Abstract
Background
Neuropathic pain is associated with significant co-morbidity, including anxiety and depression, which impact considerably on the overall patient experience. However, pain co-morbidity symptoms are rarely assessed in animal models of neuropathic pain. To improve the clinical validity of a widely used rodent model of traumatic peripheral neuropathy, we have investigated fear-avoidance- and depression-related behaviours in nerve-injured and sham-operated mice over a 4 week period.
Methods
Male C57BL/6J mice were subjected to partial sciatic nerve ligation (PSNL) or sham surgery and were assessed on days 7, 14, and 28 after operation. Withdrawal thresholds to punctate mechanical and cooling stimuli were measured. Mice were tested on the novel open-field and elevated plus-maze tests for fear-avoidance behaviour, and on the tail suspension test for depression-related behaviour.
Results
Hypersensitivity to punctate mechanical and cool stimuli was evident up to day 28 after PSNL. However, there was no change in fear-avoidance- or depression-related behaviours regardless of interval after-surgery.
Conclusion
These data demonstrate that pain behaviour in nerve-injured C57BL/6J mice was not associated with alterations in emotion-related behaviours.
Keywords: mouse; pain, chronic; pain, neuropathic; pain, psychological variables; research, animal
There is a significant co-morbidity between neuropathic pain and various neuropsychiatric disorders, including anxiety and depression.1-5 The high prevalence of these disorders and associated clinical burden among patients with chronic pain is well recognized, although the evidence for common pathophysiological mechanisms is only now beginning to emerge.4 6 A recent study found symptoms of depression and anxiety in the majority of patients suffering from lesions of a peripheral nerve or nerve root,2 with one study finding a prevalence rate for depression approaching 100% among chronic pain patients.7 In turn, there is evidence for the deleterious impact of affective illness on both the diagnosis and prognosis of neuropathic pain.4 8 9
Rodent models have proved valuable in identifying pathophysiological mechanisms and therapeutic targets for emotional10 and pain disorders.11 12 However, relatively few studies have investigated the potential effects of neuropathic pain on measures of fear-avoidance- and depression-related behaviours in rodents. Kontinen and colleagues13 found that spinal nerve ligation-induced neuropathic pain did not alter fear-avoidance behaviours in the open-field and elevated plus-maze (EPM) tests in rats tested 2 weeks after injury. In contrast, a recent study in mice showed that persistent inflammatory pain induced by intraplantar injection of complete Freund's adjuvant and nerve injury caused by partial sciatic nerve ligation (PSNL) led to increased fear-avoidance behaviours on the light–dark exploration test and EPM 4 weeks later.14 Similarly, we have recently observed increased fear-avoidance behaviour in rat models of traumatic, viral, and cytokine-induced neuropathic pain as assessed in the open-field test.15 16
To further clarify the relationship between pain and emotion, the present study used an established murine model of neuropathic pain to investigate the effects of pain on fear-avoidance- and depression-related behaviours. C57BL/6J mice underwent unilateral PSNL and were then tested on the novel open-field, EPM, and tail suspension tests (TSTs) at intervals of 7, 14, or 28 days after surgery. We hypothesized that traumatic peripheral neuropathy would be associated with increased fear-avoidance and depression-related behaviours, and that these effects would be more pronounced as the chronicity of pain increased.
Methods
Animals and surgical procedure
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) with mean weight of 25.6 g (range 20.8–30.2 g) were randomly allocated to one of two experimental groups: PSNL (n=36) and sham procedure (n=36). Nerve-injured and sham animals were examined behaviourally at one of three time points: day 7, 14, and 28 after operation (n=12 per group, i.e. separate groups of animals were used at each time point). Surgery was performed under general anaesthesia with isoflurane 1.0–2.0% and nitrous oxide 60% in oxygen. Briefly, the left (ipsilateral) sciatic nerve was exposed just above its trifurcation and one-third–half of the nerve trunk tightly ligated using 7.0 non-absorbable silk suture (Mersilk, Ethicon).11 The wound was closed in layers (4-0 Mersilk, Ethicon), and animals were allowed to recover. In sham animals, the sciatic nerve was exposed, but not ligated. Mice were housed four per cage in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 06.00 hours). Experimental procedures were performed in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the local Animal Care and Use Committee.
Stimulus-evoked reflex withdrawal tests
Before surgery, the baseline threshold value for hind paw withdrawal in response to punctate mechanical stimulation was measured in conscious animals with graded von Frey filaments (Stoelting Co., Wood Dale, IL, USA). Threshold response was defined by the filament that caused active paw withdrawal at least three times in every five applications.17 Baseline measurements were also taken for hind paw withdrawal responses to an acetone drop applied to the plantar surface of each hind paw.18 The response was scored 0–3 based on the following scale: 0=no response; 1=brief flick of hind limb; 2=hind limb raised for >1 s; and 3=biting and licking of hind paw. There were 12 animals per group (as described in Methods). For each animal, 2 readings were taken per paw. This gave a total of 24 readings per paw. To allow comparisons within groups and among groups, the mean of 24 readings was calculated (i.e. 2 per animal). The standard error of this mean was then derived. Animals were re-tested on days 6, 13, and 27 after operation for responses to von Frey filaments and acetone to establish the development of neuropathy (i.e. development of hypersensitivity to mechanical and cool stimulation). Animals that did not demonstrate significant (P<0.05, paired t-test) reduction in sensory thresholds compared with baseline values were excluded from the study. Reflex withdrawal testing was performed in the morning under controlled environmental conditions and was consistent across all groups tested.
Novel open-field
The novel open-field test was conducted on the morning after postoperative stimulus-evoked reflex withdrawal testing.19 The apparatus was a square arena (39×39×35 cm) with opaque white Plexiglas walls and floor, which was evenly illuminated to ~20 lux. The mouse was placed in a corner and allowed to explore freely for 15 min. Total distance travelled, time spent in the (20×20 cm) centre, and entries into the centre were measured using the Ethovision videotracking system (Noldus Information Technology, Leesburg, VA, USA).
Elevated plus-maze
The EPM test was conducted 3 h after the open-field test.19 The apparatus consisted of two open arms (30×5 cm; 55 lux) and two closed arms (30×5×15 cm; 5 lux), extending from a 5×5 cm central area and elevated 49.5 cm from the ground (San Diego Instruments, San Diego, CA, USA). The walls were made from black Acrylonitrile Butadiene Styrene (ABS) plastic and the floor from white ABS plastic. A 0.5 cm raised lip around the perimeter of the open arms prevented mice from falling off the maze. The mouse was placed in the centre facing an open arm and allowed to explore the apparatus for 5 min. Time spent and entries into the open and closed arms were measured using the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA, USA).
Tail suspension test
The TST19 20 was conducted last, on the morning after testing in the EPM. The rationale for this being that it may be a more stressful test. The mouse was securely fastened by the end of the tail to a flat surface that was suspended in a visually isolated white Plexiglas box (40 cm3) and manually observed for the presence or absence of immobility (cessation of limb movements) every 5 s during the last 4 min of a 6 min session.
Statistical analysis
Statistical analysis was performed using Sigmastat (Jandel Scientific Software version 2.0). A three-way analysis of variance (anova), followed where appropriate by post-hoc tests, was used to compare ipsilateral or contralateral paw withdrawal response thresholds at baseline with those measured after operation, and for ipsilateral compared with contralateral paws at each time point. For statistical comparisons in the integrated behavioural paradigms, a two-way anova followed by post-hoc tests was used for all inter- and intra-groups. Significance level was taken at P<0.05. Variance is expressed as standard error of the mean (sem).
Results
Behavioural reflex sensitivity
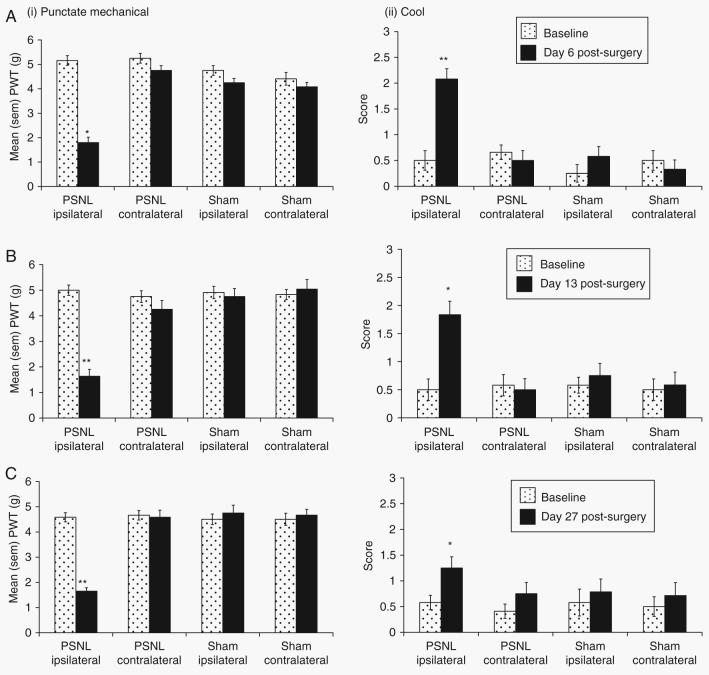
There was a significant interaction between surgery (PSNL vs sham), foot side (contralateral vs ipsilateral), and time (baseline vs postoperative day) on reflex withdrawal to punctate mechanical stimulation on each postoperative day (F1,282=14.68, P<0.01). Post-hoc tests demonstrated that PSNL animals had significantly lower pain thresholds on the ipsilateral side on days 6 (P<0.05), 13, (P<0.01), and 27 (P<0.01) compared with sham-operated animals (Fig. 1). There were no effects of surgery or time on the contralateral side. Three-way anova also found an interaction between surgery, foot side, and time on withdrawal to a cooling stimulus that was significant for day 13 (F1,22=5.89, P<0.05) and approached statistical significance on day 6 (F1,22=4.09, P=0.0556). On day 27, there was a main effect of surgery on withdrawal to the cool stimulus (F1,22=5.43, P<0.05). Post-hoc analyses showed that there was a greater response to the cool stimulus on the ipsilateral side in PSNL animals on days 6 (P<0.01), 13 (P<0.05), and 27 (P<0.05) compared with sham-operated animals.
Fig 1.
Behavioural reflex sensitivity. Development of hypersensitivity to (i) punctate mechanical and (ii) cool stimuli in the ipsilateral hind paw of partial sciatic nerve ligated (PSNL) (n=12 per group) vs sham-operated (n=12 per group) mice on days (a) 6; (b) 13; and (c) 27 post-surgery. Statistical analysis was performed using the three-way anova followed by post-hoc tests where appropriate. *P<0.05 or **P<0.01 for ipsilateral response threshold compared with sham ipsilateral values. At all time points, PSNL injured animals developed significant sensitivity to both tests compared with sham-operated animals.
Novel open-field
There was a main effect of surgery (PSNL vs sham) on percentage time spent in the central area of the open-field (F1,66=6.63, P<0.05). However, on post-hoc analysis, there were no significant differences for any of the specific postoperative days (Fig. 2a). There were main effects of both surgery (F1,66=4.054, P<0.05) and postoperative day (F2,66=4.278, P<0.05), but no interaction on frequency of entries into the centre of the open-field. Both PSNL-injured and sham-operated mice entered the central area significantly (P<0.05) more often on day 14 than on either day 7 or day 28 after operation (Fig. 2b). There was a main effect of postoperative day, but not surgery, for total distance travelled in the open-field (F2,66=13.036, P<0.01). Post-hoc analyses revealed that greater distance was travelled on day 14 compared with days 7 and 28 (P<0.05), regardless of surgical condition (Fig. 2c).
Fig 2.
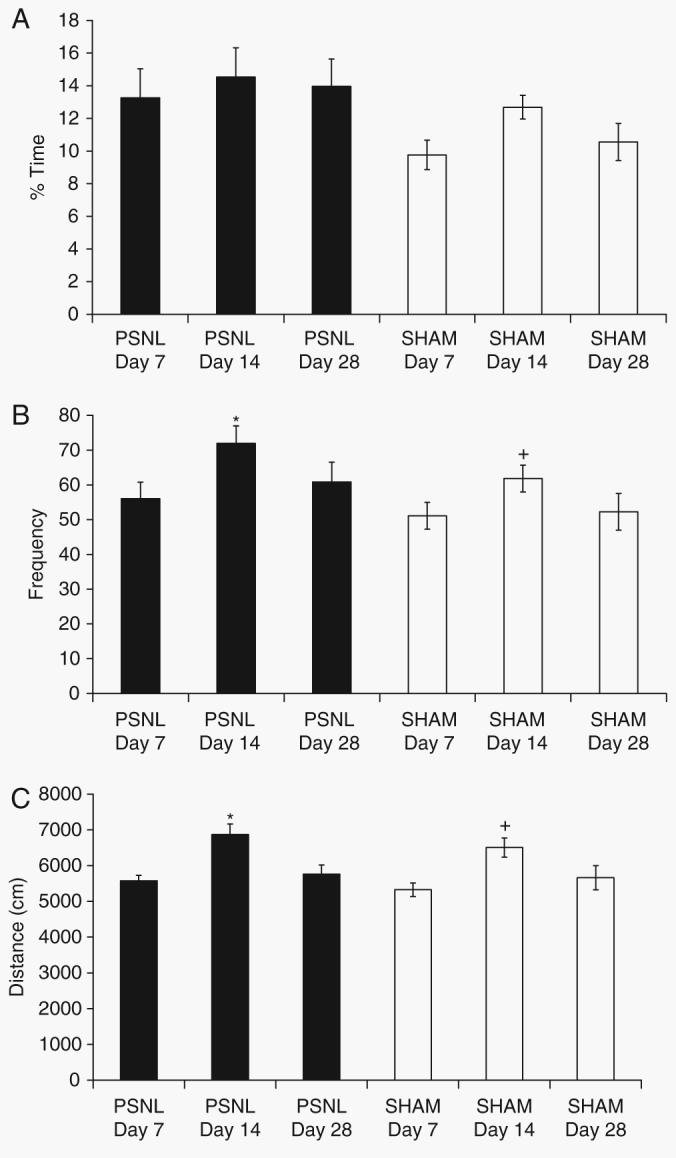
Open-field test. Outcome measures during a 15 min testing session were (a) percentage time spent in the inner zone (10×10 cm); (b) frequency of entry into inner zone; and (c) total distance travelled in the whole arena (40×40 cm). Data are shown for PSNL and sham-operated mice on days 7, 14, and 28 post-surgery. Statistical analysis was performed using the two-way anova followed by post-hoc tests where appropriate. *P<0.05 compared with PSNL-operated animals on days 7 and 28 post-surgery; + P<0.05 compared with sham-operated animals on days 7 and 28 post-surgery.
Elevated plus-maze
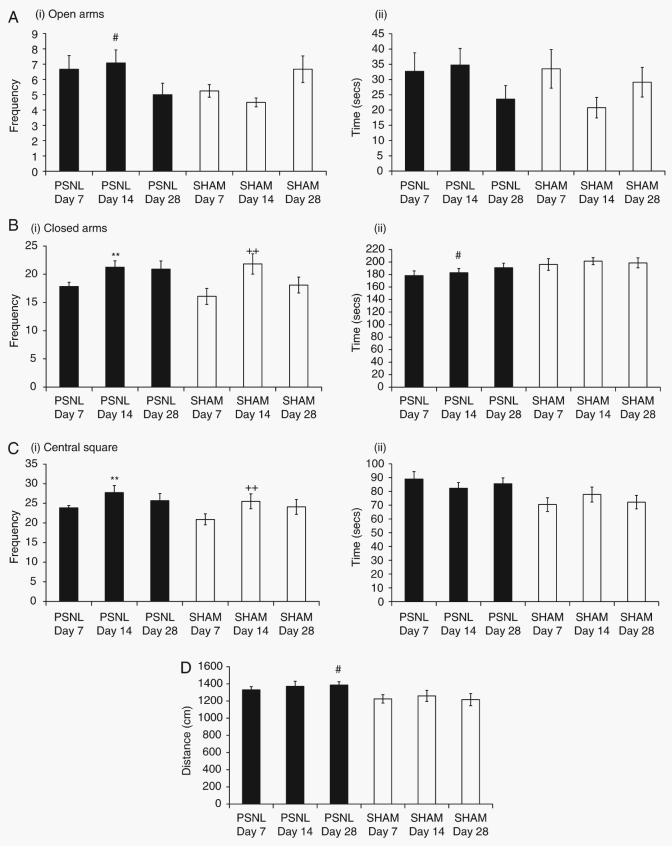
There was a significant interaction between surgery (PSNL vs sham) and postoperative day of testing for frequency of open arm entries (F2,66=4.663, P<0.01). Post-hoc analysis revealed that PSNL animals made significantly (P<0.05) more entries into the open arms than did sham-operated animals on day 14 after operation, but not on the other days (Fig. 3a), However, there were no main effects or interactions on the time spent in the open arms. There was a significant effect of postoperative day, but not surgery, on closed arm entries (F2,66=5.68, P<0.05), with significantly more entries into the closed arms on day 14 than day 7 regardless of surgical condition (Fig. 3b). In addition, there was a main effect of surgery on the time spent in the closed arms (F1,66=5.774, P<0.05). Post-hoc analysis revealed that on day 14 after operation, PSNL-operated animals spent significantly (P<0.05) less time in the closed arms than sham-operated animals (Fig. 3b). There was a main effect of postoperative day on frequency of entries into the centre square (F2,66=3.356, P<0.05), and post-hoc analysis revealed that mice= made significantly (P<0.05) more entries here on day 14 compared with day 7 regardless of surgery (Fig. 3c). There was a main effect of surgery on the time spent in the centre square (F1,66=9.315, P<0.01). Post-hoc analysis revealed that PSNL mice generally spent more time in the centre square than sham-operated mice (Fig. 3c).
Fig 3.
Elevated plus-maze test. Outcome measures during a 5 min testing session were: (a) (i) frequency of entry into the open arms and (ii) time spent in the open arms; (b) (i) frequency of entry into the closed arms and (ii) time spent in the closed arms; (c) (i) frequency of entry into the central square and (ii) time spent in the central square; and (d) total distance travelled in the maze. Statistical analysis was performed using the two-way anova followed by post-hoc tests where appropriate. #P<0.05 compared with sham; **P<0.05 compared with day 7 PSNL; and ++ P<0.05 compared with day 7 sham.
Finally, there was a main effect of surgery, but not postoperative day, on the total distance travelled throughout the EPM (F1,66=8.455, P<0.01). Post-hoc tests showed that on postoperative day 28, PSNL-operated mice generally travelled significantly (P<0.05) further than sham-operated animals (Fig. 3d).
Tail suspension test
There was a main effect of postoperative day, but not surgery, for per cent immobility in the TST (F2,54=4.016, P<0.05). Post-hoc analyses revealed that mice demonstrated significantly (P<0.05) less immobility on day 29 than on day 15 after operation (Fig. 4).
Fig 4.
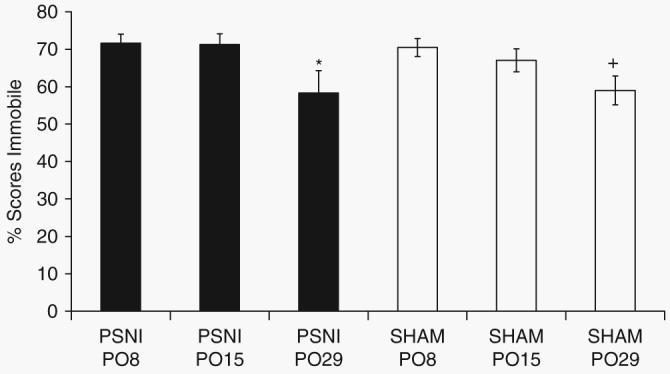
Tail suspension test. Immobility scores were measured on days 8, 15, and 29 post-surgery. No difference in immobility between PSNL and sham-operated animals was observed at any time point. There was significantly (P<0.05) less immobility on day 29 than at earlier time points post-surgery. Statistical analysis was performed using the two-way anova followed by post-hoc tests where appropriate. *P<0.05 compared with PSNL day 15; + P<0.05 compared with sham day 15.
Discussion
In humans, neuropathic pain is associated with significant co-morbidity, including anxiety and depression, which impact considerably on the overall patient experience and quality of life.1 2 To date, reports of investigations of the presence of such co-morbid behaviour in rodent models of neuropathic pain are limited and somewhat conflicting.21 Therefore, we have used three well-characterized behavioural paradigms to determine whether traumatic peripheral nerve injury-induced neuropathic pain behaviour in male C57BL/6J mice is associated with increased fear-avoidance- and depression-related behaviour
Firstly, we chose to utilize two well-validated measures of fear-avoidance behaviour: the novel open-field test and the EPM. Both tests are based upon conflict between an innate aversion to exposed, well-lit spaces and a tendency to explore novel environments.10 As these tests may therefore detect subtle differences in the behaviour, we felt it necessary to investigate behavioural responses in both paradigms. Results showed that although hypersensitivity to punctate mechanical and cool stimulation was sustained for 28 days after partial sciatic nerve injury, there was no evidence of increased fear-avoidance behaviour after PSNL in either test. In fact, mice that demonstrated an increased sensitivity in stimulus-evoked hind limb reflex tests after PSNL tended to demonstrate evidence of reduced fear-avoidance behaviour, in that they showed more approach behaviour into potentially aversive environments. Specifically, PSNL-injured animals spent a greater amount of time in the centre of the open-field arena compared with sham-operated mice. No clear differences were seen on measures of fear-avoidance behaviour in the EPM. Thus, these findings therefore do not support our hypothesis that traumatic peripheral neuropathy is associated with increased fear-avoidance behaviour, and that this effect is more pronounced as the chronicity of pain is increased.
Secondly, we used a pharmacologically validated test for depression-related behaviour; the TST. The TST23 24 is frequently used to assess depression-related behaviour, validated by its sensitivity to clinically effective antidepressants that cause mice to actively and persistently engage in escape-directed behaviours compared with non-treated controls.10 Depression may be a major symptom associated with chronic neuropathic pain.2 22 However, there was no evidence of depression-related behavioural abnormalities in nerve-injured animals in this test. Again, these findings fail to support our original hypothesis that mice displaying significant pain behaviour will also show signs of co-morbidity depression-related behaviours. Taken together, these data may suggest that conventional murine models of pain do not fully recapitulate the range of symptoms displayed by humans suffering from neuropathic pain, at least to the extent emotional symptoms can be measured in mice.
In contrast to our findings, a recent study in C57BL/6J mice showed increased fear-avoidance behaviours on the light–dark exploration test and EPM 4 weeks after intraplantar injection of complete Freund's adjuvant or PSNL.14 The reason for these discrepancies is not clear, but may reflect differences in experimental protocols and paradigm design (e.g. it is not clear whether separate animals were used at each of the four time points examined or whether the same animals were re-tested at different time points, which is likely to reduce validity of behavioural testing).25
Another potentially salient factor in the study of fear-avoidance and pain in mice is the presence of marked strain differences. Data from strain comparison studies in tests of fear-avoidance demonstrate a significant strain effect on baseline activity,26 and a similar strain difference exists for various tests of nociceptive sensitivity.27 For example, the 129S1 strain tends to be the most sensitive to von Frey hair stimulation at baseline and develops the greatest magnitude of sensitivity after peripheral nerve injury. However, on threshold withdrawal from a heat stimulus (Hargreave's test), 129S1 mice are less sensitive than C57BL/6J mice. Interestingly, after peripheral nerve injury, 129S1 mice develop a significantly greater hypersensitivity compared with C57BL/6J mice.27 In contrast, in an alternative test of ongoing pain, the formalin test, 129S1 mice show reduced pain behaviour compared with C57BL/6J mice.27 This suggests that the strain used must be taken into consideration when modelling human conditions, including pain and fear-avoidance behaviour. This raises the possibility that genetic factors influence the effects of neuropathic pain on measures of fear-avoidance- and depression-related behaviour, and it would be of interest to compare effects of our pain model across different strains.
Another factor to consider is potential species differences between rats and mice. A number of rat models of pain have been shown to be associated with increased fear-avoidance behaviour in the open-field paradigm.15 16 Therefore, when assessing the suitability of integrated behavioural paradigms for the detection of pain behaviour, we must consider the suitability of the tests with regard to species as it appears that there is not only a strain-specific, but also a species-specific effect on fear-avoidance behaviour. Our findings suggest that mice, though widely used in paradigms of fear-avoidance- and depression-related behaviour, may not be a suitable species for the investigation of neuropathic pain co-morbidity. A better species for this purpose may be rats15 16 in which pharmacologically sensitive fear-avoidance behaviour, characterized by increased thigmotaxsis (‘wall-hugging’) and reduced entry into the centre of an open-field arena, is correlated with mechanical hypersensitivity.
In conclusion, we have shown that in contrast to previous findings,14 C57BL/6J mice displaying nerve injury-induced pain-like behaviour, as determined by standard hind paw withdrawal reflex tests, do not display increased fear-avoidance or depression-related behaviour for up to 4 weeks after injury. In contrast, neuropathic mice tended towards less fear-avoidance or depressed-related state in all paradigms assessed. This behaviour may be a correlation of altered endogenous systems known to be associated with fear-avoidance and stress states such as the opioid or serotonergic systems. However, the involvement of such mechanisms in the behaviour we have tested is yet to be determined and merits further investigation. This suggests, however, that these tests could be utilized to assess the involvement of fear-avoidance mechanisms in the endogenous control of pain and co-morbid behaviour.
Acknowledgements
This work was supported by The Wellcome Trust (London Pain Consortium) and the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program.
References
- 1.Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Rosberg K, Kvarnstrom A, Kinnman E, Gordh T, Nordfors LO, Kristofferson A. Peripheral neuropathic pain—a multidimensional burden for patients. Eur J Pain. 2001;5:379–89. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5:9–27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- 4.Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7:403–12. doi: 10.1007/s11940-005-0032-0. [DOI] [PubMed] [Google Scholar]
- 5.Micó JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–54. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich-Lai YM, Xie W, Meij JT, Dolgas CM, Yu L, Herman JP. Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav. 2006;88:67–76. doi: 10.1016/j.physbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18–34. [PubMed] [Google Scholar]
- 8.Verma S, Gallagher RM. The psychopharmacologic treatment of depression and anxiety in the context of chronic pain. Curr Pain Headache Rep. 2002;6:30–9. doi: 10.1007/s11916-002-0021-x. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher RM, Verma S. Managing pain and comorbid depression: a public health challenge. Semin Clin Neuropsychiatry. 1999;4:203–20. doi: 10.153/SCNP00400203. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Holmes A. Model organisms: the ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 11.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–18. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 13.Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–6. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- 14.Narita M, Kaneko C, Miyoshi K, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–50. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 15.Hasnie FS, Parker S, Breuer J, Rice ASC. The influence of analgesic therapy and varicella zoster concentration on mechanical hypersensitivity in a rat model of zoster-associated pain; IASP 11th World Congress on Pain Abstracts; 2005. p. P37. [Google Scholar]
- 16.Wallace VC, McMahon SB, Rice ASC. The characterisation of rodent models of HIV-gp120 and antiretroviral-associated painful peripheral neuropathy; IASP 11th World Congress on Pain Abstracts; 2005. p. P44. [Google Scholar]
- 17.Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–33. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlton SM, Lekan HA, Kim SH, Chung JM. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain. 1994;56:155–66. doi: 10.1016/0304-3959(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–14. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 20.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 21.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–5. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Worz R. Pain in depression – depression in pain. Pain Clin Updates: IASP. 2003;11:1–2. [Google Scholar]
- 23.Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–92. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 24.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459:221–30. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- 26.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Mogil JS, Wilson SG, Bon K, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]