Abstract
A murine stromal cell line (OP9-DL1) expressing a notch ligand, Delta-like-1, has been shown to be able to drive the differentiation of both murine and human hematopoietic progenitors into T cells in vitro. Further studies showed that hematopoietic progenitors transduced by a retroviral vector to express a human CD8 T-cell receptor (TCR) followed by an OP9-DL1 monolayer coculture could generate antigen-specific cytotoxic T lymphocytes in vitro. It remains unknown if a similar method could be applied to produce CD4 helper T cells. In this report, we show that murine adult bone marrow (BM) cells transduced with an OT2 CD4 TCR and cocultured with OP9 stromal cells expressing Delta-like-1 can differentiate into antigen-specific CD4 T cells in vitro. These cells are capable of inducing the expression of T-cell activation markers and producing cytokines upon stimulation. We have also constructed a new stromal cell line (OP9-DL1-IAb) ectopically expressing a murine major histocompatibility complex class II protein, I-Ab, in OP9-DL1 cells. This new line could accelerate the development of TCR-transduced BM cells into CD4 T cells, resulting in cells with an improved capacity to respond to T-cell stimulation to secrete cytokines. Taken together, we demonstrate a general and potentially useful method to generate autologous antigen-specific CD4 helper T cells in vitro from easily accessible BM cells.
Introduction
Although the immune system can deal with most pathogens well, certain microbial infections, such as HIV [1], can directly target immune cells, preventing the host from generating an effective pathogen-specific immunity. Similarly, the immune system can often fail to suppress the growth of tumors, largely due to a low number of tumor-reactive T cells and/or an energy of T cells that is induced by tumor cells [2]. Thus, it is conceivable that immunotherapy by direct provision of a large quantity of functional T cells through adoptive transfer (termed passive T-cell immunotherapy or adoptive T-cell therapy) can be a viable approach to treat certain infectious diseases and cancers [2,3]. To carry out such a therapy, we need a sufficient amount of antigen-reactive T cells for transfer. One approach is to use allogeneic effector T cells from appropriate donors [4]. Adoptive transfer of these donor T cells has been shown to be able to treat some leukemia-like diseases [5–7]. However, the low availability of these donor cells limits the widespread application of this type of treatment. Moreover, patients usually suffer from severe toxicity due to graft-versus-host disease (GVHD). An improved approach for obtaining T cells is through the in vitro expansion of patients' own lymphocytes [2]. One example is the autologous transfer of in vitro expanded tumor-infiltrating lymphocytes (TILs) for the treatment of solid melanoma tumors [2]. Due to patient-to-patient variation, the major limitation of this approach is that isolation and selection of good quality T cells from patients is not always successful; therefore, the treatment will be limited to a small number of patients. Thus, it is of great interest to develop methods to reliably generate large numbers of antigen-specific T cells in vitro, which could address many of these described limitations.
One of the popular methods for developing T cells from hematopoietic progenitors in vitro is the use of fetal thymic organ culture [8]. But this method is expensive and difficult to scale up, making it impractical for generating a sufficient amount of T cells for adoptive transfer. Recently, an OP9-based coculture system capable of supporting the hematopoietic differentiation of progenitor cells into functional T cells has been reported [9–11]. OP9 is a stromal cell line derived from the macrophage CSF-deficient osteopetrotic mouse [12,13]. It was found that overexpression of a notch ligand, Delta-like-1, in OP9 cells resulted in the generation of a new cell line (OP9-DL1) that could drive the differentiation of both murine and human hematopoietic progenitors into T cells in vitro [9]. Thus far, murine progenitors isolated from fetal liver [9] or adult bone marrow (BM) [14,15], and human progenitors isolated from umbilical cord blood [16], pediatric BM [17], or postnatal thymus [18], have all been shown to be able to differentiate into T cells using the OP9-DL1 co-culture system, although the yield of T cells from adult BM was reported to be extremely low [15]. Subsequent studies showed that retrovirus-mediated transfer of a human CD8 T-cell receptor (TCR) into human hematopoietic progenitors derived from umbilical cord blood [19] or postnatal thymus [18] followed by OP9-DL1 monolayer coculture demonstrated a promising method of in vitro generation of antigen-specific cytolytic T lymphocytes (CTLs). It remains to be tested if antigen-specific CD4 helper T cells can be generated using a similar approach.
Many studies have shown that CD4 helper T cells play an indispensable role in orchestrating CTLs (CD8 T cells) to mount efficient immune responses by providing “cognate help” [20,21]. This “cognate help” role of CD4 T cells has also been found to be necessary in tumor immunotherapy; it has been proposed that the lack of an effective means of engaging CD4 helper cells is one of the major reasons for our inability to achieve a robust and long-lived CTL response against cancer cells [22,23]. This may support the argument that adoptive transfer of antigen-specific CD4 T cells, along with CD8 T cells, could represent an ideal approach for generating productive antigen-specific immune responses, although care needs to be taken to ensure that CD4 T cells with negative regulatory function, such as CD4+CD25+Foxp3+ regulatory T cells [24], are depleted from the CD4 population before transfer. In this study, we tested the concept of in vitro generation of antigen-specific CD4 T cells by coculturing OP9-DL1 cells with adult BM cells transduced to express a CD4 TCR. Considering that OP9 cells lack the expression of major histocompatibility complex (MHC) class II molecules, which may limit its ability to support CD4 T-cell development [10], we also investigated the effect of CD4-TCR/MHC-class-II interactions on the generation of functional CD4 cells by ectopic expression of I-Ab in OP9-DL1 cells (designated as OP9-DL1-IAb). We demonstrated that CD4 TCR retroviral transfer could be used to engineer adult BM cells to produce antigen-specific CD4 T cells in vitro. Moreover, the existence of CD4-TCR/MHC-class-II interactions in the coculture system could improve the functional response of these cells to generate cytokines.
Materials and Methods
Mice
Six- to eight-week-old female C57BL/6 (B6) mice were purchased from Charles River Breeding Laboratories. All animal procedures were approved by the Department of Animal Resources of the University of Southern California.
Construct preparation
The cDNAs of the alpha and beta chains of the murine I-Ab were PCR-amplified from appropriate mouse clones (ATCC mammalian gene collection, ATCC numbers 10324451 for alpha chain and 10470166 for beta chain) using specific primers (alpha chain: sense, 5′-CGCCGAGATCTCTCGAGATGCCGCGCAGCAGAGCTCTGATTC-3′, antisense, 5′-GGCGGAATTCTCATAAAGGCCCTGGGTGTCTGGAGGTG-3′; beta chain: sense, 5′-CACAACCATGGCTCTGCAGATCCCCAGCCTCC-3′, antisense, 5′-GCAGGTCGACTCACTGCAGGAGCCCTGCTGGAGGAG-3′; restriction sites for alpha chain, BglII and EcoRI, and for beta chain, NcoI and SalI, are shown by underlining). The resulting alpha chain was inserted downstream of the viral LTR promoter in the MIG retroviral vector [25], followed by insertion of the beta chain downstream of internal ribosome entry site (IRES) to replace the EGFP gene. We designated the final construct MIAb.
Cell lines
The OP9-MIG and OP9-DL1 cell lines were described previously by Schmitt et al. [9]. An ecotropic murine leukemia virus glycoprotein (Eco)-pseudotyped MIAb retrovirus (MIAb/Eco) was generated by cotransfecting HEK293T cells with the retroviral packaging vector pCL-Eco and a plasmid expressing a mouse MHC class II protein (MIAb) using a standard calcium phosphate precipitation technique. OP9-MIG or OP9-DL1 expressing I-Ab were generated by spin infection of OP9-MIG or OP9-DL1 with MIAb/Eco retroviruses in the presence of 10 μg/mL polybrene for 90 min at 2,500 rpm and 25°C using a Sorvall Legend 7 centrifuge. The efficiency of infection was determined by staining and flow cytometry analysis using Phycoerythrin (PE)-conjugated anti-mouse I-Ab antibody (clone AF6-120.1, BD Biosciences) (around 50%). Cells expressing I-Ab were sorted by FACS sorting, and then subcloned to generate clonal cell lines, OP9-MIG-IAb and OP9-DL1-IAb. All of the OP9-derived cell lines were cultured in a 10-cm dish in OP9 medium (αMEM (Gibco) supplemented with 20% FBS (Sigma), 10 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM glutamine).
Mouse bone marrow stem cells and OP9 cells coculture
Retroviral vector MOT2 was generated by linking cDNAs encoding OT2 TCR α and β chains with an IRES and cloning this into the MSCV-based retroviral vector under control of the viral LTR promoter [26]. BM cells were harvested from B6 female mice treated with 5-FU for 5 days to enrich the stem cells. Collected BM cells were cultured for 4 days in RPMI 1640 medium (Cellgro) containing 10% FBS (Sigma), 10 U/mL of penicillin, 100 μg/mL of streptomycin and 2 mM glutamine with 20 ng/mL recombinant murine IL-3 (Peprotech), 50 ng/mL recombinant murine IL-6 (Peprotech) and 50 ng/mL recombinant murine stem cell factor (Peprotech). On day 2 and day 3, the cells were spin infected with MOT2/Eco retrovirus for 90 min at 2,500 rpm and 25°C as described above. On day 4, the transduced BM cells were collected and transferred onto 80–90% confluent OP9-MIG, OP9-DL1, OP9-MIG-IAb, or OP9-DL1-IAb cell monolayers in 10-cm dishes with OP9 medium supplemented with 5 ng/mL murine IL-7 (Peprotech) and 5 ng/mL human Flt-3 ligand (Peprotech). BM cells were collected for surface marker analysis and transferred to new dishes containing OP9-MIG, OP9-DL1, OP9-MIG-IAb, or OP9-DL1-IAb at the indicated time points.
Flow cytometry
Surface staining was performed by blocking the cells with anti-mouse CD16/CD32 (clone 2.4G2, BD Pharmingen) followed by incubation with fluorochrome-conjugated antibodies. FITC-, PE- or PE-Cy5- conjugated antibodies specific for mouse Sca-1, CD117 (c-Kit), CD4, CD8, CD25, CD44, CD69, CD62L, Thy1, CD45R/B220, TCRVα2, and TCRVβ5.1,5.2 were purchased from BD Biosciences. Intracellular staining of TCRVα2 and TCRVβ5.1,5.2 was performed using the Cytofix/Cytoperm Kit from BD Pharmingen and following the manufacturer's protocol. The mouse Foxp3 intracellular staining kit from eBioscience was used for the regulatory T-cell analysis. All of the flow cytometry analysis was done with a FACSort (BD Bioscience) instrument.
T-cell stimulation and functional assays
For the anti-CD3/CD28 costimulation assay, a 24-well dish was precoated with 300 μL/well of anti-mouse CD3 (5 μg/mL in PBS, BD Pharmingen) at 4°C overnight. On the next day, OP9-cocultured cells were seeded at 1 × 106 cells per well in 1 mL of T-cell culture medium (RPMI 1640 medium (Cellgro) containing 10% FBS (Sigma), 10 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM glutamine) in presence of 1 μg/mL anti-mouse CD28 (BD Pharmingen) at 37°C in a humidified 5% CO2 incubator. Three days later, the culture supernatants were collected for IFN-γ and IL-2 ELISA assays and the stimulated cells were collected for flow cytometry analysis. For the peptide-based stimulation assay, 1 × 106 single positive (SP) CD4 T cells were seeded per well together with 2 × 106 spleen cells harvested from naïve B6 female mice as antigen-presenting cells (APCs) loaded with 1 μg/mL ovalbumin peptide (OVAp329–337, designated as OVAp2) for 3 days at 37°C in a 5% CO2 incubator and were then analyzed.
IFN-γ and IL-2 ELISA
ELISA plates (96-well) were precoated with 50 μL per well of 1 μg/mL anti-mouse IFN-γ or anti-mouse IL-2 (BD Pharmingen) in carbonate buffer at 4°C overnight. The plates were then washed six times with deionized water (DI water) followed by blocking with 100 μL per well of dilution buffer [2% borate buffered saline (BBS) and 0.002% sodium azide) for 30 min at 37°C. After six washes, sample supernatants were 2-fold serially diluted in dilution buffer, added to the plates at a final volume of 50 μL per well, and incubated for 3 h at 37°C. The plates were then washed ten times and incubated for 45 min at room temperature (RT) with 50 μL per well of 1 μg/mL biotinylated detecting antibody (BD Pharmingen) in dilution buffer. After ten washes, streptavidin-conjugated horseradish peroxidase (1:200 in dilution buffer) (R&D systems) were added at 50 μL per well for 30 min at RT. Finally, the plates were washed ten times, 50 μL per well of tetramethylbenzidine (TMB) substrate solution (KPL) was added, and the plates were incubated for 5–30 min at RT. The reaction was stopped by adding 50 μL per well of 2 M H2SO4. The absorbance at the wavelength of 450 nm (OD 450) was measured using a plate reader (Molecular Devices).
Statistical analysis
The significance of the difference between groups in the experiments measuring cytokine production was evaluated by analysis of variance followed by a one-tailed Student's t-test.
Results
Construction of OP9 cells expressing a MHC class II protein
Our initial interest was to generate antigen-specific CD4 T cells in vitro using an OP9-DL1 culture system. Direct introduction of cDNAs of a TCR into human hematopoietic progenitor cells (HPCs) by retroviral transduction and further culture of the cells on OP9-DL1 is a method that has been shown to generate functional antigen-specific CD8 T cells [18,19]. We wanted to investigate whether this approach could be used to produce antigen-specific CD4 T cells. In recognition of the fact that OP9 cells lack the expression of MHC class II molecules, we were also interested in studying whether enforced expression of a MHC class II molecule on OP9 cells could affect the development and function of antigen-specific CD4 T cells generated in this in vitro culture system.
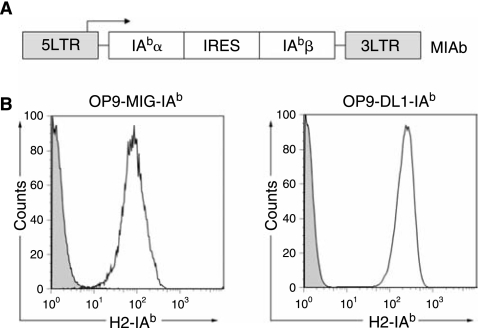
We chose the well-defined OT2 TCR as a model CD4 TCR in this study [27]. This TCR recognizes residues 329–337 of chicken ovalbumin presented by I-Ab, a murine MHC class II molecule. We analyzed OP9 stromal cells for their expression of MHC class II proteins by flow cytometry and found that surface expression of I-Ab was undetectable (Fig. 1B, control). Thus, we decided to evaluate the role of ectopic expression of I-Ab in OP9-DL1 stromal cells on the generation of OT2 CD4 T cells in vitro. The alpha and beta chains of cDNAs of murine I-Ab were linked by an IRES sequence and cloned into a murine stem cell virus-based retroviral vector [25] (Fig. 1A). Retrovirus encoding two chains of I-Ab was used to transduce OP9-DL1 cells and the cells that expressed a high level of I-Ab were sorted. The sorted cells were sub-cloned and one clone, designated OP9-DL1-IAb, was selected as a stromal line for the rest of the in vitro culture study (Fig. 1B, right). A control OP9-MIG-IAb cell line lacking the expression of Delta-like-1 was similarly generated using an OP9-MIG (OP9 transduced to express GFP alone) cell line (Fig. 1B, left).
FIG. 1.
Construction of OP9 stromal cells to express murine MHC class II protein (I-Ab) by retroviral transduction. (A) Schematic representation of a retroviral construct encoding α and β chains of murine I-Ab. Abbreviations: LTR, long-terminal repeats; IRES, internal ribosome entry site. Expression is driven by the viral LTR promoter. (B) Flow cytometry analysis of OP9 stromal cell lines (OP9-MIG-IAb and OP9-DL1-IAb) for their expression of I-Ab by surface staining. Solid line: OP9-MIG-IAb or OP9-DL1-IAb cells; shaded area: parental OP9-MIG or OP9-DL1 cells.
Generation of TCR-positive T cells using adult bone marrow progenitors in OP9 coculture systems
It has been shown that primitive fetal liver cells can be used as hematopoeitic progenitor cells to efficiently generate T lymphocytes when cocultured with OP9-DL1 stromal cells in the presence of IL-7 and Flt3-L [9]. Although adult BM cells were also reported to be able to give rise to T cells using similar culture conditions [14,15,28,29], the yield was much lower than that of fetal liver cells [14,15,28,29]. We repeated this culture process using either bulk or sorted (Lin−Sca-1+c-Kit+) BM cells from wild-type mice (4–6 weeks old) and compared their capacities to generate T cells with either OP9-DL1 or OP9-DL1-IAb cells. B lymphocyte differentiation of marrow cells was completely blocked when the cells were cultured with either stromal cell lines (OP9-DL1 or OP9-DL1-IAb) (data not shown). Generation of TCR-positive cells [either double positive (DP) or SP stage] was barely detectable (data not shown), which is consistent with the report by Huang et al. [15]. No marked difference in T cell development was observed using either bulk or sorted marrow cells. We found that expression of I-Ab in the OP9-DL1 cells did not significantly alter the differentiation patterns of marrow cells under our detection conditions (data not shown). It is not too surprising that the adult BM cells behaved differently from the fetal progenitor cells and could not efficiently differentiate to TCR-positive T cells in the current coculture system, as several studies have shown that different environments were required for adult and fetal lymphopoiesis [15,30–32]. It seems possible that the current culture conditions have not been optimized for adult marrow cells and identification of new conditions such as cytokine concentration [15,28] and stromal coculture environments could potentially improve the efficiency of the process. One of the key steps in generating a T cell is the successful rearrangement of the TCR genes. Thus, we reasoned that the delivery of a prearranged TCR gene into adult BM cells might facilitate T cell development under the OP9-DL1 culture condition.
Generation of TCR-specific CD4 T cells in OP9 coculture systems
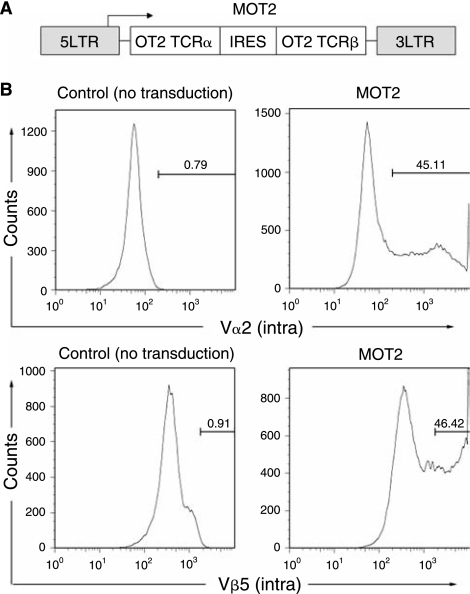
We next investigated the idea of generating TCR-specific CD4 helper T cells in OP9 coculture systems by retroviral introduction of a CD4 TCR gene to adult BM cells followed by coculture with OP9 cells expressing Delta-like-1 and/or a murine MHC class II protein. We used a mouse stem cell virus-based retroviral vector encoding the alpha and beta chains of an I-Ab-restricted OT2 CD4 TCR gene linked by an IRES sequence to deliver the TCR gene into the adult BM cells (Fig. 2A, designated MOT2) [33]. The transgene expression was controlled by the viral LTR promoter. We harvested adult BM cells enriched with HPCs from wild-type mice and exposed them to MOT2 vectors. Intracellular staining was performed to measure the transduction efficiency. The OT2 TCR uses Vα2 and Vβ5 TCR family chains and we used the antibodies specific for these chains for flow cytometry analysis. Approximately 45% of the total BM cells were modified to coexpress OT2 TCR alpha and beta chains (Fig. 2B).
FIG. 2.
Retroviral transduction of adult bone marrow (BM) cells to express OT2 CD4 T-cell receptor (TCR). (A) Schematic representation of a retroviral construct encoding α and β chains of OT2 TCR. Abbreviations: LTR, long-terminal repeats; IRES, internal ribosome entry site. Expression was driven by the viral LTR promoter. (B) Flow cytometry analysis of viral transduced BM cells for their expression of OT2 TCR by intracellular staining for TCR α chain using anti-Vα2 antibody and for TCR β chain using anti-Vβ5 antibody. Nontransduced BM cells were included as negative controls.
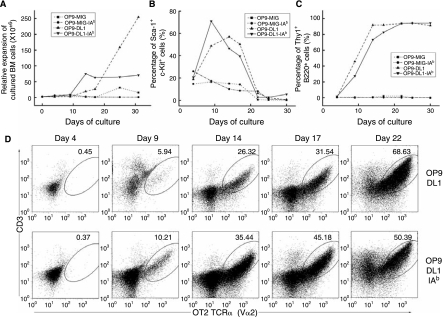
The transduced cells were subsequently cocultured on various OP9 cells (OP9-MIG, OP9-MIG-IAb, OP9-DL1, and OP9-DL1-IAb). Cell expansion, which is evidence of hematopoietic differentiation, was observed for cells cultured on OP9-MIG, OP9-DL1, and OP9-DL1-IAb, but not on OP9-MIG-IAb cells (Fig. 3A). The distinct kinetics and rate of cell expansion on the various stromal cells was also detected (Fig. 3A). OP9-MIG only supported limited expansion and the expansion did not start until day 17 of coculture. Significant expansion by both OP9-DL1 and OP9-DL1-IAb was observed after day 9 of coculture. However, MOT2-transduced BM cells cocultured on OP9-DL1 exhibited a stronger expansion (>4-fold) than the OP9-DL-IAb coculture. An apparent plateau in cellularity was obtained for the OP9-DL1-IAb coculture after an initial 2-week culture. It seems that OP9-DL1-IAb stromal cells have a reduced capacity to support expansion of adult BM cells, probably because of their elevated potency in facilitating hematopoietic differentiation (see below).
FIG. 3.
Coculture of OT2 TCR-transduced BM cells with various OP9 cells (OP9-MIG, OP9-IAb, OP9-DL1, and OP9-DL1-IAb). Results shown is one representative set of data from three independent experiments. (A) The expansion rate of transduced BM cells following coculture on OP9 cells. Total cellularity from OP9-MIG (▪), OP9-IAb (•), OP9-DL1 (▴), or OP9-DL1-IAb (▾) coculture was measured at the indicated time points. (B) Phenotypic analysis of Sca-1 and c-Kit double-positive transduced BM cells following coculture on OP9 cells. Percentages of Sca-1+c-Kit+ cells from various OP9 cells [the labeling was the same as (A)] were determined by flow cytometry analysis during the course of coculture at the indicated time points. (C) Phenotypic analysis of committed T-cell population (Thy1+B220+) from transduced BM cells following coculture on OP9 cells. Percentages of Thy1+B220+ cells from various OP9 cells [the labeling was the same as (A)] were determined by flow cytometry analysis during the course of coculture at the indicated time points. (D) The dot plots show the flow cytometry analysis of surface coexpression of CD3 and OT2 TCR alpha chain from transduced BM cells following coculture on either OP9-DL1 or OP9-DL1-IAb at the indicated time points.
We monitored the surface markers of the transduced BM cells developing in the cocultures. The markers (Sca-1+c-Kit+) were used to track the possible presence of hematopoietic progenitors. We found that both OP9-DL1 and OP9-DL1-IAb could initially increase the percentage of Sca-1+c-Kit+ cells, followed by a gradual decline (Fig. 3B). Most Sca-1+c-Kit+ cells disappeared after 3 weeks of coculture, indicating that the majority of the hematopoietic progenitors had undergone differentiation. The markers B220−Thy1+ were employed to monitor the progress of T-cell differentiation. As expected, OP9 cells lacking the expression of a notch ligand could not support hematopoietic T-cell differentiation. In contrast, most cells developed on either OP9-DL1 or OP9-DL1-IAb cells were committed to the T-cell lineage after 2-week cocultures (Fig. 3C).
We analyzed the TCR expression by costaining the CD3 and OT2 TCR alpha chains. For MOT2-transduced BM cells cultured on control OP9 cells lacking the expression of Delta-like-1 (either OP9-MIG or OP9-IAb), we could not detect the surface expression of the OT2 TCR even after 3 weeks of coculture (data not shown). In contrast, OT2 TCR expression was readily detectable as early as on day 9 of coculture on transduced cells differentiating on either OP9-DL1 or OP9-DL1-IAb stromal cells (Fig. 3D).
Interestingly, it appeared that the expression of I-Ab could facilitate T-cell development (5.94% of OT2 in OP9-DL1 vs. 10.21% of OT2 in OP9-DL1-IAb on day 9). After 3 weeks of coculture, more than 50% of the total cells were OT2 TCR-positive. It should be noted that virtually all of the CD3+ cells expressed the transduced TCR, suggesting that the rearrangement of endogenous TCR could be a limiting step for in vitro T-cell development and the ectopic provision of prearranged TCR could partially overcome this limitation.
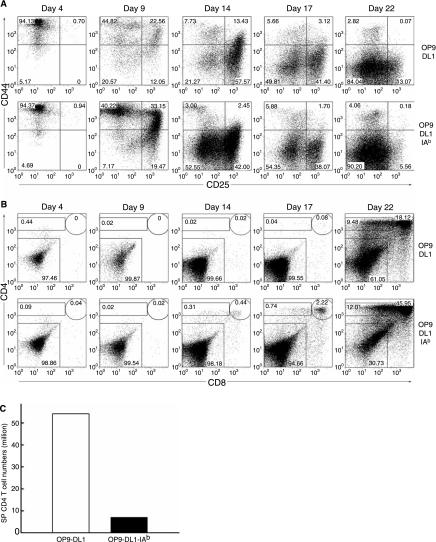
The phenotypic progression from CD25−CD44+ (DN1) to CD25+CD44+ (DN2) to CD25+CD44− (DN3), and finally CD25−CD44− [DN4, also called pre-DP (double positive) [34]], is a characteristic differentiation pattern for early immature T-cell precursors that are double-negative (DN) for CD4 and CD8 expressions [34–37]. Previous study showed that different DN differentiation kinetics occurred when fetal liver and adult BM were used as the progenitor cells cocultured on OP9-DL1 cells [15]. We carefully monitored this aspect of differentiation and found that both OP9-DL1 and OP9-DL1-IAb could support the progressive differentiation of TCR-transduced BM progenitors from DN1 to DN4 (Fig. 4A). At day 17 of both cocultures, TCR-transduced cells were predominately DN3 and DN4. At the same time point and the same conditions of the culture, we (data not shown) and others [29] found that DN2 and DN3 dominated when wild-type, nontransduced BM cells were cultured with Delta-like-1-expressing OP9 cells, suggesting that the enforced expression of prearranged TCR genes could accelerate the development of DN cells in such in vitro culture conditions. We also noticed that expression of I-Ab could facilitate the DN differentiation, as the major population (52%) of cells cocultured with OP9-DL1-IAb had advanced to the DN4 stage by day 14 of coculture, compared to only 21% of the cells cocultured with OP9-DL1 (Fig. 4A).
FIG. 4.
Development of CD4 T cells from TCR-transduced BM cells cocultured with either OP9-DL1 or OP9-DL1-IAb cells. Results shown is one representative set of data from three independent experiments. (A) Phenotypic analysis of double-negative development of transduced BM cells following coculture with OP9 cells at indicated time points by surface costaining of CD25 and CD44 expressions. (B) Phenotypic analysis of CD4 and CD8 expressions on transduced BM cells cocultured with OP9 cells at indicated time points by surface costaining of CD4 and CD8. (C) The total numbers of SP CD4 T cells generated from OP9 cocultures. The two million BM cells were transduced with OT2 vector and then the OP9 cocultures were initiated.
Flow cytometry analysis was further performed to track the expression of CD4 and CD8 on MOT2-transduced cells at various time points during the coculture period (Fig. 4B). Transduced BM cells cocultured on either OP9-DL1 or OP9-DL1-IAb could give rise to CD8+CD4+ immature DP T cells as early as on day 17 or day 14 of coculture, respectively, indicating that expression of a MHC class II protein is dispensable for differentiation into DP cells under this culture condition. However, the presence of I-Ab had the effect of promoting differentiation and resulted in DP cells (44%) accounting for the majority of cultured MOT2-transduced cells in OP9-DL1-IAb after a 3-week coculture (Fig. 4B). Despite potential help from the early expression of TCR and the presence of I-Ab, this differentiation process is still much slower than that of the fetal liver progenitors, in which DP cells can be detected as early as after 1 week of coculture [9]. In addition to DP cells, we also detected a significant population of mature SP CD4 T cells (9.48% from OP9-DL1 coculture; 12.01% from OP9-DL1-IAb coculture). Approximately 54 and 7 million of SP CD4 T cells were obtained from OP9-DL1 and OP9-DL1-IAb cocultures, respectively (original cell input of adult BM cells was 2 million; Fig. 4C). These CD4 T cells predominantly (>95%) expressed the OT2 TCR, detected by staining using antibodies against TCR Vα2 chain and Vβ5 chain (data not shown). No regulatory T-cell populations could be identified from these SP CD4 T cells generated from both cocultures by costaining of CD4, Vβ5, and Foxp3 (data not shown).
Functional analysis of TCR-specific CD4 T cells generated by OP9 cocultures
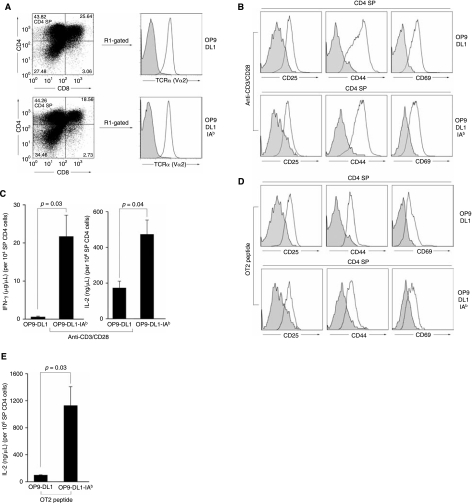
In light of the fact that mature SP CD4 T cells emerged in OP9 cocultures, we went on to evaluate whether these T cells could undergo TCR signaling in response to stimulation. Adult BM progenitor cells, transduced by a MOT2 vector and cultured with either OP9-DL1 or OP9-DL1-IAb for about 3–4 weeks, were seeded in an anti-CD3-coated plate and stimulated with anti-CD28 antibody. We observed that these T cells proliferated under the condition of this polyclonal anti-CD3/CD28 stimulation (data not shown), indicating that these cells might have acquired the feature of functional maturity. Following the stimulation with antibodies, the SP CD4 T cells were greatly expanded (from 9–10% to 43–44%) (Figs. 4B and 5A). When we gated on these SP CD4 T cells, we found that the majority of them expressed the OT2 TCR, as assessed by TCR Vα2 staining (Fig. 5A). The level of OT2 TCR expression was similar for the CD4 T cells cocultured either on OP9-DL1 or OP9-DL1-IAb cells. Phenotypic analysis of these T cells showed that these stimulated cells displayed the typical activated T-cell markers (CD25+CD44+CD69+), as compared to nonstimulated control cells (Fig. 5B). IFN-γ and IL-2 ELISAs were performed to measure the capacity of these CD4 T cells to secrete cytokines upon polycloncal stimulation. As shown in Fig. 5C, IFN-γ and IL-2 secretions were detected for CD4 T cells generated from both cocultures; no production of either IFN-γ or IL-2 was detected for nonstimulated cells (data not shown). Interestingly, we found that CD4 T cells from the OP9-DL1-IAb coculture produced significantly more IFN-γ (>35-folds) than that of the OP9-DL1 coculture. The same trend was observed for IL-2 secretion, although to a lesser extent.
FIG. 5.
Functional analysis of antigen-specific CD4 T cells generated from TCR-transduced BM cells cocultured with either OP9-DL1 or OP9-DL1-IAb cells. Cocultured BM cells were stimulated for 2 days with either anti-CD3/CD28 antibodies (A–C) or with antigen-presenting cells pulsed with OVAp2 peptide (D and E). (A) Flow cytometry analysis of the expansion of SP CD4 T cells upon stimulation for 3 days. OT2 TCR expression was analyzed by gating on the CD4+CD8− population (R1-gated, solid line). Shaded area: staining with an isotype control antibody. (B and D) Flow cytometry analysis of surface expression of CD25, CD44, and CD69 before stimulation (shaded area) or after stimulation (solid line) on gated SP CD4 T cells. IFN-γ (C and E) and IL-2 (C) productions in response to anti-CD3/CD28 stimulation were determined by ELISA.
To determine if these CD4 T cells generated in vitro could mount antigen-specific immune responses, we cultured these TCR-transduced and SP-sorted cells with OVAp2 peptide-loaded APCs harvested from a mouse spleen. MOT2-transduced cells were able to recognize OVAp2-pulsed APCs, as evidenced by the appearance of T-cell activation markers (CD25+CD44+CD69+) (Fig. 5D). IL-2 secretion was observed for peptide-stimulated cells (Fig. 5E); no IL-2 secretion was detected for either nonstimulated cells or cells cocultured with APCs without peptide-pulsing (data not shown). The cells were able to respond to a peptide concentration of 1 μg/mL, which was equivalent to the sensitivity of the response of OT2 transgenic T cells to the OVAp2 peptide [26,33]. Similar to the observation from polyclonal stimulation, markedly higher production of IL-2 was detected for T cells developed on OP9-DL1-IAb cells, as compared to IL-2 secreted from cells generated on OP9-DL1 coculture. However, no production of IFN-γ could be detected for TCR-transduced T cells generated either from the OP9-DL1 or OP9-DL1-IAb coculture.
Discussion
The purpose of this study is to evaluate a strategy to generate antigen-specific CD4 helper T cells in vitro by culturing adult BM cells transduced to express a CD4 TCR with OP9 stromal cells expressing a notch ligand, Delta-like-1. In vitro generation of antigen-specific CD4 T cells is of great interest to both fundamental study of T-cell biology and practical immunotherapeutic applications. For instance, there is accumulating evidence showing that CD4 T cells play an indispensable role in antitumor immunity [20–23,38–40]. The availability of a large quantity of CD4 T cells recognizing the tumor antigen can potentially enhance the current therapy of adoptive transfer of antitumor CD8 T cells to achieve maximal therapeutic efficacy [3,41]. We focused on adult BM cells for this study because from the therapeutic point of view, they represent the most easily accessible HPCs and can be frequently and safely isolated.
Our method relies on a recently reported OP9-DL1 coculture system, in which the ectopic expression of a notch ligand in a BM stromal cell line, OP9, was found to be sufficient to support the development of hematopoietic progenitors into T cells in vitro [9–11]. However, OP9-DL1 cells lack the expression of MHC class II molecules, which may limit their capacity to support the development of CD4 T cells [10]. This prompted us to also evaluate the effect of the expression of MHC class II proteins in OP9-DL1 cells on generating antigen-specific CD4 T cells. It was reported that hematopoietic progenitors derived from fetal liver had dramatically different kinetics of differentiation from that of progenitors originating from adult BM [15,30–32]. We verified that the yield of T cells generated from bulk adult BM cells cocultured on OP9-DL1 cells was extremely low; most of the development of the cells was halted at the DN2 and DN3 stages [15]. When adult progenitor cells were transduced to express prearranged OT2 TCR alpha and beta chains, a majority of the cells (>70%) were committed to the T-cell lineage within 2 weeks of cocultures. CD3 and TCR expressions were detected as early as on day 9 of cocultures. The increased commitment of T cells is likely due to the earlier expression of the OT2 TCR, leading to the bypass of endogenous TCR rearrangement. This observation is consistent with the result by van Lent et al. [18], in which earlier expression of a human TCR in human hematopoietic progenitors from postnatal thymus drastically enhanced T-cell development.
Several interesting features were observed in T-cell development in the TCR transduction cocultures with the expression of the murine MHC class II protein, I-Ab. We first observed different expansion kinetics in the presence of I-Ab. Transduced BM cells started marked expansion earlier (day 9) in OP9-DL1-IAb coculture, but quickly reached a plateau at day 14. In contrast, a slower expansion occurred in the OP9-DL1 coculture at day 10, followed by a rapid expansion after day 17. We therefore observed a reduced expansion capacity of OP9 cells upon an enforced expression of I-Ab; it remains to be determined whether the interaction between the OT2 TCR and I-Ab plays a role in limiting hematopoietic expansion. It was also found that I-Ab expression in the OP9-DL1 cell line could accelerate T-cell development. Approximately 10% of BM cells became CD3+OT2+ T cells by day 9 of the OP9-DL1-IAb coculture, compared to around 6% of these T cells appearing in the OP9-DL1 coculture. A close examination of the development of DN cells revealed that 52% of OP9-DL1-IAb-cocultured BM cells reached the DN4 stage by day 14, whereas only 21% of the OP9-DL1-cocultured cells were able to arrive at the same stage. Similar acceleration was also observed in the development of single-positive cells. A majority of the BM cells (>45%) turned into CD4+CD8+ SP cells in the OP9-DL1-IAb coculture at day 22, while only 18% of the cells from the OP9-DL1 coculture displayed the SP phenotype. Further experiments are needed in order to elucidate the exact reason for the accelerated development, although we suspect that TCR signaling induced by I-Ab engagement may partially contribute to such an observation.
In vitro culture of OT2 TCR-transduced BM cells resulted in a large majority (∼90%) of OT2 TCR-expressing CD4 T cells. Such a dominant representation of TCR-specific T cells was also reported previously when human CD8 TCRs were introduced to hematopoietic progenitors [18]. In fact, similar over-representation of TCR-cloned T cells was observed in both TCR-transgenic mice [42] and TCR-retrogenic mice (mice reconstituted by hematopoietic stem cells transduced by a retroviral vector carrying a TCR gene) [26,33,43]. In contrast, transduction of mature T cells with TCR-retroviral vectors can only generate a modest population of antigen-specific T cells [44–46]. The mispairing between the alpha and beta chains of the transduced TCR with the beta and alpha chains of endogenous TCRs was proposed to be the major reason for the low efficiency of redirecting the specificity of mature T cells by the TCR transduction method [47,48]. Thus, our result and others' results [18,19] clearly suggest that modification of hematopoietic progenitors with a TCR-retroviral vector represents an efficient way to generate antigen-specific T cells both in vitro [18,19] and in vivo [26,33,43].
We tested the functional properties of SP CD4 T cells generated from the TCR transduction cocultures. Upon polyclonal anti-CD3/CD28 stimulation, these cells proliferated, displayed typical surface markers of activated T cells, and secreted the cytokines IFN-γ and IL-2, indicating that these T cells reached functional maturity and were able to initiate TCR signaling in response to stimulation. As compared to CD4 T cells developed from the OP9-DL1 coculture, a much higher production of cytokines (IFN-γ and IL-2) were detected for T cells generated from the OP9-DL1-IAb coculture, suggesting that the provision of interaction between the OT2 TCR and I-Ab in the coculture might enhance the functional development of CD4 T cells. To examine whether our OP9 coculture could generate regulatory T cells, which might contribute to the observed discrepancy of cytokine production of cells cultured on OP9-DL1 versus those cultured on OP9-DL1-IAb, we performed intracellular staining of Foxp3 and could not detect any population of T cells that were CD4+Vβ5+Foxp3+. More experiments are underway to identify the molecular difference between these two groups of T cells and to elucidate the detailed molecular mechanism for the enhanced function. In response to peptide stimulation, we observed antigen-specific responses by these SP CD4 T cells, as manifested by both activation marker staining and IL-2 secretion. Similar to the results of polyclonal stimulation, markedly higher production of IL-2 was observed for OT2 CD4 T cells cocultured with OP9-DL1-IAb. Interestingly, under the current condition of antigen-specific stimulation, we were unable to detect the production of IFN-γ We speculate that the lack of certain costimulatory signals [49,50] during the stimulation may result in the failure of IFN-γ secretion and are designing experiments to test this hypothesis.
In conclusion, we demonstrated an example of in vitro generation of antigen-specific CD4 T cells by retroviral transduction of adult BM cells to express a CD4 TCR. Ectopic expression of a MHC class II protein could accelerate the development of TCR-transduced BM cells, resulting in functionally improved antigen-specific CD4 T cells. Although our data shows that this represents a promising approach to obtain CD4 T cells with desired specificity in vitro, there is much left to learn about this stromal system we engineered. Will the provision of MHC class II molecules on OP9-DL1 have a similar effect on other hematopoietic progenitors, such as fetal liver cells? We did not observe a significantly altered development pattern for nontransduced BM cells cocultured with either OP9-DL1 or OP9-DL1-IAb. Could this be simply due to the inefficient nature of coculture using BM cells, disallowing the opportunity to capture the difference? We are addressing this question by coculturing wild-type progenitors from fetal livers. We are also in the process of fully examining the functional potential of these CD4 T cells by adoptive transfer of these cells to mice followed by the measurement of the immune response upon antigen-specific immunization.
Acknowledgments
We thank April Tai and Lili Yang for critical reading of the manuscript. This work was supported by a National Institute of Health grant AI068978.
References
- 1.Douek DC. Picker LJ. Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 2.Dudley ME. Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L. Powell DJ., Jr. Rosenberg SA. Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb HJ. Holler E. Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol. 1997;9:139–145. doi: 10.1097/00001622-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bollard CM. Aguilar L. Straathof KC. Gahn B. Huls MH. Rousseau A. Sixbey J. Gresik MV. Carrum G. Hudson M. Dilloo D. Gee A. Brenner MK. Rooney CM. Heslop HE. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney CM. Smith CA. Ng CY. Loftin SK. Sixbey JW. Gan Y. Srivastava DK. Bowman LC. Krance RA. Brenner MK. Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 7.Khanna R. Bell S. Sherritt M. Galbraith A. Burrows SR. Rafter L. Clarke B. Slaughter R. Falk MC. Douglass J. Williams T. Elliott SL. Moss DJ. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceredig R. Jenkinson EJ. MacDonald HR. Owen JJ. Development of cytolytic T lymphocyte precursors in organ-cultured mouse embryonic thymus rudiments. J Exp Med. 1982;155:617–622. doi: 10.1084/jem.155.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt TM. Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 10.Zuniga-Pflucker JC. T-cell development made simple. Nat Rev Immunol. 2004;4:67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
- 11.de Pooter R. Zuniga-Pflucker JC. T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol. 2007;19:163–168. doi: 10.1016/j.coi.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Wiktor-Jedrzejczak W. Bartocci A. Ferrante AW., Jr. Ahmed-Ansari A. Sell KW. Pollard JW. Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida H. Hayashi S. Kunisada T. Ogawa M. Nishikawa S. Okamura H. Sudo T. Shultz LD. Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt TM. de Pooter RF. Gronski MA. Cho SK. Ohashi PS. Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 15.Huang J. Garrett KP. Pelayo R. Zuniga-Pflucker JC. Petrie HT. Kincade PW. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol. 2005;175:4858–4865. doi: 10.4049/jimmunol.175.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Motte-Mohs RN. Herer E. Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 17.De Smedt M. Hoebeke I. Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis. 2004;33:227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 18.van Lent AU. Nagasawa M. van Loenen MM. Schotte R. Schumacher TN. Heemskerk MH. Spits H. Legrand N. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. J Immunol. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y. Parkhurst MR. Zheng Z. Cohen CJ. Riley JP. Gattinoni L. Restifo NP. Rosenberg SA. Morgan RA. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen EM. Lemmens EE. Wolfe T. Christen U. von Herrath MG. Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 21.Shedlock DJ. Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 22.Pardoll DM. Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S. CD4+ T lymphocytes: a critical component of antitumor immunity. Cancer Invest. 2005;23:413–419. [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Van Parijs L. Refaeli Y. Lord JD. Nelson BH. Abbas AK. Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 26.Yang L. Qin XF. Baltimore D. Van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc Natl Acad Sci USA. 2002;99:6204–6209. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnden MJ. Allison J. Heath WR. Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 28.Balciunaite G. Ceredig R. Fehling HJ. Zuniga-Pflucker JC. Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 29.Zakrzewski JL. Kochman AA. Lu SX. Terwey TH. Kim TD. Hubbard VM. Muriglan SJ. Suh D. Smith OM. Grubin J. Patel N. Chow A. Cabrera-Perez J. Radhakrishnan R. Diab A. Perales MA. Rizzuto G. Menet E. Pamer EG. Heller G. Zuniga-Pflucker JC. Alpdogan O. van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 30.Douagi I. Vieira P. Cumano A. Lymphocyte commitment during embryonic development, in the mouse. Semin Immunol. 2002;14:361–369. doi: 10.1016/s1044532302000702. [DOI] [PubMed] [Google Scholar]
- 31.Crompton T. Outram SV. Buckland J. Owen MJ. Distinct roles of the interleukin-7 receptor alpha chain in fetal and adult thymocyte development revealed by analysis of interleukin-7 receptor alpha-deficient mice. Eur J Immunol. 1998;28:1859–1866. doi: 10.1002/(SICI)1521-4141(199806)28:06<1859::AID-IMMU1859>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho TL. Mota-Santos T. Cumano A. Demengeot J. Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7– mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L. Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrie HT. Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey DI. Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 36.Ceredig R. Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 37.Bhandoola A. von Boehmer H. Petrie HT. Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura T. Iwakabe K. Sekimoto M. Ohmi Y. Yahata T. Nakui M. Sato T. Habu S. Tashiro H. Sato M. Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxevanis CN. Voutsas IF. Tsitsilonis OE. Gritzapis AD. Sotiriadou R. Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164:3902–3912. doi: 10.4049/jimmunol.164.7.3902. [DOI] [PubMed] [Google Scholar]
- 40.Marzo AL. Kinnear BF. Lake RA. Frelinger JJ. Collins EJ. Robinson BW. Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 41.Moeller M. Haynes NM. Kershaw MH. Jackson JT. Teng MW. Street SE. Cerutti L. Jane SM. Trapani JA. Smyth MJ. Darcy PK. Adoptive transfer of gene-engineered CD4+ helper T cells induces potent primary and secondary tumor rejection. Blood. 2005;106:2995–3003. doi: 10.1182/blood-2004-12-4906. [DOI] [PubMed] [Google Scholar]
- 42.von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 43.Arnold PY. Burton AR. Vignali DA. Diabetes incidence is unaltered in glutamate decarboxylase 65-specific TCR retrogenic nonobese diabetic mice: generation by retroviral-mediated stem cell gene transfer. J Immunol. 2004;173:3103–3111. doi: 10.4049/jimmunol.173.5.3103. [DOI] [PubMed] [Google Scholar]
- 44.Kessels HW. Wolkers MC. van den Boom MD. van der Valk MA. Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 45.Morgan RA. Dudley ME. Wunderlich JR. Hughes MS. Yang JC. Sherry RM. Royal RE. Topalian SL. Kammula US. Restifo NP. Zheng Z. Nahvi A. de Vries CR. Rogers-Freezer LJ. Mavroukakis SA. Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heemskerk MH. Hagedoorn RS. van der Hoorn MA. van der Veken LT. Hoogeboom M. Kester MG. Willemze R. Falkenburg JH. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109:235–243. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 47.Cohen CJ. Zhao Y. Zheng Z. Rosenberg SA. Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuball J. Dossett ML. Wolfl M. Ho WY. Voss RH. Fowler C. Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroczek RA. Mages HW. Hutloff A. Emerging paradigms of T-cell costimulation. Curr Opin Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Ward RC. Kaufman HL. Targeting costimulatory pathways for tumor immunotherapy. Int Rev Immunol. 2007;26:161–196. doi: 10.1080/08830180701365941. [DOI] [PubMed] [Google Scholar]