Abstract
Garlic-derived organosulfur compounds (OSCs) are highly effective in affording protection against chemically-induced pulmonary carcinogenesis in animal models. We now demonstrate that garlic constituent diallyl trisulfide (DATS) suppresses viability of cultured human lung cancer cell lines H358 (a non-small cell lung cancer cell line) and H460 (a large cell lung cancer cell line) by causing G2-M phase cell cycle arrest and apoptotic cell death. On the other hand, a normal human bronchial epithelial cell line BEAS-2B was significantly more resistant to growth inhibition and apoptosis induction by DATS compared with lung cancer cells. We also found that even a subtle change in the OSC structure could have a significant impact on its biological activity. For example, DATS was significantly more effective than either diallyl sulfide or diallyl disulfide against proliferation of lung cancer cells. The DATS-mediated G2-M phase cell cycle arrest was explained by down-regulation of cyclin-dependent kinase 1 (Cdk1) and cell division cycle 25C protein expression leading to accumulation of Tyr15 phosphorylated (inactive) Cdk1. The DATS-induced apoptosis correlated with induction of proapoptotic proteins Bax, Bak, and BID, and a decrease in the expression of anti-apoptotic proteins Bcl-2 and Bcl-xL in lung cancer cells but not in BEAS-2B. Knockdown of Bax and Bak proteins conferred significant protection against DATS-induced apoptotic cytoplasmic histone-associated DNA fragmentation. On the other hand, BID protein was dispensable for DATS-induced apoptosis. In conclusion, the present study indicates that Bax and Bak proteins are critical targets of DATS-induced apoptosis in human lung cancer cells.
Keywords: Diallyl trisulfide, lung cancer, Bax, Bak, apoptosis
INTRODUCTION
Epidemiological data continues to support the premise that dietary intake of Allium vegetables (e.g., garlic) may lower the risk of different types of malignancies [You et al., 1989; Gao et al., 1999; Fleischauer et al., 2000; Hsing et al., 2002]. Anticarcinogenic effect of Allium vegetables is attributable to organosulfur compounds (OSCs), which are released upon processing (e.g., cutting or chewing) of these vegetables [Block, 1992]. Allium vegetable-derived OSCs including diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) have been shown to inhibit cancer in animal models induced by a variety of carcinogens [Wargovich, 1987; Sparnins et al., 1988; Hong et al., 1992; Reddy et al., 1993; Hu et al., 1997]. For example, oral gavage of 200 mg DAS/kg body weight for 3 days prior to the challenge with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), significantly decreased the incidence of NNK-induced lung tumors and the tumor multiplicity in female A/J mice [Hong et al., 1992]. Dietary feeding of 100 ppm and 200 ppm of DADS suppressed the incidence and multiplicity of azoxymethane-induced invasive colon cancer in rats [Reddy et al., 1993]. The naturally occurring OSCs are also effective in affording protection against benzo[a]pyrene-induced forestomach and pulmonary carcinogenesis in mice [Sparnins et al., 1988; Hu et al., 1997]. The mechanisms by which OSCs inhibit chemically-induced cancer are well-explained and involve an increase in expression of phase 2 carcinogen detoxifying enzymes including glutathione transferases and inhibition of cytochrome P450-dependent monooxygenases [Brady et al., 1991; Hu et al., 1996; Singh et al., 1998]. We have also shown previously that oral gavage of 16.5 and 33 μmol DADS (in 0.1 mL soybean oil) to female athymic mice, three times/week beginning the day of subcutaneous tumor cell injection, significantly inhibits growth of H-ras oncogene transformed NIH 3T3 cells without causing weight loss or any other side effects [Singh, 2001].
More recent studies including those from our laboratory have revealed that naturally-occurring OSCs can inhibit growth of cancer cells by causing cell cycle arrest and apoptosis induction [Sundaram and Milner, 1996; Knowles and Milner, 2000; Hong et al., 2000; Nakagawa et al., 2001; Filomeni et al., 2003; Xiao et al., 2004; Xiao et al., 2005; Wu et al., 2005; Hosono et al., 2005; Herman-Antosiewicz and Singh, 2004; Herman-Antosiewicz and Singh, 2005; Xiao and Singh, 2006; Antosiewicz et al, 2006; Kim et al., 2007; Herman-Antosiewicz et al., 2007a; Herman-Antosiewicz et al., 2007b]. The cell cycle arrest and apoptosis induction by DADS was first documented by Milner and colleagues in human colon cancer cells [Sundaram and Milner, 1996; Knowles and Milner, 2000]. For example, treatment of HCT-15 human colon cancer cells with 100 and 500 μM DADS for 24 h or 48 h resulted in apoptotic DNA fragmentation [Sundaram and Milner, 1996]. We have shown recently that DATS at 10, 20, and/or 40 μM concentrations suppresses growth of PC-3, DU145 and LNCaP human prostate cancer cells in culture by causing apoptosis [Xiao et al., 2004; Kim et al., 2007]. The DATS-induced apoptosis in prostate cancer cells at 20 and 40 μM concentration correlated with generation of reactive oxygen species and activation of c-Jun N-terminal kinase [Xiao et al., 2004; Kim et al., 2007]. Sakamoto et al. [1997] reported suppression of human lung cancer A549 cell growth in the presence of DADS (50 and 100 μM, 24 h exposure) and DATS (10 and 50 μM, 24 h exposure). Interestingly, the apoptotic DNA fragmentation in A549 cells was evident even at 1 and 5 μM DATS concentrations [Sakamoto et al., 1997].
The present study demonstrates that DATS suppresses growth of cultured H358 and H460 human lung cancer cells by causing apoptotic cell death. Interestingly, an immortalized normal human bronchial epithelial cell line (BEAS-2B) is significantly more resistant to growth inhibition and apoptosis induction by DATS compared with H358 or H460 cells. The DATS-induced apoptosis in human lung cancer cells is mediated by induction of Bax and Bak proteins.
MATERIALS AND METHODS
Reagents
The garlic-derived OSCs including DAS (purity ~97%), DADS (purity ~80%), DATS (purity ~97%), dipropyl sulfide (DPS; purity ~97%), and dipropyl disulfide (DPDS; purity ~98%) were purchased from LKT Laboratories (St. Paul, MN) or Sigma-Aldrich (Milwaukee, WI). Tissue culture media and fetal bovine serum were from GIBCO (Grand Island, NY), Cell Death Detection ELISA kit was from Roche Applied Sciences (Mannheim,Germany), and RNaseA was from Promega (Madison, WI). The antibodies against cyclinB1 (monoclonal, cat. #sc-245), cyclin-dependent kinase 1 (Cdk1; monoclonal, cat. #sc-8395), cell division cycle 25C (Cdc25C; monoclonal, cat. #sc-13138), poly-(ADP-ribose)-polymerase (PARP; polyclonal, cat. #sc-7150), Bax (monoclonal, cat. #sc-7480), Bak (polyclonal, cat. #sc-832), Bcl-xL (monoclonal, cat. #sc-8392), and BID (polyclonal, cat. #sc-6538) were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-Bcl-2 antibody (monoclonal, cat. #M0887) was from DAKOCytomation (Carpinteria, CA). The anti-Tyr15 phosphorylated Cdk1 (affinity isolated, cat. #C0228) and anti-actin (monoclonal, cat. #A5441) antibodies were from Sigma (St. Louis, MO).
Cell Culture and Cell Survival Assay
The human non-small cell lung cancer cell line H358, the human large cell lung cancer cell line H460, and the immortalized normal human bronchial epithelial cell line BEAS-2B were obtained from American Type Culture Collection (Manassas, VA). The H460 cell line was originally derived from the pleural fluid of a male patient with large cell cancer of the lung. These cells express p53 mRNA at levels comparable to normal human lung without any gross structural abnormalities in DNA. The H358 is a non-small cell lung cancer cell line that was established from tumor tissue of a male patient. This cell line has homozygous deletion of p53. The BEAS-2B cell line was isolated from normal human bronchial epithelium obtained from autopsy of a non-cancerous individual and immortalized by infection with an adenovirus 12-SV40 virus hybrid. The BEAS-2B cell line has been used extensively as a representative normal bronchial epithelial cell line. Monolayer cultures of H358 and H460 cells were maintained in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, antibiotic mixture (10 ml/liter), 10 mM HEPES, 1 mM sodium pyruvate, 1.5 g/L bicarbonate and 4.5 g/L glucose. The BEAS-2B cell line was cultured in Bronchial Epithelial Cell Basal Medium (Cambrex, Walkersville, MD; Cat# CC-3171). The mouse embryonic fibroblasts (MEFs) derived from wild-type (WT) and BID knockout mice (BID-KO) mice and immortalized by transfection with the SV40 genomic DNA were a generous gift from the late Dr. Stanley Korsmeyer (Dana Farber Cancer Institute, Boston, MA). The MEFs derived from WT and BID-KO mice were maintained as described by Wei et al. [2001]. Culture of each cell line was maintained at 37°C in an atmosphere of 95% air and 5% CO2. Effect of OSCs on survival of H358, H460, and BEAS-2B cells was determined by trypan blue dye exclusion assay. Briefly, 0.5–1×104 cells in 1 ml of complete medium were plated and allowed to attach by overnight incubation. The medium was replaced with fresh complete medium containing desired concentrations of the OSCs, and the plates were incubated for 24 h, 48 h or 72 h at 37°C. Stock solutions of the OSCs were prepared in dimethyl sulfoxide (DMSO), and an equal volume of DMSO (final concentration 0.05%) was added to the controls. At the end of the incubation, both floating and adherent cells were collected, suspended in 30 μl of 0.07% trypan blue solution, and counted under an inverted microscope using a hemocytometer.
Analysis of Cell Cycle Distribution
The effect of the DATS treatment on cell cycle distribution was determined by flow cytometry after staining the cells with propidium iodide as described by us previously [Herman-Antosiewicz and Singh, 2005]. Briefly, 3×105 cells were seeded in T25 flasks and allowed to attach by overnight incubation. The medium was replaced with fresh complete medium containing desired concentrations of DATS (or DMSO for controls; final concentration 0.05%) and the flasks were incubated for 4, 8 or 16 h at 37°C. Both floating and adherent cells were collected, washed with phosphate buffered saline (PBS) and fixed with 70% ethanol overnight at 4°C. The cells were then treated with 80 μg/ml RNaseA and 50 μg/ml propidium iodide for 30 min and analyzed using a Coulter Epics XL Flow cytometer. Percentage of cells in different phase of the cell cycle was computed for DMSO-treated control and DATS-treated cultures.
Immunoblotting
The cells were treated with DATS (or DMSO) as described above, and washed twice with ice-cold PBS. The cells were lysed as described by us previously [Xiao et al., 2003]. The cell lysate was cleared by centrifugation at 14,000×g for 15 min at 4°C. For Western blot analysis, 10–90 μg of lysate protein was subjected to sodium-dodecyl sulfate polyacrylamide gel electrophoresis (6 to 12.5%) and the proteins were transferred onto membrane. After blocking with 5% nonfat dried milk in Tris buffered saline, the membrane was incubated with the desired primary antibody for 1 h at room temperature or overnight at 4°C. The membrane was then treated with appropriate peroxidase-conjugated secondary antibody and the bands were visualized using enhanced chemiluminescence method. Each membrane was stripped and reprobed with antibodies against actin to correct for differences in protein loading. Quantitation of the immunoreactive bands was performed using ImageQuaNT (version 4.2a) software (Molecular Dynamics, Sunnyvale, CA). Immunoblotting was performed at least twice using independently prepared lysate to ensure reproducibility of the results.
Determination of Apoptosis
The proapoptotic effect of DATS was assessed by fluorescence microscopy to score apoptotic cells with condensed and fragmented DNA after staining the cells with 4′,6-diamidino-2-phenylindole (DAPI) or quantitation of cytoplasmic histone-associated DNA fragmentation. The DAPI assay was performed as described by us previously [Xiao et al., 2004]. Quantitation of cytoplasmic histone-associated DNA fragmentation was performed using a kit from Roche Applied Sciences according to the manufacturer’s recommendations.
RNA Interference of Bax and/or Bak
RNA interference of Bax was performed using a Signal Silence ®Bax siRNA (cat. #6321) purchased from Cell Signaling Technology. RNA interference of Bak was performed using a Bak targeted siRNA (cat. #sc-29786) procured from Santa Cruz Biotechnology. A non-specific control siRNA was purchased from Qiagen (Valencia, CA; cat. #1022076). Briefly, H460 cells (5×104) were seeded in 6-well plates and allowed to attach by overnight incubation. The cells were transfected with 100 nmol/L of siRNA using Oligofectamine. Twenty-four hours after transfection, the cells were treated with DMSO (control) or desired concentrations of DATS for 24 h. Both floating and adherent cells were collected, washed with PBS, and processed for analysis of cytoplasmic histone-associated DNA fragmentation or immunoblotting of the desired protein as described above.
RESULTS
DATS Inhibited Viability of Human Lung Cancer Cells
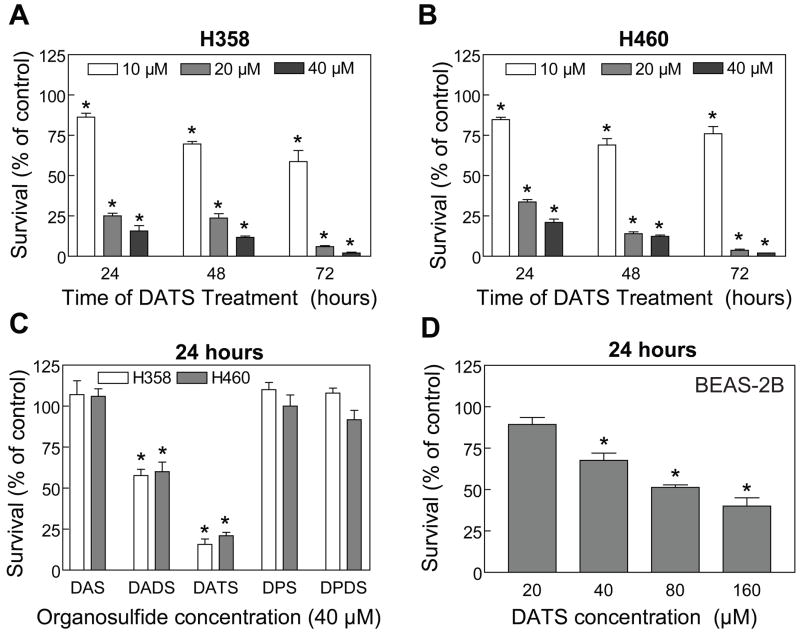
We determined the effects of DAS, DADS, DATS, DPS and DPDS on cell survival using a pair of well-characterized human lung cancer cell lines (H460 and H358) as a model. The above OSCs were selected not only because together they account for >90% of total oil-soluble volatile sulfur compounds in fresh garlic but also to gain insight into the contribution of allyl groups and the number of sulfur atoms on antiproliferative effect of these compounds. It has been estimated that 1 g of fresh garlic bulb contains up to 1.1 mg of DATS, between 530–610 μg of DADS, and up to 100 μg of DAS [reviewed in Shukla and Kalra, 2007]. The effects of the OSCs on H358 and H460 cell viability was determined by trypan blue dye exclusion assay and the results are summarized in Fig. 1A-C. Viability of both H358 (Fig. 1A) and H460 cells (Fig. 1B) was decreased significantly upon treatment with DATS in a concentration- and time-dependent manner with an IC50 of <20 μM (24 h drug exposure). The DADS was relatively less effective compared with DATS against proliferation of H358 and H460 cells. For example, a twenty-four hour treatment of H358 cells with 40 μM DATS resulted in an approximate 84% decrease in cell viability, whereas >50% of the cells survived under similar conditions of DADS treatment (Fig. 1C). Comparable results were observed in H460 cells (Fig. 1C). The viability of H358 or H460 cells was not affected in the presence of other OSCs (DAS, DPS and DPDS) at 40 μM concentration (Fig 1C). We selected DATS for further studies mainly because of its higher potency against lung cancer cells compared with other OSCs.
Figure 1.
Trypan blue dye exclusion assay for effects of the OSCs on viability of H358 and H460 human lung cancer cells and immortalized normal human bronchial epithelial cell line BEAS-2B. The H358 and H460 cell lines were treated with either DMSO (final concentration 0.05%) or the indicated concentrations of the OSCs for specified time-points. Cell viability assay for the BEAS-2B cell line was performed following 24 h drug-exposure. Cell viability assays for each compound were performed twice in triplicate to ensure reproducibility of the results. Data from a representative experiment (mean ± SE) are shown in panels A-D. *Significantly different (P<0.05) compared with control by one-way ANOVA followed by Dunnett’s test.
Next, we raised the question of whether DATS-mediated growth inhibition was selective for lung cancer cells, which is a highly desirable feature of potential cancer preventive and therapeutic agents. We addressed this question by determining the effect of DATS treatment on viability of BEAS-2B cells. As can be seen in Fig. 1D, the BEAS-2B cell line was significantly more resistant to growth inhibition by DATS compared with H358 or H460 cells. For example, viability of H460 and H358 cells was decreased by about 79% and 84%, respectively, upon a 24 h treatment with 40 μM DATS (Fig. 1C). The survival of BEAS-2B cells was decreased only by about 32% by a similar DATS treatment (Fig. 1D).
DATS Treatment Caused G2-M Phase Cell Cycle Arrest in Human Lung Cancer Cells and Normal Bronchial Epithelial Cells
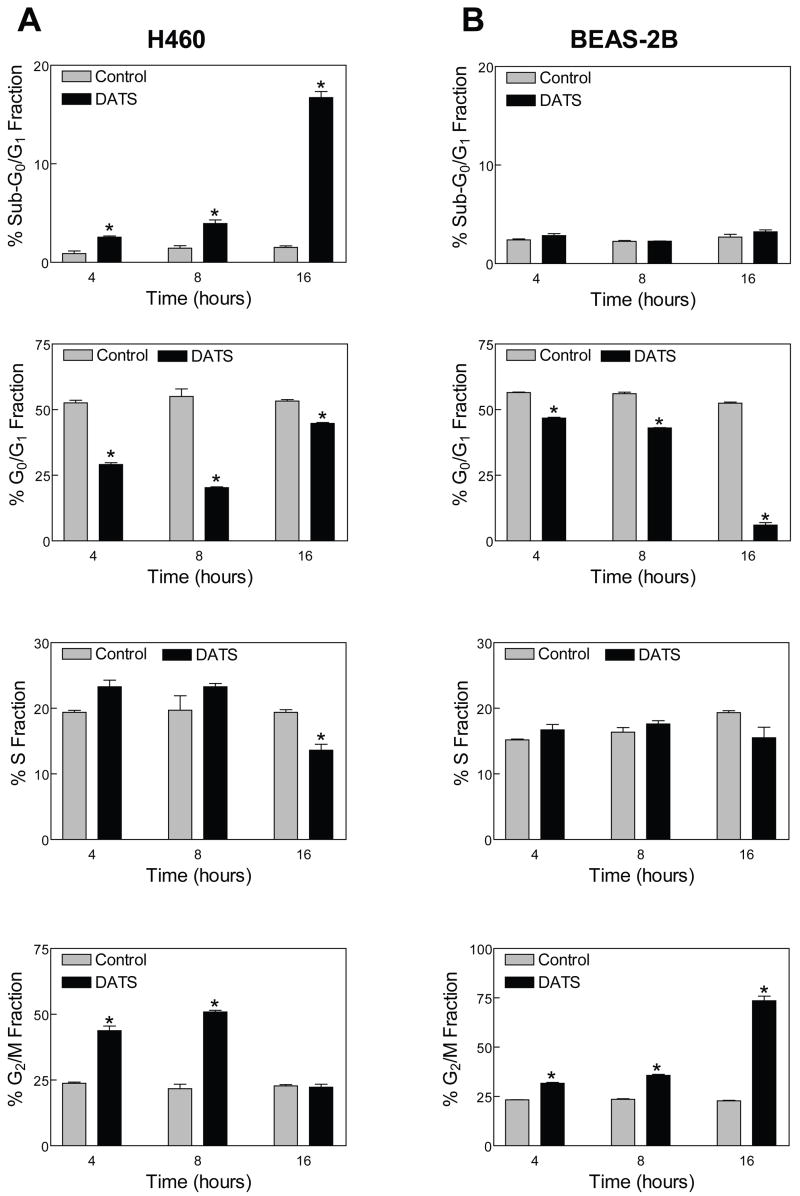
To gain insight into the mechanism for growth suppressive effect of DATS, we determined its effect on cell cycle distribution. As can be seen in Fig. 2A, exposure of H460 cells to 40 μM DATS for 4 and 8 h resulted in a statistically significant enrichment of G2-M fraction. The DATS-mediated G2-M phase cell cycle arrest in both H460 (Fig. 2A) and H358 cells (results not shown) was evident as early as 4 h after treatment and accompanied by a decrease in mainly G0-G1 phase cells. The cell cycle arrest caused by DATS in both cell lines was maintained for at least up to 8 h. The G2-M phase arrest was abolished at the 16 h time point. However, the cells treated for 16 h with DATS exhibited a significant increase in fraction of sub-diploid (Sub-G0-G1) apoptotic cells. Interestingly, the DATS-mediated G2-M phase arrest, but not the enrichment of sub-diploid cells, was observed in BEAS-2B cells (Fig. 2B).
Figure 2.
Cell cycle distribution in H460 (A) and BEAS-2B (B) cells treated with DMSO (final concentration 0.05%) or 40 μM DATS for the indicated time periods. In panels A-B, columns, mean (n= 3); bars, SE. *Significantly different (P<0.05) compared with the corresponding DMSO-treated control by t-test. Similar results were observed in at least 2 independent experiments.
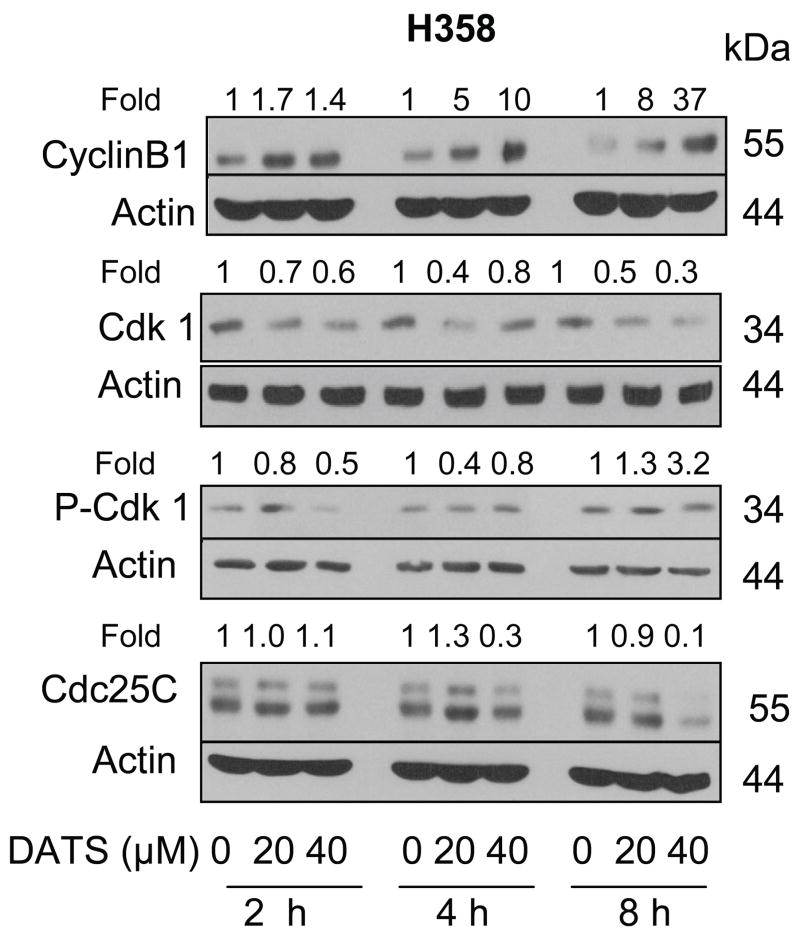
We determined the effect of DATS treatment on levels of proteins involved in regulation of G2-M transition, including cyclinB1, Cdk1, Cdc25C and phospho-(Tyr15)-Cdk1 (inactive) [Hartwell and Kastan, 1994; Draetta and Eckstein, 1997] using H358 cells to gain insight into the mechanism of cell cycle arrest. The DATS treatment caused an increase in the level of cyclinB1 in a concentration and time-dependent manner (Fig. 3). On the other hand, protein levels of Cdk1 and Cdc25C were decreased upon treatment of H358 cells with DATS. The net result of these effects was accumulation of inactive (Tyr15 phosphorylated) Cdk1.
Figure 3.
Immunoblotting for Cyclin B1, Cdk1, P-Cdk1, and Cdc25C using lysates from H358 cells treated with DMSO (final concentration 0.05%) or 20 and 40 μM DATS for the indicated time periods. The blots were stripped and re-probed with anti-actin antibody to normalize for differences in protein loading. Densitometric quantitation after correction for actin loading control is shown on top of each band. Immunoblotting for each protein was performed at least twice using independently prepared lysates.
DATS Tretament Selectively Caused Apoptosis in Human Lung Cancer Cells
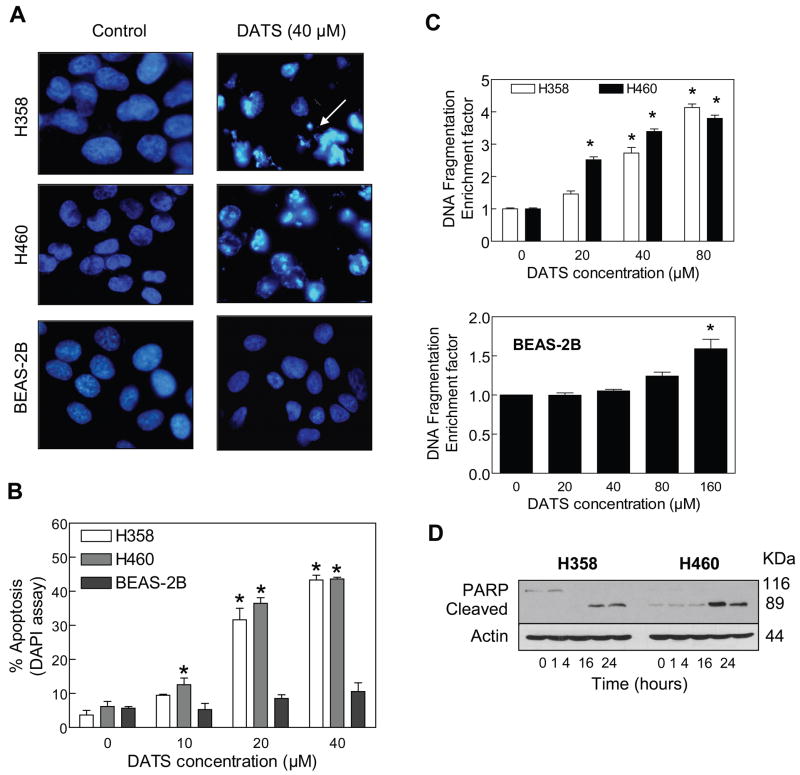
Suppression of cancer cell growth by many natural products including cruciferous vegetable constituent sulforaphane and traditional oriental medicine constituents, correlates with their ability to caused apoptotic cell death [Choi and Singh, 2005; Singh et al., 2007; Hahm et al., 2008]. Because analysis of cell cycle distribution indicated appearance of sub-diploid cells in DATS-treated cultures, we proceeded to characterize proapoptotic effect of DATS by DAPI assay. As can be seen in Fig. 4A, 24 h exposure of H358 and H460 cells to 40 μM DATS resulted in enrichment of apoptotic cells with condensed chromatin (identified by arrow). In agreement with the results of cell viability assay, apoptotic cells with condensed chromatin were rarely seen in DATS-treated BEAS-2B cultures (Fig. 4A). Apoptotic cells with condensed chromatin were scored from cultures of DMSO-treated control and DATS-treated cells and the results are summarized in Fig 4B. The fraction of apoptotic cells increased upon treatment with DATS in a concentration-dependent manner in both H358 and H460 cells (Fig. 4B).
Figure 4.
A, DAPI staining for H358, H460 and BEAS-2B cells treated for 24 h with DMSO (control) or 40 μM DATS. Apoptotic cells with condensed chromatin (arrow) were clearly visible in DATS-treated H358 and H460 cells, but not in DATS-treated BEAS-2B cells. B, Quantitation of apoptotic cells with condensed chromatin (DAPI assay) in H358, H460 and BEAS-2B cells treated for 24 h with DMSO (control) or the indicated concentrations of DATS. Columns, mean (n= 4); bars, SE. *Significantly different (P<0.05) compared with the corresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test. Similar results were observed in at least 2 independent experiments. C, Cytoplasmic histone-associated DNA fragmentation in H358, H468 and BEAS-2B cells following 24 h treatment with DMSO (control) or the indicated concentrations of DATS. Columns, mean (n= 3); bars, SE. *Significantly different (P<0.05) compared with the corresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test. D, Immunoblotting for PARP cleavage using lysates from H358 and H460 cells treated with 40 μM DATS for the indicated time periods. The blot was stripped and re-probed with anti-actin antibody to normalize for differences in protein loading. Immunoblotting for PARP was performed at least twice using independently prepared lysates.
Proapoptotic effect of DATS was confirmed by quantitation of cytoplasmic histone-associated DNA fragmentation. The DATS-treated H358 and H460 cells exhibited significant increase in cytoplasmic histone-associated DNA fragmentation compared with corresponding DMSO-treated controls (Fig. 4C). However, a statistically significant increase in cytoplasmic histone-associated DNA fragmentation relative to DMSO-treated control in BEAS-2B cells was observed only at 160 μM DATS concentration (Fig. 4C). The DATS treatment resulted in cleavage of PARP, another hallmark of apoptotic, in both H358 and H460 cells (Fig. 4D).
Effect of DATS Treatment on Levels of Bcl-2 Family Proteins in Human Lung Cancer Cells
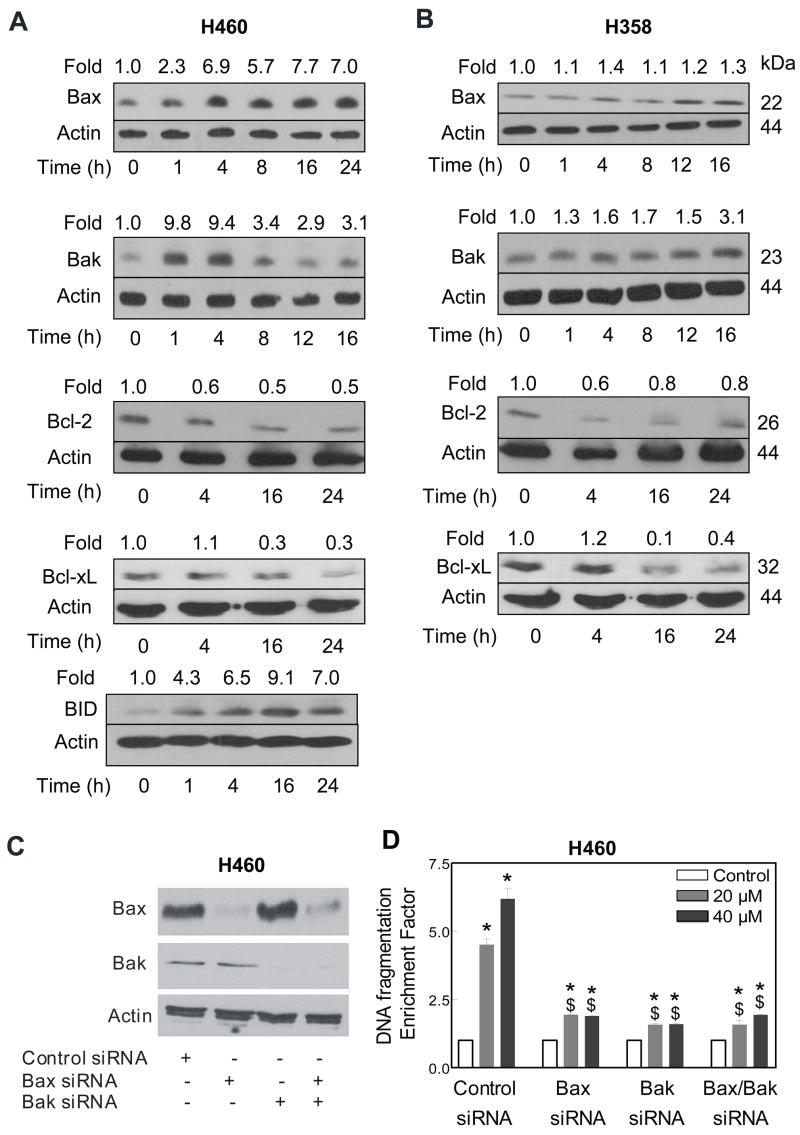
The Bcl-2 family proteins have emerged as critical regulators of mitochondria-mediated apoptosis by functioning as either promoters (e.g., Bax and Bak) or inhibitors (e.g., Bcl-2 and Bcl-xL) of the cell death process [Oltvai et al., 1993; Kiefer et al., 1995; Chao and Korsmeyer, 1998]. We proceeded to test the involvement of Bcl-2 family proteins in regulation of DATS-induced apoptosis. The DATS treatment resulted in induction of Bax and Bak proteins in both H460 (Fig. 5A) and H358 cells (Fig. 5B). The levels of anti-apoptotic proteins Bcl-2 and Bcl-xL were markedly decreased on treatment of H460 and H358 cells with DATS (Fig. 5A,B). In addition, a marked increase in the level of BID protein was evident in DATS-treated H460 cells (Fig. 5A).
Figure 5.
Immunoblotting for Bax, Bak, Bcl-2, Bcl-xL and/or BID proteins using lysates from H460 (A) and H358 (B) cells treated with 40 μM DATS for the indicated time periods. The blots were stripped and re-probed with anti-actin antibody to normalize for differences in protein loading. Immunoblotting for each protein was performed at least twice using independently prepared lysates to ensure reproducibility of the results. C, Immunoblotting for Bax and/or Bak proteins using lysates from H460 cells transiently transfected with a control siRNA and Bax and/or Bak-targeted siRNA. The blot was stripped and re-probed with anti-actin antibody to ensure equal protein loading. D, Cytoplasmic histone-associated DNA fragmentation in H460 cells transiently transfected with a control siRNA or Bax and/or Bak-targeted siRNA and treated for 24 h with either DMSO (control) or the indicated concentrations of DATS. Columns, mean (n= 3); bars, SE. *Significantly different (P<0.05) compared with the corresponding DMSO-treated control, and $significantly different (P<0.05) compared with the control siRNA transfected cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Similar results were observed in 2 independent experiments.
Bak/Bax Knockdown Conferred Significant Protection Against DATS-induced Apoptosis in H460 Cells
Because DATS treatment caused an increase in protein levels of Bax and Bak in both cell lines, we hypothesized that these proteins might be critical mediators of DATS-induced apoptosis. We used Bax and Bak targeted siRNA to test this hypothesis. As can be seen in Fig. 5C, the levels of Bax and Bak proteins were decreased by more than 90% in H460 cells transiently transfected with Bax- and Bak-targeted siRNA. The cytoplasmic histone-associated DNA fragmentation resulting from 24 h treatment of H460 cells with 20 and 40 μM DATS was statistically significantly lower in H460 cells with knockdown of Bax and/or Bak proteins compared with cells transiently transfected with a control non-specific siRNA (Fig. 5D).
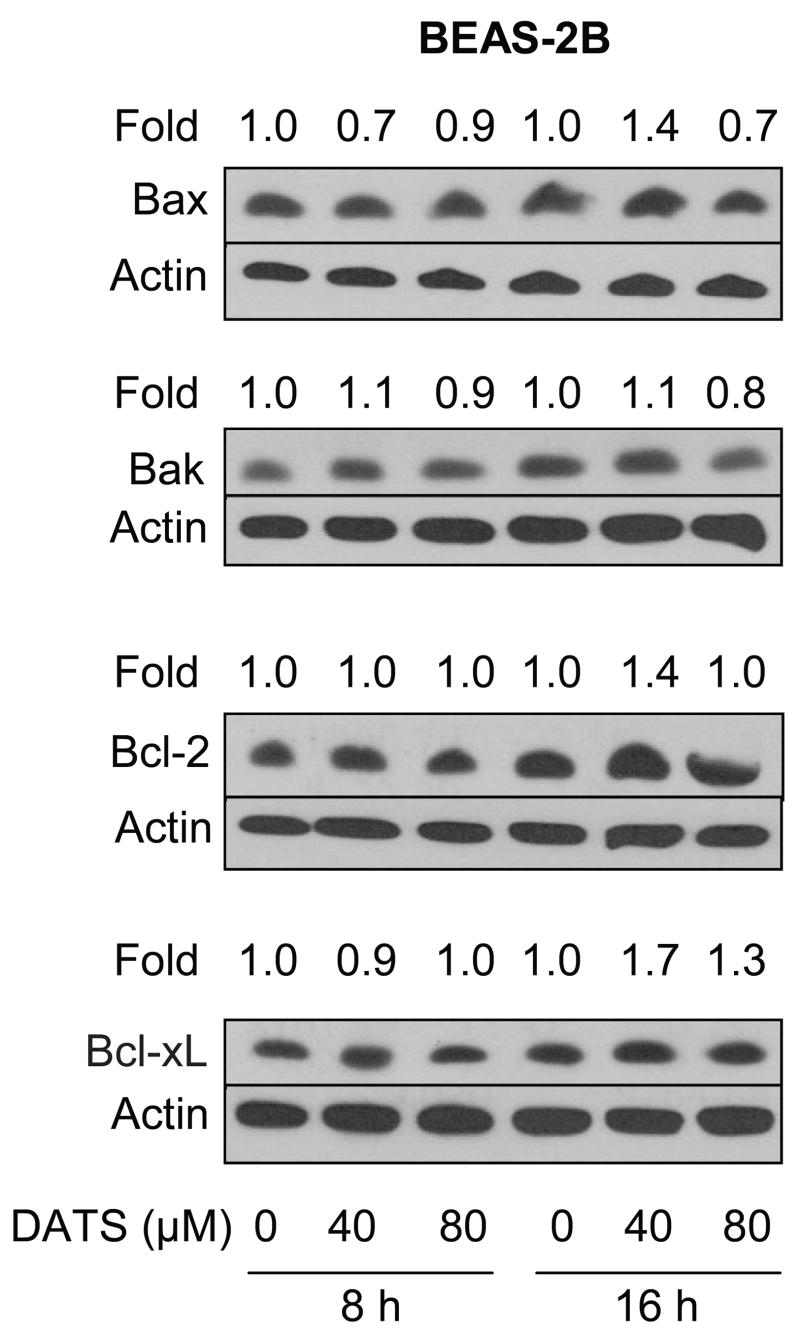
Effect of DATS Treatment on Levels of Bcl-2 Family Proteins in BEAS-2B Cells
To gain insight into the mechanism of differential sensitivity towards DATS-induced apoptosis between lung cancer cells and BEAS-2B (Fig. 4A,B), we determined the effect of DATS treatment on levels of Bcl-2 family proteins (Bax, Bak, Bcl-2, Bcl-xL) using BEAS-2B cell line. As can be seen in Fig. 6, DATS treatment failed to cause induction of Bax or Bak expression in BEAS-2B cells even at 80 μM concentration. Similarly, unlike lung cancer cells (Fig. 5A,B), DATS-mediated down-regulation of Bcl-2 or Bcl-xL protein level was not observed in BEAS-2B cells (Fig. 6).
Figure 6.
Immunoblotting for Bax, Bak, Bcl-2, and Bcl-xL proteins using lysates from BEAS-2B cells treated with DMSO (control) or 40 and 80 μM DATS for 8 or 16 h. The blots were stripped and re-probed with anti-actin antibody to normalize for differences in protein loading. Immunoblotting for each protein was performed twice using independently prepared lysates to ensure reproducibility of the results.
BID Protein Was Dispensable in DATS-induced Apoptosis
Because DATS treatment caused an increase in the protein level of BID at least in H460 cells (Fig. 5A), we designed experiments to determine the role of BID in DATS-induced apoptosis using SV40 immortalized MEFs derived from WT and BID knockout (BID-KO) mice. Initially, we determined the effect of DATS treatment on levels of BID protein by immunoblotting. The DATS treatment resulted in a modest increase in the level of BID protein in the MEFs derived from WT mice (Fig. 7A). We compared sensitivities of SV40 immortalized MEFs derived from WT and BID-KO mice towards DATS-induced apoptosis and the results are shown in Fig. 7B. The MEFs derived from BID-KO mice did not exhibit significant resistance towards DATS-induced cytoplasmic histone associated DNA fragmentation (Fig. 7B) or accumulation of sub-diploid cells (Fig. 7C). The knockout cells, if available, are priceless and highly useful alternative to siRNA technology for molecular analysis of signaling pathways. Because wild-type and BID knockout cells are more or less equally sensitive to DATS-mediated apoptosis in two-independent assays of apoptosis induction, similar results are highly likely in experiments with knockdown of the BID protein.
Figure 7.
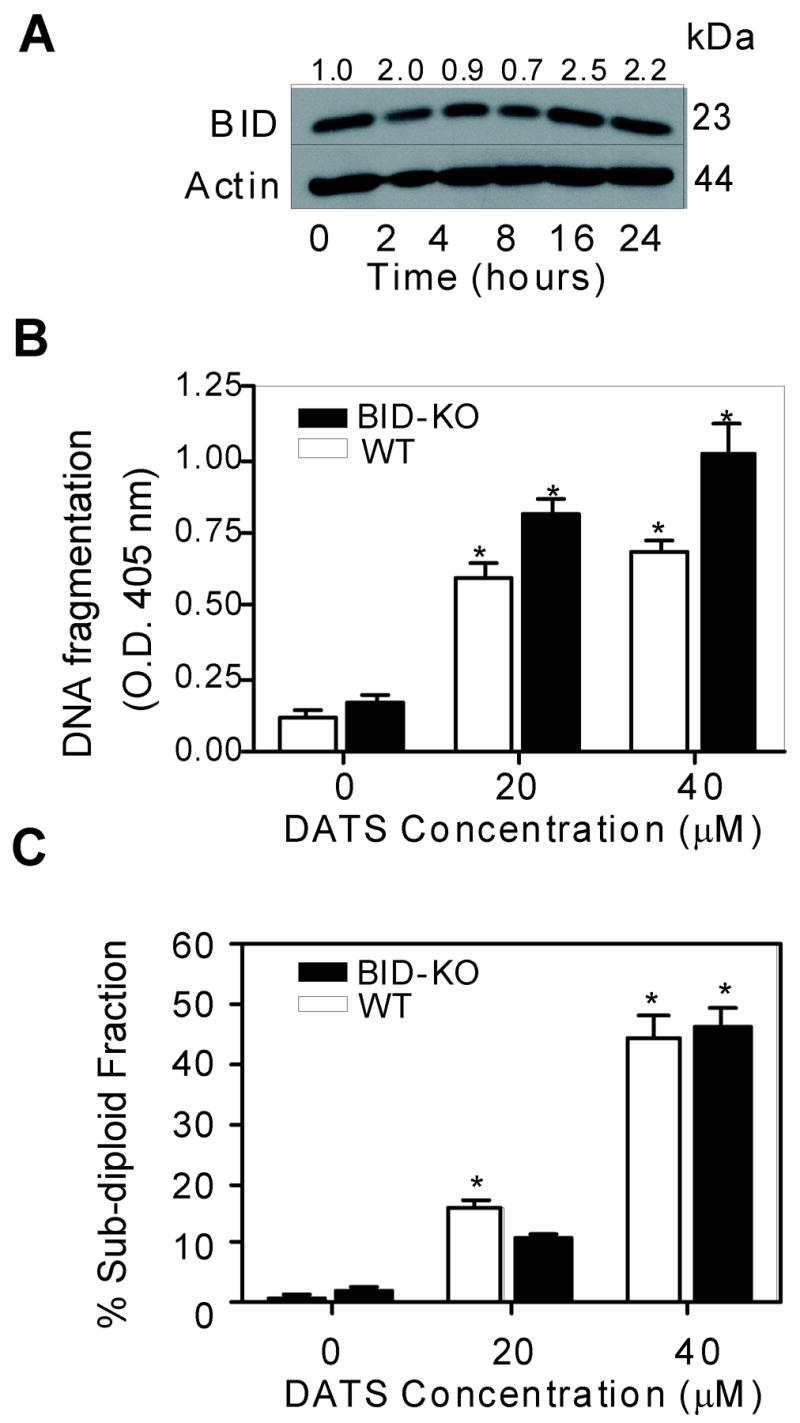
A, Immunoblotting for BID protein using lysates from the MEFs derived from WT mice and treated with 40 μM DATS for the indicated time periods. The blot was stripped and reprobed with anti-actin antibody to normalize for differences in protein loading. Immunoblotting was performed twice using independently prepared lysates. B, Cytoplasmic histone-associated DNA fragmentation in the MEFs derived from WT or BID-KO mice following 24 h treatment with either DMSO (control) or the indicated concentrations of DATS. C, Percentage of sub-G0/G1 apoptotic fraction in the MEFs derived from WT or BID-KO mice following 24 h treatment with either DMSO (control) or the indicated concentrations of DATS. Columns, mean (n= 3); bars, SE. *Significantly different (P<0.05) compared with the corresponding DMSO-treated control by one-way ANOVA followed by Dunnett’s test.
Discussion
Lung cancer is a major health concern for men and women worldwide [Jemal et al., 2006]. Non-small cell lung carcinoma represents 75–80% of all lung cancer cases and is often refractory to therapy. We demonstrate that DATS effectively inhibits proliferation of a non-small cell lung cancer cell line and a large cell lung cancer cell line. We can also conclude that p53, which is implicated in apoptotic response to diverse stimuli [Sheikh and Fornace, 2000], is not required for apoptosis in our model because H358 (p53 null) and H460 (wild-type p53) cells are more or less equally sensitive towards DATS-mediated apoptosis. It is interesting to note that the BEAS-2B cell line is significantly more resistant to growth suppression and apoptosis induction by DATS. These results suggest that DATS may selectively target lung cancer cells but spare normal bronchial epithelium, which is a highly desirable property of potential anticancer agents. We also found that while DATS is a relatively more effective inhibitor of lung cancer cell proliferation compared with DADS, the remaining naturally-occurring sulfur compounds (DAS, DPS, and DPDS) are practically inactive. These results point towards significant contribution of both the allyl group and the number of sulfur atoms in antiproliferative effect of the OSCs against human lung cancer cells. These results are comparable to those observed in human prostate cancer cells [Xiao et al., 2004]. However, the possibility that the difference in potency of allyl sulfides against proliferation of human lung and prostate cancer cell lines is due to differential volatilities of these compounds cannot be ignored. Further studies are needed to systematically explore this possibility.
The DATS treatment causes G2-M phase cell cycle arrest in both lung cancer cells and BEAS-2B cells. Interestingly, the G2-M arrested lung cancer cells, but not the BEAS-2B, are able to escape the cell cycle arrest but driven to apoptotic DNA fragmentation by about 16 h of treatment. It is possible that the G2-M arrest is a priming mechanism for the initiation of the cell death process in the lung cancer cells. Alternatively, the possibility that lung cancer cells experience mitotic catastrophe and activate apoptosis, whereas the normal cells have mechanisms to avoid these fates cannot be ruled out. Even though further studies are needed to experimentally validate this hypothesis, we have shown previously that DATS treatment causes mitotic catastrophe in human prostate cancer cells [Herman-Antosiewicz and Singh, 2005].
Eukaryotic cell cycle progression involves sequential activation of Cdks whose activation is dependent upon their association with corresponding cyclins [Hartwell and Kastan, 1994]. A complex between Cdk1 (also known as p34cdc2) and cyclinB1 plays an important role in regulation of G2-M transition [Hartwell and Kastan, 1994]. Activity of Cdk1/cyclinB1 kinase is negatively regulated by reversible phosphorylations at Thr14 and Tyr15 of Cdk1 [Hartwell and Kastan, 1994]. Dephosphorylation of Thr14 and Tyr15 of Cdk1 and hence activation of the Cdk1/cyclinB1 kinase is catalyzed by Cdc25 family of dual-specificity phosphatases [Draetta and Eckstein, 1997]. We found that the DATS-mediated G2-M phase cell cycle arrest in H358 cells correlates with a decrease in the protein levels of Cdk1 and Cdc25C, which is likely to inhibit activity of the Cdk1/cyclinB1 kinase complex. Consistent with this prediction, the DATS treatment causes an increase in the levels of Tyr15 phosphorylated (inactive) Cdk1. From these results, it is reasonable to interpret that down-regulation of Cdk1 and Cdc25C proteins expression accounts for DATS-induced cell cycle arrest in lung cancer cells.
Growth inhibitory and proapoptotic effects of DATS in lung cancer cells (present study) were observed at 10–40 μM concentrations. Such concentrations may be achievable in humans based on a recent pharmacokinetics study in rats [Sun et al., 2006]. Peak plasma concentration of DATS in rats was shown to be about 31 μM following administration of 10 mg DATS via a jugular vein cannula [Sun et al., 2006]. In a human intervention study, oral administration of 200 mg of DATS in combination with 100 μg selenium every other day for one month did not cause any harmful side effects [Li et al., 2004]. We have shown previously that oral gavage of 6 μmol DATS to male nude mice, three times per week (Monday, Wednesday, and Friday), significantly retards growth of PC-3 human prostate cancer xenografts without causing weight loss or any other side effects [Xiao et al., 2006].
The mechanism for DATS-induced apoptosis has been studied in various cellular systems including human colon and prostate cancer cells [reviewed by Herman-Antosiewicz and Singh, 2004; Herman-Antosiewicz et al., 2007a]. However, it was unclear if the DATS-induced apoptosis signaling in lung cancer cells resembled that in other cellular systems. The results of the present study point towards noticeable differences in apoptosis signaling between lung and other cancer cells. For example, we have shown previously that the apoptosis induction by 40 μM DATS in human prostate cancer cells is accompanied by c-Jun N-terminal kinase-mediated phosphorylation of Bcl-2, which is evidenced by appearance of immunoreactive bands with reduced electrophoretic mobility [Xiao et al., 2004]. On the other hand, phosphorylation of Bcl-2 (retardation of electrophoretic mobility) upon treatment with 40 μM DATS (16–24 h treatment) is not evident in H358 or H460 cells (Fig. 5A,B). Some similarities are also evident in DATS-induced apoptosis mechanisms between human prostate and lung cancer cells. Similar to the results of the present study, DATS treatment (40 μM) causes an increase in the protein levels of Bax and Bak in LNCaP cell line [Kim et al., 2007]. Moreover, the DATS-mediated suppression of PC-3 xenograft growth in vivo is accompanied by up-regulation of Bax and Bak proteins [Xiao et al., 2006]. Bax and Bak knockdown confers significant protection against DATS-induced apoptosis in both lung [present study] and LNCaP cells [Kim et al., 2007]. Moreover, apoptosis caused by DAS (20 μM) and DADS (5 μM) correlates with a modest increase in protein level of Bax in H1299 and H460 lung cancer cells [Hong et al., 2000]. Accordingly it is reasonable to conclude that Bax and Bak are critical mediators of DATS-induced apoptosis regardless of the origin of the cancer cells. Critical role of Bax and Bak in regulation of DATS-induced apoptosis is also substantiated by the results in BEAS-2B cells. The BEAS-2B cells are significantly more resistant towards DATS-mediated apoptosis compared with lung cancer cells (Fig. 4A,B) and DATS treatment fails to cause induction of Bax or Bak protein level in this cell line even at 80 μM concentration (Fig. 6). It is important to point out that mutation in Bak and Bax gene have been shown to cause resistance towards apoptosis induction by certain stimuli [Kondo et al., 2000; Ionov et al., 2000; LeBlanc et al., 2002].
In conclusion, the present study indicates that DATS treatment inhibits growth of cultured human lung cancer cells irrespective of their p53 status. On the other hand, an immortalized normal human bronchial epithelial cell line is significantly more resistant towards DATS-mediated growth suppression and apoptosis induction compared with lung cancer cells. We also provide experimental evidence to indicate that Bax and Bak are critical mediators of DATS-induced apoptosis signaling in human lung cancer cells. Finally, the results of the present study favor a mechanistic hypothesis that DATS-induced apoptosis in human lung cancer cells may primarily be mediated by the mitochondria-caspase-9 pathway.
Acknowledgments
We thank Dr. Stanley J. Korsmeyer for generous gift of the MEFs derived from WT and BID-KO mice.
Grant sponsor: This investigation was supported in part by the National Cancer Institute grant CA113363 and the Lung Cancer SPORE grant CA904544.
References
- Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. c-Jun NH2-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–5386. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- Block E. The organosulfur chemistry of the genus Allium- implications for the organic chemistry of sulfur. Angew Chem Int Ed Engl. 1992;31:1135–1178. [Google Scholar]
- Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, Yang CS. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol. 1991;4:642–647. doi: 10.1021/tx00024a008. [DOI] [PubMed] [Google Scholar]
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta. 1997;1332:M53–M63. doi: 10.1016/s0304-419x(96)00049-2. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–5949. [PubMed] [Google Scholar]
- Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. 2000;72:1047–1052. doi: 10.1093/ajcn/72.4.1047. [DOI] [PubMed] [Google Scholar]
- Gao CM, Takezaki T, Ding JH, Li MS, Tajima K. Protective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high epidemic area in Jiangsu Province, China. Jpn J Cancer Res. 1999;90:614–621. doi: 10.1111/j.1349-7006.1999.tb00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14:1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Powolny AA, Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin. 2007a;28:1355–1364. doi: 10.1111/j.1745-7254.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable-derived organosulfur compounds: a review. Mutation Res. 2004;555:121–131. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28528. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A, Stan SD, Hahm ER, Xiao D, Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic. Mol Cancer Ther. 2007b;6:1249–1261. doi: 10.1158/1535-7163.MCT-06-0477. [DOI] [PubMed] [Google Scholar]
- Hong YS, Ham YA, Choi JH, Kim J. Effects of allyl sulfur compounds and garlic extract on the expressions of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines. Exp Mol Med. 2000;32:127–134. doi: 10.1038/emm.2000.22. [DOI] [PubMed] [Google Scholar]
- Hong JY, Wang ZY, Smith TJ, Zhou S, Shi S, Pan J, Yang CS. Inhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lung. Carcinogenesis. 1992;13:901–904. doi: 10.1093/carcin/13.5.901. [DOI] [PubMed] [Google Scholar]
- Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of β-tubulin. J Biol Chem. 2005;280:41487–41493. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, Fraumeni JF., Jr Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst. 2002;94:1648–1651. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- Hu X, Benson PJ, Srivastava SK, Mack LM, Xia H, Gupta V, Zaren HA, Singh SV. Glutathione S-transferases of female A/J mouse liver and forestomach and their differential induction by anti-carcinogenic organosulfides from garlic. Arch Biochem Biophys. 1996;336:199–214. doi: 10.1006/abbi.1996.0550. [DOI] [PubMed] [Google Scholar]
- Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher RJ, Zaren HA, Awasthi S, Awasthi YC, Singh SV. Induction of glutathione S-transferase π as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer. 1997;73:897–902. doi: 10.1002/(sici)1097-0215(19971210)73:6<897::aid-ijc23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA. 2000;97:10872–10877. doi: 10.1073/pnas.190210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles LM, Milner JA. Diallyl disulfide inhibits p34(cdc2) kinase activity through changes in complex formation and phosphorylation. Carcinogenesis. 2000;21:1129–1134. [PubMed] [Google Scholar]
- Kondo S, Shinomura Y, Miyazaki Y, Kiyohara T, Tsutsui S, Kitamura S, Nagasawa Y, Nakahara M, Kanayama S, Matsuzawa Y. Mutations of the bak gene in human gastric and colorectal cancers. Cancer Res. 2000;60:4328–4330. [PubMed] [Google Scholar]
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, Sun PH, Xie XY. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chinese Med J. 2004;117:1155–1160. [PubMed] [Google Scholar]
- Nakagawa H, Tsuta K, Kiuchi K, Senzaki H, Tanaka K, Hioki K, Tsubura A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis. 2001;22:891–897. doi: 10.1093/carcin/22.6.891. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res. 1993;53:3493–3498. [PubMed] [Google Scholar]
- Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer. 1997;29:152–156. doi: 10.1080/01635589709514617. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Fornace AJ. Role of p53 family members in apoptosis. J Cell Physiol. 2000;182:171–181. doi: 10.1002/(SICI)1097-4652(200002)182:2<171::AID-JCP5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Kalra N. Cancer Chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Singh SV. Impact of garlic organosulfides on p21(H-ras) processing. J Nutr. 2001;131:1046s–1048s. doi: 10.1093/jn/131.3.1046S. [DOI] [PubMed] [Google Scholar]
- Singh SV, Choi S, Zeng Y, Hahm E, Xiao D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate-dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;67:7439–7449. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H: quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998;244:917–920. doi: 10.1006/bbrc.1998.8352. [DOI] [PubMed] [Google Scholar]
- Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131–134. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- Sun X, Guo T, He J, Zhao M, Yan M, Cui F, Deng Y. Simultaneous determination of diallyl trisulfide and diallyl disulfide in rat blood by gas chromatography with electron-capture detection. Pharmazie. 2006;61:985–988. [PubMed] [Google Scholar]
- Sundaram SG, Milner JA. Diallyl disulfide induces apoptosis of human colon tumor cells. Carcinogenesis. 1996;17:669–673. doi: 10.1093/carcin/17.4.669. [DOI] [PubMed] [Google Scholar]
- Wargovich MJ. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethylhydrazine-induced colon cancer. Carcinogenesis. 1987;8:487–489. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic Bax and Bak: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XJ, Kassie F, Mersch-Sundermann V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat Res. 2005;579:115–124. doi: 10.1016/j.mrfmmm.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-jun N-terminal kinase and extracellular- signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Brisson M, Lazo JS, Singh SV. Diallyl trisulfide-induced G2-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species dependent destruction and hyperphosphorylation of Cdc25C. Oncogene. 2005;24:6256–6268. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- Xiao D, Lew KL, Kim Y, Zeng Y, Hahm E, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;15:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- Xiao D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533–540. doi: 10.1093/carcin/bgi228. [DOI] [PubMed] [Google Scholar]
- Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF, Jr, Wang TG. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989;18:162–164. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]