;99:1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- 96.Engel T, Hernandez F, Avila J, Lucas JJ. J. Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le Corre S, Klafki HW, Plesnila N, Hubinger G, Obermeier A, Sahagun H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM. Proc. Natl. Acad. Sci. USA. 2006;103:9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, Terada S, Hamamura T, Trojanowski JQ, Lee VM, Kuroda S. Acta Neuropathol. 2005;110:547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 99.Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Proc. Natl. Acad. Sci. USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryves WJ, Harwood AJ. Biochem. Biophys. Res. Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 101.Phiel CJ, Klein PS. Annu. Rev. Pharmacol. Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 102.Stambolic V, Ruel L, Woodgett JR. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 103.Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. J. Biol. Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 104.Cohen P, Goedert M. Nat. Rev. Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 105.Meijer L, Flajolet M, Greengard P. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Patel DS, Dessalew N, Iqbal P, Bharatam PV. Curr. Protein Pept. Sci. 2007;8:352–364. doi: 10.2174/138920307781369409. [DOI] [PubMed] [Google Scholar]
- 107.Huang HC, Klein PS. Curr. Drug Targets. 2006;7:1389–1397. doi: 10.2174/1389450110607011389. [DOI] [PubMed] [Google Scholar]
- 108.Pallas M, Verdaguer E, Jorda EG, Jimenez A, Canudas AM, Camins A. Med. Hypotheses. 2005;64:120–123. doi: 10.1016/j.mehy.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 109.Knockaert M, Greengard P, Meijer L. Trends Pharmacol. Sci. 2002;23:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 110.De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Eur. J. Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 111.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 112.Vita M, Abdel-Rehim M, Olofsson S, Hassan Z, Meurling L, Siden A, Siden M, Pettersson T, Hassan M. Eur. J. Pharm. Sci. 2005;25:91–103. doi: 10.1016/j.ejps.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 113.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. J. Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Biochim. Biophys. Acta. 2007;1772:473–483. doi: 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 115.Zhang M, Li J, Chakrabarty P, Bu B, Vincent I. Am. J. Pathol. 2004;165:843–853. doi: 10.1016/S0002-9440(10)63347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kesavapany S, Zheng YL, Amin N, Pant HC. Biotechnol. J. 2007;2:978–987. doi: 10.1002/biot.200700057. [DOI] [PubMed] [Google Scholar]
- 117.Wang JZ, Grundke-Iqbal I, Iqbal K. Eur. J. Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li M, Makkinje A, Damuni Z. Biochemistry. 1996;35:6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- 119.Li M, Makkinje A, Damuni Z. J. Biol. Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 120.Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. FEBS Lett. 2004;566:261–269. doi: 10.1016/j.febslet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 121.Li SP, Deng YQ, Wang XC, Wang YP, Wang JZ. J. Pineal. Res. 2004;36:186–191. doi: 10.1111/j.1600-079x.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 122.Li XC, Wang ZF, Zhang JX, Wang Q, Wang JZ. Eur. J. Pharmacol. 2005;510:25–30. doi: 10.1016/j.ejphar.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 123.Wang DL, Ling ZQ, Cao FY, Zhu LQ, Wang JZ. J. Pineal. Res. 2004;37:11–16. doi: 10.1111/j.1600-079X.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 124.Zhu LQ, Wang SH, Ling ZQ, Wang DL, Wang JZ. J. Pineal. Res. 2004;37:71–77. doi: 10.1111/j.1600-079X.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 125.Hoyer S. Adv. Exp. Med. Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- 126.de la Torre JC. Neurol. Res. 2004;26:517–524. doi: 10.1179/016164104225016254. [DOI] [PubMed] [Google Scholar]
- 127.Salkovic-Petrisic M, Hoyer S. J. Neural Transm. Suppl. 2007:217–233. doi: 10.1007/978-3-211-73574-9_28. [DOI] [PubMed] [Google Scholar]
- 128.Craft S. Curr. Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 129.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Ann. Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. FEBS Lett. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schubert D. Ageing Res. Rev. 2005;4:240–257. doi: 10.1016/j.arr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 132.Yanagisawa M, Planel E, Ishiguro K, Fujita SC. FEBS Lett. 1999;461:329–333. doi: 10.1016/s0014-5793(99)01480-5. [DOI] [PubMed] [Google Scholar]
- 133.Planel E, Miyasaka T, Launey T, Chui DH, Tanemura K, Sato S, Murayama O, Ishiguro K, Tatebayashi Y, Takashima A. J. Neurosci. 2004;24:2401–2411. doi: 10.1523/JNEUROSCI.5561-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.d'Abramo C, Ricciarelli R, Pronzato MA, Davies P. J. Neurochem. 2006;98:1068–1077. doi: 10.1111/j.1471-4159.2006.03931.x. [DOI] [PubMed] [Google Scholar]
- 135.Hu SH, Yang YP, Zhang MX, Gong CX. Prog. Biochem. Biophys. 2007;34:533–537. [Google Scholar]
- 136.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Exp. Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 137.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 138.Iyer SP, Hart GW. Biochemistry. 2003;42:2493–2499. doi: 10.1021/bi020685a. [DOI] [PubMed] [Google Scholar]
- 139.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. J. Biol. Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 140.Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Greig IR, Vocadlo DJ. J. Am. Chem. Soc. 2007;129:635–644. doi: 10.1021/ja065697o. [DOI] [PubMed] [Google Scholar]
- 141.Zhu X, Avila J, Perry G, Smith MA. Am. J. Pathol. 2007;170:1457–1459. doi: 10.2353/ajpath.2007.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]