Abstract
We examined the analgesic properties of endomorphin-2 expressed in DRG neurons transduced with a non-replicating herpes simplex virus (HSV)-based vector containing a synthetic endomorphin-2 gene construct. HSV-mediated endomorphin-2 expression reduced nocisponsive behaviors in response to mechanical and thermal stimuli after injection of complete Freund’s adjuvant (CFA) into the paw, and reduced peripheral inflammation measured by paw swelling after injection of CFA. The analgesic effect of the vector was blocked by either intraperitoneal or intrathecal administration of naloxone methiodide, blocking peripheral and central μ opioid receptors, respectively. Endomorphin-2 vector injection also reduced spontaneous pain-related behaviors in the delayed phase of the formalin test and in both CFA and formalin models suppressed spinal c-fos expression. The magnitude of the vector-mediated analgesic effect on the delayed phase of the formalin test was similar in naïve animals and in animals with opiate tolerance induced by twice daily treatment with morphine, suggesting that there was no cross-tolerance between vector-mediated endomorphin-2 and morphine. These results suggest that transgene-mediated expression of endomorphin-2 in transduced DRG neurons in vivo acts both peripherally and centrally through mu opioid receptors to reduce pain perception.
Keywords: Inflammatory pain, Opiates, Endomorphin-2, Gene therapy, Herpes simplex virus
1. Introduction
Opioid receptors in the spinal dorsal horn play a critical role in modulating nociceptive neurotransmission, with mu opioid receptors accounting for the majority (approximately 70%) of the opiate binding activity in the spinal cord (Besse et al., 1990a, b), and the most potent opiate drugs act as mu opioid receptor ligands. Leu- and metenkephalin are endogenous opioids that are released from inhibitory interneurons in the dorsal horn of spinal cord, but both of these peptides have a substantially higher affinity for delta than for mu opioid receptors (Zadina et al., 1997). A pair of peptides (endomorphin-1 and endomorphin-2) isolated originally from brain lack the canonical Tyr-Gly-Gly-Phe sequence common to other endogenous opioids, but have a 1000-fold selectivity for mu opioid receptors over delta opioid receptors (Chapman et al., 1997; Stone et al., 1997; Zadina et al., 1997).
We and others have previously shown that recombinant herpes simplex virus (HSV)-based vectors can be used to deliver genes from peripheral application to dorsal root ganglion (DRG) neurons in vivo, and that an HSV vector engineered to express enkephalin can be used to modify nociceptive signal transmission and provide an analgesic effect in rodent and primate models of inflammatory, neuropathic and cancer-related pain (Braz et al., 2001; Goss et al., 2002; Goss et al., 2001; Hao et al., 2003; Meunier et al., 2005; Wilson et al., 1999; Yeomans et al., 2004; Yeomans et al., 2006). In light of the predominance of mu receptors in the spinal cord, we subsequently constructed a recombinant replication-defective HSV vector to express endomorphin-2 (Wolfe et al., 2007), and demonstrated that this endomorphin-2-expressing vector reduces nocisponsive behaviors in the spinal nerve ligation model of neuropathic pain; the effect of vector-mediated endomorphin-2 in vivo was blocked by the intrathecal administration of the mu-selective antagonist CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr amide) (Wolfe et al., 2007). In the current study we tested the effect of the endomorphin-2-expressing vector in the complete Freund’s adjuvant and formalin tests of inflammatory pain, and used those models to examine the site of action of transgene-mediated endomorphin and the interaction of the endomorphin effect with morphine.
2. Material and methods
2.1. Vectors
The construction and characterization of the endomorphin-2 expressing vector (QHEND) and the control vector (Q0ZHG) has been reported in detail (Wolfe et al., 2007). The UL41 targeting endomorphin-2 (EM-2) expression cassette plasmid was recombined into a replication-defective HSV backbone, QOZ, a derivative of QOZHG (Chen et al., 2000). QOZHG was derived from the previously described mutant vectors TOZ.1 and d106 (Hobbs and DeLuca, 1999; Krisky et al., 1998). The viral immediate early gene enhancer elements were removed from the IE gene promoters of ICP22 and ICP47 to abrogate their expression in the ICP4 and ICP27 deleted background of d106. In addition, d106 contributed an HCMV:EGFP cassette substituted for the UL54 (ICP27) coding sequence while TOZ.1 contributed an ICP0:lacZ cassette inserted in the UL41 locus of QOZHG. The EM-2-EGFP expression cassette was then recombined into the UL41 locus of QOZ and LacZ negative- EGFP positive, recombinants identified on 7b cells. The HCMV promoter in the context of an HSV backbone drives transient transgene expression (Puskovic et al., 2004) and with other transgenes has been shown to produce 3–4 weeks of antinociception (Hao et al., 2003).
2.2. Animal experiments
Male Sprague–Dawley rats weighing 225–250 g were housed one to four per cage approximately 7 days prior to the beginning of the study, with free access to food and water and maintained on a 12:12, light:dark schedule at 21 °C and 60% humidity. All housing conditions and experimental procedures were approved by the University Animal Care and Use Committee and were conducted in accordance with the ethical guidelines of the International Association for the Study of Pain (Zimmermann, 1983).
For intrathecal administration, intrathecal catheters were implanted under isoflurane anesthesia. A polyethylene (PE-10) catheter filled with 0.9% saline was advanced 8 cm caudally through an incision in the atlanto-occipital membrane to position its tip at the level of the lumbar enlargement. The rostral tip of the catheter was passed subcutaneously, externalized on top of the skull, and sealed with a stainless-steel plug. Animals showing neurological deficits after implantation were excluded. Animals were used within 5 days after implantation of the catheter.
The endomorphin-2 expressing or control vector was inoculated in the paw and 30 μl of vector QHEND or Q0ZHG at a concentration of 4 × 108 plaque forming units/ml was injected subcutaneously in the plantar surface of the left hind paw. In the current study we used two models of inflammatory pain: complete Freund’s adjuvant (CFA) and formalin test. In the CFA model vector inoculation was performed 3 days prior to injection CFA. In the formalin test studies the vector was inoculated 1 week prior to formalin injection and behavioral test. All behavioral tests were carried out in the morning.
2.3. Complete Freund’s adjuvant (CFA) model of inflammatory pain
Complete Freund’s adjuvant (CFA, 0.05 ml, Sigma, MO, USA) was injected subcutaneously into the plantar surface of the left hindpaw of awake animals. The CFA injection produced an intense tissue inflammation of the hindpaw. Pain-related behaviors were assessed by evaluation of mechanical threshold, thermal hyperalgesia, and weight bearing as described below. Peripheral inflammation was assessed by measurement of paw edema.
2.4. Mechanical threshold
Animals were placed in transparent plastic cubicles on a mesh floor for an acclimatization period of at least 30 min on the morning of the test day. Mechanical allodynia was determined by assessing paw withdrawal to von Frey hairs of graded tensile strength. A series of calibrated von Frey filaments were presented serially to the hind paw in ascending order of strength, with each filament applied for 6 s with sufficient force to cause slight bending against the paw. A positive response was defined as rapid withdrawal and/or licking of the paw immediately upon application of the stimulus, which was then followed by application of the next finer von Frey filament. After a negative response, the next higher von Frey filament was applied. Animals that did not respond to a pressure of 15.1 g were assigned to this cut-off value. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the up-down method (Chaplan et al., 1994; Dixon, 1980).
2.5. Thermal hyperalgesia
The latency to hind paw withdrawal from a thermal stimulus was determined by exposing the plantar surface of the hind paw to radiant heat using a modified Hargreaves thermal testing device (Hargreaves et al., 1988). Rats were placed in individual enclosures on a glass plate maintained at 30 °C and a radiant thermal stimulus was positioned underneath the glass plate directly under the hind paw. Activation of the bulb simultaneously activated a timer, and both were immediately turned o. by paw withdrawal or at the 20 s cut-off time. Three independent measurements were made at 5 min intervals; the data reported represents the average of the three measurements.
2.6. Assessment of hind paw weight bearing
Difference in distribution of weight-bearing between the left and right (contralateral control) hindpaws was utilized as an index of paw discomfort in the paw CFA model. An incapacitance tester (Linton Instrumentation, Stevenage, UK) was employed for determination of hind paw weight distribution as described previously (Bove et al., 2003). Rats were placed in an angled plexiglass chamber positioned so that each hind paw rested on a separate force plate. The force exerted by each hind limb (measured in grams) was averaged over a 10 s period. Data was presented as the difference in weight bearing between the left paw and right (contralateral control) paw.
2.7. Measurement of paw edema
Ipsilateral and contralateral hindpaw volume was determined by plethysmometry (Ugo Basile, Comerio, Italy) as described previously (Helyes et al., 2004) on the morning of the test day. The plethysmometer consists of two vertical interconnected water-filled Perspex cells, the larger of which is used to measure volume displacement produced by the rat paw. The water level in the smaller tube, which contains a transducer, follows that of the larger one and is therefore proportional to the volume of water into which the paw is dipped. The transducer is used to display the volume of the paw in cm3. Edema is expressed as the percentage increase in the volume of the treated (left) paw relative to that of the control (right) paw at each time interval.
2.8. Morphine tolerance
In order to assess the effect of vector-mediated endomorphin expression in the setting of tolerance, tolerance to morphine was induced by administration of intraperitoneal morphine (Sigma), 10 mg/kg (8 a.m. and 8 p.m.) twice daily. The development of tolerance to morphine was established by evaluating the antinociceptive effect of morphine 1 day before the start of morphine treatment (naïve rats responding within 2–5 s were included in further testing), and 1 hr after morphine administration on day 1 and day 7 of morphine treatment.
2.9. Tail-flick test
In the experiments involving animals rendered tolerant to morphine, the development of tolerance was confirmed by tail-flick test. The warm water tail-flick test was performed by placing the distal 1/3 tail in a heated water bath maintained at 55 °C. The latency to the first sign of a rapid tail-flick or tail withdrawal was taken as the behavioral end point. A 12-s cut-off was employed to avoid tissue damage. All of the animals tested demonstrated tolerance; they were then tested for the effect of the vector in nociceptive pain by the formalin test.
2.10. Formalin test
The analgesic effect of vector-mediated endomorphin-2 was compared between naïve and morpine-tolerant rats using the formalin test. Rats were inoculated with either vector Q0ZHG or vector QHEND 3 days prior to the start of morphine treatment, and at 7 days of morphine treatment the response to intraplantar formalin was compared between Q0ZHG and QHEND-inoculated animals, tolerant to morphine as established by tail-flick. The formalin test was carried out as follows. Fifty microliters of 5% formalin was injected subcutaneously into the plantar surface of the right hind paw using 27-gauge needle. Animals were then placed in a clear Plexiglas cylinder (20 × 30 cm) with a mirror below the floor at a 45° angle to allow for unencumbered observation during the test. The number of flinches per minute was counted 1 and 5 min after formalin injection (phase 1) and then at 5 min intervals thereafter for 60 min (phase 2) as described previously (Hao et al., 2002).
2.11. Immunohistochemistry
In CFA model, immunohistochemical expression of spinal c-Fos was investigated as described previously (Hao et al., 2002). A gentle touch was applied once every 4 s for 10 min with the flat surface of the experimenter’s thumb to the rat paw (Ma and Woolf, 1996). Each stimulus was moved from the middle of the foot to the distal footpad over the course of 2 s in a manner that would not elicit a flexion reflex in normal rats. Two hours after completion of stimulation or formalin injection, the animals were perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The spinal cord was postfixed, cryoprotected and 35 μm transverse cryosections of L4–5 segments incubated overnight at 4 °C with anti-c-Fos (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by biotinylated goat anti-rabbit immunoglobulin G, (1:100; Vector Laboratories, Burlingame, CA, USA) and detected with diaminobenzidine (Vectastain Elite ABC Kit and DAB Kit, Vector Laboratories). Six to ten sections from each animal were randomly selected and the expression of Fos-LI was evaluated by a blinded observer counting the number of immunopositive cells in the gray matter of ipsilateral spinal cord. For purposes of laminar distribution, regions were defined as superficial dorsal horn (laminae I–II), nucleus proprius (laminae III–IV), neck of the dorsal horn (laminae V–VI) and laminae VII–X.
3. Drugs
For study of the central effect of opioid antagonism on the vector expressing EM-2, 3 days after CFA naloxone methiodide (10 μg/rat, Sigma, St. Louis, MO) was delivered with a microsyringe in a total volume of 10 μl followed immediately by 10 μl of saline to flush the catheter. For study of the peripheral effect of opioid antagonism on the vector expressing EM-2, intraperitoneal naloxone methiodide (10 mg/kg in 1 ml saline, Sigma, St. Louis, MO) was administered 3 days after CFA. Morphine sulfate (Baxter, Deerfield, IL) was given intraperitoneally for morphine tolerance. All drugs were dissolved in saline.
3.1. Statistics
The statistically significant differences between groups was evaluated by analysis of variance (ANOVA) followed by Fisher’s PLSD post hoc test (StatView 5.0, SAS Institute, Cary, NC, USA). For repeated measures of behavioral function, the general linear model for repeated measures was used to identify significant effects of treatment condition on the behavioral measure (SPSS Inc., Chicago, IL, USA). All data are presented as mean ± SEM.
4. Results
Because no sequences corresponding to the predicted proendomorphin gene are present in human databases, we constructed an endomorphin-2 expressing vector containing the N-terminal signal sequence of human preproenkephalin (PENK) followed by a pair of EM-2 coding elements each flanked by dibasic cleavage sites. Each EM-2 moiety contains a C-terminal glycine residue extension (YPFFG) processed by PAM to yield the Cterminal amidated EM-2 peptides (YPFF-NH2). These coding elements were fused in frame with the EGFP open reading frame to allow efficient cleavage and provide a fluorescent biomarker (Wolfe et al., 2007). We confirmed the identity of the released peptide by combined HPLC/RIA and mass spectroscopy of peptides released from primary DRG neurons transduced with the vector (Wolfe et al., 2007). Expression in DRG in vivo following subcutaneous inoculation of the vector was confirmed by ELISA of transduced DRG (Wolfe et al., 2007).
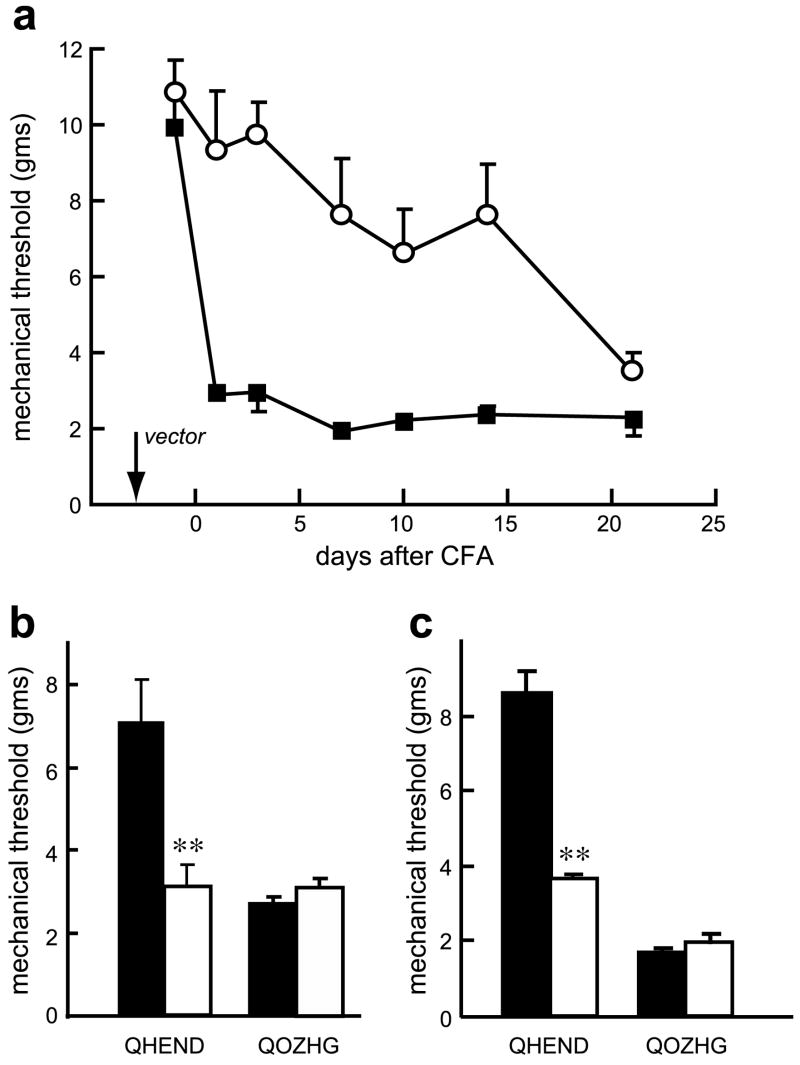
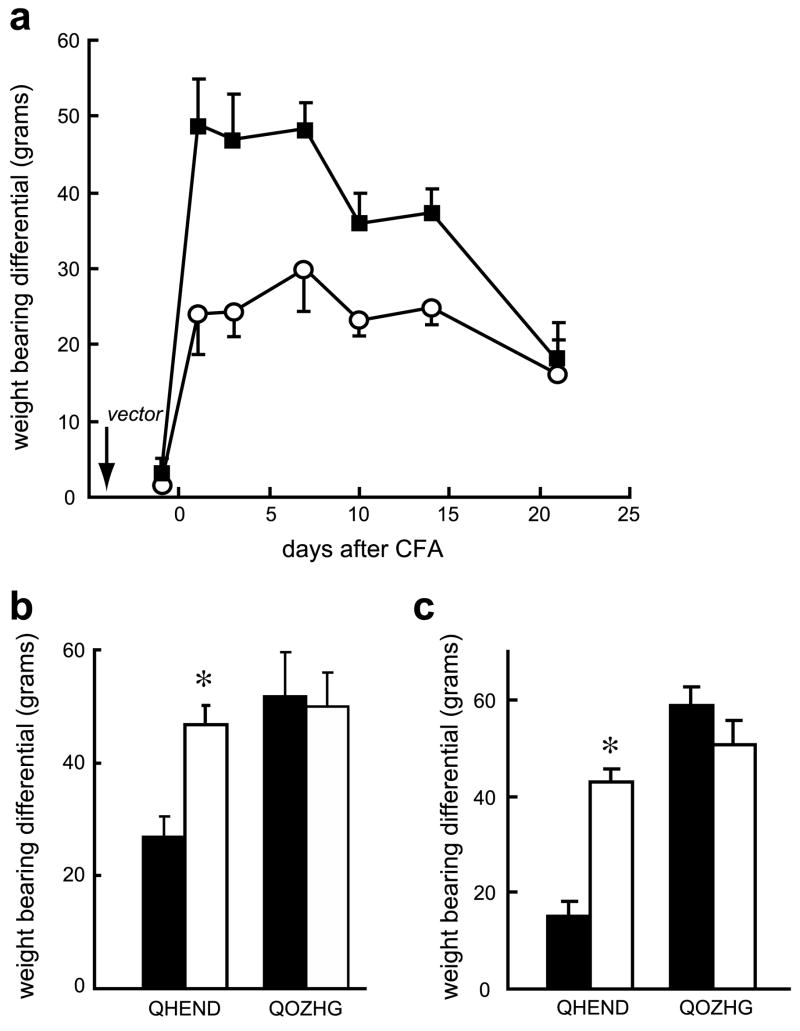
Inoculation of the endomorphin-2-expressing HSV vector 3 days prior to the subcutaneous injection of CFA into the footpad resulted in a substantial and significant reduction of mechanical allodynia measured using von Frey filaments (Fig. 1a). The vector-mediated effect persisted at least 2 weeks after vector inoculation, consistent with the known kinetics of transgene expression driven by the human cytomegalovirus immediate early promoter. The vector-mediated analgesic effect was blocked by either intrathecal (Fig. 1b) or intraperitoneal (Fig. 1c) naloxone methiodide, suggesting that the effect of vector-produced endomorphin-2 was mediated by a combination of central and peripheral sites of action. Mechanical sensitivity was also assessed by spontaneous weight-bearing measured with an incapacitance tester. Rats inoculated with the control vector and injected with CFA showed a 50 g differential in spontaneous weight bearing between the normal and CFA inoculated hind limb, while the differential in animals inoculated with the endomorphin-2-expressing vector peaked at about 25 g (Fig. 2a). This differential was largely eliminated by intrathecal (Fig. 2b) or intraperitoneal (Fig. 2c) injection of naloxone methiodide. Intrathecal or intraperitoneal administration of saline did not affect mechanical allodynia nor thermal hyperalgesia (data not shown).
Fig. 1.
QHEND reduces mechanical allodynia in the CFA model of inflammatory pain in naïve rats. Inflammatory injury was induced by injection of CFA and mechanical allodynia tested using von Frey filaments. Inoculation of QHEND (open circles) but not QOZHG (filled squares) 3 days prior to CFA significantly reduced mechanical allodynia (a); P < 0.01, general linear model, repeated measures test (SPSS); n = 6 animals per group. The antiallodynic effect of QHEND was reversed by intrathecal (10 μg) (b) or intraperitoneal (10 mg/kg) (c) naloxone-methiodide (Nal-M) tested 3 days after CFA; behavioral testing was carried out 30 min after administration of Nal-M; **P < 0.01 pre Nal-M (filled bars) vs post Nal-M (open bars), t-test; n = 6 animals per group.
Fig. 2.
QHEND reduces tactile allodynia measured by differential weight-bearing in naïve rats. Inoculation of QHEND (open circles) but not QOZHG (filled squares) subcutaneously 3 days prior to CFA significantly increased the ability of the animals to bear weight on the CFA-injected paw (a); P < 0.01, general linear model, repeated measures test (SPSS); n = 6 animals per group. This effect was reversed by intrathecal (b) or intraperitoneal (c) Nal-M tested 3 days after CFA; behavioral testing was carried out 30 min after administration of Nal- M; *P < 0.05 pre Nal-M (filled bars) vs post Nal-M (open bars), t-test; n = 6 animals per group.
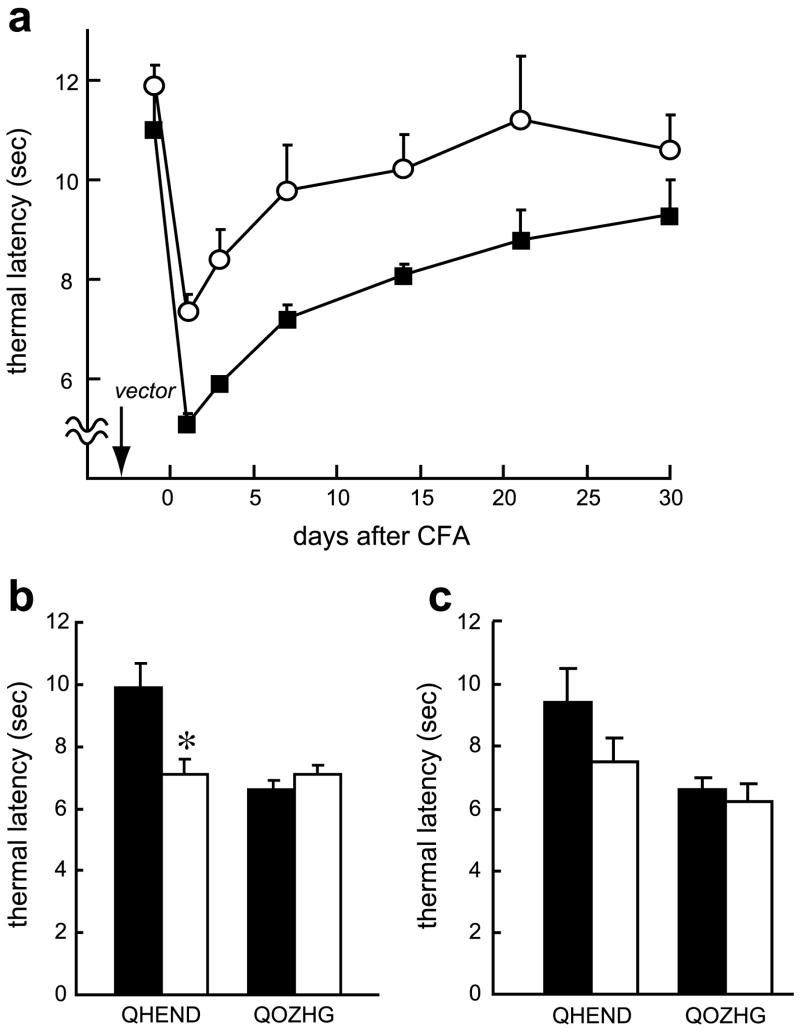
CFA administration induced thermal hyperalgesia that was prolonged over the course of a few weeks. Animals inoculated with the endomorphin-2-expressing vector showed a substantial and statistically significant reduction in thermal hyperalgesia over the course of several weeks (Fig. 3a), an effect that was substantially blocked by naloxone methiodide administered by intrathecal injection (Fig. 3b); the effect of intraperitoneal naloxone methiodide was not statistically significant (Fig. 3c).
Fig. 3.
QHEND reduces thermal hyperalgesia in the CFA model of inflammatory pain in naïve rats. Inoculation of QHEND (open circles) but not QOZHG (filled squares) 3 days prior to CFA prolongs thermal latency (a); P < 0.01, general linear model, repeated measures test (SPSS); n = 6 animals per group. The anti-hyperalgesic effect was reversed by intrathecal Nal-M (b) although the effect of intraperitoneal Nal-M failed to reach statistical significance (c), *P < 0.05 pre Nal-M (filled bars) vs post Nal-M (open bars), t-test; n = 5–6 animals per group. Nal-M was administered 3 days after CFA; behavioral testing was carried out 30 min after administration of Nal-M.
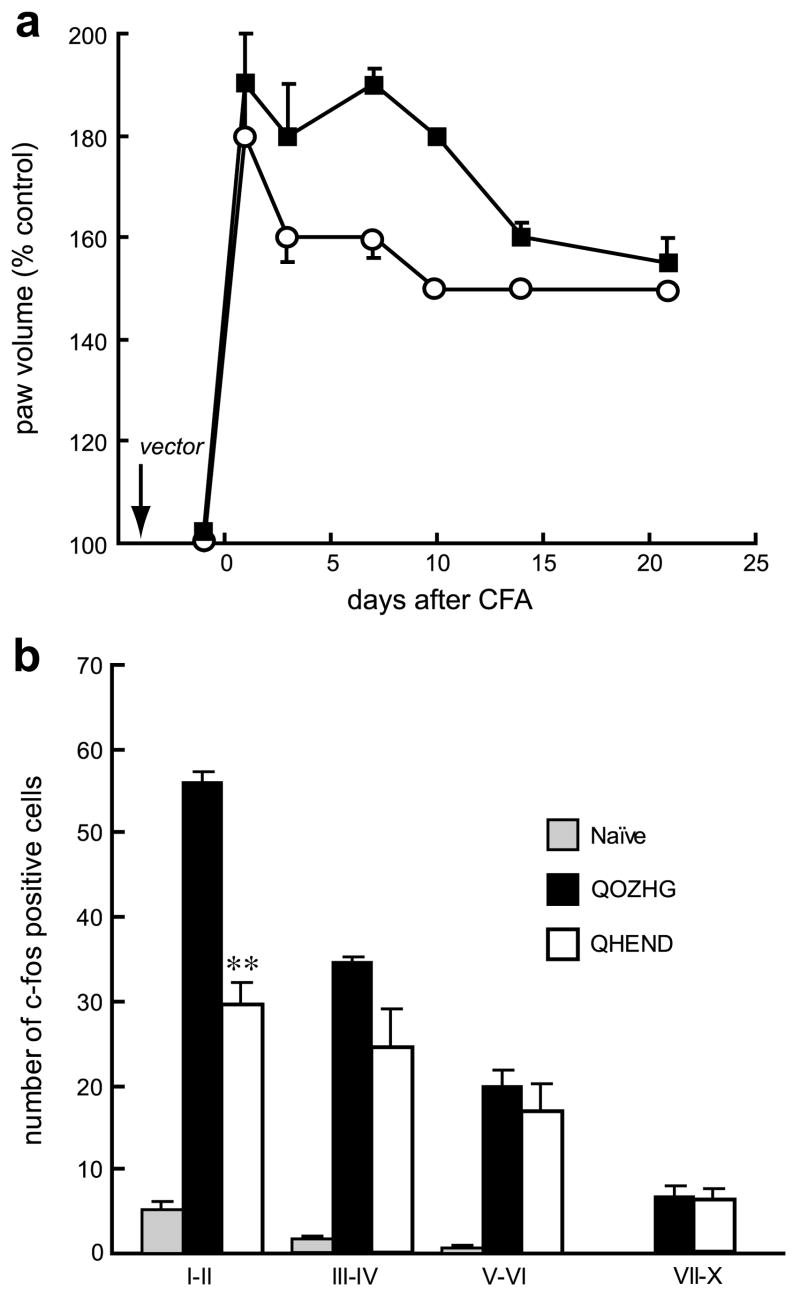
We found that vector-mediated endomorphin-2 expression reduced peripheral inflammation measured by paw swelling after CFA injection in the paw (Fig. 4a) although the duration of this effect of vector-mediated endomorphin-2 was shorter than the duration of the analgesic effect of the vector. Vector-mediated endomorphin-2 expression also reduced the number of c-fos positive cells in laminae I–II of dorsal horn evoked by gentle touch stimulation to injured paw for 10 min 2 hours before sacrifice (Fig. 4b).
Fig. 4.
QHEND reduces peripheral inflammation after CFA in naïve rats. Injection of CFA injection resulted in an increase in paw volume, measured by plethysmometer. Inoculation of QHEND (open circles) but not QOZHG (filled squares) 3 days prior to CFA significantly decreased the volume of the CFA-injected paw (a), p < 0.01, General linear model, repeated measure (SPSS); n = 6 animals per group. Expression of c-fos in laminae I–II of dorsal horn evoked by gentle touch stimulation to the injured paw 3 days after CFA was reduced in animals inoculated with QHEND. **P < 0.01 QHEND vs Q0ZHG, ANOVA; n = 4–5 animals per group.
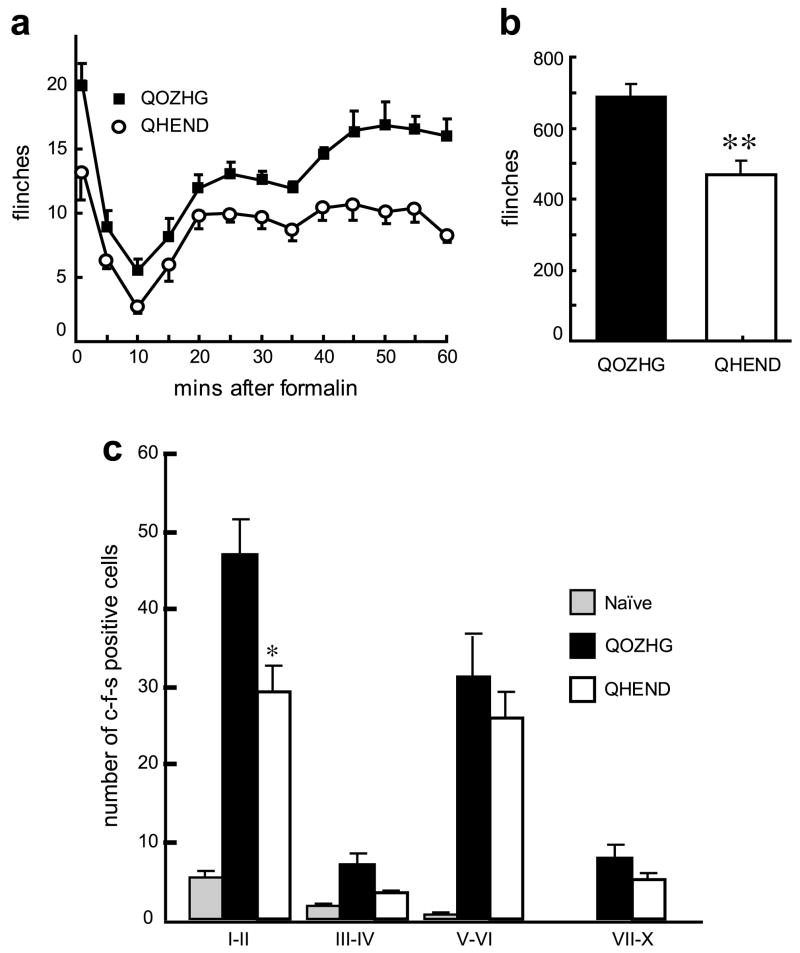
We also examined the effect of the vector in the delayed phase of the formalin model of inflammatory pain. Subcutaneous inoculation of the endomorphin-2- expressing vector 1 week prior to injection of formalin ipsilaterally reduced spontaneous flinching during the delayed phase of the formalin test (Fig. 5a and b). The reduction in spontaneous flinching was reflected in a significant reduction in c-fos positive cells in the dorsal horn of spinal cord in QHEND vector-inoculated compared to control vector-inoculated animals (Fig. 5c).
Fig. 5.
(a) Inoculation of QHEND but not QOZHG into the hindpaw 1 week prior to formalin testing significantly reduced flinching after injection of formalin in the paw in naïve rats; P < 0.01, general linear model, repeated measures test (SPSS); n = 5–6 animals per group. (b) The sum of flinches in phase 2 of the formalin test was significantly reduced in animals inoculated with QHEND; **P < 0.01 QHEND vs Q0ZHG, t-test. (c) QHEND inoculation reduced c-fos expression after injection of formalin; *P < 0.05 QHEND vs Q0ZHG, ANOVA; n = 3–5 animals per group.
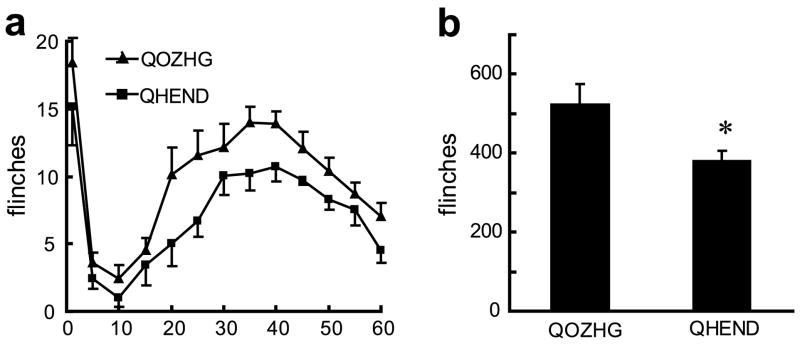
Rats rendered tolerant to morphine by injection of 10 mg/kg twice a day for 7 days develop tolerance to the effect of morphine. These rats showed a substantially diminished response to 10 mg/kg morphine in the delayed phase of the formalin test, equivalent to the response of naïve animals to only 1.2 mg/kg of morphine (data not shown). Animals injected with the vector and rendered tolerant to morphine by twice daily injection of morphine prior to formalin test showed a response to vector in the delayed phase of the formalin test that was of the same magnitude as the effect seen in naïve animals (Fig. 6 a and b compared to Fig. 5a and b), indicating that vector-produced endomorphin-2 has similar analgesic potency in morphine tolerant animals as in naïve animals.
Fig. 6.
(a) In animals rendered tolerant to morphine by twice daily injections of morphine (confirmed by tail-flick test, data not shown) QHEND continued to provide an analgesic effect in the formalin test; general linear model, repeated measures test, P < 0.05, (SPSS); n = 7–8 animals per group. (b) The sum of flinches in phase 2; *P < 0.05 QHEND vs Q0ZHG, t-test.
5. Discussion
We have previously reported on the characterization of the endomorphin-2-expressing vector, and the effect of vector-mediated endomorphin-2 expression in the spinal nerve ligation model of neuropathic pain (Wolfe et al., 2007). The results of the current study extend those results to demonstrate that the endomorphin-2-expressing HSV vector provides an analgesic effect in two different models of inflammatory pain. The analgesic effect was reversed by naloxone methiodide injected intraperitoneally or intrathecally. And finally, the magnitude of the vector-mediated effect was unchanged in animals rendered tolerant to the effect of morphine.
There is extensive evidence to demonstrate that spinal cord excitability is directly influenced by descending inputs originating in higher centers (e.g. periaqueductal gray) and that the descending projections (noradrenergic, serotonergic and opioid) result in release inhibitory neuropeptides in the spinal cord (Basbaum and Fields, 1984). The inhibitory effect at the spinal cord may result from direct post-synaptic inhibition or through activation of interneurons (Basbaum and Fields, 1984). In addition to postsynaptic spinal effects, there is considerable evidence for an opioid-mediated presynaptic effects that reduce neuropeptide release from primary afferents (Yaksh, 1997). Spinal opioid receptors are located both presynaptically on the central terminals of small diameter nociceptive afferents and postsynaptically on interneurons and on the dendrites of second order projection neurons, and are also expressed in immune cells that infiltrate sites of tissue injury. Presynaptic opioid receptors are also present on axon terminals of unmyelinated and small myelinated fibers in the skin, and the number of these peripheral opioid receptors may be increased by inflammation (Hassan et al., 1993; Schafer et al., 1995; Stein, 1995). The clinical effects of transgene-mediated endomorphin-2 expression could result from presynaptic effects that selectively decrease excitatory neurotransmitter release from nociceptive afferents, or from post-synaptic effects resulting in hyperpolarization of second order neurons in the spinal cord. The observation that most of the analgesic effects of vector-mediated endomorphin-2 could be reversed both by intrathecal (10 μg) or intraperitoneal (10 mg/kg) naloxone methiodide suggests that the effect of endomorphin-2 expression from transduced DRG neurons is derived from both central effects at the level of the dorsal horn as well as from effects occurring peripherally on the sensory nerve terminals in the skin.
Tissue injury heightens the sensitivity of nociceptors to both thermal and mechanical stimuli, a phenomenon that results in pain, from the production and release of chemical mediators from the primary sensory terminal and from non-neural cells. Some inflammatory mediators directly activate nociceptors, while others act together to produce a sensitization of the somatosensory nervous system (Scholz and Woolf, 2002). Nocicpetor activation contributes a component of “neurogenic” inflammation, an efferent function of the nociceptor whereby release of neurotransmitters, notably substance P and calcitonin gene related peptide (CGRP), from the peripheral terminal induces vasodilation and plasma extravasation of proteins and fluid from postcapillary venules, as well as activation of non-neuronal cells, including mast cells and neutrophils (Julius and Basbaum, 2001) that in turn contribute elements to the inflammatory milieu inducing peripheral sensitization. This complex relationship between inflammation (edema) and nociception can be interrupted by vector-mediated EM-2 inhibition of the primary nociceptive afferents.
Our observation that the vector-mediated peptide produced by transduction of DRG in vivo may have peripheral effects is consistent with the observations by Braz et al with a similar HSV vector expressing pro-enkephalin that the transgene product is transported both peripherally and centrally from transduced DRG neurons (Antunes Bras et al., 1998) and that the analgesic effects of HSV vector-mediated release of enkephalin in a rodent model of adjuvant-induced polyarthritis could be reversed by the intraperitoneal administration of 3 mg/kg/day of naloxone methiodide (Braz et al., 2001). A central site of action is supported by microdialysis and superfusion studies demonstrating central release of GABA from afferent terminals in spinal cord of animals transduced with a similar HSV vector expressing glutamic acid decarboxylase (Liu et al., 2004). The substantial and statistically significant reduction in paw swelling may also be related to peripheral effects of endomorphin-2, as opiates have been shown to inhibit both neurogenic and carrageenen-induced plasma extravasation in the skin (Barber, 1993; Joris et al., 1990). Alternatively, it is possible that presynaptic inhibition of the primary afferent inhibits the peripheral release of nerve-derived inflammatory mediators such as substance P or CGRP (Pohl et al., 1989), although at least one study suggests that mu-selective agonists may not possess such effects (Mauborgne et al., 1987).
The only vector-mediated effect that was reversed by intrathecal but not by intraperitoneal naloxone methiodide was the effect of the vector on thermal hyperalgesia in the CFA model (Fig. 3c). There are two possible explanations for this observation. It is possible that studies of a larger group of animals would reveal the result to be statistically significant. The alternate possibility is that the endomorphin-2 produced reduction in thermal hyperalgesia is mediated by central opiate receptors post-synaptic to the DRG.
The magnitude of the analgesic effect in the CFA model of inflammatory pain is substantial, and persists for at least 2 weeks (Fig. 1). The time course of the anti-allodynic effect is similar (though not identical) to that which we have observed in other studies of HSV-mediated transgene expression in models of inflammatory and neuropathic pain (Goss et al., 2002; Hao et al., 2006; Hao et al., 2003; Liu et al., 2004). This time course is consistent with the time course of expression of other transgenes whose expression was similarly driven by the human cytomegalovirus immediate early promoter in the context non-replicating HSV vectors (Puskovic et al., 2004). A longer duration of analgesic effect, up to 7 weeks, has been reported in experiments that were performed using an HSV vector defective only in the HSV thymidine kinase gene (Wilson et al., 1999). Deletion of thymidine kinase renders the recombinant vector incapable of replicating in neurons, but it is possible that viral replication in the epithelium by that recombinant may account for the different time course reported using that vector. In any case, the prolonged duration of the effect is consistent with the observation we have made in other models of HSV-mediated gene transfer that the animals do not develop tolerance to the vector-mediated effect.
Immunocytochemical detection of c-fos in spinal neurons has been widely used as a marker to identify populations of spinal cord neurons activated by noxious stimuli, or by innocuous stimulation in animals with chronic neuropathic or inflammatory pain (Catheline et al., 1999, 2001; Ma and Woolf, 1996). After injection of either formalin or CFA c-fos immunoreactive neurons are found in laminae I–II and V–VI in the spinal dorsal horn, corresponding to the laminar distribution of the unmyelinated (C) and thinly myelinated (Aδ) nociceptive afferents (Presley et al., 1990). The addition of gentle touch in the CFA model further activates non-nociceptive afferents, resulting in increased c-fos expression in laminae III–IV, in the distribution of the afferent terminals of touch-sensitive (Aβ) fibers. The observation that vector-mediated expression of endomorphin-2 suppressed spinal c-fos is consistent with the behavioral studies, and serves as a histologic correlate of the behavioral results.
The vector-mediated effect of endomorphin-2 was unchanged in animals rendered tolerant to morphine. Most of the opioid analgesics used clinically selectively bind to mu opioid receptors. Subtle but significant differences in the pharmacology of different drugs has led to the identification of a family of mu receptors (naloxonazine- sensitive and naloxonazine-insensitive mu receptors) by pharmacological and receptor binding approaches (Pasternak, 2004; Wolozin and Pasternak, 1981) with incomplete cross-tolerance across receptor subtypes (Pasternak, 2001). Using molecular biological techniques some 11 different MOR-1 variants have been identified that differ in their C-terminal sequence, and show marked differences in response to morphine and endomorphin-2 when assessed functionally by GTPγS stimulation assay (Bolan et al., 2004), although the relationship of splice variants to pharmacologic subtypes remains to be established. Our results nonetheless are consistent with a previous report that endomorphin-2 produces an antinociceptive effect in rat tail flick and paw pressure tests in rats tolerant to chromic morphine (Labuz et al., 2002).
These results demonstrate analgesic efficacy in a model of inflammatory pain and a prolonged course of action. Further studies will be required to determine if a combination of enkephalin- and endomorphin-expressing HSV vectors provide a more robust effect, and to establish whether the strategy of gene transfer to DRG using HSV vectors may prove to be useful in the treatment of patients with intractable pain.
Acknowledgments
This work was supported by grants from the NINDS (DJF, MM, JCG), NIDA (SH), and the Department of Veterans Affairs (DJF and MM). We acknowledge the excellent technical assistance of Vikram Thakur and Shue Liu.
References
- Antunes Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F, Pohl M. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- Barber A. Mu- and kappa-opioid receptor agonists produce peripheral inhibition of neurogenic plasma extravasation in rat skin. Eur J Pharmacol. 1993;236:113. doi: 10.1016/0014-2999(93)90233-8. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990a;521:15. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic location of mu, delta and kappa opioid receptors in the superficial layers of the dorsal horn of the rat spinal cord. Prog Clin Biol Res. 1990b;328:183. [PubMed] [Google Scholar]
- Bolan EA, Pan YX, Pasternak GW. Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse. 2004;51:11. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]
- Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Effects of opioid receptor antagonists on the effects of i.v. morphine on carrageenin evoked c-Fos expression in the superficial dorsal horn of the rat spinal cord. Brain Res. 1999;824:105. doi: 10.1016/s0006-8993(99)01207-x. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat: a Fos study. Pain. 2001;92:389. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chapman V, Diaz A, Dickenson AH. Distinct inhibitory effects of spinal endomorphin-1 and endomorphin-2 on evoked dorsal horn neuronal responses in the rat. Br J Pharmacol. 1997;122:1537. doi: 10.1038/sj.bjp.0701594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li J, Mata M, Goss J, Wolfe D, Glorioso JC, et al. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J Virol. 2000;74:10132. doi: 10.1128/jvi.74.21.10132-10141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X-P, et al. Herpes vector-mediated expression of proenkephalin reduces pain-related behavior in a model of bone cancer pain. Ann Neurol. 2002;52:662. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- Hao S, Takahata O, Mamiya K, Iwasaki H. Sevoflurane suppresses noxious stimulus-evoked expression of Fos-like immunoreactivity in the rat spinal cord via activation of endogenous opioid systems. Life Sci. 2002;71:571. doi: 10.1016/s0024-3205(02)01704-6. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect. Pain. 2003;102:135. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, et al. Anti-inflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund’s adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Hobbs IInd WE, DeLuca NA. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris J, Costello A, Dubner R, Hargreaves KM. Opiates suppress carrageenan-induced edema and hyperthermia at doses that inhibit hyperalgesia. Pain. 1990;43:95. doi: 10.1016/0304-3959(90)90054-H. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Krisky DM, Wolfe D, Goins WF, Marconi PC, Ramakrishnan R, Mata M, et al. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5:1593. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- Labuz D, Przewlocki R, Przewlocka B. Cross-tolerance between the different mu-opioid receptor agonists endomorphin-1, endomorphin-2 and morphine at the spinal level in the rat. Neurosci Lett. 2002;334:127. doi: 10.1016/s0304-3940(02)01121-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67:307. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Mauborgne A, Lutz O, Legrand JC, Hamon M, Cesselin F. Opposite effects of delta and mu opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem. 1987;48:529. doi: 10.1111/j.1471-4159.1987.tb04125.x. [DOI] [PubMed] [Google Scholar]
- Meunier A, Latremoliere A, Mauborgne A, Bourgoin S, Kayser V, Cesselin F, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Multiple opiate receptors: deja vu all over again. Neuropharmacology. 2004;47(Suppl 1):312. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Pohl M, Lombard MC, Bourgoin S, Carayon A, Benoliel JJ, Mauborgne A, et al. Opioid control of the in vitro release of calcitonin gene-related peptide from primary afferent fibres projecting in the rat cervical cord. Neuropeptides. 1989;14:151. doi: 10.1016/0143-4179(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC, et al. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol Ther. 2004;10:67. doi: 10.1016/j.ymthe.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Schafer M, Imai Y, Uhl GR, Stein C. Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not muopioid receptor transcription in dorsal root ganglia. Eur J Pharmacol. 1995;279:165. doi: 10.1016/0014-2999(95)00150-j. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Stone LS, Fairbanks CA, Laughlin TM, Nguyen HO, Bushy TM, Wessendorf MW, et al. Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and -2. Neuroreport. 1997;8:3131. doi: 10.1097/00001756-199709290-00025. [DOI] [PubMed] [Google Scholar]
- Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci USA. 1999;96:3211. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Hao S, Hu J, Srinivasan R, Goss J, Mata M, et al. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain. 2007;133:29. doi: 10.1016/j.pain.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA. 1981;78:6181. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997;41:94. doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Jones T, Laurito CE, Lu Y, Wilson SP. Reversal of ongoing thermal hyperalgesia in mice by a recombinant herpesvirus that encodes human preproenkephalin. Mol Ther. 2004;9:24. doi: 10.1016/j.ymthe.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Lu Y, Laurito CE, Peters MC, Vota-Vellis G, Wilson SP, et al. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;13:589. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]