Abstract
Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODN) is a recent class of immunostimulatory adjuvants that includes unmethylated CpG dinucleotide sequences similar to those commonly found in bacterial DNA. CpG ODN specifically triggers toll like receptor 9 (TLR9), which is found within phagoendosomes of antigen presenting cells (APCs) such as dendritic cells (DCs). CpG ODN triggers activation and maturation of DCs and helps to increase expression of antigens. CpG ODN can be used to induce polarized Th1 type immune responses. Several studies have shown that antigens and CpG ODN must be co-localized in the same APC to generate the most potent therapeutic antigen-specific immune responses. Delivery vehicles can be utilized to ensure co-delivery of antigens and CpG ODN to the same APCs and to significantly increase uptake by APCs. These strategies can result in antigen-specific immune responses that are 5 to 500-fold greater than administration of antigen alone. In this review, we discuss several recent and innovative strategies to co-delivering antigens and CpG ODN adjuvants to APCs. These approaches include the utilization of conjugate molecules, multi-component nanorods, liposomes, biodegradable microparticles, pulsatile release chips and cell-microparticle hybrids.
Keywords: delivery systems, antigen, CpG ODN, adjuvant, vaccine, controlled release
1. Introduction
Immunization with antigens alone produces poor immunity. By co-administrating the antigen with an adjuvant, better immune responses can be generated [1-3]. This occurs because the adjuvants act as immunostimulatory agents. The severe inflammation induced by many adjuvants, such as killed bacteria, negates their potential for human application [4]. Those adjuvants that are currently approved by the FDA, such as alum, are not very efficient. Technologies that incorporate adjuvants to increase the immunogenicity of antigens has been recently cited as one of the top ten technologies that will significantly impact global health [5]. Although the mechanism of adjuvants is not fully understood, it is believed that they promote specific types of immune responses, such as antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) [6]. Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODN), when used as an adjuvant, results in much stronger CD8+ responses than antigen delivery alone [3, 7-10].
2. CpG ODN as an adjuvant
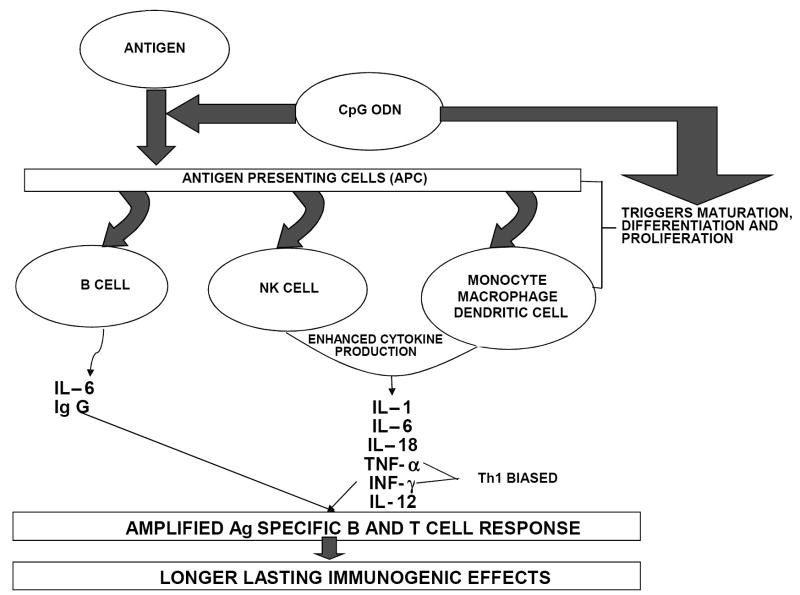
CpG ODN with sequence patterns like those found in bacterial DNA activate natural killer cells to secrete interferon-γ (IFN-γ) and generate a cell-mediated immune response [11]. The specific sequence motif present in bacterial DNA that is responsible for triggering these immune responses is the unmethylated CpG dinucleotide flanked by two 5’ purines and two 3’ pyrimidines [12, 13]. CpG ODN are taken up by cells via adsorptive endocytosis and bind to the toll-like receptor 9 (TLR9) present within the endolysosomes in the intracellular compartment of B cells and plasmacytoid dendritic cells (pDCs) [14-16]. The binding triggers cell signaling pathways that induce leukocyte gene expression and cytokine secretion. CpG ODN triggers an immunostimulatory cascade inducing the maturation, differentiation and proliferation of multiple immune cells including B and T lymphocytes, macrophages, natural killer (NK) cells and monocytes/macrophages that produce interleukin (IL)- 1, 6, 12 and 18, interferon-γ (INF-γ) and tumor necrosis factor- α (TNF-α) (Figure 1) [17-19]. Rapid induction of these immune responses and production of Th1 related cytokines is a critical step to controlling the early spread of pathogens [5].
Figure 1.
Schematic showing immunostimulatory cascade triggered by co-delivery of antigen and CpG ODN.
3. Rationale and need for devices that co-deliver antigens and CpG ODN
While CpG ODN exhibits potent immunostimulatory effects, the rapid degradation and ineffective delivery into the intracellular compartments of APCs are major obstacles to improving its efficacy [20]. When antigen is administered alone, it elicits strong Th2 type immune responses. A significant shift in the isotype of antibody response can be achieved by co-administering antigen and CpG ODN [7, 12, 21, 22]. Addition of CpG ODN has been reported to result in a significant increase in production of IgG2a antibody, increasing the IgG2a: IgG1 ratio over nine-fold [12]. This enhanced Th1 type immune response is essential for counteracting intracellular pathogens including choriomeningitis virus, hepatitis B virus and tetanus toxoid [12, 23-25]. For example, co-administration of CpG ODN and hepatitis B surface antigen (HBsAg) vaccine to the same site of the muscle significantly enhanced the antibody response [5]. In contrast, when CpG ODN was administered separately following the administration of the vaccine, it did not induce any significant improvement in immunostimulatory effects over the administration of vaccine alone [5]. These studies highlight the importance of delivery devices that protect CpG ODN from enzymatic degradation, improve targeting of CpG ODN to the endolysosomes, ensure that both CpG ODN and antigen are co-delivered to the same APC, and provide long-term immunity. Here, we review several different delivery systems that are being developed for CpG ODN to meet some of these challenges.
4. Approaches for co-delivering antigens and CpG ODN
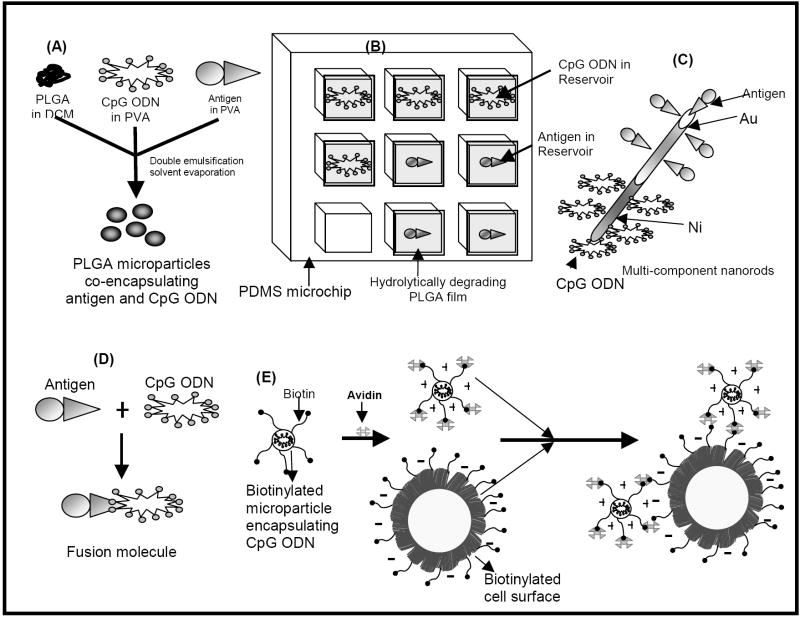
We and others have shown that CpG ODN and antigens can be delivered to the same APCs using a number of different strategies. These include the utilization of multicomponent metallic nanorods [1], biodegradable microparticles and nanoparticles [26, 27] including cationic microparticles [28-30], pulsatile releasing drug delivery chips [31], and cell-microparticle hybrids [32] (Figure 2). Perhaps the simplest approach to co-delivering antigens and CpG ODN is to chemically conjugate them together thereby ensuring that both components will enter the same cell [12, 33-36].
Figure 2.
Schematic showing some recent innovative strategies to co-delivering antigen and CpG-ODN. These include (A) PLGA microparticles co-entrapping antigen and CpG ODN, (B) pulsatile delivery of antigen and CpG ODN from PDMS microchips with biodegradable PLGA seals, (C) multi-component nanorods functionalized with antigen and CpG ODN in spatially-defined regions, (D) antigen-CpG ODN conjugate molecules and (E) Cell-microparticle hybrids.
4.1 CpG ODN and antigen conjugates
One approach to crosslinking antigens and CpG ODN has been to utilize the biotin-avidin interaction to link the two molecules together. The cross-linked molecule elicited a ten-fold increase in immune responses when compared to the use of antigen alone [12]. Furthermore, the effect was eliminated upon treating the complex with DNAse. This confirmed that the increase in immune response was due to the presence of the ODN bearing the CpG motif [12]. The conjugate exhibited a higher preferential uptake by APCs over the unconjugated mixture of antigen and ODN. This was attributed to the CpG ODN binding to receptors expressed by the APCs that enabled a significant increase in endocytosis of the conjugated antigen. Conjugation of the CpG ODN to antigen is also reported to enhance cross-presentation [37, 38]. Cross-presentation refers to the ability of certain APC’s to process and present extracellular pathogens to cytotoxic T cells (CD8 T cells). CpG ODN-antigen conjugate molecules demonstrate much higher efficacy than mixtures of antigen and CpG ODN at equivalent concentrations [35, 39].
We have synthesized CpG ODN-antigen conjugate molecules by covalently linking CpG ODN to the model antigen ovalbumin (OVA) [39]. Figure 3 shows the schematic for the synthesis of the conjugate molecule. In this approach, the phosphothiolated sulfhydryl modified ODN containing the CpG motif was linked to the amino terminal of the l- lysine residues on OVA using N-hydroxysuccinimide (NHS) chemistry. In addition to providing the aforementioned advantages of a conjugate molecule, chemical modification of the ODN backbone by phosphothiolation as used in this study has been shown to significantly reduce degradation by nuclease enzymes and hence increase the half life in-vivo [40]. Additionally, the chemically modified form of CpG ODN has been shown to retain excellent immunostimulatory activity on APCs. We measured IFN-γ production by pulsing DCs generated from bone marrow of wild C57BL mice with CpG ODN–OVA conjugate molecules, OVA/alum or OVA and CpG ODN in solution. Splenocytes harvested from naive transgenic C57BL/6 OT-1 mice were co-cultured with the treated DCs for a further 24 hours and the supernatant from the co-culture was analyzed for the presence of IFN-γ by ELISA. The CpG ODN-OVA conjugate molecule generated a 16-fold higher IFN-γ response in comparison to co-cultures that were pulsed with CpG ODN and OVA in solution (Table I).
Figure 3.
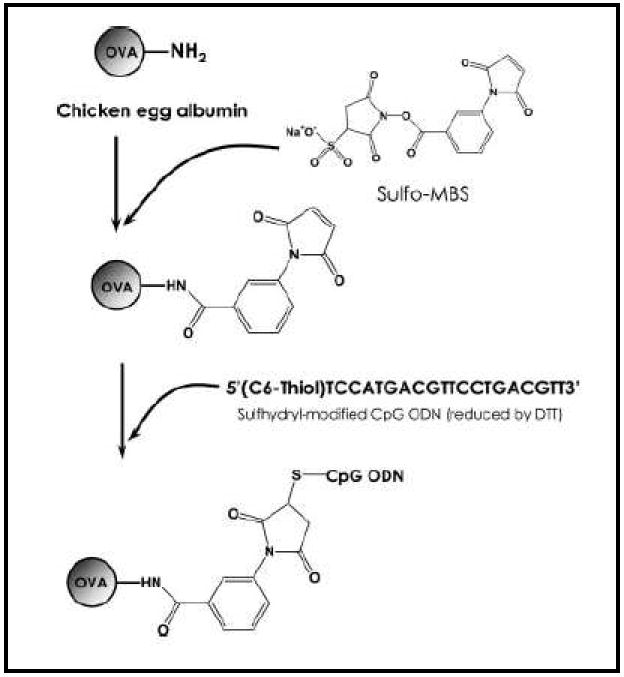
Schematic showing synthesis of the OVA-CpG ODN conjugate molecule. Reproduced with permission from [39]. Copyright © Wiley-VCH Verlag CmbH & Co. KHaA.
Table I.
IFN-γ measurements of DCs pulsed with antigen-CpG ODN conjugates as co-delivery vehicle and solution as co-delivery vehicle of CpG ODN and OVA. Reproduced with permission from [39]. Copyright © Wiley-VCH Verlag CmbH & Co. KgaA.
| DC + OT-1 Cells | IFN-γ ± SE (pg/mL) |
|---|---|
| OVA-CpG fusion | 862 ± 21 |
| OVA and Alum | None detected |
| OVA + CpG | 53 ± 13 |
| Untreated DCs | None detected |
A chemical conjugate of CpG ODN to an allergen, Amb a1, for the prophylactic treatment of allergic rhinitis and asthma was synthesized by Tighe et al. [41]. Figure 4 shows the IgG2a levels in mice after intradermal injection of the conjugate or a physical mixture of the two. Table II represents the cytokine profile of the activated spleen cells of mice immunized by the antigen alone, the conjugate molecule and a mixture of Amb a1 and alum. Figure 4 and Table II show that conjugation of Amb a1 to CpG ODN induced the strongest Th1 type immune response and induction of a 10-fold higher IFN-γ response. The enhanced immunogenicity of Amb a1-CpG ODN conjugates was even more pronounced in rabbits and monkeys. These species formed high titers of IgG antibodies to the Amb a1-CpG ODN conjugate but failed to respond to the antigen alone [41]. CpG ODN facilitates a higher uptake of the conjugate molecule by APCs and produced a polarized TH1 type response by translocation of the conjugate to lysosomal-associated membrane protein (Lamp-1) positive endosomal-lysosomal compartments. Another factor that may be contributing to the increased efficacy is that conjugating CpG ODN to antigen shifts antigen uptake from inefficient fluid phase pinocytosis to efficient DNA receptor-mediated endocytosis. Even non-stimulatory ODNs linked to antigen, enhanced uptake although the presence of the stimulatory CpG ODN was essential for triggering maturation of the DCs [38]. For example, conjugation of CpG ODN to OVA was shown to increase OVA uptake in B cells by 40-fold, which in turn, led to upregulation of co-stimulatory molecules and cytokines such as IL-12 [36]. CpG ODN-antigen conjugates have significant potential for inducing immune responses to weak antigens or lower doses of antigen used in prophylactic allergen immunotherapeutic applications [38, 42].
Figure 4.
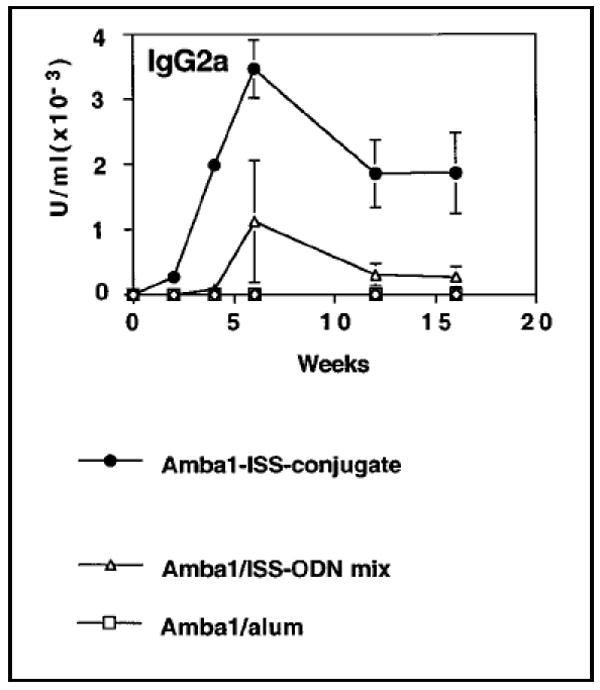
Graph showing IgG2a antigen (Amb a1) specific IgG2a profile generated by antigen-immunostimulatory ODN (ISS ODN) conjugate, antigen-ISS ODN free solution mixture and antigen-alum mixture. Reproduced with permission from [41]. Copyright © Elsevier B.V.
Table II.
Cytokine profile of antigen (Amb a1) activated spleen cells with and without adjuvant immunostimulatory ODN (ISS ODN) as a conjugate molecule and mixture forms. Reproduced with permission from [41]. Copyright © Elsevier B.V.
| Antigen used for immunization | IFN-γ (pg/mL) | IL-5 (pg/mL) |
|---|---|---|
| Amb a 1 | <10 | 630 ± 150 |
| Amb a 1-ISS conjugate | 8340 ± 2170* | <10* |
| Amb a 1-mODN conjugate | <10 | 500 ± 150 |
| Amb a 1/ISS-ODN mix | 170 ± 110 | 610 ± 280 |
| Amb a 1/alum | 490 ± 240 | 4410 ± 880 |
| Saline (naive) | <10 | <10 |
4.2 Multicomponent nanorods
Ballistic delivery of antigens to the sub-dermal layers (that contain an abundance of APCs such as Langerhans cells) using the gene gun can stimulate strong antigen-specific immune responses [43-45]. The gene gun has been used to primarily deliver single functional gold particles. We developed a multi-component nanorod that could deliver CpG ODN and antigens to the same cells when bombarded into the skin using the gene gun. The nanorods were fabricated by electrodeposition into alumina templates with an array of cylindrical pores [46]. An evaporated silver film on one side of the template served as the working electrode in a three-electrode configuration [1, 46-48]. A thin layer of silver was electrodeposited into the template to ensure easy release of the nanorods from the template. Gold segments were deposited prior to nickel segments to prevent erosion of the nickel layers during silver removal. The silver layers were dissolved using nitric acid and the alumina template was then dissolved using potassium hydroxide.
Nanorod length could be controlled by the length and strength of the potential applied and the composition could be controlled by the metal ion solution used to deposit the metal segments [1, 46-48]. Using chemical moieties that bind selectively to either gold or nickel, we attached plasmid DNA (pDNA) constructs bearing the unmethylated CpG sequence or the antigen OVA to the different segments [1]. The antigens and DNA molecules were bound to the same nanorod in spatially defined regions [46]. This was achieved by converting a small proportion of the primary amine groups of the model antigen OVA into sulfhydryl groups. The OVA was then bound to the gold segments of the nanorods through a thiolate linkage. Electrostatic interactions were used to bind DNA to the nickel segments by suspending the dual component nanorods in a 0.1 M solution of 3-[(2-aminoethyl)dithio]-propionic acid (AEDP). The carboxylic acid terminus of AEDP binds to the native oxide on the nickel segments. This results in the surface presentation of primary amine groups spaced by a reducible disulfide linkage. In the reducing environment of the cell, the disulfide linkage between the plasmid/CpG and the nanowire is cleavable to enhance release of the plasmid/CpG. When both OVA and CpG motifs were bound to the same nanorod, we observed a 10-fold increase in the CD8+ T-cell response in comparison to OVA delivery on nanorods alone (Figure 5). These nanorods are expected to have significant potential for co-delivery of CpG ODN and antigens using ballistic delivery methods such as the gene gun.
Figure 5.
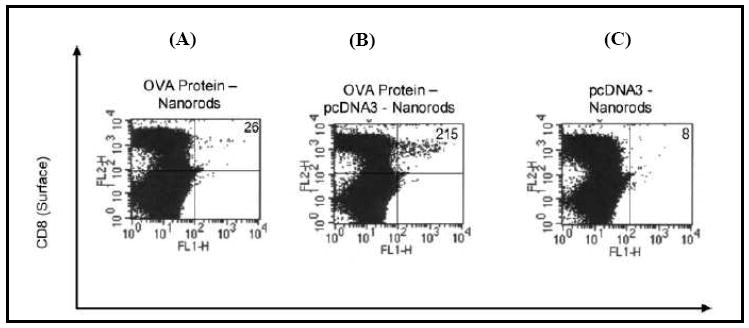
Ten-fold increase in ovalbumin-specific CD8+ responses are observed in C57BL/6mice immunized with nanorods co-functionalized with OVA and CpG motif (B) in comparison to nanorods functionalized only with OVA (A) or CpG motif (C) alone. Reproduced and adapted with permission from [1]. Copyright © IOP.
4.3 Liposomes
Liposomes were amongst the first strategies developed for co-delivering antigens and CpG ODN [49, 50]. Liposomes are artificial closed vesicles composed of concentric lipid bilayers that are separated by aqueous regions and have been utilized as delivery systems for several anti-cancer drugs, proteins and DNA. Liposomal delivery systems have been used as carriers for immunoadjuvants to induce immune responses against a variety of antigens [49, 50]. Like biodegradable polyesters including poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and polylactic-co-glycolic acid (PLGA), liposomes are safe and biodegradable making them attractive vehicles for co-delivery of CpG ODN and antigens [49, 50]. Liposomal vehicles have been used to deliver CpG ODN alone and to co-deliver CpG ODN and antigens for therapy of diseases such as leishmaniasis [51, 52].
Current therapy against leishmaniasis shows limited effect and the available drugs bear a high toxicity potential. Studies show that recovery and protection against further infection are mainly dependent on the induction of Th1 type immune responses and hence a strategy co-delivering CpG ODN with antigen would be a promising vaccine against all forms of human leishmaniasis [52]. Liposomes co-encapsulating CpG ODN and antigens have been prepared by a dehydration-rehydration vesicle (DRV) method [53]. This method involves the lipid phase consisting of distearoylphosphatidylcholine (DSPC) and cholesterol dissolving in a chloroform-methanol mixture. The lipid film formed upon solvent evaporation is then hydrated to form multilamellar vesicles (MLV). The rLmSTI1 antigen and CpG ODN were added to empty vessels and converted to 100 nm unilamellar vesicles using high pressure extrusion. The liposomes were then lyophilized and rehydrated. The Th1 biased antigen-specific antibody response and the ratio of IgG2a/IgG1 response in mice sera is shown in Figure 6A and 6B, respectively. The co-delivery approach resulted in the highest levels of IgG2a antibodies and the highest ratio of IgG2a/IgG1 antibodies in comparison to the groups immunized with a solution form of the components or the antigen and CpG ODN administered alone. Additionally, the number of viable parasites (L. major) was quantified in spleen of different groups of mice after challenge with different formulations (Figure 7). The lowest number of live parasites was seen in the group of mice immunized with the liposomal formulation co-encapsulating antigen and CpG ODN. These results show that liposomal delivery of CpG ODN with antigen may be a suitable strategy to enhance Th1 type immune responses and induce protection against several pathogens [51, 54, 55].
Figure 6.
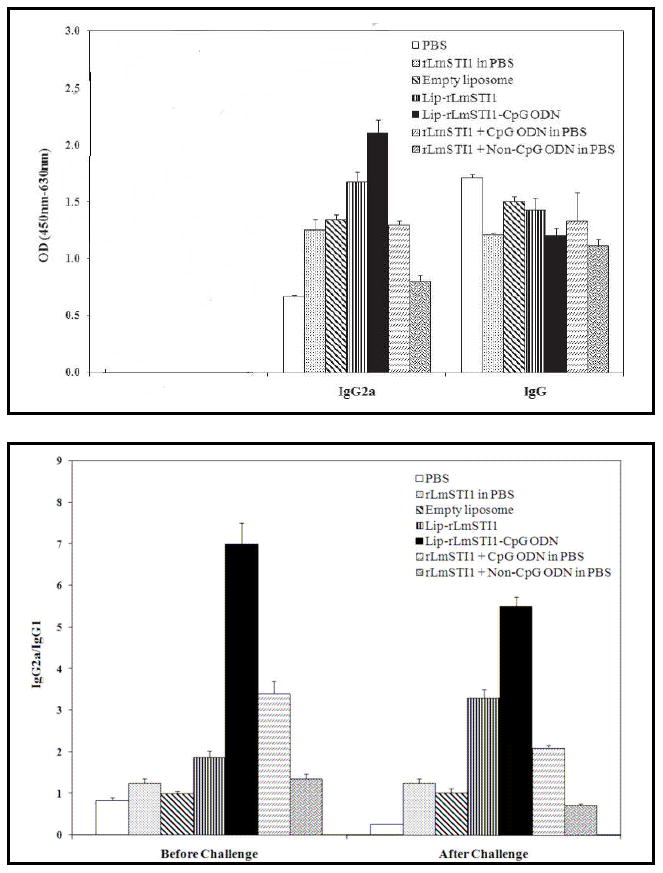
Bar charts showing IgG2a and IgG levels (A) and ratio of IgG2a/IgG1 (B) in sera of immunized mice at the end of 14 weeks. The mice were immunized with antigen alone (rLMSTI1 in PBS), empty liposomes, liposomes encapsulating antigen alone (Lip-rLMSTI1),liposomes co-encapsulating both antigen and CpG ODN (Lip-rLMSTI1-CpG ODN), both antigen and CpG ODN in solution (rLMSTI1 + CpG ODN in PBS) or liposomes entrapping antigen alone and co-administering CpG ODN in solution (rLmSTI1 + Non-CpG ODN in PBS). Reproduced with permission from [52]. Copyright © The American Society for Microbiology.
Figure 7.
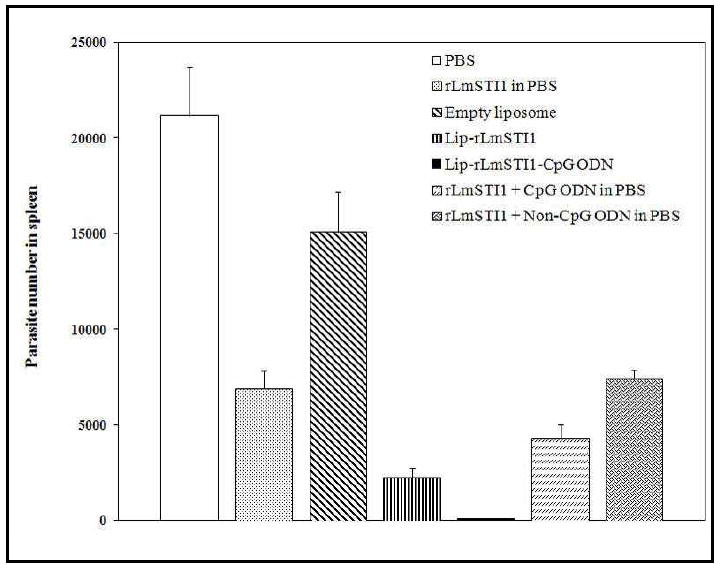
Viable parasite (L. major) in spleen of different groups of mice immunized with antigen alone (rLMSTI1 in PBS), empty liposomes, liposomes encapsulating antigen alone (Lip-rLMSTI1), liposomes co-encapsulating both antigen and CpG ODN (Lip-rLMSTI1-CpG ODN), antigen and CpG ODN in solution (rLMSTI1 + CpG ODN in PBS) or liposomes entrapping antigen alone and co-administering CpG ODN in solution (rLmSTI1 + Non-CpG ODN in PBS). Reproduced with permission from [52]. Copyright © The American Society for Microbiology.
4.4 Biodegradable microparticles
Biodegradable microparticles fabricated from FDA approved polymers such as PLGA have shown great potential for protein, peptide and DNA delivery over the last two decades [27]. Microparticle-based vaccines have been shown to stimulate strong immune responses and this therapeutic approach has been applied for the prevention and treatment of a variety of diseases including tetanus, influenza, hepatitis and cancer [27, 56-58]. One of the most attractive advantages of using PLGA microparticle systems in the area of vaccine delivery is the ability to develop a single-shot vaccine [59, 60]. For example, a three-injection schedule for prevention of hepatitis B infection has been converted into a single shot therapy by employing PLA and PGA microparticles encapsulating hepatitis B surface antigen (HBsAg). In addition, microparticles have also been shown to enhance the immunogenicity of encapsulated antigens due to their particulate nature [61]. Antigen-loaded PLGA microparticles generate a strong Th1 type immune response even against poor immunogens [27]. We have shown that incubating microparticles with DCs triggers activation and maturation, which are key steps in initiating the immune response against target antigens [26].
Microparticles are fabricated from biodegradable polymers like PLA, PGA and PLGA using techniques such as emulsification/solvent evaporation [62-64]. Several studies have shown that careful control over the formulation parameters such as surfactant concentration and stirring speed can be used to optimize loading levels and particle sizes [26, 65-69]. These systems can be delivered orally or to mucosal membranes (e.g. nasal and vaginal) and can protect CpG ODN from enzymatic degradation by nucleases [26]. Additionally, studies have shown that particles up to 10 μm in size are preferentially internalized by APCs thus functioning as suitable delivery vehicles for the co-delivery of antigen and CpG ODN [26]. This efficient internalization by APCs is attributed to the fact that microparticle carriers have comparable dimensions to the pathogens that the immune system has evolved to combat [70]. We and other have adopted these desirable properties to target APCs, co-deliver antigen and CpG ODN to the same APC, and generate stronger Th1 type antigen-specific immune responses with reduced toxicity [26, 71-73].
For example, we have developed a PLGA-based microparticle delivery system that co-entraps OVA as the model antigen and CpG ODN as the adjuvant [26]. The PLGA microparticles were formed using a double emulsion solvent evaporation technique. In this approach, a solution of OVA and CpG ODN is dissolved in a 1% w/v aqueous solution of poly (vinyl alcohol) (PVA) and is rapidly emulsified using ulltrasonication into an external oily phase comprising of dichloromethane (DCM) to form the first emulsion. This O/W emulsion is subsequently added to an external aqueous phase consisting of 1% w/v PVA to form the second emulsion. Following evaporation of DCM, the microparticles are collected by centrifugation and lyophilized. The microparticles exhibited an average size of 1.4 μm with a loading efficiency of 23 % for the antigen and 33- 35 % for CpG ODN.
Particle uptake by dendritic cells was assessed by flow cytometry using rhodamine-loaded microparticles. Figure 8 shows the efficient uptake of the microparticles by DCs with 92% of DCs having internalized particles by the end of 16 hours. Additionally, the microparticle system entrapping the antigen and CpG ODN generated a 20-fold higher INF-γ production by T-cells in comparison to the aqueous solution vehicle for co-delivery of OVA and CpG ODN.
Figure 8.
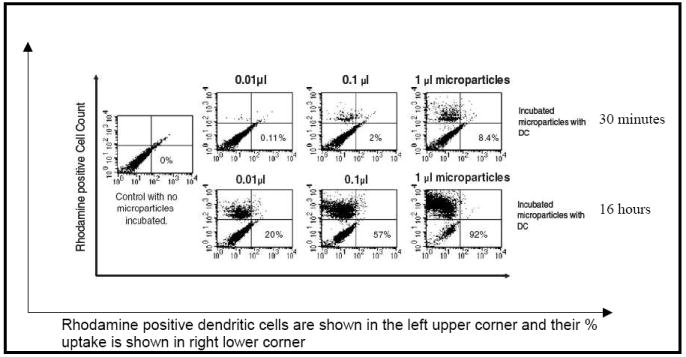
Uptake of rhodamine-loaded PLGA microparticles by DC2.4 dendritic cells (DCs) at the end of 30 minutes and 16 hours as measured by flow cytometry. Reproduced with permission from [26]. Copyright © Lippincott Williams & Wilkins.
We studied the IgG levels using ELISA and plotted the response over a 10 week course (Figure 9). Microparticles co-entrapping OVA and CpG ODN generated significant increases in antigen-specific antibody responses in comparison to empty microparticles or CpG ODN and OVA co-administered in an aqueous solution (Figure 9). These results highlight the substantial improvement in the immune stimulatory effects that can be achieved when the antigen and adjuvant are co-delivered to the same cell at the same time in a microparticle carrier.
Figure 9.

Graphs showing data from (A) ELISA assay of anti-OVA IgG response in mouse serum. Mice were inoculated twice on day 1 (week 0) and 14 (week 2) with either empty microparticles (MP), microparticle entrapping OVA alone (MP OVA), or microparticle co-encapsulating OVA and CpG ODN (MP CpG OVA), or with OVA administered as solution) or OVA and CpG co-administered in solution and (B) serial dilution in phosphate buffered saline of week 4 serum from mice immunized at week 0 and week 2. The positive control is a monoclonal anti-Ova antibody (4A9). The negative control is pretreated mouse serum. Reproduced with permission from [26]. Copyright © Lippincort Williams & Wilkins.
4.4.1 Alginate Microparticles
Rebelatto et al. have demonstrated that intranasal immunization is the most effective way to induce mucosal immunity as antibodies resulting from parenteral administration do not effectively reach mucosal surfaces, which is the primary entry point of infectious agents into the host body [74]. Antigens loaded into particles are more effective than soluble antigens for intranasal delivery because of greater endocytosis of the antigen by the mucosal- associated lymphoid tissue (MALT). Additionally, alginate when used as the particulate carrier has been shown to act as an adjuvant in animal species [74].
Tafaghodi and co-workers have coupled the advantages of alginate as the particulate carrier for tetanus toxoid (TT) and CpG ODN as adjuvant by fabricating alginate microparticles co-encapsulating these two components [75]. The microparticles prepared by an emulsion solvent precipitation technique were tested for their immunostimulatory effects by intranasal administration in white albino rabbits. Figure 10 shows the serum anti-TT IgG titres up to 12 weeks stimulated by antigen and CpG ODN in solution, antigen loaded microparticles, antigen and CpG ODN co-encapsulated microparticles and blank microparticles. Co-encapsulation of CpG ODN and antigen greatly enhanced IgG A titers when compared to microparticles loaded with antigen alone. Figure 11 shows the serum anti-TT antitoxin titers from these formulations. Intranasal administration of these microparticles in four human volunteers did not result in nasal irritation and did not result in hemolytic effects on the erythrocytes.
Figure 10.
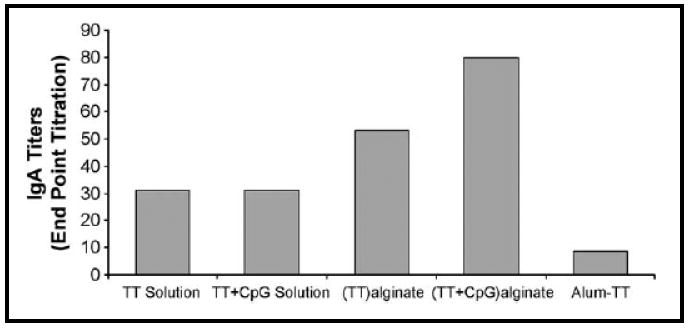
Nasal Lavage anti-IgG A titers in rabbits immunized with intranasal formulations of antigen and CpG ODN in solution, alginate microparticles loaded with antigen alone, alginate microparticles co-encapsulating antigen and CpG ODN or antigen co-delivered with the alum as adjuvant. Reproduced with permission from [75]. Copyright © Elsevier B.V.
Figure 11.
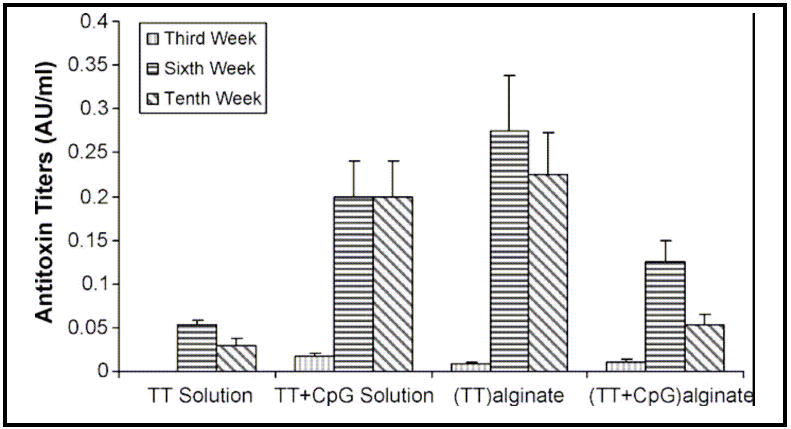
Serum antitoxin titers in rabbits immunized with intranasal formulations of antigen with or without CpG ODN in solution, alginate microparticles loaded with antigen alone, or alginate microparticles co-encapsulating antigen and CpG ODN [75]. Copyright © Elsevier B.V.
Co-encapsulation of CpG ODN with TT generates larger sized particles in comparison to the microparticles loaded with antigen alone. These larger microparticles resist translocation to the regional lymph nodes and are retained in the MALT. This enhances the uptake of the microparticles by the cells in the MALT, thereby inducing potent mucosal immunostimulatory responses.
In a modification to the above approach, hepatitis B surface antigen (HBsAg) was adsorbed on alginate-coated chitosan nanoparticles and co-administered with CpG ODN-loaded nanoparticles. This combination generated the highest humoral mucosal immune response and the highest IFN-γ activity relative to all control groups. In this approach, chitosan acts as the core for the carrier system and alginate is used to tailor a desired release profile for the antigen [76]. These studies highlight the potential of alginate-based microparticles for co-delivering CpG ODN and antigen. This is an approach that has shown particular promise for mucosal routes of administration.
4.4.2 Cationic microparticles
A derivation of the biodegradable microparticle delivery approach is to prepare cationic microparticles that can bind CpG ODN to the surface of the microparticle. The adsorption is facilitated by an electrostatic interaction between the negative charge on the ODN backbone and a positive charge on the cationic microparticle surface [77]. For example, anthrax vaccine (AVA) formulated in alum was used as a model antigen and co-administered with CpG ODN adsorbed onto the surface of cationic PLGA microparticles and compared with co-delivery with the free form of CpG ODN. Figures 12A and 12B show the IgG2a levels and toxin neutralizing activity (TNA) titers, respectively, over a period of 16 weeks in mice. The microparticle system with adsorbed CpG ODN co-delivered with AVA showed a 50-fold higher IgG2a anti-PA specific antibody activity and the highest TNA response. Figure 13 shows the survival rate of the mice vaccinated with various formulations. The mice immunized with the microparticles with adsorbed CpG ODN co-delivered with AVA showed greater than 80% survival even at the most susceptible stage of infection (2 weeks). In contrast, other formulations including the antigen alone and the antigen administered with the free form of CpG ODN failed to elicit a protective immune response and resulted in a mortality rate of 90%. Dissociation of the free CpG ODN from the antigen due to free diffusion from a solution form is inhibited by adsorption on the surface of PLGA microparticles ensuring localized delivery of the antigen and adjuvant to the APCs.
Figure 12.
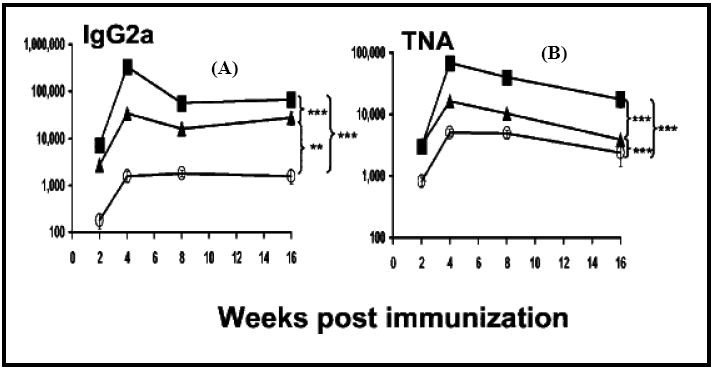
Graph showing IgG2a anti-PA antibody titers (A), and toxin neutralizing activity (TNA) (B), in anthrax-vaccinated mice. Male A/J mice were immunized intraperitoneally with AVA (O) with free CpG ODN (▲) or PLGA-adsorbed CpG ODN (■). Reproduced with permission from [77].Copyright © The American Center for Microbiology.
Figure 13.
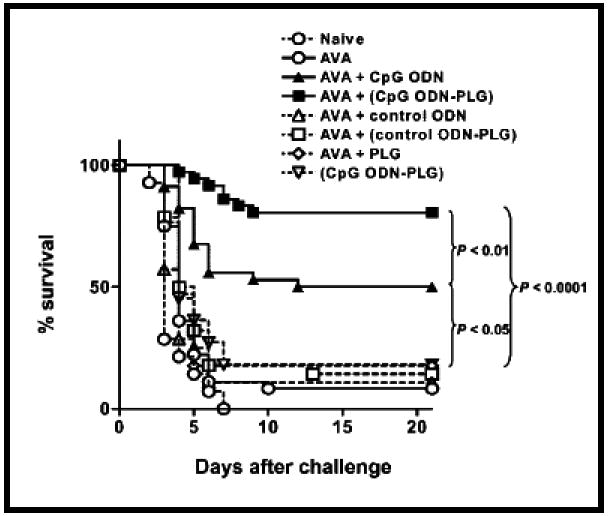
Graph showing survival of vaccinated mice. A/J mice were immunized intraperitoneally with anthrax vaccine (AVA) plus free CpG ODN (AVA + CpG ODN), AVA and PLGA-adsorbed CpG ODN (AVA + CpG ODN-PLG), AVA alone (AVA) or PLGA. Reproduced with permission from [77]. Copyright © The American Center for Microbiology.
Other cationic microparticles that can potentially be used to deliver CpG ODN and antigens include biodegradable microparticles that have been functionalized with cetyltrimethylammoniumbromide (CTAB) [78], cetyldimethylethylammonium bromide (CDAB), dimethyl dioctadecyl ammonium bromide (DDAB) [79], 1,2-dioleoyl-1,3-trimethylammoniopropane (DOTAP), cationic DDAB [79], poly(L-lysine) (PLL) [80-83], polyamidoamine (PAMAM) dendrimers [28], polyethylenimine [72, 84-95] and chitosan [96]. The advantage of these cationic microparticles is that they also have the potential to provide sequential release of antigen and CpG ODN, which could be used to enhance the immune response further [28]. Another approach that can be used to provide sequential release of CpG ODN and antigens is pulsatile release systems.
4.5 Pulsatile Delivery Systems
Pulsatile delivery systems release drugs in bursts or periodic intervals, separated by time intervals of little or no drug release [97, 98]. For certain active agents, such as hormones, it has been well established that pulsatile release offers advantages over sustained release because pulsatile release profiles more strongly mimic the body’s natural release profiles [97, 98].
Vaccines are traditionally administered as an initial single shot of the antigen and/or adjuvant followed by repeated booster shots to optimize protective immunity [99] Pulsatile release systems could offer the possibility of single shot vaccines if the initial and booster release of the antigen can be achieved from the same system. Medlicott et al. and Powell et al. have advocated the need to develop a delivery system that can carry both the antigen and adjuvant in the same delivery vehicle and yet provide differential release of the antigen and adjuvant to maintain high antibody titers for prolonged periods of time for protective immunity [98, 99]. Additional features of such an “autoboost” vehicle would ideally be reduced toxicity and maintenance of antigen and CpG ODN integrity until final release so as to elicit maximal antigen-specific immune responses [99].
Several systems that provide pulsatile release of drugs and hormones have been investigated. These include delivery systems that respond to changes in pH [100], temperature [101], electric [102] and magnetic fields [103] or exposure to triggers such as ultrasound [104, 105], enzymes [106] or light [107]. More recently, promising approaches for pulsatile release of drugs have been developed using silicon-based microchips with wells that can provide release of single or multiple chemicals in response to an electrical stimulus [108-110]. We have developed a poly(dimethylsiloxane) (PDMS) chip with multiple reservoir wells that are covered with biodegradable seals that can provide pulsatile release of CpG ODN and antigens [31].
We selected PDMS as the reservoir component as it is a highly flexible and robust material that can be effectively molded. PLGA polymer films of varying composition and thickness were used as seals to the wells. The composition, molecular weight and thickness of the PLGA films were all parameters used to control the degradation rate of the seals. For example, thicker films degraded faster than thinner films. It was found that the film degradation rate can be used to fine-tune the release of CpG ODN over day length periods [31, 111].
Figure 14 shows the pulsatile release profile from a chip incorporating two doses of a model antigen OVA and CpG ODN. CpG ODN and OVA could be delivered in sequential pulses 4-6 days apart and the entire sequence repeated after 18 days providing the necessary booster dose to maintain high antibody titers [31]. The ability to provide repeated sequential release of CpG oligonucleotides and antigens, suggests significant potential for this device in vaccinations or applications that require defined complex release patterns.
Figure 14.
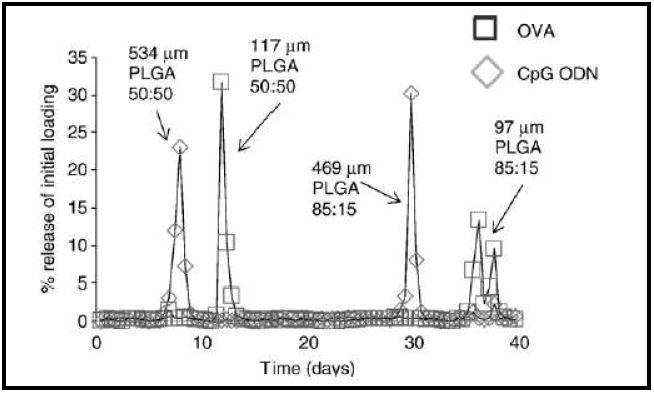
Graph showing pulsatile release of OVA and CpG ODN from PDMS microchips with hydrolytically degrading PLGA seals. Reproduced with permission from [31]. Copyright © Elsevier B.V.
4.6 Cell-microparticle Hybrids
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that functions as white blood cell growth factor [112]. GM-CSF has been shown to stimulate the production of white blood cells following chemotherapy. GM-CSF has a critical role in maturation and functioning of APCs like dendritic cells and converts Langerhans cells of the skin to immunostimulatory APCs [113, 114]. Co-delivery of the free form of CpG ODN and soluble GM-CSF as an aqueous solution triggers an enhanced production of antigen specific antibodies [115]. In comparison to soluble antigen, whole tumor cells serve as effective vaccine vehicles because they carry a complete complement of tumor cell antigens that can overcome resistant mutations and tumor cell variants [2]. Irradiated tumor cells engineered to secrete GM-CSF are safe and stimulate potent antitumor immunity in mice [116]. As described in the earlier sections, an approach that can deliver CpG ODN localized with the antigen in a non-solution type vector ensures both components are delivered to the same APC and this can significantly amplify the antigen-specific immune response [12].
We have recently developed a novel hybrid system that conjugates whole tumor cells with microparticles entrapping CpG ODN using the biotin-avidin binding mechanism (Figure 15) [117]. Native sialic acid residues on the cell surface are converted to non-native aldehydes using periodate treatment [118]. The aldehyde groups are then conjugated to biotin hydrazide via an amide linkage to produce biotin-functionalized cell surfaces. Particles prepared from polylactic acid-polyethylene glycol-biotin [119] or chitosan (unpublished data) are then assembled with the biotin presenting cells using avidin as a bridging molecule. Figure 16A shows a light microscopy image of multiple cell-microparticle hybrids cultured on a well plate and Figure 16B shows a scanning electron microscopy (SEM) image of a single hybrid [117]. In order to ensure that transfection of tumor cells to express GM-CSF does not inhibit their ability to form a hybrid, we carried out the assembly process using rhodamine-loaded microparticles with cells that had been previously transfected to express the model reporter green fluorescent protein (GFP). Figure 16C shows that prior transfection does not diminish the ability of cells to form a hybrid. Such a co-delivery approach to deliver irradiated tumor cells expressing GM-CSF with microparticles encapsulating CpG ODN in a localized manner to APCs would be a potential mechanism to augment anti-tumor activity and achieve a significant increase in the antigen-specific immune response. We are currently investigating this system for effective vaccination against solid tumors.
Figure 15.
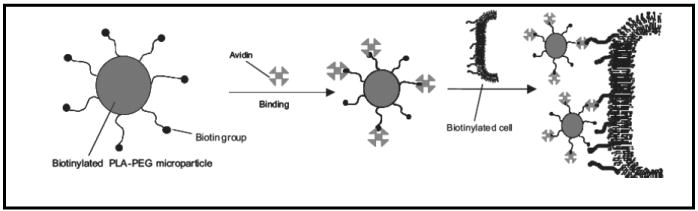
Schematic showing the avidin-biotin mediated self-assembly of hybrid cell-microparticle carrier systems for potential co-delivery of antigen and CpG ODN. Reproduced with permission from [117]. Copyright © Wiley-VCH Verlag CmbH & Co. KGaA.
Figure 16.
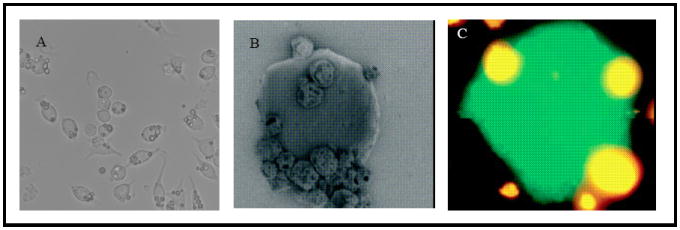
(A) Light microscopy image of multiple cell-microparticle hybrids cultured on a well plate, (B) SEM image of a cell-microparticle hybrid and (C) rhodamine-loaded microparticles immobilized on the surface of a cell expressing green fluorescent protein. Reproduced with permission from [117]. Copyright © Wiley-VCH Verlag CmbH & Co. KGaA.
5. Conclusions and future challenges
The strategies for co-delivering antigens and CpG ODN described in this review have the potential to improve the efficacy of CpG ODN as an adjuvant against a wide range of diseases. These include asthma [120], anthrax [77], neuroblastoma [2], lymphoma [121], and prostate cancer amongst others [122, 123]. Future approaches to enhancing the efficacy of CpG ODN include co-administering with agonists for different toll-like receptors and delivering CpG ODN as an adjuvant to other vaccines currently in development [124]. For example, CpG ODN has been shown to enhance the efficacy of adenovirus-based prostate cancer vaccines and vaccines based on irradiated neuroblastoma cells that express GM-CSF [2, 122, 123]. In these studies, delivery vehicles are being utilized that further improve the enhancements to the antigen-specific immune response generated by CpG ODN.
Approaches that ensure co-delivery of CpG ODN and antigen to the same APC result in stronger immunogenic responses including enhancement in speed and duration of immune response, modulation of the isotype of antigen-specific antibody response and increase in the immunogenicity of weak antigens. Delivery systems described in this review have demonstrated targeted delivery of CpG ODN to the intracellular compartments in APCs. The exact vehicle used for delivery of CpG ODN is dependent on the application and the route of administration. For example, multi-component metallic nanorods are uniquely suited to ballistic delivery [1, 46].
Future studies are expected to continue to develop delivery vehicles that are tailored to the application, dosing profile required and route of administration. Ongoing clinical studies have demonstrated safety and activity of CpG ODN in humans [125] but some of the areas that still need to be addressed are a more precise understanding of how different classes of ODN regulate the immunostimulatory cascade, better monitoring of the long-term safety of CpG ODN, identification of the optimal dose and duration of vaccine therapy relative to the type of vehicle being used and blocking or reducing immunosuppressive responses. Several recent studies have shown that the choice of material used for the delivery vehicle can also modulate the immune response. This is a potential parameter that could be used to further enhance the efficacy of the CpG ODN class of immunological adjuvants.
Acknowledgments
We gratefully acknowledge support from the American Cancer Society (IRG-77004-28), the National Cancer Institute at the National Institutes of Health (1R21CA133456-01), the Pharmaceutical Research and Manufacturers of America Foundation and partial support from Public Health Service grant number P50 CA09724-04 from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salem AK, Hung CF, Kim TW, Wu TC, Searson PC, Leong KW. Multi-component nanorods for vaccination applications. Nanotechnology. 2005;16(4):484–487. [Google Scholar]
- 2.Sandler AD, Chihara H, Kobayashi G, Zhu XY, Miller MA, Scott DL, Krieg AM. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Research. 2003;63(2):394–399. [PubMed] [Google Scholar]
- 3.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raychaudhuri S, Rock KL. Fully mobilizing host defense: Building better vaccines. Nature Biotechnology. 1998;16(11):1025–1031. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 5.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. Journal of Controlled Release. 2004;97(1):1–17. doi: 10.1016/j.jconrel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z, Leong KW. Controlled delivery of antigens and adjuvants in vaccine development. Journal of Pharmaceutical Sciences. 1996;85(12):1261–1270. doi: 10.1021/js9602812. [DOI] [PubMed] [Google Scholar]
- 7.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304(5673):1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 8.Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, Weiner GJ. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. Journal of Immunology. 2000;165(11):6244–6251. doi: 10.4049/jimmunol.165.11.6244. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nature Medicine. 2003;9(7):831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique Palindromic Sequences in Synthetic Oligonucleotides Are Required to Induce Inf and Augment Inf-Mediated Natural-Killer Activity. Journal of Immunology. 1992;148(12):4072–4076. [PubMed] [Google Scholar]
- 12.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunological Reviews. 2004;199(1):201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. Cpg Motifs in Bacterial-DNA Trigger Direct B-Cell Activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 14.Roman M, MartinOrozco E, Goodman S, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nature Medicine. 1997;3(8):849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 15.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. Journal of Immunology. 1996;157(5):2116–2122. [PubMed] [Google Scholar]
- 16.Sun SQ, Zhang XH, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CgG DNA. Journal of Experimental Medicine. 1998;188(12):2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowdery JS, Krieg AM, Hooker NA, Mildenstein KL, Chace JH. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Journal of Allergy and Clinical Immunology. 1997;99(1):1970–1970. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 18.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of interleukin-12 and tumor necrosis factor-alpha. Cellular Immunology. 1996;167(1):72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 19.Yi AK, Chace JH, Cowdery JS, Krieg AM. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. Journal of Immunology. 1996;156(2):558–564. [PubMed] [Google Scholar]
- 20.Sands H, Goreyferet LJ, Cocuzza AJ, Hobbs FW, Chidester D, Trainor GL. Biodistribution and Metabolism of Internally H-3 Labeled Oligonucleotides .1. Comparison of a Phosphodiester and a Phosphorothioate. Molecular Pharmacology. 1994;45(5):932–943. [PubMed] [Google Scholar]
- 21.Chu RS, Askew D, Harding CV. Immunobiology of Bacterial Cpg-DNA. 2000;247:199–210. doi: 10.1007/978-3-642-59672-8_14. [DOI] [PubMed] [Google Scholar]
- 22.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. Faseb Journal. 1998;12(4):A612–A612. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCluskie MJ, Davis HL. Cutting edge: CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. Journal of Immunology. 1998;161(9):4463–4466. [PubMed] [Google Scholar]
- 24.Oxenius A, Martinic MMA, Hengartner H, Klenerman P. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. Journal of Virology. 1999;73(5):4120–4126. doi: 10.1128/jvi.73.5.4120-4126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeberger A, Wagner C, Zemann A, Luhrs P, Kutil R, Goos M, Stingl G, Wagner SN. CpG motifs are efficient adjuvants for DNA cancer vaccines. Journal of Investigative Dermatology. 2004;123(2):371–379. doi: 10.1111/j.0022-202X.2004.23208.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. Journal of Immunotherapy. 2007;30(5):469–478. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 27.Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. Journal of Controlled Release. 2002;85(13):247–262. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XQ, Intra J, Salem AK. Conjugation of polyamidoamine dendrimers on biodegradable microparticles for nonviral gene delivery. Bioconjugate Chemistry. 2007;18:2068–2076. doi: 10.1021/bc070116l. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Briones M, Ott G, O’Hagan D. Cationic microparticles: A potent delivery system for DNA vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(2):811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M, Ott G, Kazzaz J, Ugozzoli M, Briones M, Donnelly J, O’Hagan DT. Cationic microparticles are an effective delivery system for immune stimulatory CpG DNA. Pharmaceutical Research. 2001;18(10):1476–1479. doi: 10.1023/a:1012269226066. [DOI] [PubMed] [Google Scholar]
- 31.Intra J, Glasgow JM, Mai HQ, Salem AK. Pulsatile release of biomolecules from polydimethylsiloxane (PDMS) chips with hydrolytically degradable seals. Journal of Controlled Release. 2008;127(3):280–287. doi: 10.1016/j.jconrel.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Krishnamachari Y, Pearce ME, Salem AK. Self-assembly of cell-microparticle hybrids. Advanced Materials. 2008;20(5):989–993. [Google Scholar]
- 33.Datta SK, Takabayashi K, Raz E. The therapeutic potential of antigen-oligonucleotide conjugates. Therapeutic Oligonucleotides. 2003;1002:105–111. doi: 10.1196/annals.1281.022. [DOI] [PubMed] [Google Scholar]
- 34.Cho HJ, Takabayashi K, Cheng PM, Nguyen MD, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nature Biotechnology. 2000;18(5):509–514. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 35.Datta SK, Takabayashi K, Raz E. Therapeutic Oligonucleotides. 2003:105–111. doi: 10.1196/annals.1281.022. [DOI] [PubMed] [Google Scholar]
- 36.Heit A, Schmitz F, O’Keeffe M, Staib C, Busch DH, Wagner H, Huster KM. Protective CD8 T cell immunity triggered by CpG-protein conjugates competes with the efficacy of live vaccines. Journal of Immunology. 2005;174(7):4373–4380. doi: 10.4049/jimmunol.174.7.4373. [DOI] [PubMed] [Google Scholar]
- 37.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. Journal of Immunology. 2004;172(3):1501–1507. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 38.Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S, Lipford GB, Vabulas RM, Wagner H. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. European Journal of Immunology. 2002;32(8):2356–2364. doi: 10.1002/1521-4141(200208)32:8<2356::AID-IMMU2356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XQ, Dahle CE, Weiner GJ, Salem AK. A comparative study of the antigen-specific immune response induced by co-delivery of CpG ODN and antigen using fusion molecules or biodegradable microparticles. Journal of Pharmaceutical Sciences. 2007;96(12):3283–3292. doi: 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- 40.Butler M, Stecker K, Bennett CF. Cellular distribution of phosphorothioate oligodeoxynucleotides in normal rodent tissues. Laboratory Investigation. 1997;77(4):379–388. [PubMed] [Google Scholar]
- 41.Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, Raz E. Conjugation of immunostimulatory DNA to the short ragweed allergen Amb a 1 enhances its immunogenicity and reduces its allergenicity. Journal of Allergy and Clinical Immunology. 2000;106(1):124–134. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- 42.Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. Journal of Immunology. 2000;164(11):5575–5582. doi: 10.4049/jimmunol.164.11.5575. [DOI] [PubMed] [Google Scholar]
- 43.Trimble C, Lin CT, Hung CF, Pai S, Juang J, He LM, Gillison M, Pardoll D, Wu L, Wu TC. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(2526):4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 44.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8(+)T cells after gene gun immunization. Journal of Experimental Medicine. 1998;188(6):1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CT, Trimble C, Hung CF, Pi S, Juang J, He LM, Gillison M, Pardoll D, Lai CH, Chang TC, Wu L, Wu TC. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Journal of Immunotherapy. 2003;26(6):S9–S9. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 46.Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nature Materials. 2003;2(10):668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- 47.Salem AK, Chen M, Hayden J, Leong KW, Searson PC. Directed assembly of multisegment Au/Pt/Au nanowires. Nano Letters. 2004;4(6):1163–1165. [Google Scholar]
- 48.Salem AK, Chao J, Leong KW, Searson PC. Receptor-mediated self-assembly of multi-component magnetic nanowires. Advanced Materials. 2004;16(3):268–271. [Google Scholar]
- 49.Badiee A, Davies N, McDonald K, Radford K, Michiue H, Hart D, Kato M. Enhanced delivery of immunoliposomes to human dendritic cells by targeting the multilectin receptor DEC-205. Vaccine. 2007;25(25):4757–4766. doi: 10.1016/j.vaccine.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 50.Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Advanced Drug Delivery Reviews. 2001;50(12):143–156. doi: 10.1016/s0169-409x(01)00154-5. [DOI] [PubMed] [Google Scholar]
- 51.Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier M-P. Effective induction of CD8+ T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine. 2003;21(23):3319–3329. doi: 10.1016/s0264-410x(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 52.Badiee A, Jaafari MR, Samiei A, Soroush D, Khamesipour A. Co-encapsulation of CpG ODN with rLmSTI1 in liposome enhances immune response and protection in immunized BALB/c mice against leishmaniasis. 2008:CVI.00413–00407. doi: 10.1128/CVI.00413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayer LD, Bally MB, Hope MJ, Cullis PR. Techniques for Encapsulating Bioactive Agents into Liposomes. Chemistry and Physics of Lipids. 1986;40(24):333–345. doi: 10.1016/0009-3084(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 54.Jiao XM, Wang RYH, Qiu Q, Alter HJ, Shih JWK. Enhanced hepatitis C virus NS3 specific Th1 immune responses induced by co-delivery of protein antigen and CpG with cationic liposomes. Journal of General Virology. 2004;85:1545–1553. doi: 10.1099/vir.0.79896-0. [DOI] [PubMed] [Google Scholar]
- 55.Whitmore MM, Li S, Falo L, Huang L. Systemic administration of LPD prepared with CpG oligonucleotides inhibits the growth of established pulmonary metastases by stimulating innate and acquired antitumor immune responses. Cancer Immunology Immunotherapy. 2001;50(10):503–514. doi: 10.1007/s002620100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta PN, Khatri K, Goyal AK, Mishra N, Was SP. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. Journal of Drug Targeting. 2007;15(10):701–713. doi: 10.1080/10611860701637982. [DOI] [PubMed] [Google Scholar]
- 57.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28(35):5344–5357. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Goforth R, Salem AK, Zhu X, Miles S, Zhang XQ, Lee J, Sandler AD. Immune Stimulatory Antigen Loaded Particles Combined with Depletion of Regulatory T-cells Induce Potent Tumor Specific Immunity in a Mouse Model of Melanoma. Cancer Immunology, Immunotherapy. 2008 doi: 10.1007/s00262-008-0574-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaganathan KS, Singh P, Prabakaran D, Mishra V, Vyas SP. Development of a single-dose stabilized poly(D,L-lactic-co-glycolic acid) microspheres-based vaccine against hepatitis B. Journal of Pharmacy and Pharmacology. 2004;56(10):1243–1250. doi: 10.1211/0022357044418. [DOI] [PubMed] [Google Scholar]
- 60.Singh M, Li XM, McGee JP, Zamb T, Koff W, Wang CY, Ohagan DT. Controlled release microparticles as a single dose hepatitis B vaccine: Evaluation of immunogenicity in mice. Vaccine. 1997;15(5):475–481. doi: 10.1016/s0264-410x(97)00225-9. [DOI] [PubMed] [Google Scholar]
- 61.Gupta RK, Chang AC, Siber GR. Modulation of the Immune Response to Vaccine Antigens. 1998;92:63–78. [Google Scholar]
- 62.Lassalle V, Ferreira ML. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromolecular Bioscience. 2007;7(6):767–783. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 63.Krishnamachari Y, Madan P, Lin SS. Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. International Journal of Pharmaceutics. 2007;338(12):238–247. doi: 10.1016/j.ijpharm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Kumar PS, Ramakrishna S, Saini TR, Diwan PV. Influence of microencapsulation method and peptide loading on formulation of poly(lactide-co-glycolide) insulin nanoparticles. Pharmazie. 2006;61(7):613–617. [PubMed] [Google Scholar]
- 65.Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. Journal of Pharmaceutical Sciences. 2008;97(7):2448–2461. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- 66.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. Journal of Controlled Release. 2008;125(3):193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Pean JM, Venier-Julienne MC, Boury F, Menei P, Denizot B, Benoit JP. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. Journal of Controlled Release. 1998;56(13):175–187. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 68.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. International Journal of Pharmaceutics. 2002;245(12):109–121. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 69.Román BS, Irache JM, Gómez S, Tsapis N, Gamazo C, Espuelas MS. Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70(1):98–108. doi: 10.1016/j.ejpb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharmaceutical Research. 2002;19(6):715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 71.Lavelle EC. Lectins and microparticles for enhanced oral vaccination. Methods. 2006;38(2):84–89. doi: 10.1016/j.ymeth.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Oster CG, Kim N, Grode L, Barbu-Tudoran L, Schaper AK, Kaufmann SHE, Kissel T. Cationic microparticles consisting of poly(lactide-co-glycolide) and polyethylenimine as carriers systems for parental DNA vaccination. Journal of Controlled Release. 2005;104(2):359–377. doi: 10.1016/j.jconrel.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Zhang XQ, Intra J, Salem AK. Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods. Journal of Microencapsulation. 2008;25(1):1–12. doi: 10.1080/02652040701659347. [DOI] [PubMed] [Google Scholar]
- 74.Rebelatto MC, Guimond P, Bowersock TL, HogenEsch H. Induction of systemic and mucosal immune response in cattle by intranasal administration of pig serum albumin in alginate microparticles. Veterinary Immunology and Immunopathology. 2001;83(12):93–105. doi: 10.1016/s0165-2427(01)00370-1. [DOI] [PubMed] [Google Scholar]
- 75.Tafaghodi M, Tabassi SAS, Jaafari MR. Induction of systemic and mucosal immune responses by intranasal administration of alginate microspheres encapsulated with tetanus toxoid and CpG-ODN. International Journal of Pharmaceutics. 2006;319(12):37–43. doi: 10.1016/j.ijpharm.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 76.Borges O, Cordeiro-da-Silva A, Tavares J, Santarem N, de Sousa A, Borchard G, Junginger HE. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69(2):405–416. doi: 10.1016/j.ejpb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 77.Xie H, Gursel I, Ivins BE, Singh M, O’Hagan DT, Ulmer JB, Klinman DM. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infection and Immunity. 2005;73(2):828–833. doi: 10.1128/IAI.73.2.828-833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh M, Ugozzoli M, Briones M, Kazzaz J, Soenawan E, O’Hagan DT. The effect of CTAB concentration in cationic PLG microparticles on DNA adsorption and in vivo performance. Pharmaceutical Research. 2003;20(2):247–251. doi: 10.1023/a:1022327305369. [DOI] [PubMed] [Google Scholar]
- 79.Wasan EK, Reimer DL, Bally MB. Plasmid DNA is protected against ultrasonic cavitation-induced damage when complexed to cationic liposomes. J Pharm Sci. 1996;85(4):427–433. doi: 10.1021/js9504752. [DOI] [PubMed] [Google Scholar]
- 80.Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Influence of formulation parameters on the characteristics of poly(D, L-lactide-co-glycolide) microspheres containing poly(L-lysine) complexed plasmid DNA. Journal of Controlled Release. 1999;60(23):279–286. doi: 10.1016/s0168-3659(99)00076-0. [DOI] [PubMed] [Google Scholar]
- 81.Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Preparation and characterization of poly (D,L-lactide-co-glycolide) microspheres for controlled release of poly(L-lysine) complexed plasmid DNA. Pharmaceutical Research. 1999;16(4):509–513. doi: 10.1023/a:1018862827426. [DOI] [PubMed] [Google Scholar]
- 82.Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Stability of poly(L-lysine)-complexed plasmid DNA during mechanical stress and DNase I treatment. Pharmaceutical Development and Technology. 1999;4(4):491–498. doi: 10.1081/pdt-100101386. [DOI] [PubMed] [Google Scholar]
- 83.Gebrekidan S, Woo BH, DeLuca PP. Formulation and in vitro transfection efficiency of poly (D, L-lactide-co-glycolide) microspheres containing plasmid DNA for gene delivery. AAPS PharmSciTech. 2000;1(4):E28. doi: 10.1208/pt010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang XQ, Intra J, Salem AK. Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods. Journal of Microencapsulation. 2008;25:1–12. doi: 10.1080/02652040701659347. [DOI] [PubMed] [Google Scholar]
- 85.Bivas-Benita M, Romeijn S, Junginger HE, Borchard G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58(1):1–6. doi: 10.1016/j.ejpb.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Rosa G, Bochot A, Quaglia F, Besnard M, Fattal E. A new delivery system for antisense therapy: PLGA microspheres encapsulating oligonucleotide/polyethyleneimine solid complexes. International Journal of Pharmaceutics. 2003;254(1):89–93. doi: 10.1016/s0378-5173(02)00689-0. [DOI] [PubMed] [Google Scholar]
- 87.dos Santos ALG, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, Schmaler J, Besnard M, Behar-Cohen F, Fattal E. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. Journal of Controlled Release. 2006;112(3):369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Kasturi SP, Qin H, Thomson KS, El-Bereir S, Cha SC, Neelapu S, Kwak LW, Roy K. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. Journal of Controlled Release. 2006;113(3):261–270. doi: 10.1016/j.jconrel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Kasturi SP, Sachaphibulkij K, Roy K. Covalent conjugation of polyethyleneimine on biodegradable microparticles for delivery of plasmid DNA vaccines. Biomaterials. 2005;26(32):6375–6385. doi: 10.1016/j.biomaterials.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 90.Manuel WS, Zheng J, Hornsby PJ. Transfection by polyethyleneimine-coated microspheres. Journal of Drug Targeting. 2001;9(1):15. doi: 10.3109/10611860108995629. [DOI] [PubMed] [Google Scholar]
- 91.Moffatt S, Cristiano RJ. Uptake characteristics of NGR-coupled stealth PEI/pDNA nanoparticles loaded with PLGA-PEG-PLGA tri-block copolymer for targeted delivery to human monocyte-derived dendritic cells. International Journal of Pharmaceutics. 2006;321(12):143–154. doi: 10.1016/j.ijpharm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Moffatt S, Cristiano RJ. PEGylated J591 mAb loaded in PLGA-PEG-PLGA tri-block copolymer for targeted delivery: In vitro evaluation in human prostate cancer cells. International Journal of Pharmaceutics. 2006;317(1):10–13. doi: 10.1016/j.ijpharm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Nam YS, Kang HS, Park JY, Park TG, Han SH, Chang IS. New micelle-like polymer aggregates made from PEI-PLGA diblock copolymers: micellar characteristics and cellular uptake. Biomaterials. 2003;24(12):2053–2059. doi: 10.1016/s0142-9612(02)00641-5. [DOI] [PubMed] [Google Scholar]
- 94.Sutton D, Durand R, Shuai XT, Gao JM. Poly(D, L-lactide-co-glycolide)/poly(ethylenimine) blend matrix system for pH sensitive drug delivery. Journal of Applied Polymer Science. 2006;100(1):89–96. [Google Scholar]
- 95.Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine (PEI)-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. Journal of Controlled Release. 2008 doi: 10.1016/j.jconrel.2008.1004.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ravi Kumar MN, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25(10):1771–1777. doi: 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 97.Vogelhuber W, Rotunno P, Magni E, Gazzaniga A, Spruss T, Bernhardt G, Buschauer A, Gopferich A. Programmable biodegradable implants. Journal of Controlled Release. 2001;73(1):75–88. doi: 10.1016/s0168-3659(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 98.Medlicott NJ, Tucker IG. Pulsatile release from subcutaneous implants. Advanced Drug Delivery Reviews. 1999;38(2):139–149. doi: 10.1016/s0169-409x(99)00013-7. [DOI] [PubMed] [Google Scholar]
- 99.Powell MF. Drug delivery issues in vaccine development. Pharmaceutical Research. 1996;13(12):1777–1785. doi: 10.1023/a:1016064504346. [DOI] [PubMed] [Google Scholar]
- 100.Siegel RA, Falamarzian M, Firestone BA, Moxley BC. Ph-Controlled Release from Hydrophobic Poly-Electrolyte Copolymer Hydrogels. Journal of Controlled Release. 1988;8(2):179–182. [Google Scholar]
- 101.Bae YH, Okano T, Hsu R, Kim SW. Thermosensitive Polymers as on-Off Switches for Drug Release. Makromolekulare Chemie-Rapid Communications. 1987;8(10):481–485. [Google Scholar]
- 102.Kwon IC, Bae YH, Kim SW. Electrically Erodible Polymer Gel for Controlled Release of Drugs. Nature. 1991;354(6351):291–293. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 103.Edelman ER, Kost J, Bobeck H, Langer R. Regulation of Drug Release from Polymer Matrices by Oscillating Magnetic-Fields. Journal of Biomedical Materials Research. 1985;19(1):67–83. doi: 10.1002/jbm.820190107. [DOI] [PubMed] [Google Scholar]
- 104.Kost J, Leong K, Langer R. Ultrasound-Enhanced Polymer Degradation and Release of Incorporated Substances - (Controlled Release Drug Delivery Systems) Proceedings of the National Academy of Sciences of the United States of America. 1989;86(20):7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim HJ, Matsuda H, Zhou HS, Honma I. Ultrasound-triggered smart drug release from a poly(dimethylsiloxane)-mesoporous silica composite. Advanced Materials. 2006;18(23):3083–3086. [Google Scholar]
- 106.Fischelghodsian F, Brown L, Mathiowitz E, Brandenburg D, Langer R. Enzymatically Controlled Drug Delivery. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(7):2403–2406. doi: 10.1073/pnas.85.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathiowitz E, Cohen MD. Polyamide Microcapsules for Controlled Release .5. Photochemical Release. Journal of Membrane Science. 1989;40(1):67–86. [Google Scholar]
- 108.Ito Y, Hasuda H, Morimatsu M, Takagi N, Hirai Y. A microfabrication method of a biodegradable polymer chip for a controlled release system. Journal of Biomaterials Science-Polymer Edition. 2005;16(8):949–955. doi: 10.1163/1568562054414621. [DOI] [PubMed] [Google Scholar]
- 109.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nature Materials. 2003;2(11):767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 110.Santini JT, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 111.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(,-lactide-co-glycolide) nano- and microparticles. Journal of Controlled Release. 2003;92(12):173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 112.Weiner GJ, Woodridge JE, Liu H, Dahle CE, Krieg AM. Immunostimulatory CpG oligodeoxynucleotide is effective as an adjuvant in inducing production of anti-idiotype antibody and is synergistic with GMCSF. Blood. 1996;88(10):348–348. [Google Scholar]
- 113.Benz R, Goede JS, Parlier V, Mühlematter D, Jotterand M, Fehr J. G-CSF-induced remission in two cases of acute myeloid leukemia. Leukemia Research. 2008;32(7):1148–1152. doi: 10.1016/j.leukres.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Ohno R. Granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor in the treatment of acute myeloid leukemia and acute lymphoblastic leukemia. Leukemia Research. 1998;22(12):1143–1154. doi: 10.1016/s0145-2126(98)00117-9. [DOI] [PubMed] [Google Scholar]
- 115.Kwissa M, Kroger A, Hauser H, Reimann J, Schirmbeck R. Cytokine-facilitated priming of CD8+T cell responses by DNA vaccination. Journal of Molecular Medicine-Jmm. 2003;81(2):91–101. doi: 10.1007/s00109-002-0395-6. [DOI] [PubMed] [Google Scholar]
- 116.Nair RE, Jong YS, Jones SA, Sharma A, Mathiowitz E, Egilmez NK. L-12+GM-CSF microsphere therapy induces eradication of advanced spontaneous tumors in her-2/neu transgenic mice but fails to achieve long-term cure due to the inability to maintain effector T-cell activity. Journal of Immunotherapy. 2006;29(1):10–20. doi: 10.1097/01.cji.0000175489.19314.d2. [DOI] [PubMed] [Google Scholar]
- 117.Krishnamachari Y, Pearce ME, Salem AK. Self-assembly of cell-microparticle hybrids. Advanced Materials. 2008;20(5):989–993. [Google Scholar]
- 118.Sinclair J, Salem AK. Rapid localized cell trapping on biodegradable polymers using cell surface derivatization and microfluidic networking. Biomaterials. 2006;27(9):2090–2094. doi: 10.1016/j.biomaterials.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 119.Salem AK, Cannizzaro SM, Davies MC, Tendler SJB, Roberts CJ, Williams PM, Shakesheff KM. Synthesis and characterisation of a degradable poly(lactic acid)-poly(ethylene glycol) copolymer with biotinylated end groups. Biomacromolecules. 2001;2(2):575–580. doi: 10.1021/bm010030+. [DOI] [PubMed] [Google Scholar]
- 120.Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, Krieg AM. Cutting edge: Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. Journal of Immunology. 1998;160(6):2555–2559. [PubMed] [Google Scholar]
- 121.Warren TL, Wittrock C, Weiner GJ. CpG DNA as monotherapy for lymphoma: Linking innate and adaptive immunity. Blood. 2002;100(11):203A–203A. [Google Scholar]
- 122.Lubaroff DM, Karan D, Andrews MP, Acosta A, Abouassaly C, Sharma M, Krieg AM. Decreased cytotoxic T cell activity generated by co-administration of PSA vaccine and CpG ODN is associated with increased tumor protection in a mouse model of prostate cancer. Vaccine. 2006;24(3536):6155–6162. doi: 10.1016/j.vaccine.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 123.Lubaroff DM, Konety BR, Link BK, Madsen TM, Shannon ME, Ecklund DJ, O’Donnell MA, Ratliff TL, Williams RD. Adenovirus PSA (Ad5-PSA) gene therapy for prostate cancer: A phase I clinical trial. Journal of Urology. 2003;169(4):395–395. [Google Scholar]
- 124.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunology. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, Laframboise C, Al Adhami MJ, Khaliq Y, Seguin I, Cameron DW. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004;22(2324):3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]