Abstract
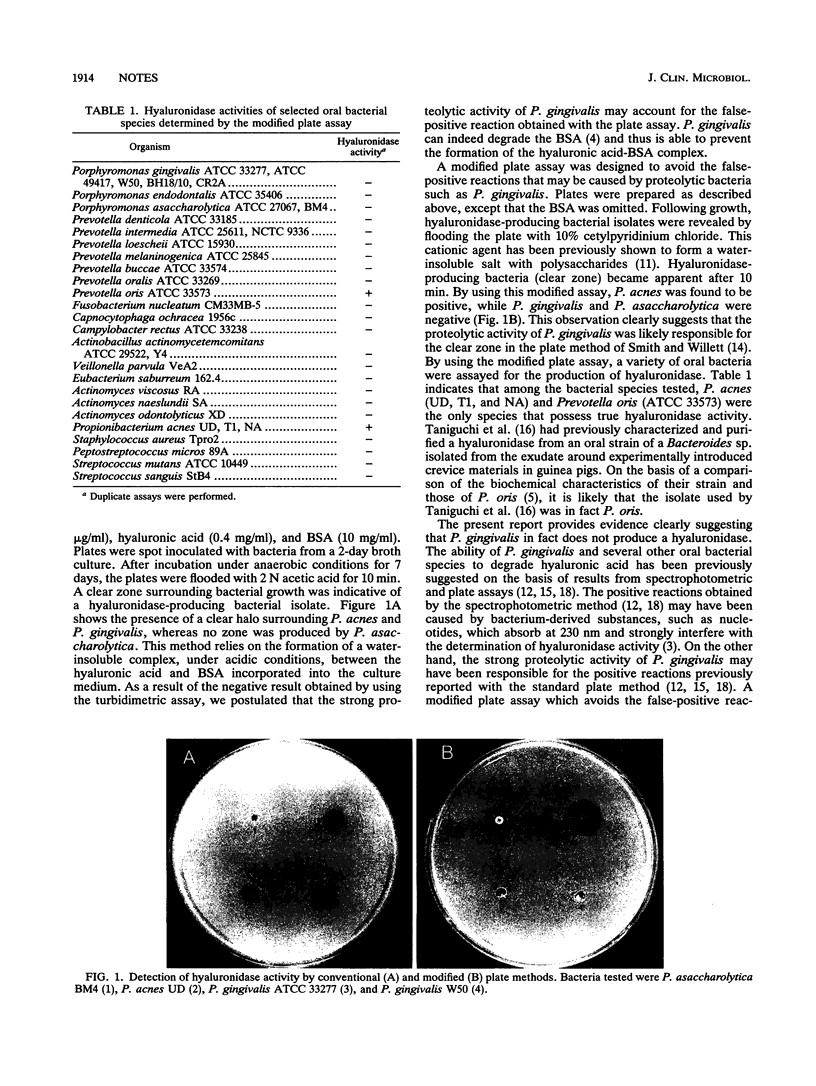
The aim of the present study was to evaluate the ability of Porphyromonas gingivalis to degrade hyaluronic acid. No hyaluronidase activity was detected using a turbidimetric method, whereas a standard plate assay showed a positive reaction for P. gingivalis. We postulated that the high proteolytic activity of P. gingivalis may account for this observation. A modified plate assay was designed to avoid false-positive reactions caused by proteolytic bacteria. The new assay, based on the formation of a water-insoluble salt between hyaluronic acid and the polyanion cetylpyridinium chloride, indicated that P. gingivalis does not have hyaluronidase activity. By this modified plate method, it was found that among 24 different oral bacterial species tested, Propionibacterium acnes and Prevotella oris were the only species that possess hyaluronidase activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fitzgerald T. J., Gannon E. M. Further evidence for hyaluronidase activity of Treponema pallidum. Can J Microbiol. 1983 Nov;29(11):1507–1513. doi: 10.1139/m83-232. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A. The hyaluronidase associated with Treponema pallidum facilitates treponemal dissemination. Infect Immun. 1987 May;55(5):1023–1028. doi: 10.1128/iai.55.5.1023-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham E., Holland K. T., Gowland G., Cunliffe W. J. Purification and partial characterization of hyaluronate lyase (EC 4.2.2.1) from Propionibacterium acnes. J Gen Microbiol. 1979 Dec;115(2):411–418. doi: 10.1099/00221287-115-2-411. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Willett N. P. Rapid plate method for screening hyaluronidase and chondroitin sulfatase-producing microorganisms. Appl Microbiol. 1968 Sep;16(9):1434–1436. doi: 10.1128/am.16.9.1434-1436.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen E. K., Hentges D. J. Hydrolytic enzymes of anaerobic bacteria isolated from human infections. J Clin Microbiol. 1981 Aug;14(2):153–156. doi: 10.1128/jcm.14.2.153-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Fujimura S., Takeuchi K., Nakamura T. Purification and characterization of mucopolysaccharidase from an oral strain of Bacteroides sp. Appl Environ Microbiol. 1983 Dec;46(6):1252–1257. doi: 10.1128/aem.46.6.1252-1257.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipler L. S., Embery G. Glycosaminoglycan-depolymerizing enzymes produced by anaerobic bacteria isolated from the human mouth. Arch Oral Biol. 1985;30(5):391–396. doi: 10.1016/0003-9969(85)90065-2. [DOI] [PubMed] [Google Scholar]