Abstract
Tissue morphogenesis remains one of the least understood problems in cell and developmental biology. There is a disconnect between the mechanisms that apply to two-dimensional (2D) cultures and those seen in vivo. Three-dimensional (3D) culture presents a complex stimulus triggering cellular responses that are only partially understood. We compared 2D and 3D cultures of human mesenchymal stem cells in the presence of mitogen-activated protein kinase kinase (MEK) inhibitor, PD98059, to determine the role of extracellular signal-related kinase (ERK) in collagen-induced differentiation. 3D collagen I culture enhanced and accelerated the osteogenic differentiation of human mesenchymal stem cells (hMSC). Contrary to 2D results, the addition of PD98059 induced a significant amplification of osteogenic gene expression and matrix mineralization in 3D cultures. The inhibition of ERK altered cell-mediated compaction, proliferation, and resulted in the development of distinct tissue microstructure. Therefore, we suggest that the ability to reorganize collagen in 3D is an important step in ERK-mediated osteogenic differentiation. This work aims to propose a correlation between osteogenic differentiation and hMSC-directed collagen I remodeling. We present a potential mechanistic link (ERK) through which the three dimensionality of an engineered tissue acts to differentially induce and maintain cellular phenotype during tissue development.
Introduction
Human mesenchymal stem cells (hMSC) are a popular model for studies of development due to their potential to differentiate into cells representing all three germ layers. Differentiation occurs in response to both soluble and insoluble cues that induce programs of transcriptional and phenotypic modification [1,2]. For example, hMSC maintained in two dimension produce bone nodules in response to dexamethasone, but do not form mature bone [3,4]. However, the role that hMSC play in tissue development remains largely unknown.
There is a disconnect between the mechanisms that apply to two-dimensional (2D) cultures and those seen in vivo [5]: signaling pathways are derived from a planar, rigid surface that does not exist in vivo. Three-dimensional (3D) in vitro culture, using extracellular matrix (ECM) proteins as a differentiation stimulus, could provide a link to bridge these two environments. 3D culture offers the opportunity to integrate 2D biochemical mechanisms with environmental factors found in vivo. As an additional advantage, 3D culture permits remodeling and collagen I fibrillogenesis in the ECM and coupled with second harmonic generation (SHG) has the potential to associate this fibrillogenesis with gene expression. SHG microscopy allows for the visualization of nanoperiodic, noncentrosymmetric structure with the use of high-intensity light without the need for labeling of the collagen network [6–8]. Tracking of SHG signal allows for the analysis of collagen I fibril alignment and consolidation [9,10]. It allows the examination of the potential relationship between such remodeling and hMSC differentiation.
Cells interact with the same ECM differently when grown in 2D or 3D culture [11,12]. In 2D culture systems both fibroblasts and epithelial cells proliferate profusely and share a spindle-like morphology [13]. Fibroblasts grown in floating collagen gels lose this proliferative nature and adopt a dendritic morphology and behavior similar to that of differentiated connective tissue [14]. Conversely, epithelial cells placed in floating collagen gels reorganize to form higher-order mammary structure and produce milk proteins [15–17]. Cells plated on rigid, 2D substrates develop strong adhesions characterized by the recruitment of focal adhesion kinase (FAK) to form punctate focal adhesions and the upregulation of Y397 phosphorylation on FAK. 3D matrix adhesions in vitro, as opposed to these traditional focal adhesions, recruit different structural and signaling proteins to the adhesion site which alters signal propagation and downregulates phosphorylation of the FAK Y397 residue [18]. A loss in phosphorylation at this site is also observed in vivo [18] and may be responsible for the dramatic alteration of cellular behavior in response to a 3D matrix as compared to their 2D counterparts of a similar composition [18]. These studies underscore the fact that we do not fully understand the way cells transduce and interpret 3D information.
Extracellular signal-related kinase (ERK) is a member of the mitogen-activated protein kinase (MAPK) family. ERK is tied to both integrin and growth factor signaling and controls a range of cellular behaviors including proliferation, apoptosis, and differentiation. As such, ERK is a strong candidate to be the discriminating switch point between lineages and the integration of 3D information. Collagen I induction of hMSC osteogenic differentiation in 2D is mediated by the integrin activation of ERK [19]. Inhibition of ERK results in decreased hMSC differentiation potential in several systems and along several lineages including osteogenic, neural, and mesodermal [20–22]. Conversely, however, the inhibition of this pathway in bovine satellite cells promotes myogenic differentiation [23]. The duality of ERK in 2D, both as a promoter and inhibitor of differentiation, further implicates it as an important switch in the process of cellular differentiation and maturation. Activated indirectly by matrix adhesion signaling, ERK then becomes a potential link between matrix interaction and cellular behavior, and consequently, a mechanism for 3D cell-matrix–induced osteogenesis will require a role for ERK. It is the goal of this work to investigate the role of ERK in collagen I remodeling during the osteogenic differentiation of hMSC.
Materials and Methods
hMSC were purchased from Lonza Group Ltd. (Allendale, NJ). hMSC tissue culture medium (DMEM) was purchased from Mediatech (Cellgro, Herndon, VA) and fungizone/penicillin G/streptomycin sulfate (FPS) from Hyclone (Fisher Scientific, Fair Lawn, NJ). Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Woodland, CA). Trypsin-EDTA and agarose were obtained from Sigma Chemical Co. (St. Louis, MO). Purified, lyophilized bovine collagen I was purchased from MP Biomedicals (Solon, OH) and solubilized collagen I from calf skin was purchased from Sigma Chemical Co. (St. Louis, MO). The MAPK kinase (MEK) inhibitor, PD98059, was purchased from Calbiochem (San Diego, CA). The TRIzol reagent for RNA isolation was purchased from Invitrogen (Carlsbad, CA) and QuantiTect® SYBR® Green One Step RT-PCR Kit from Qiagen (Valencia, CA). Primers were ordered from Integrated DNA Technologies (Coralville, IA). The phospho-ERK TiterZyme®EIA Enzyme Immunometric Assay Kit was ordered from Assay Designs (Ann Arbor, MI). Hematoxylin, eosin, and alizarin red S were purchased from Sigma Chemical Co. (St. Louis, MO). Hoechst 33258 dye was purchased from Molecular Probes Inc. (Eugene, OR) and Proteinase K from Promega Inc. (Madison, WI). Unless otherwise specified, other standard reagents were obtained from Fisher Scientific (Fair Lawn, NJ).
Cell culture
hMSC are provided by Lonza and are donated by healthy males and nonpregnant females between 18 and 45 years of age. All donors must have negative clinical laboratory tests for HIV; hepatitis B and hepatitis C; normal vital signs and hematology values; and a negative medical history for heart disease, kidney disease, cancer, bleeding ulcers, diabetes, jaundice, liver disease, hepatitis, and epilepsy. In addition, the donors must maintain a weight not more than 10% above the normal body weight for their height, have no blood or bleeding disorders, and are not taking any prescription medicines other than those deemed allowable. The hMSC used in this study were obtained from three individual donors fitting the above criteria.
Cryopreserved hMSC were grown according to the manufacturer's instructions. hMSC were cultured in 1 × Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 10,000 U/mL FPS. Medium was changed every 3 days and cultures were incubated at 37°C in a humidified atmosphere containing 95% air and 5% CO2. Cells were detached using trypsin-EDTA and passaged into fresh culture flasks upon reaching confluence. hMSC were used between passages 6 and 8. In preparation for the incorporation in 3D constructs, cells were washed with PBS, detached with trypsin-EDTA, collected, and counted using a Beckman Coulter Counter. Cells for 2D control samples were prepared in the same manner and plated in a 6-well plate at a density of 2.3 × 104 cells/cm2. For collagen-coated samples, solubilized collagen was adsorbed to the surface of the 6-well plate and incubated at 37°C for at least 1 h. Following adsorption of the collagen to the tissue culture plastic, the surfaces were rinsed with sterile PBS and cells plated. ERK activity was controlled through the inhibition of its upstream activator MEK using PD98059 dissolved in DMSO. Cells were preincubated with PD98059 at a concentration of 50 μM for 15 min and then plated for 2D culture or encapsulated within a collagen hydrogel for 3D culture as described below. Constructs were grown in PD98059-supplemented medium for the given time points. The final DMSO concentration never exceeded 0.1% and the same amount of the DMSO vehicle was added to control samples.
Collagen gel preparation
Three-dimensional collagen I gels were prepared by mixing cells with the following reagents: DMEM (14%), FBS (10%), 5× concentration DMEM (16%), 0.1 N NaOH (10%), and 4 mg/mL collagen I (50%). The final collagen concentration was 2 mg/mL within each construct. Constructs of a volume of 1.0 mL were made in 12-well plates, and the cellular density was kept constant at 1.0 × 106 cells/mL/ECM. The constructs were incubated at 37°C for 30 min, released from the wells, and incubated in DMEM. Images of the constructs were taken at days 0, 1, 3, and 7, and construct areas were recorded for the analysis of compaction.
DNA assay
Cell number was assessed at different time points to quantify cell recovery (day 0), to characterize cell proliferation (day 15), and to normalize calcium assays (day 21). To extract the cells, the constructs were freeze-dried and digested in Proteinase K at 55°C for 16–20 h, breaking apart the matrix and allow for cellular release. DNA content was analyzed using the Hoechst 33258 dye and samples were diluted and loaded into a 96-well plate in duplicate. Following the addition of the DNA binding, dye samples were incubated (protected from light) for 15 min. Fluorescence was quantified using a fluorescence microplate reader (BioTek Instruments, Inc., Winooksi, VT) at an excitation wavelength of 350 nm and an emission wavelength of 460 nm. The DNA content of each 3D construct was converted to cell number based on cell and DNA standards.
RNA isolation
RNA was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was isolated at days 1, 3, 7, and 14 for initial studies to determine optimal time point for gene detection. Experiments involving the inhibition of ERK were performed at day 7. Constructs were homogenized in the TRIzol reagent using the TissueRuptor power homogenizer. Isolation was performed according to the manufacturer's instructions, and total isolated RNA was dissolved in RNase/DNase free water and stored at −20°C. Total RNA was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE).
Quantitative real-time RT-PCR
Quantitative RT-PCR was performed using the LightCycler® 480 Real-Time PCR System (Roche, Pleasonton, CA) and the QuantiTect® SYBR® Green RT-PCR Kit with HotStar Taq DNA polymerase. 1× QuantiTect SYBR Green, 0.5 μM Primer F and Primer R, and 0.5 μL/reaction QuantiTect RT Mix were combined with sample RNA to yield a 20 μL reaction volume. Primers used for the amplification of differentiation marker genes were designed using OligoPerfect™ (Invitrogen, Carlsbad, CA) and purchased through IDT Technologies (Coralville, IA). RT-PCR was performed according to the following protocol defined by the manufacturer: RT 20 min at 50°C, 20°C/s ramp, PCR activation 15 min at 95°C, 20°C/s ramp, 35–55 cycles of [denaturation 15 s at 94°C, 20/s ramp, annealing 20–30 s at 50–60°C, 20°C/s ramp, extension 30 s 72°C, 2°C/s ramp]. The markers of cell phenotype used in this study are listed in Table 1 and represent the osteogenic, chondrogenic, adipogenic, and myogenic lineages. All samples were loaded in duplicate and normalized to total RNA content and to the performance of the housekeeping gene, GAPDH. Fold differences in gene expression were relative to hMSC cultured on tissue culture plastic and calculated using the δδCt method [24].
Table 1.
Primers Used for Amplification of Differentiation Marker Genes
| Target | Primer sequences |
|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F 5′-CGACCACTTTGTCAAGCTCA-3′ R 5′-AGGGGTCTACATGGCAACTG-3′ |
| Bone sialoprotein (BSP) | F 5′-CTGCTTCCTCACTCCAGGAC-3′ R 5′-GATTGCTTCCTCTGGCAGTC-3′ |
| Osteocalcin (BGLAP) | F 5′-GACTGTGACGAGTTGGCTGA-3′ R 5′-CTGGAGAGGAGCAGAACTGG-3′ |
| Runt-related transcription factor 2 (RUNX2) | F 5′-TTACTTACACCCCGCCACTC-3′ R 5′-CACTCTGGCTTTGGGAAGAG-3′ |
| Osterix (OSX) | F 5′-GCCAGAAGCTGTGAAACCTC-3′ R 5′-GCTGCAAGCTCTCCATAACC-3′ |
| Collagen I (COL I) | F 5′-GACGTCCTGGTGAAGTTGGT-3′ R 5′-ACCAGGGAAGCCTCTCTCTC-3′ |
| Collagen II (COL II) | F 5′-GTGAAGACGTGAAAGACTGC-3′ R 5′-CTCCAGGTTCTCCTTTCTGT-3′ |
| Aggrecan (AGG) | F 5′-ACACCAGTTTGTTGAAGTG-3′ R 5′-CTCCACTGACCTCAGCTATG-3′ |
| Myogenin (MYO) | F 5′-CAAGTGAAACGGTTTGAGAG-3′ R 5′-GGAAGGTTCCCAATATTCAC-3′ |
| Dystrophin (DYST) | F 5′-GAGCTATGCCTACACACAGG-3′ R 5′-TCTTTCACCACTCCACATC-3′ |
| Fatty acid binding protein 4 (FAB4) | F 5′-CTGCAGAGACAGGAAAGTC-3′ R 5′-GTTCAATGCGAACTTCAGTC-3′ |
| Peroxisome proliferator-activated receptor γ (PPARG) | F 5′-CAAGCCCTTCACTACTGTTG-3′ R 5′-GCTTTATCTCCACAGACACG-3′ |
Quantification of ERK activity
Cells were serum deprived in 1% FBS, DMEM overnight before plating. Cells were plated as indicated above at concentrations of 10 × 103/cm2 and 1 × 106/cm3 for 2D and 3D samples, respectively. Protein was extracted from 3D (days 1 and 3) and 2D samples (1h) in ice-cold RIPA buffer supplemented with a protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). 3D samples were homogenized in RIPA buffer using the TissueRuptor power homogenizer and clarified by centrifugation at 4°C for 2 h at 4000g. Samples were assayed using the TiterZyme®EIA phospho-ERK 1/2 Elisa Assay Kit according to the manufacturer's instructions. Briefly, samples were diluted in assay buffer to normalize for cell number and incubated for 1 h in a 96-well plate. Following antibody and substrate incubation, the samples were read on a plate reader at an optical density of 450 nm. The concentration of pERK (pg/μL) was quantified against a standard curve and normalized by cell number as determined by a DNA assay.
Tissue morphology—hematoxylin and eosin (H&E) and alizarin red S
At day 21, samples were washed twice with PBS and fixed in 10% formaldehyde for 1 h at room temperature. Samples were dehydrated in 10% sucrose overnight to prepare for sectioning. Dehydrated collagen I gels were embedded in O.C.T. compound (Sakura, Torrance, CA), sectioned (10 mm) in a cryostat (Sakura, Torrance, CA) and positioned on to Superfrost-plus microscope slides (Fisher Scientific, Fair Lawn, NJ). H&E staining was performed on the mounted samples according to the following protocol: 100% ethanol (EtOH) (1 min), 95% EtOH (1 min), 70% EtOH (1 min), 50% EtOH (1 min), distilled water (5 min), hematoxylin (5 min), rinsed in distilled water until clear, tap water (5 min), distilled water (3 min), eosin (10 min), 95% EtOH (5 min) three times, 100% EtOH (5 min) three times, and xylene (10 min). Following staining, the samples were covered with a coverslip and stored at 4°C for imaging.
For mineral detection, samples were washed twice with excess dH2O and incubated in the presence of 40 mM alizarin red solution (pH 4.1) for 20 min at room temperature with shaking. Samples were washed four times with excess dH2O with shaking for 5 min per wash and coverslipped. All images were acquired using a Zeiss AxioImager (Carl Zeiss, Inc., Thornwood, NY) at a 40× magnification.
Second harmonic generation
Collagen I constructs were harvested at day 21 and sectioned as described above. Frozen sections were imaged using a Zeiss LSM 510 two photon confocal microscope (Zeiss Inc., Thornwood, NY). Samples were excited by a two photon laser at 820 nm and emissions were collected at 480 nm. Images were taken at a 40× magnification.
Statistical analysis
All experiments were repeated a minimum of three times, and the representing data are presented as mean ± SEM. Statistical analyses were performed using Student's unpaired t-test, and a p-value ≤0.05 was considered significant.
Results and Discussion
3D collagen I culture enhances and accelerates the osteogenic differentiation of hMSCs
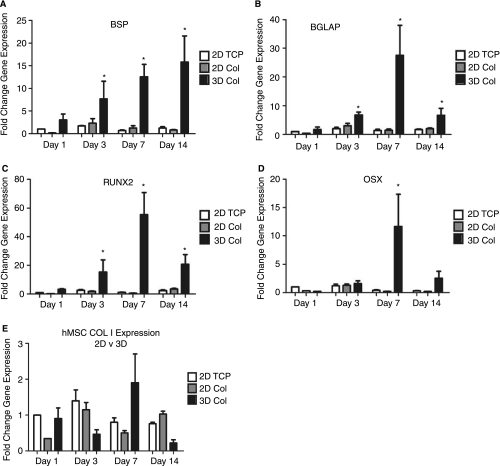
The addition of a third dimension to hMSC culture may alter the differentiation behavior in response to the osteoinductive stimulus. 3D matrix adhesions change the way in which cells interact with the collagen matrix and alter the signal transduction patterns that define behavior. Enhanced osteogenic differentiation of hMSC was observed when embedded in collagen I hydrogels and cultured for 1, 3, 7, and 14 days (Fig. 1). The increase in the expression of hMSC in the 3D collagen I microenvironment was significant when compared to 2D tissue culture plastic (TCP) and 2D collagen I-coated (Col) controls. 2D culture of hMSC on collagen I-coated surfaces increases their osteogenic potential after two weeks in culture [25]. The temporal pattern of gene expression suggests that 3D collagen I was able to both enhance and accelerate the osteogenic differentiation of hMSC. Collagen I showed no significant increase in gene expression over 2D controls, which is attributed to the abundance of collagen I present in the surrounding matrix that may downregulate production of the endogenous protein [26]. These results highlight that hMSCs have differentiated to a greater degree in response to their 3D environment. Likewise, 3D culture of fibroblasts and epithelial cells differ significantly when cultured in 2D and 3D environments. Both cell types exhibit significant differentiation and adoption of a mature phenotype when cultured in 3D environment [14,16,17]. The upregulation of osteogenic gene expression in hMSC likewise implies that dimension and spatial organization play a positive role in differentiation.
FIG. 1.
3D culture of human mesenchymal stem cells (hMSCs) in collagen I hydrogels results in enhanced osteogenic differentiation. hMSC cultured in 3D collagen I hydrogels were assayed for the expression of five markers of osteogenic differentiation at days 1, 3, 7, and 14. (A) Bone sialoprotein (BSP), (B) osteocalcin (BGLAP), (C) runt-related transcription factor 2 (RUNX2), (D) osterix (OSX), and (E) collagen I (COL I). Samples are normalized to GAPDH and shown as a fold increase in gene expression from hMSC plated on 2D TCP at day 1. *Statistical significance p < 0.05, n = 3 relative to 2D hMSC plated on TCP.
Collagen I induces a range of gene responses in hMSCs
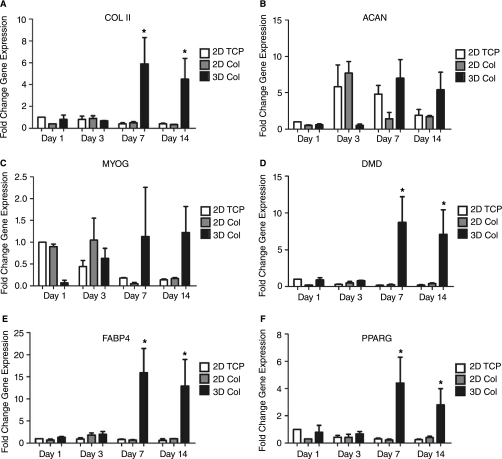
The potential osteogenic differentiation of hMSC described above presumes the downregulation of genetic markers representative of alternative lineages [27]. However, 3D culture alone may not be specific for osteogenic differentiation. In fact, in addition to the increase in osteogenic gene makers we saw some increase in chondrogenic, myogenic, and adipogenic genes (Fig. 2). The heterogeneous response suggests that hMSC are integrating the external stimuli in a way that preserves multipotency and additional signals are necessary for full lineage commitment. Traditionally, the specific osteogenic differentiation of hMSC is initiated by the addition of beta glycerophosphate, ascorbate, and dexamethasone. Addition of these stimuli into the system studied here would, however, overwhelm the physiology of the system masking the subtle developmental events that are occurring in response to cellular interaction with the collagen I matrix. In addition, some reports indicate toxicity upon use of these supplements in vivo [28,29]. The development of a system to physiologically alter the specific cellular phenotype over an extended time course would be of benefit to the applications of bone tissue engineering and cellular engraftment.
FIG. 2.
3D collagen I induces a range of human mesenchymal stem cell (hMSC) gene response. hMSC cultured in 3D collagen I hydrogels were assayed for the expression of markers of the chondrogenic, myogenic, and adipogenic lineages to assess nonosteogenic differentiation in response to the 3D collagen I stimulus. Chondrogenic: (A) collagen II (COL II) and (B) aggrecan (ACAN); myogenic: (C) myogenin (MYOG) and (D) dystrophin (DMD); and adipogenic: (E) fatty acid binding protein 4 (FABP4) and (F) peroxisome proliferator-activated receptor γ (PPARG). Samples are normalized to GAPDH and shown as a fold increase in gene expression from hMSC plated on 2D TCP at day 1. *Statistical significance p ≤ 0.05, n = 3 relative to 2D hMSC plated on TCP.
A significant level of adipogenesis may have occurred given the detection of highly induced levels of FABP4 and PPARγ. Upon encapsulation within 3D collagen I hydrogels the morphology of hMSC changes dramatically. Cells initially adopt a rounded morphology and experience increased cytoskeletal spreading over time. hMSC within 3D space never adopt the rigid, spread morphologies of cells grown in 2D space. These changes in cell shape may be transduced into differentiation signals through the actin cytoskeleton irrespective of the biochemistry of the substrate [30]. The adoption of a rounded morphology promotes adipogenesis in hMSC and can be inhibited through the activation of RhoA, a regulator of cytoskeletal tension [31]. Consequently, cell shape changes when cultured in 3D may be responsible for the promotion of hMSC adipogenesis observed in this study.
One important driver of cellular behavior and differentiation is the mechanical interaction between cells and their microenvironment [32–36]. Matrix elasticity specifically regulates the differentiation of hMSC presumably through the mechanosensing capabilities of focal adhesions. An increasingly rigid substrate leads to enhanced osteogenic potential while more elastic matrices promote myogenic and neurogenic marker expression [32]. The use of biophysical cues as a means of controlling cellular behavior has not been employed in this study but may alter the levels of gene expression. For instance, cells may reorganize the matrix yielding an environment with an elastic modulus closer to that of native tissue [13]. Newly formed 3D collagen I gels are compliant and this may be preserving hMSC plasticity.
The inhibition of ERK by the MEK inhibitor PD98059 alters cell-mediated compaction and proliferation
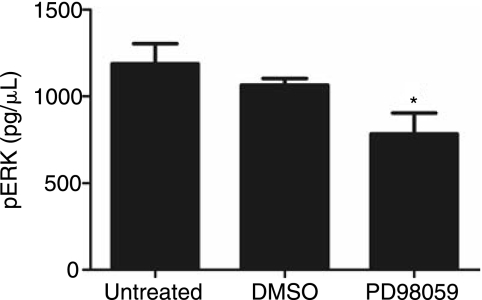
Previous 2D studies have examined the mechanism by which collagen I induces the osteogenic differentiation of hMSC and assigned a positive role for ERK in the induction of osteogenic gene expression [19,37,38]. The addition of a MEK inhibitor (PD98059) into 2D collagen I culture results in the downregulation of ERK activity and blocks osteogenic gene expression [19,22]. Our observation of increased expression of osteogenic gene markers when hMSC are cultured in 3D collagen I (Fig. 1) implicates either a quantitative increase in the amount of ERK signaling or a different mode of signal transduction. To determine whether the same pathway was being followed in 2D and 3D, samples were cultured with PD98059 for 7 days. Addition of the drug results in a statistically significant decrease in ERK activity as measured by an ELISA assay for phospho-ERK in cell samples after day 1 (Fig. 3).
FIG. 3.
The addition of the MEK inhibitor PD98059 decreases ERK activity. ERK activity was measured using an ELISA assay kit and quantified as pg/μL of phosphorylated ERK (pERK). Cell lysate was harvested in RIPA supplemented with protease inhibitors. Lysate was loaded into the assay and normalized to cell number. pERK was quantified through detection of substrate conversion at an OD of 450 nm. *Statistical significance p ≤ 0.05, n = 3.
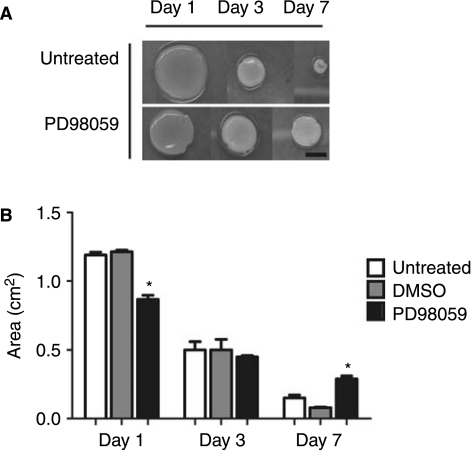
The coupling of cells with 3D matrix drives differentiation. 3D collagen I gels undergo a compaction in response to cell-mediated traction and remodeling forces [13]. Remodeling of hydrogels occurs at the cell interface and can be detected by the organization of collagen fibers in close proximity to cytoskeletal components using confocal microscopy [39] (data not shown). With the addition of PD98059, the collagen I hydrogels showed significantly different compaction profiles over the 7-day time course of the study (Fig. 4). The largest compaction event is observed by day 1, where gels compact to much less than 50% of their original area. By day 7, the gels have completed their compaction coincident with the first major peak in osteogenic gene expression. The focal adhesions rearrange in 3D, leading to changes in the magnitude of the forces that the cells exert on their external environment [40]. Although direct remodeling of the matrix occurs at the cell surface in 3D, the question remains as to how it contributes to osteogenic differentiation of hMSC. Rearrangement of collagen I does not occur in 2D cultures of hMSC, but it serves as a dramatic stimulus of osteogenesis in 3D [13,40].
FIG. 4.
(A) and B) PD98059 alters the compaction of collagen gels. hMSC were embedded and cultured within collagen I hydrogels and observed over time. The area of each gel was calculated by measurement of the x and y diameter at days 1, 3, and 7. Area was calculated using the following equation: A = πr2. *Statistical significance p ≤ 0.05, n ≥ 12.
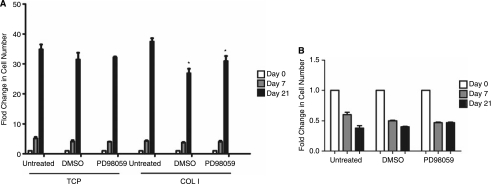
Compared to 2D, cells cultured in 3D collagen I do not proliferate (Fig. 5). Untreated, DMSO, and PD98059 cultures were grown for 0, 7, and 21 days. In 2D, hMSC grew rapidly except when cultured with PD98059. A loss of proliferation, as observed in the 3D samples, is a prerequisite of terminal differentiation in osteogenesis [14,41,42]. This is also consistent with 3D culture of other cells types, including fibroblasts, epithelial cells, rat aortic smooth muscle cells, and hMSC, all of which stop proliferating when cultured in 3D [14,16,17,41]. hMSC have an initial viability of approximately 80% (data not shown) and maintain this high level of viability across the time course observed in this study [43]. In addition, when fibroblasts were cultured in 3D collagen I matrices, compaction of the matrix resulted in the conversion of integrin-mediated survival signals into proapoptotic signals [44,45]. This regulation of ECM tension, therefore, regulates cell fate, and apoptosis may be required for the removal of unneeded cells from the construct: in a fashion similar to the final stages of wound repair [46]. Taken together, these factors may help to explain the drop in cell number and presumed decrease in proliferation rates seen in 3D culture.
FIG. 5.
(A) PD98059 does not affect 3D human mesenchymal stem cell proliferation. Cellular number was assessed in both 2D and 3D conditions with and without the drug, PD98059, at days 0, 7, and 21. (A) 2D cell numbers at each time point were assessed by counting adherent cells. (B) 3D cell number was assessed using the DNA Hoescht assay. *Statistical significance p ≤ 0.05, n = 3. Error bars in (A) are too tight to be resolved.
Inhibition of ERK amplifies the osteogenic differentiation of hMSCs in 3D culture
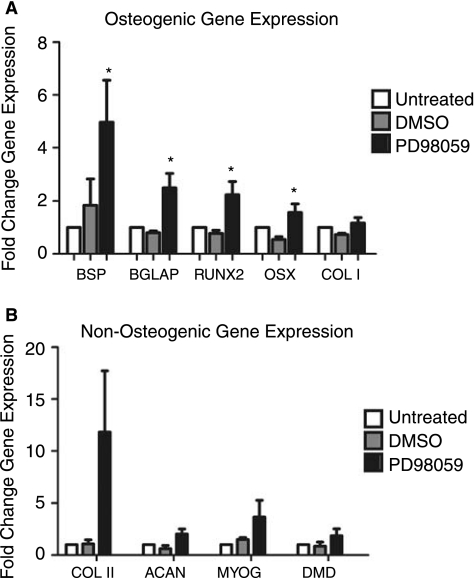
Osteogenic gene expression was enhanced in PD98059-treated gels at day 7 (Fig. 6). This time point represented peak expression in untreated controls (Fig. 1). Enhanced expression of the osteogenic gene markers is significant and conflicts 2D data in which inhibition of ERK prevented the collagen I–induced osteogenic differentiation of hMSC [19]. This enhancement, therefore, implies a fundamentally different role played by ERK in 3D collagen I-induced differentiation. These contrasting roles for ERK suggest that it may act as a molecular switch for differentiation in response to dimensionality cues. Day 7 expression of the chondrogenic and myogenic lineage markers was not statistically significant. Adipogenic markers were undetectable under PD98059 culture conditions by qRT-PCR (Ct ≥ 35). This phenomenon has been termed gene focusing in 2D culture of stem cells and may be further evidence of the lineage commitment necessary for terminal differentiation [47]. 3D hMSC culture is complex and the nature of the collagen I gel may be a platform to allow additional processes, such as remodeling to occur, that further drives cellular differentiation.
FIG. 6.
(A) PD98059 amplifies the osteogenic differentiation of hMSC in 3D collagen I culture. hMSC embedded in 3D collagen I were cultured for 7 days and then assayed for (A) osteogenic (BSP, BGLAP, RUNX2, OSX, COL I), (B) chondrogenic (COL II, ACAN), and myogenic (MYOG, DMD) lineage markers. Evaluated gene expression was normalized to GAPDH and expressed here as fold gene expression over 2D TCP controls. *Statistical significance p ≤ 0.05, n ≥ 3.
The discussion of the role of ERK in adipogenesis in the literature supports mixed conclusions. Published work indicates that ERK is necessary for the differentiation of the mesoderm, with decreased ERK activity resulting in the decrease of both osteogenesis and adipogenesis [19,48,49]. In contrast, additional reports indicate that increased ERK activity promotes osteogenesis and inhibits adipogenesis, indicating a reversed role for ERK as stated above [50]. To substantiate this point, ERK has been shown to play opposite roles in differentiation as a result of 3D culture conditions, in particular during the tube formation of endothelial cells in collagen I matrices [51]. These contrasting roles for ERK implicate it as a dynamic signaling protein that may play different roles at different times during development.
ERK inhibition results in increased matrix mineralization after 21 days in 3D collagen I culture
The development of an osteoblastic phenotype is characterized by the deposition of mineral into the collagenous matrix [3,52]. Figure 7A–C shows diffuse but positive alizarin red S staining for extracellular calcium in controls. Consistent with the previous studies, culture in 3D collagen I resulted in an increase in calcium deposition. With the addition of PD98059, staining increased and revealed punctate calcification nodules (arrows). With the inhibition of ERK in 3D culture, we observe a significant change in hMSC behavior from that which is observed in 2D culture. The reorganization of the matrix may promote efficient mineral deposition. The presence of fibrillar collagen promotes BSP-driven mineralization as gelatin was unable to enhance bone healing to the same extent as collagen gels [53]. Increased mineralization as induced by PD98059, therefore, implies that the matrix rearrangement induced by ERK inhibition may be promoting fiber organization and alignment. This may be because PD98059 promotes compaction of the matrix and perhaps a rearrangement of the collagen microstructure.
FIG. 7.
(A) PD98059 increases the deposition of mineral by human mesenchymal stem cells (hMSCs) in 3D collagen I culture. hMSCs were again embedded in 3D collagen I and cultured for 21 days to assess matrix mineralization. Frozen sections were mounted and stained using alizarin red S (ARS) stain for calcium deposition. Scale bars represent 20 μm and images were taken at 40× magnification.
The inhibition of ERK results in the development of distinct tissue microstructure
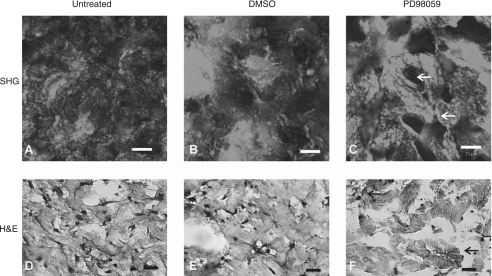
The transduction of signal in response to insoluble matrix cues must be dependent on the structure and orientation of the collagen network. Analysis of this network using SHG multiphoton confocal microscopy (Fig. 8A–C) showed the presence of densely organized collagen I (arrows) when cultured with PD98059. When a collagen solution gels, the dissolved monomers condense and link laterally to form large fibers. The large fibers themselves are not linked but instead are an entanglement or mesh-work that allows them to slip and slide past each other [13]. The active reorganization of this naive matrix, by hMSC, results in the development of higher-order collagen fiber structures which can be detected by SHG. SHG signal represents the presence of fibers oriented in the plane of the polarized light [6–8]. Therefore, the increased signal intensity observed in PD98059 samples may be indicative of higher levels of collagen I organization. This level of organization or fibrillogenesis includes the alignment, bundling, and consolidation of collagen I fibers. This remodeling may be the cause of 3D induced enhancement of osteogenic differentiation.
FIG. 8.
(A) PD98059 promotes the rearrangement of the tissue microstructure. Human mesenchymal stem cells were embedded in 3D collagen I and cultured for 21 days to assess tissue morphology. Samples were dehydrated, sectioned using a cryostat, and mounted for imaging. (A–C) Sections of untreated, DMSO, and PD98059 samples were stained with H&E to distinguish cellular microarchitecture within the developing “tissue.” (D–F) Additional mounted sections were imaged using SHG confocal microscopy to evaluate the degree of collagen I network organization. Samples were all excited at a wavelength of 820 nm and signal was collected at 410 nm equally for each sample. Images are representative of a larger set, n ≥ 3 and were taken at a 40× magnification. Scale bars represent 20 μm.
The changes seen in the collagen network are reflected in a more global manner using H&E. H&E staining (Fig. 8D–F) demonstrates the presence of a new tissue microstructure in PD98059-treated samples. PD98059 samples contain areas of highly consolidated cuboidal cell clusters that adopt distinct 3D shapes (arrow). In contrast, controls maintained a diffuse distribution of cells that are characteristically elongated.
The role of ERK in differentiation appears to have fundamentally changed in the transition from 2D to 3D culture. This change is reflected in the reorganization by both the cells and their surrounding matrix. Whereas cells in 2D and untreated 3D culture remain elongated, cells in treated 3D culture adopt a more cuboidal cell shape consistent with that found in mature osteoblasts. 3D matrix adhesions alter focal adhesion signaling and may drive the switch between the positive and negative roles of ERK in osteogenesis [54]. Additional mechanisms may couple with, or inhibit, the classic 2D MAPK signaling. Matrix remodeling and changes in tissue microstructure may potentially be a critical inducer of hMSC differentiation.
Taken together, these data suggest a correlation between osteogenic differentiation and hMSC-directed collagen I remodeling. ERK represents a potential mechanistic link through which the three dimensionality of a tissue acts to differentially induce and maintain cellular phenotype during tissue development. Identifying the mechanisms by which these events occur allows for a better understanding of disease progression as well as provides decision guides for the engineering of cell and tissue phenotype. The clinical success of regenerative techniques is dependent on the ability of engineered biomaterials to induce cellular interaction with the host via implanted cell differentiation and engraftment. To achieve this within a controlled and well-defined system requires this fundamental understanding of the interplay between cells and their 3D microenvironment.
Acknowledgment
This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases #1RO1 AR053231, August 2006–July 2010.
References
- 1.Jaiswal RK. Jaiswal N. Bruder SP. Mbalaviele G. Marshak DR. Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Jaiswal N. Haynesworth SE. Caplan AI. Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 4.Haynesworth SE. Goshima J. Goldberg VM. Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 5.Baksh D. Song L. Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freund I. Deutsch M. Sprecher A. Connective tissue polarity. Optical second-harmonic microscopy, crossed-beam summation, and small-angle scattering in rat-tail tendon. Biophys J. 1986;50:693–712. doi: 10.1016/S0006-3495(86)83510-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohler W. Millard AC. Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- 8.Theodossiou T. Rapti GS. Hovhannisyan V. Georgiou E. Politopoulos K. Yova D. Thermally induced irreversible conformational changes in collagen probed by optical second harmonic generation and laser-induced fluorescence. Lasers Med Sci. 2002;17:34–41. doi: 10.1007/s10103-002-8264-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim BM. Eichler J. Reiser KM. Rubenchik AM. Da Silva LB. Collagen structure and nonlinear susceptibility: effects of heat, glycation, and enzymatic cleavage on second harmonic signal intensity. Lasers Surg Med. 2000;27:329–335. doi: 10.1002/1096-9101(2000)27:4<329::aid-lsm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Schenke-Layland K. Riemann I. Damour O. Stock UA. Konig K. Two-photon microscopes and in vivo multi-photon tomographs—powerful diagnostic tools for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2006;58:878–896. doi: 10.1016/j.addr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang F. Hansen RK. Radisky D. Yoneda T. Barcellos-Hoff MH. Petersen OW. Turley EA. Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver VM. Lelievre S. Lakins JN. Chrenek MA. Jones JC. Giancotti F. Werb Z. Bissell MJ. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen JA. Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 14.Grinnell F. Ho CH. Tamariz E. Lee DJ. Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissell MJ. Radisky DC. Rizki A. Weaver VM. Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry G. Lee EY. Farson D. Koval M. Bissell MJ. Collagenous substrata regulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Exp Cell Res. 1985;156:487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- 17.Emerman JT. Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 18.Geiger B. Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 19.Salasznyk RM. Klees RF. Hughlock MK. Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 20.Fierro F. Illmer T. Jing D. Schleyer E. Ehninger G. Boxberger S. Bornhauser M. Inhibition of platelet-derived growth factor receptor beta by imatinib mesylate suppresses proliferation and alters differentiation of human mesenchymal stem cells in vitro. Cell Prolif. 2007;40:355–366. doi: 10.1111/j.1365-2184.2007.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunath T. Saba-El-Leil MK. Almousailleakh M. Wray J. Meloche S. Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 22.Simmons CA. Matlis S. Thornton AJ. Chen S. Wang CY. Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 23.Kook SH. Son YO. Choi KC. Lee HJ. Chung WT. Hwang IH. Lee JC. Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem. 2008;309:133–141. doi: 10.1007/s11010-007-9651-y. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Salasznyk RM. Williams WA. Boskey A. Batorsky A. Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner HA. Integrin signaling in fibrosis and scleroderma. Curr Rheumatol Rep. 1999;1:28–33. doi: 10.1007/s11926-999-0021-5. [DOI] [PubMed] [Google Scholar]
- 27.Salasznyk RM. Klees RF. Westcott AM. Vandenberg S. Bennett K. Plopper GE. Focusing of gene expression as the basis of stem cell differentiation. Stem Cells Dev. 2005;14:608–620. doi: 10.1089/scd.2005.14.608. [DOI] [PubMed] [Google Scholar]
- 28.Bruder SP. Jaiswal N. Ricalton NS. Mosca JD. Kraus KH. Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355(Suppl.):S247–S256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 29.Ng PC. Lam CW. Wong GW. Lee CH. Cheng PS. Fok TF. Chan IH. Wong E. Cheung K. Lee SY. Changes in markers of bone metabolism during dexamethasone treatment for chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F49–F54. doi: 10.1136/fn.86.1.F49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S. Chen CS. Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBeath R. Pirone DM. Nelson CM. Bhadriraju K. Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 32.Engler AJ. Sen S. Sweeney HL. Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Hinz B. Celetta G. Tomasek JJ. Gabbiani G. Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasek JJ. Gabbiani G. Hinz B. Chaponnier C. Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa S. Pawelek P. Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182:572–582. doi: 10.1016/0014-4827(89)90260-7. [DOI] [PubMed] [Google Scholar]
- 36.Wozniak MA. Desai R. Solski PA. Der CJ. Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salasznyk RM. Klees RF. Williams WA. Boskey A. Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salasznyk RM. Klees RF. Boskey A. Plopper GE. Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5. J Cell Biochem. 2007;100:499–514. doi: 10.1002/jcb.21074. [DOI] [PubMed] [Google Scholar]
- 39.Brown RA. Prajapati R. McGrouther DA. Yannas IV. Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Wozniak MA. Modzelewska K. Kwong L. Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Hong H. McCullough CM. Stegemann JP. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28:3824–3833. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stegemann JP. Hong H. Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 43.Batorsky A. Liao J. Lund AW. Plopper GE. Stegemann JP. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng. 2005;92:492–500. doi: 10.1002/bit.20614. [DOI] [PubMed] [Google Scholar]
- 44.Xia H. Nho RS. Kahm J. Kleidon J. Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 45.Tian X. Rusanescu G. Hou W. Schaffhausen B. Feig LA. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 2002;21:1327–1338. doi: 10.1093/emboj/21.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daley WP. Peters SB. Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 47.Ward DF., Jr Salasznyk RM. Klees RF. Backiel J. Agius P. Bennett K. Boskey A. Plopper GE. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells Dev. 2007;16:467–480. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- 48.Constant VA. Gagnon A. Yarmo M. Sorisky A. The antiadipogenic effect of macrophage-conditioned medium depends on ERK1/2 activation. Metabolism. 2008;57:465–472. doi: 10.1016/j.metabol.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Liao QC. Li YL. Qin YF. Quarles LD. Xu KK. Li R. Zhou HH. Xiao ZS. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J Cell Biochem. 2008. (Epub ahead of print April. ). [DOI] [PMC free article] [PubMed]
- 50.Chen TH. Chen WM. Hsu KH. Kuo CD. Hung SC. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;355:913–918. doi: 10.1016/j.bbrc.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 51.Yang B. Cao DJ. Sainz I. Colman RW. Guo YL. Different roles of ERK and p38 MAP kinases during tube formation from endothelial cells cultured in 3-dimensional collagen matrices. J Cell Physiol. 2004;200:360–369. doi: 10.1002/jcp.20025. [DOI] [PubMed] [Google Scholar]
- 52.Kadiyala S. Young RG. Thiede MA. Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 53.Xu L. Anderson AL. Lu Q. Wang J. Role of fibrillar structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials. 2007;28:750–761. doi: 10.1016/j.biomaterials.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 54.Cukierman E. Pankov R. Stevens DR. Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]