Abstract
Objective. To determine the first signs and symptoms, and the clinical, biological and radiological characteristics of patients with early SpA.
Methods. A total of 150 SpA patients were selected from 2367 listed in REGISPONSER (Registro Español de Espondiloartritis de la Sociedad Española de Reumatología). The inclusion criterion was a disease course of ⩽2 yrs from the onset of symptoms or the appearance of the first sign of disease.
Results. Forty-six patients had AS, 51 psoriatic SpA (Ps-SpA), 43 uSpA, 5 ReA, 4 IBD arthropathy and 1 JCA. The mean age at onset of symptoms and at diagnosis was higher in Ps-SpA group (48.1 ± 13.6 and 48.5 ± 13.6 yrs) than in AS group (38.1 ± 12.8 and 38.9 ± 12.7 yrs) and uSpA group (36.3 ± 11.5 and 36.9 ± 11.4 yrs). The most frequent signs or symptoms were back pain: 72% AS group and 56% uSpA group. Lower limb arthritis was the first symptom in 57% Ps-SpA patients, 35% uSpA patients and 20% AS patients; upper limb arthritis was the first symptom in 53% Ps-SpA group and <16% of the remainder. Compared with longer duration disease, at onset, AS patients report upper limb arthritis more frequently and uSpA patients report more of enthesitis. Early radiological sacroiliitis was observed in all AS patients, of whom 54% had Grade II, 39% had Grade III and 7% had Grade IV.
Conclusions. In our population, the first manifestations of SpA were low back pain and SI syndrome in AS and uSpA patients and peripheral arthritis in the Ps-SpA group. We can find early SI joint affectation in AS patients.
Keywords: SpAs, AS, uSpA, SpA's cohort, Epidemiology, Disease registry
Introduction
The inflammatory rheumatic diseases affecting the spine are included in the heterogeneous group of SpA. The group is formed by AS (the most common), psoriatic SpA (Ps-SpA), IBD arthropathy (IBDA), ReA, uSpA and JCA. They all share clinical manifestations (the most important being inflammatory back pain), radiological manifestations (sacroiliitis) and genetic traits (such as a strong association with HLA-B27) [1]. The global prevalence of these entities ranges from 0.23% to 1.8% [2].
Despite the remarkable increase in our knowledge of the epidemiological, clinical and therapeutic aspects of these diseases, we know very little about their onset pattern. In fact, the delay in diagnosis (6.5 ± 8.4 yrs in Spain) [3] in patients suffering from this condition means that it is difficult to determine how they progress between onset and the first clinical manifestations. Patient registries can provide information on recently diagnosed patients and enable us to avoid the memory bias that frequently burdens population studies. In April 2004, the Spanish Spondyloarthropathy Study Group of the Spanish Society of Rheumatology (GRESSER) began the National Registry of Spondyloarthritis [Registro Español de Espondiloartritis de la Sociedad Española de Reumatología (REGISPONSER)] [3]. Today, it has more than 2000 patients (150 of whom have <2 yrs of disease duration). Data are available on patient characteristics, clinical aspects, radiological presentation and therapeutic response. This registry has been the basis for describing the clinical, socio-demographic, analytical, radiological and metrological features of patients with SpA in Spain. We consider that the information provided by our cohort could add to our knowledge of the early stages of SpA.
The REGISPONSER-Early cohort was created and followed to the present day in an attempt to fill some of the gaps in our knowledge of this condition.
In this article, we will try to clarify about the beginning of these diseases and how the memory bias influences the knowledge of the onset.
Patients and methods
Patients
We selected 150 SpA patients from the 2367 listed in REGISPONSER (National Registry of Spondyloarthropathy: http://biobadaser.ser.es/cgi-bin/regisponser/index.html). The inclusion criteria for the register were: fulfilment of the classification criteria from the European Spondyloarthropathy Study Group (ESSG) [4], blood tests available within 15 days of the inclusion visit and a complete radiographic study within the previous year, and agreement to complete all self-administered questionnaires. This project was approved by the Committee of Ethical and Sanitary Investigation of the ‘Reina Sofía’ University Hospital according to the fundamental principles established in the Declaration of Helsinki, in the council of Europe Convention for Human Rights and every patient signed an informed patient consent at inclusion in REGISPONSER.
For this study, we selected patients with a disease course of ⩽2 yrs from the onset of symptoms or the appearance of the first sign attributable to the disease, including musculoskeletal signs/symptoms (inflammatory back pain as defined by Calin criteria [5], SI syndrome, dactylitis, enthesitis according to MASES—Maastricht Ankylosing Spondylitis Enthesitis Score assessment criteria [6], cervical pain, lower limb arthritis and upper limb arthritis) and the extra-articular-related pathology psoriasis. Forty-six (30.7%) of the 150 patients fulfilled the modified New York criteria for AS, 51 (34%) had Ps-SpA, 43 (28.7%) had uSpA, 5 (3.3%) had ReA, 4 (2.7%) had IBDA and 1 (0.7%) had JCA.
In order to streamline the statistical analysis, only three subgroups were formed, namely, those with the largest number of patients (AS, Ps-SpA and uSpA). These 140 SpA patients constitute the REGISPONSER-Early cohort and whose characteristics are provided in this article.
We also selected 1383 SpA patients from REGISPONSER who had >10 yrs of evolution from the onset of signs/symptoms attributable to the disease and with the three main diagnoses named before. Of the total 1383 patients, 1074 (77.7%) patients had AS, 187 (13.5%) had Ps-SpA and 122 (8.8%) had uSpA. This subgroup is called as ‘REGISPONSER-Late’ in this article.
Data collection
Data collection was given in detail in one of our previous paper [3].
Statistical analysis
A descriptive analysis was made of the epidemiological, clinical, radiographic and analytical variables according to the different diagnoses.
Comparisons of continuous variables between groups were made by analysis of variance, and the Tukey or Games–Howell tests were used for post hoc testing. Kruskal–Wallis test for ordinal variables and Mann–Whitney for post hoc test were used. Categorical variables were compared using the chi-square test. Differences with a P-value of <0.05 were considered to be statistically significant.
Results
The clinical characteristics of this cohort are presented in Table 1. We found a higher percentage of women in the uSpA group than in the AS and Ps-SpA groups (without statistical difference) and we observed a greater presence of HLA-B27 in the AS and uSpA groups (P < 0.001). The mean age at onset of symptoms and age at diagnosis were higher in the Ps-SpA than in the AS and uSpA groups (P < 0.001). Total REGISPOSER-Early sub-cohort media of age at onset compared with REGISPONSER-Late media was also higher (41.2 ± 13.6 vs 26.7 ± 10.5, P < 0.001). Ps-SpA first's signs/symptoms were mostly upper and lower limb arthritis (53 and 57%, respectively) and 82% presented psoriasis at the onset of SpA. The diagnosis delay in this series of patients was <1 yr in all groups.
Table 1.
Characteristics of patients, by specific diagnosis
| AS (n = 46) | Ps-SpA (n = 51) | uSpA (n = 43) | P | Total (n = 140) | |
|---|---|---|---|---|---|
| Men, n (%) | 33 (72) | 31 (61) | 25 (58) | 0.36 | 89 (64) |
| Age, yrs | 39.5 ± 12.7 | 49.3 ± 13.6* | 37.8 ± 11.4* | <0.001 | 42.6 ± 13.6 |
| Age at diagnosis, yrs | 38.9 ± 12.7 | 48.5 ± 13.6* | 36.9 ± 11.4** | <0.001 | 41.8 ± 13.6 |
| Age at onseta, yrs | 38.1 ± 12.8 | 48.1 ± 13.6* | 36.3 ± 11.5** | <0.001 | 41.2 ± 13.7 |
| Time of evolutiona, yrs | 1.5 ± 0.6 | 1.2 ± 0.7 | 1.5 ± 0.7 | 0.20 | 1.4 ± 0.7 |
| HLA-B27 positive, n (%) | 33/40 (82) | 8/35 (23)* | 25/38 (66)** | <0.001 | 66/113 (58) |
| Disease durationb, yrs | 0.7 ± 0.7 | 0.9 ± 0.7 | 0.9 ± 0.7 | 0.30 | 0.8 ± 0.7 |
| Diagnosis delay, yrs | 0.8 ± 0.6 | 0.4 ± 0.6* | 0.6 ± 0.7 | 0.005 | 0.6 ± 0.6 |
Results are expressed as a mean ± s.d. unless otherwise specified. Significance was obtained using the chi-square test for qualitative variables and analysis of variance for quantitative variables (Tukey as post hoc test). aFrom the first signs/symptoms attributable to the disease. bFrom the date of the diagnosis. *Significant differences (P < 0.05) vs AS. **Significant differences (P < 0.05) vs Ps-SpA.
First clinical manifestations
The first clinical manifestations attributable to the disease by each group and compared with REGISPONSER-Late cohort are presented in Table 2. The initial most common symptom was inflammatory back pain, both globally and by group; SI syndrome, defined as alternating pain that affects the buttocks, was the initial symptom in 46% of the AS patients and in 35% of the uSpA patients. Dactylitis was not observed at onset in patients with AS, was present in 10% of all Ps-SpA cases and in 7% of uSpA cases. Enthesitis was observed more frequently at the onset of uSpA (28%) than in the other groups (<13%; P = 0.046).
Table 2.
First signs/symptoms attributable to the disease
| AS |
Ps-SpA |
uSpA |
P |
|||||
|---|---|---|---|---|---|---|---|---|
| ≤2 yrs (n = 46) | >10 yrs (n = 1074) | ≤2 yrs (n = 51) | >10 yrs (n = 187) | ≤2 yrs (n = 43) | >10 yrs (n = 122) | ≤2 yrs | >10 yrs | |
| Low back pain | 33 (72) vs 769 (72) P = 0.98 | 13 (26)*vs 31 (17)*P = 0.15 | 24 (56)**vs 62 (51)*,**P = 0.57 | <0.001 | <0.001 | |||
| SI syndrome | 21 (46) vs 443 (41) P = 0.55 | 6 (12)*vs 17 (9)*P = 0.59 | 15 (35)**vs 35 (29)*,**P = 0.45 | 0.001 | <0.001 | |||
| Neck pain | 3 (6) vs 121 (11) P = 0.31 | 1 (2) vs 14 (7) P = 0.20 | 2 (5) vs 6 (5)*P = 1 | 0.54 | 0.038 | |||
| Dactylitis | 0 vs 12 (1) P = 1 | 5 (10) vs 17 (9)*P = 0.79 | 3 (7) vs 3 (3)**P = 0.18 | 0.10 | <0.001 | |||
| Arthritis, lower limbs | 9 (20) vs 176 (16) P = 0.57 | 29 (57)*vs 131 (70)*P = 0.08 | 15 (35)**vs 40 (33)*,**P = 0.80 | <0.001 | <0.001 | |||
| Arthritis, upper limbs | 7 (15) vs 37 (3) P < 0.001 | 27 (53)*vs 106 (57)*P = 0.63 | 7 (16)**vs 13 (11)*,**P = 0.33 | <0.001 | <0.001 | |||
| Enthesitis | 6 (13) vs 75 (7) P = 0.14 | 5 (10) vs 15 (8) P = 0.78 | 12 (28)**vs 18 (15)*P = 0.05 | 0.046 | 0.010 | |||
Dates are expressed as n (%). Significance obtained by the chi-square test for contingency tables. *Significant differences (P < 0.05) vs AS within each group of time of evolution. **Significant differences (P < 0.05) vs Ps-SpA within each group of time of evolution. Comparison of REGISPONSER-Early vs ‘REGISPONSER-Late’.
Arthritis was the first symptom in 67% of the patients, and was more frequent in the lower limbs (38%) than in the upper limbs (29%). The rating by group reveals that the frequency was significantly higher in Ps-SpA patients than in uSpA and AS patients (P < 0.001).
Comparing these first manifestations with REGISPONSER-Late we can see that patients with early AS report upper limb arthritis more frequently (15% vs 3%, P < 0.001) and those with early uSpA present with more of enthesitis (28% vs 15%, P = 0.05); other comparisons did not reach statistically significant differences.
Clinical features at inclusion
Clinical manifestations at inclusion in the registry, metrological data and radiological characteristics are presented in Table 3. Enthesitis affected 26% of the uSpA and 24% of the AS patients with a lower frequency in the other SpA. Uveitis was present in 9% of the AS patients and 7% of the uSpA patients. Peripheral joint was more frequent in the Ps-SpA group (51%) than in the other groups (21% uSpA and 20% AS; P < 0.001).
Table 3.
Characteristics of patients on inclusion, by specific diagnosis
| AS (n = 46) | Ps-SpA (n = 51) | uSpA (n = 43) | P | Total (n = 140) | |
|---|---|---|---|---|---|
| Peripheral arthritis, n (%) | 9 (20) | 26 (51)* | 9 (21)** | <0.001 | 44 (31) |
| Enthesitis, n (%) | 11 (24) | 7 (14) | 11 (26) | 0.49 | 29 (21) |
| Uveitis, n (%) | 4 (9) | 1 (2) | 3 (7) | 0.33 | 8 (6) |
| Clinic form (axial-mixed), n(%) | 44 (96) | 15 (29)* | 31 (72)*,** | <0.001 | 90 (64) |
| BASFI | 2.8 ± 2.6 | 2.8 ± 2.5 | 2.4 ± 2.3 | 0.74 | 2.7 ± 2.4 |
| Chest expansion, cm | 4.9 ± 2.6 | 4.0 ± 1.5 | 5.1 ± 2.0 | 0.06 | 4.7 ± 2.2 |
| Schober modified, cm | 4.2 ± 2.6 | 4.6 ± 1.8 | 5.8 ± 3.3* | 0.016 | 4.9 ± 2.7 |
| Occiput-to-wall distance, cm | 1.7 ± 3.5 | 1.0 ± 2.4 | 0.6 ± 1.8 | 0.16 | 1.1 ± 2.7 |
| Finger-to-floor distance, cm | 13.6 ± 12 | 12.8 ± 12.5 | 8.2 ± 9.0* | 0.06 | 11.6 ± 11.4 |
| Cervical rotation 20°–70°, n (%) | 10 (22) | 9 (18) | 3 (7) | 0.13 | 22 (16) |
Results are expressed as the mean ± s.d., unless otherwise specified. Significance obtained by the chi-square test for qualitative variables and analysis of variance for quantitative variables (Games–Howell as post hoc test). *Significant differences (P < 0.05) vs AS. **Significant differences (P < 0.05) vs Ps-SpA.
Chest expansion was lower in Ps-SpA patients than in AS and uSpA patients (P = 0.06). Lumbar flexibility, as measured by the Schober's test, was preserved in all groups, with the highest values in the uSpA group (P = 0.016). The occiput-to-wall distance was higher in the AS group than in the Ps-SpA and uSpA groups (P = 0.16). The finger-to-floor distance was greater in the AS group (slightly greater than that of the Ps-SpA group and somewhat greater than that of the uSpA group; P = 0.06). Cervical rotation was between 20° and 70° in 22% of the AS patients, 18% of the Ps-SpA patients and 7% of the uSpA patients (P = 0.13). No patients had a rotation of <20°.
Disease activity
The analysis of disease activity parameters at inclusion is presented in Table 4 (no statistical difference was found in this analysis). We found that nocturnal pain measured by the visual analogue scale (VAS) was greater in patients with AS than in those with uSpA and Ps-SpA; activity levels valued by the patient with the VAS were slightly higher in the Ps-SpA group. The physician's global assessment measured by VAS was almost similar in the three main groups, and somewhat lower in the group with less frequent SpA. The ESR and levels of CRP were slightly higher in the Ps-SpA group than in the AS and uSpA groups. As for the functional status of physical and mental components measured according to the SF-12 questionnaire [7], the averages of the three main groups showed no major differences; a lower score (which means a better functional status) was observed in the less frequent SpA group.
Table 4.
Parameters defining the disease activity of patients, by specific diagnosis
| AS (n = 46) | Ps-SpA (n = 51) | uSpA (n = 43) | P | Total (n = 140) | |
|---|---|---|---|---|---|
| Nocturnal back pain, VAS 0–10 cm | 3.8 ± 3.0 | 3.1 ± 3.3 | 2.9 ± 2.3 | 0.31 | 3.2 ± 2.9 |
| Patient global assessment, VAS 0–10 cm | 4.3 ± 2.6 | 4.8 ± 2.7 | 4.2 ± 2.8 | 0.49 | 4.4 ± 2.7 |
| Physician global assessment, VAS 0–10 cm | 3.2 ± 2.3 | 3.4 ± 2.4 | 3.3 ± 2.1 | 0.92 | 3.3 ± 2.2 |
| ESR, mm/h | 16.2 ± 14.2 | 19.6 ± 18.4 | 17.4 ± 15.1 | 0.59 | 17.8 ± 16 |
| CRP, mg/l normal <5 mg/l | 6.1 ± 11.1 | 12.1 ± 19.2 | 8.0 ± 11.1 | 0.13 | 8.8 ± 14 |
| BASDAI | 3.8 ± 2.4 | 4.6 ± 2.3 | 3.9 ± 2.6 | 0.32 | 4.1 ± 2.4 |
| Physical component SF-12 | 34.2 ± 15.2 | 36.4 ± 14.4 | 32.1 ± 15.9 | 0.39 | 34.4 ± 15 |
| Mental component SF-12 | 43.4 ± 17.7 | 45.1 ± 16.3 | 42.0 ± 19.4 | 0.70 | 43.6 ± 18 |
Results are expressed as a mean ± s.d. Significance was obtained by analysis of variance.
Radiographic scores
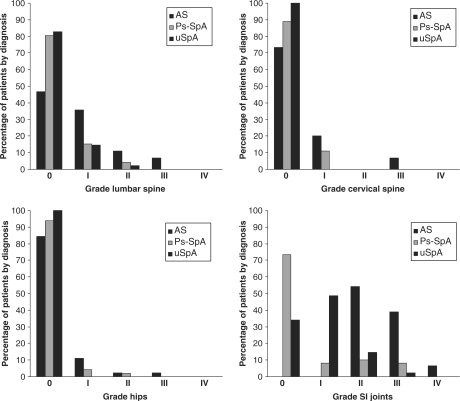
As expected, sacroiliitis was found in all patients with AS, of whom 54% had Grade II, 39% had Grade III and 7% had Grade IV sacroiliitis. In Ps-SpA group, 74% of the patients had no radiological sacroiliitis, 8% had Grade I, 10% Grade II and 8% had Grade III sacroiliitis; and no Grade IV was found. In uSpA group, 83% had Grade 0–I sacroiliitis (34 and 49%, respectively), 15% had Grade II and 2% had Grade III sacroiliitis (corresponding to one patient who did not fulfil the New York clinical criteria for AS). Low radiographic scores by Bath Ankylosing Spondylitis Radiological Index (BASRI) were found in general (Table 5, Fig. 1), but AS had always higher punctuation (P < 0.05) except on assessment of the hips vs PS-SpA (P > 0.05) except on assessment of the hip vs ps-SpA (P < 0.05).
Table 5.
Radiographic scores, by specific diagnosis
| AS (n = 46) | Ps-SpA (n = 51) | uSpA (n = 43) | P | |
|---|---|---|---|---|
| Sacroiliitis grades II–IV, n (%) | 46 (100) | 9 (18)* | 7 (17)* | <0.001 |
| Lumbar spine | 1 (0, 3) | 0 (0, 2)* | 0 (0, 1)* | <0.001 |
| Cervical spine | 1 (0, 3) | 0 (0, 1)* | 0 (0, 0)*,** | 0.001 |
| SI joints | 2 (2, 4) | 0 (0, 3)* | 1 (0, 3)*,** | <0.001 |
| BASRI-s | 3 (0, 10) | 0 (0, 4)* | 1 (0, 3)* | <0.001 |
| Hips | 0 (0, 3) | 0 (0, 2) | 0 (0, 0)* | 0.024 |
| BASRI-t | 3 (0, 10) | 0 (0, 6)* | 1 (0, 3)* | <0.001 |
Results are expressed as the median (minimum, maximum), unless otherwise specified. Significance obtained by the chi-square test for qualitative variables and Kruskal–Wallis test for ordinal variables (Mann–Whitney as post hoc test). *Significant differences (P < 0.05) vs AS. **Significant differences (P < 0.05) vs Ps-SpA.
Fig. 1.
Radiographic scores.
Comparing the AS and uSpA groups
As the tables show, there are differences in demographic and clinical characteristics, metrological data and activity indicators between these two groups of patients, although these were not statistically significant in most cases.
The metrological data in Table 3 show that, in AS patients, the mean result of the Schober's test is lower than that of uSpA patients, with a statistically significant difference (P = 0.014). The AS group has an increased finger-to-floor distance (P = 0.02), which is indicative of lower lumbar spine flexibility.
The functional degree measured by the Bath Ankylosing Spondylitis Functional Index (BASFI) was slightly higher in patients with AS, but did not reveal statistically significant differences regarding uSpA (P = 0.51). The degree of bone damage according to the BASRI for the spine (BASRI-s) and in total (BASRI-t) was significantly higher in patients with AS (P < 0.001, in both the cases), also when analysed by each component.
Discussion
Different registries developed over the last few years have proven useful for describing the epidemiological aspects, clinical patterns, response to therapy, degree of impairment in quality of life and socioeconomic impact associated with inflammatory rheumatic diseases. Similarly, a dynamic registry such as REGISPONSER is composed of a cohort with a significantly large enough number of patients to enable us to determine the progress of these diseases from onset. REGISPONSER-Early and REGISPONSER were created to provide more specific information on the different sub-cohorts of patients with SpA.
A surprising result is the average age at onset of symptoms in all early cohorts and by diagnoses, which is almost 15 yrs higher than the mean for REGISPONSER-Late sub-cohort (41.2 ± 13.7 vs 26.7 ± 12.5, respectively). A breakdown by diagnoses reveals that Ps-SpA starts later than other SpA, with almost 12 yrs’ difference (similar to REGISPONSER-Late cohort, Ps-SpA 36.2 ± 13.4, AS 25.1 ± 9.0, uSpA 26.4 ± 10.2). Although we may consider that patients could present with vague symptoms, unclear or poorly thought spondylitis many years before, here with this early assessment of the beginning of the disease and diagnosis we could assume that we are avoiding or minimizing the memory bias and that patients could remember their first symptoms better. Taking a look at other AS cohorts like GESPIC from Germany and OASIS from the Netherlands, we can see that the mean age at disease onset is higher the sooner it is assessed; however, disease onset is not clearly defined and it is often not specified in descriptive published data and we can only count with disease duration data (not specifying if it is since symptom onset or diagnosis), and recent efforts to redefine this last concept [8, 9] will help us to know if this appreciation is valid. This fact, coupled with the higher rate of SI damage over such a short period (54% of the patients with AS show Grade II sacroiliitis, 37% Grade III and 7% Grade IV) could indicate that these patients presented a short pre-radiographic pauci-symptomatic stage during which their disease was damaging the joints and bone. This contrasts with lengthy pre-radiographic phase of symptoms reported by Mau et al. [10] that radiological sacroiliitis became evident after at least 9 ± 6 yrs and radiological signs of spinal involvement after 11 ± 6 yrs mean disease duration. This confirms the difficulty to identify the beginning and maybe it is not yet possible to determine the evolution time of these diseases when it is diagnosed.
In our series, we saw that 31% of the patients met the modified New York criteria for AS [11] during the 2 yrs after the first sign or symptom attributable to the disease. Van der Heijde and co-workers [12] showed that 20% of their series of 68 patients with inflammatory spinal pain with a duration of ⩽2 yrs [Early Spondyloarthritis Clinic Cohort (ESPAC)] met the modified New York criteria. Although cohorts are not comparable, this tells us that a significant percentage of patients with SpA can be diagnosed almost at the beginning of this condition with the help of registry data and diagnostic criteria [13, 14].
Another possible explanation is that SpA that starts later has a more rapid and aggressive course, although this contrasts with previous data showing that late-onset SpA has a less aggressive or similar course [15, 16]. However, we must remember that errors in data entry can occur when working with a registry (e.g. with the onset date) and that this can affect results.
Diagnosis delay in this cohort was <1 yr both globally and in groups, much less than the 8–11 yrs reported by Feldtkeller et al. [17] in a cohort of patients with AS. In Spain, diagnosis delay has been observed to be 6.5 (± 8.4) yrs [3]. This fact highlights the importance of early diagnosis and treatment of these diseases and the effects of a delay in implementing the new treatments (physical deterioration, lower quality of life, and increased social and health care spending), as demonstrated by studies published since as early as 2004 [18, 19]. Early treatment with new biological therapies has been shown to give better results than applying them in the advanced stages [20]. Therefore, diagnostic algorithms are now being developed for patients with inflammatory spinal pain to diagnose SpA during its earliest stages [21, 22]. MRI has been proposed as a diagnostic imaging tool [20] to provide early data on inflammation involving bone; however, this test has not yet been included as a diagnostic criterion, despite its widespread use in clinical practice.
Regarding the onset symptoms in this cohort, we found that back pain was the most frequent symptom in the group as a whole. In terms of diagnostic group, arthritis of the lower limbs was more prevalent in the Ps-SpA group and the second most frequent (after back pain) among uSpA patients. Arthritis of the upper limbs was more prevalent at onset in Ps-SpA patients than in the remaining SpA patients. Onset with enthesitis was more common in uSpA patients. Dactylitis, although very rare as the first manifestation, is more frequent in Ps-SpA and uSpA patients, and we did not observe that the first manifestation of AS was dactylitis. We found some differences in our sub-cohort (REGISPONSER-Early) with respect to REGISPONSER-Late: the most relevant differences between these cohorts were related to upper limb peripheral arthritis (AS of REGISPONSER-Late patients was 3% vs 15% in REGISPONSER-Early) and enthesitis (uSpA of REGISPONSER-Late patients was 15% vs 28% in REGISPONSER-Early). The data suggest that patients with early diagnosis refer or may remember other signs/symptoms that those with longer disease do not refer/remember.
With regard to clinical expression in this cohort, we found an increased rate of peripheral joint involvement in Ps-SpA patients, and an increased rate of enthesitis in AS and uSpA patients. Although metrological measures showed a flexibility change, all groups are within the normal range and the results of the Schober's test do not appear to be altered during the first 2 yrs.
Radiological scores show that AS is the most affected SpA globally and uSpA affects SI joint more than Ps-SpA, though this last affects cervical spine more than uSpA. When the AS and uSpA groups are compared, the former presented more bone damage according to the BASRI and less flexibility according to the various metrological data. In our cohort, we observed a greater proportion of uSpA (28.6%) over the total of patients included in the registry during the first phase [3]. However, as we found no published data about cohorts with the same characteristics, we cannot make comparisons. This finding is logical, given that, by definition, uSpA corresponds to a group of diseases that meet the criteria for SpA, but not the diagnostic criteria for any defined entity. These patients may develop subsequent clinical or radiological events enabling them to be differentiated, but they can also remain in an undifferentiated state for many years.
At present, several cohorts are being formed, such as that of Heuft-Dorenbosch et al. [12], with patients suffering from inflammatory spinal pain ESPAC cohort [11] and cohorts of patients with early AS, such as the German cohort GESPIC [23]. Our study highlights the need to define the initial stages of SpA, and to carry out a close follow-up of these cohorts to expand our knowledge of the course and prognosis of this condition, and to determine the response to treatment.

Acknowledgements
M.R.-V., E.M.-G. and E.C.-E. drafted the manuscript; A.E., P.Z., R.A., J.G., J.M., X.J., C.M., E.M. and REGISPONSER working group were involved in the patient recruitment of population; P.F., E.M.-G., E.C. and M.R.-V. were involved in study design; P.F. and E.M.-G. performed the statistical analysis. All authors read the manuscript and approved the final version.
Funding: This work is partially financed by a Grant of the Fundación Española de Reumatología.
Disclosure statement: The authors have declared no conflicts of interest.
Appendix
REGISPONSER working group
Antonio Juan Mas1, Pilar Fernández Dapica2, Maria Cruz Fernández-Espartero3, Virginia Villaverde3, Maria Elia Brito Brito4, Juan Carlos Torre Alonso5, Enrique Batlle Gualda6, Eduardo Cuende Quintana7, Teresa Clavaguera Poch8, Manuel Fernández Prada9 and Enrique Judez Navarro10
1Hospital Fundación Son Llatzer, Mallorca, 2Hospital Doce de Octubre, 3Hospital Móstoles, 4Hospital Universitario Ramón y Cajal, Madrid, 5Hospital Monte Naranco, Oviedo, 6Hospital General Universitario, Alicante, 7H.U. Príncipe de Asturias, Madrid, 8Hospital de Palmaos, Girona, 9H.U. de Guadalajara and 10H. Virgen del Perpetuo Socorro, Albacete, Spain.
References
- 1.Khan MA. Update on spondyloarthropathies. Ann Intern Med. 2002;136:896–907. doi: 10.7326/0003-4819-136-12-200206180-00011. [DOI] [PubMed] [Google Scholar]
- 2.Akkoc N, Khan MA. Weisman MH, van der Heijde D, Reveille JD, editors. Epidemiology of ankylosing spondylitis and related spondyloarthropathies. In: Ankylosing spondylitis and the spondyloarthropathies. Philadelphia: Mosby. 2006:117–31. [Google Scholar]
- 3.Collantes E, Zarco P, Muñoz E, et al. Disease pattern of spondyloarthropathies in Spain: description of the first registry (REGISPONSER) Rheumatology. 2007;46:1309–15. doi: 10.1093/rheumatology/kem084. [DOI] [PubMed] [Google Scholar]
- 4.Dougados M, van der Linden S, Juhlin R, et al. The European Spondyloarthropathy Study Group preliminary criteria for the classification of spondyloartropathy. Arthritis Rheum. 1991;34:1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 5.Calin A, Porta J, Fries JF, Schurman DJ. Clinical history as a screening test for ankylosing spondylitis. J Am Med Assoc. 1977;237:2613–4. [PubMed] [Google Scholar]
- 6.Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62:127–32. doi: 10.1136/ard.62.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haywood KL, Garratt AM, Dziedzic K, Dawes PT. Generic measures of health-related quality of life in ankylosing spondylitis: reliability, validity and responsiveness. Rheumatology. 2002;41:1380–7. doi: 10.1093/rheumatology/41.12.1380. [DOI] [PubMed] [Google Scholar]
- 8.Feldtkeller E, Erlendsson J. Definition of disease duration in ankylosing spondylitis. Rheumatol Int. 2008;28:693–6. doi: 10.1007/s00296-007-0499-y. [DOI] [PubMed] [Google Scholar]
- 9.Davis JC, Dougados M, Braun J, Sieper J, van der Heijde D, van der Linden S. Definition of disease duration in ankylosing spondylitis: reassessing the concept. Ann Rheum Dis. 2006;65:1518–20. doi: 10.1136/ard.2005.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mau W, Zeidler H, Mau R, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol. 1988;15:1109–14. [PubMed] [Google Scholar]
- 11.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 12.Heuft-Dorenbosch L, Landewé R, Weijers R, et al. Performance of various criteria sets in patients with inflammatory back pain of short duration; the Maastricht Early Spondyloarthritis Clinic. Ann Rheum Dis. 2007;66:92–8. doi: 10.1136/ard.2006.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collantes E, Cisnal A, Muñoz E, et al. Validación de criterios diagnósticos y de clasificación de las espondiloartropatías: Estudio Multicéntrico en España. Rev Esp Reumatol. 1994;21:426–9. [Google Scholar]
- 14.Amor B, Dougados M, Listrat V, et al. Evaluation des criteres des spondylarthropathies d’Amor et de l’European Spondylarthropathy Study Group (ESSG) Ann Med Interne. 1991;142:85–9. [PubMed] [Google Scholar]
- 15.Calin A, Elswood J. The natural history of juvenile onset ankylosing spondylitis: a 24-year retrospective case-control study. Br J Rheumatol. 1988;27:91–3. doi: 10.1093/rheumatology/27.2.91. [DOI] [PubMed] [Google Scholar]
- 16.Calin A, Elswood J, Edmunds L. Late onset ankylosing spondylitis – a distinct disorder? Br J Rheumatol. 1990;30:69–70. doi: 10.1093/rheumatology/30.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–6. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- 18.Boonen A. A review of work-participation, cost-of-illness and cost-effectiveness studies in ankylosing spondylitis. Nat Clin Pract Rheumatol. 2006;2:546–53. doi: 10.1038/ncprheum0297. [DOI] [PubMed] [Google Scholar]
- 19.Boonen A, van den Heuvel R, van Tubergen A, et al. Large differences in cost of illness and wellbeing between patients with fibromyalgia, chronic low back pain, or ankylosing spondylitis. Ann Rheum Dis. 2005;64:396–402. doi: 10.1136/ard.2003.019711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudwaleit M, Listing J, Brandt J, Braun J, Sieper J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis. 2004;63:665–70. doi: 10.1136/ard.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52:1000–8. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 22.Heuft-Dorenbosch L, Landewé R, Weijers R, et al. Combining information obtained from MRI and conventional radiographs in order to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann Rheum Dis. 2005;64:1703–9. doi: 10.1136/ard.2005.044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraliakos X, Listing J, Rudwaleit M, Brandt J, Sieper J, Braun J. Radiographic progression in patients with ankylosing spondylitis after 2 years of treatment with the tumour necrosis factor alpha antibody infliximab. Ann Rheum Dis. 2005;64:1462–6. doi: 10.1136/ard.2004.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]