Abstract
A distal symmetrical sensory peripheral neuropathy is frequently observed in people living with Human Immunodeficiency Virus Type 1 (HIV-1). This neuropathy can be associated with viral infection alone, probably involving a role for the envelope glycoprotein gp120; or a drug-induced toxic neuropathy associated with the use of nucleoside analogue reverse transcriptase inhibitors as a component of highly active anti-retroviral therapy. In order to elucidate the mechanisms underlying drug-induced neuropathy in the context of HIV infection, we have characterized pathological events in the peripheral and central nervous system following systemic treatment with the anti-retroviral agent, ddC (Zalcitabine) with or without the concomitant delivery of HIV-gp120 to the rat sciatic nerve (gp120+ddC). Systemic ddC treatment alone is associated with a persistent mechanical hypersensitivity (33% decrease in limb withdrawal threshold) that when combined with perineural HIV-gp120 is exacerbated (48% decrease in threshold) and both treatments result in thigmotactic (anxiety-like) behaviour. Immunohistochemical studies revealed little ddC-associated alteration in DRG phenotype, as compared with known changes following perineural HIV-gp120. However, the chemokine CCL2 is significantly expressed in the DRG of rats treated with perineural HIV-gp120 and/or ddC and there is a reduction in intraepidermal nerve fibre density, comparable to that seen in herpes zoster infection. Moreover, a spinal gliosis is apparent at times of peak behavioural sensitivity that is exacerbated in gp120+ddC as compared to either treatment alone. Treatment with the microglial inhibitor, minocycline, is associated with delayed onset of hypersensitivity to mechanical stimuli in the gp120+ddC model and reversal of some measures of thigmotaxis. Finally, the hypersensitivity to mechanical stimuli was sensitive to systemic treatment with gabapentin, morphine and the cannabinoid WIN 55,212-2, but not with amitriptyline. These data suggests that both neuropathic pain models display many features of HIV- and anti-retroviral-related peripheral neuropathy. They therefore merit further investigation for the elucidation of underlying mechanisms and may prove useful for preclinical assessment of drugs for the treatment of HIV-related peripheral neuropathic pain.
Keywords: HIV, anti-retroviral drugs, neuropathy, DRG, microglia
Introduction
Distal symmetrical polyneuropathy (DSP) afflicts 15-50% of people living with HIV (Verma et al., 2005; Marshall et al., 2005), 50-60% of whom have measurable sensory abnormalities (Martin et al., 2003) and on-going, paroxysmal or stimulus evoked pain (Dalakas and Pezeshkpour, 1988; Martin et al., 1999). There are two predominant (and clinically similar) settings in which painful HIV-DSP may occur. First, a disease-related DSP associated with HIV-infection per se; or secondly a drug-induced DSP associated with the use of nucleoside reverse transcriptase inhibitors (NRTI), particularly the dideoxynucleosides; zalcitabine (ddC), didanosine (ddI) and stavudine (d4T), as part of highly active anti-retroviral therapy (HAART) (Verma et al., 2005). The use of HAART has markedly increased patient survival (Beck et al., 1999) making, painful DSP an important source of morbidity in otherwise reasonably healthy HIV-infected individuals (Berger et al., 1993; Wulff et al., 2000; Dalakas et al., 2001). This limits viral suppression strategies and may precipitate abbreviation or discontinuation of anti-retroviral therapy. Therefore, it is important that analgesic strategies are developed to combat this pain. The exact pathogenesis of HIV-associated neuropathies is unclear. Features of peripheral neuropathy are apparent on examination of post-mortem peripheral nerve tissue taken from AIDS patients (Jones et al., 2005). Therefore, it is likely that HIV infection is commonly associated with axonal damage and that the clinical presentation of small fibre neuropathy is largely due to the effects of the virus. However, direct infection of the nervous system by HIV is thought to be unlikely (Michaels et al., 1988; Lipton, 1998; Jones et al., 2005). Instead, HIV-1 appears to interact with the nervous system via binding of the external envelope protein, gp120, to the chemokine receptors CXCR4 and/or CCR5 (depending on viral strain) expressed on neurons and glial cells (Pardo et al., 2001). Such interactions can result in neurotoxocity (Apostolski et al., 1993; Lavi et al., 1997; He et al., 1997; Oh et al., 2001; Keswani et al., 2003b) and peripheral axonal damage (Melli et al., 2006). In addition to gp120-modulated immunopathogenic factors (de la Monte et al., 1988; Rizzuto et al., 1995; Pardo et al., 2001; Verma et al., 2005) such neurotoxic mechanisms likely lead to the presentation of a prominently small-diameter axonal loss, associated with a ‘dying back’ pattern of axonal degeneration (for review see (Pardo et al., 2001). In contrast, certain anti-retroviral drugs may be neurotoxic via mechanisms involving mitochondrial dysfunction and toxicity (Kakuda, 2000; Dalakas et al., 2001; Keswani et al., 2003a; Joseph et al., 2004; Fiala et al., 2004). The decision to commence anti-retroviral therapy is often based on a high viral load/low CD4 count (and/or an ‘AIDS defining’ illness which is also a manifestation of a high viral load) (Gazzard et al., 2006). Consequently, at the early stage of anti-retroviral therapy (when the peripheral neuropathy usually manifests itself) there will be a period in which contemporaneous exposure to HIV-gp120 and anti-retroviral drug of choice (>1 dideoxynucleoside drug may be used although the routine use of ddC is no longer recommended in current HIV-treatment guidelines). Therefore, it is likely that although mechanistically distinct, the combination of HIV-associated pathology and neurotoxic effects of anti-retroviral drugs is synergistic for the development of painful neuropathies (Keswani et al., 2003a; White et al., 2005). Previous studies have indicated rats to be susceptible to NRTI-induced neuropathy (Schmued et al., 1996) associated with behavioural indices of neuropathic pain (Joseph et al., 2004; Bhangoo et al., 2007). Gp120 is known to induce neuropathic changes in primary sensory neurons in vitro (Oh et al., 2001; Keswani et al., 2003a; Melli et al., 2006) and recently we characterized a rodent model in which perineural administration of HIV-gp120 leads to the development of pain-like behaviour in rats as well as neuropathic changes in axons and sensory neurones (Wallace et al., 2007). However, no studies have been performed to assess the combined effects of HIV-gp120 and NRTI’s on pain behaviour and neuropathology in rats. Therefore, in an effort to further model the predominant current clinical scenario, we have, for the first time, investigated the effect of NRTI treatment on the interaction of HIV-gp120 with the peripheral nerve in the rat. To do this, we have assessed behavioural correlates of neuropathic pain and co-morbidity behaviour in rats treated concomitantly with perineural HIV-gp120 and systemic ddC as compared to either intervention alone. We have also assessed sensory neuronal phenotype, activity of non-neuronal, particularly immune, cells in the PNS and CNS and the effects on intraepidermal nerve fibre density. Furthermore, to draw parallels between these interventions and data from human studies, we have pharmacologically validated the associated behavioural sensitivity with drugs known to possess analgesic efficacy in other rodent models of neuropathic pain and from randomized controlled trials of neuropathic pain conducted in humans, including minocycline, amitriptyline, gabapentin, morphine and the cannabinoid agonist WIN-55212-2 (Hempenstall et al., 2005; Finnerup et al., 2005).
Materials and Methods
Animals and surgical methods
All experiments conformed to the British Home Office Regulations and IASP guidelines (Zimmermann, 1983). Male Wistar rats (200-250 g) (B & K, Hull, UK) were housed in a temperature-controlled environment, maintained on a 14:10 h light-dark cycle (experiments conducted in light phase) and provided with feed and water ad libitum. For rats receiving ddC treatment, 0.5 ml of ddC (Roche, Basel, Switzerland; 5, 25 or 50 mg/kg in saline) was injected i.p. three times per week for 3 weeks (Mon, Wed, Fri). This was previously established as the optimal drug delivery regime as compared to a number of regimens including; a single injection (which we found to be ineffective in producing hypersensitivity) (data not shown). For perineural HIV-gp120 administration, the technique previously described (Wallace et al., 2007) was used. Briefly, under 1-2% isoflurane anaesthesia (Abbott, UK) in O2 and N20, and aseptic surgical conditions, the left sciatic nerve was exposed in the popliteal fossa without damaging the perineurium and wrapped loosely, with a 3 × 0.5 cm2 strip of oxidized regenerated cellulose (Surgicell, Ethicon); previously soaked in 200 μl of a 0.1% rat serum albumin (RSA) in saline solution (Sigma, Poole, UK) containing 200 ng gp120-MN (Immunodiagnostics, Bedford, MA.) or for sham controls; 0.1% RSA in saline. The nerve was gently manipulated back into place and incisions closed with 4/0 sutures (Ethicon). For combined perineural HIV-gp120 + systemic ddC treatment (henceforth referred to as gp120+ddC), rats were injected with 0.5 ml of ddC (50 mg/kg in saline) at the time of surgery and three times a week thereafter for a maximum of 3 weeks. Sham controls were treated with perineural RSA and i.p. saline in the same regime as ddC treatment.
As a comparison model for nerve fibre density we used a previously characterized model of varicella zoster virus (VZV)-associated hypersensitivity (Garry et al., 2005; Hasnie et al., 2007). Methods are described in full elsewhere (Hasnie et al., 2007). In brief, primary human embryonic lung (Hel) cells (gift from J. Breuer, Royal London Hospital, UK) were inoculated with VZV (Dumas) and harvested when cells exhibited ~80% cytopathic effect (cpe) on microscopic examination. Virus-infected cells were centrifuged and re-suspended in 150 μl sterile phosphate buffer solution (Invitrogen, Paisley, UK) and 50 μl injected s.c. using a 25 gauge needle into the left (ipsilateral) hind footpad of anaesthetized rats.
Measurement of hind-limb withdrawal
Hind-paw withdrawal to sensory stimuli was measured in conscious rats as previously described (Wallace et al., 2007). Briefly, the hind paw withdrawal threshold in response to punctate static mechanical stimulation was measured using (a) graded ‘von Frey’ nylon filaments (Alan Ainsworth, UCL, London) (Chaplan et al., 1994) and (b) an electronic ‘von Frey’ device (Moller et al., 1998) of 0.5 mm2 probe tip area (Somedic Sales AB, Sweden). The time for hind-paw withdrawal in response to noxious heat was assessed using the Plantar test (Ugo Basile, Italy) (Hargreaves et al., 1988). Hypersensitivity to a cold stimulus was assessed by acetone application (Bridges et al., 2001).
Baseline measurements were obtained for all rats over the course of a week prior to surgery. All thresholds were measured by a ‘blinded’ observer. The threshold value at each time point tested was calculated as the mean ± SEM.
Open field activity
On day 21 following the first ddC injection (ddC only model) or day 14 post surgery (gp120+ddC model), rats (n = 8 per group) were placed into a 100 cm × 100 cm arena (4 lux) with a defined inner zone of 40 cm × 40 cm. Locomotion of the rats within the arena was tracked for 15 min as described previously (Hasnie et al., 2007; Wallace et al., 2007) and analysed using Ethovision software v.3 (Tracksys, Notts, UK). The total distance moved, time spent in the inner zone and number of entries into the inner zone were measured and the mean calculated. Only those rats with mechanical hypersensitivity (a change of at least 30% from baseline) were included (100% inclusion rate). For minocycline experiments, activity was measured on the final day of minocycline delivery.
Immunohistochemistry
Rats were deeply anaesthetized with pentobarbitone and transcardially perfused with heparinized saline followed by 4% paraformaldehyde (Sigma) with 1.5% picric acid (VWR, Poole, UK) in 0.1 M phosphate buffer (PB, Sigma). The L4 and L5 DRG, sciatic nerve and lumbar spinal cord were removed, post-fixed in 4% PFA for 4 h and transferred to a 15% then 30% sucrose solution in 0.1 M PB and left overnight. Tissue was embedded in OCT mounting medium (VWR) and frozen over liquid nitrogen. Cryostat sections of DRG (12 μm), sciatic nerve (15 μm) and L5 spinal cord (20 μm; level identified under light microscope) and were thaw-mounted on poly-l-lysine-coated glass slides (VWR).
For all fluorescent immunohistochemistry, sections were pre-incubated in buffer (0.1 M PBS, pH 7.4, containing 0.2% Triton X-100) containing 10% normal donkey serum for 1 h at room temperature and incubated with primary antibodies in buffer containing 4% donkey serum overnight.
The following antibodies were used: For detection of the stress-related transcription factor ATF3; rabbit anti-ATF3 (1:400; Santa Cruz Biotechnology, Santa Cruz, CA). For the detection of neuropeptides; rabbit anti-NPY (1:1000, Peninsula Laboratories Inc., Belmont, CA) and rabbit anti-galanin (1:2000; Peninsula, Cambridgeshire, UK). For the identification of cell bodies of myelinated afferents; mouse monoclonal anti-neurofilament 200 kDa (1:2000; clone N52; Sigma). For the detection of C-fibre terminals; rabbit anti-PGP 9.5 (1:1000; Ultraclone, Isle of White, UK) which recognizes the neuronal marker protein gene product 9.5 (PGP 9.5) in axons (McCarthy et al., 1995). For the staining of the chemokine CCL2; goat anti-mouse-JE (CCL2) (1:10; R&D Systems; Oxon, UK). For the staining of peripheral macrophages; mouse anti-CD68 (ED1) (1:2500; Serotec, Oxford, UK) which recognizes the CD68/ED1 glycoprotein expressed intracellularly by phagocytic macrophages (Hu and McLachlan, 2003). For the staining of satellite cells in the DRG; rabbit anti-GFAP (1:1000; Chemicon) which recognizes the GFAP intermediate filament protein that is upregulated in activated and proliferating DRG satellite cells (Hanani et al., 2005). For the staining of microglial cells; mouse anti-OX-42 (1:800; Serotec) directed against the CD11b/c protein expressed on the cell surface of microglia (Blackbeard et al., 2007). Sections were then washed in buffer and incubated with the appropriate secondary antibodies linked to either Cy3 (donkey anti-mouse-Cy3 1:300; Jackson ImmunoResearch Laboratories, Westgrove, PA) or fluorescein isothiocyanate (FITC) (donkey anti-rabbit-FITC 1:300; Jackson ImmunoResearch) for 2 h at room temperature. Three final washes in 0.1 M PBS were conducted before cover-slipping with Vecta-Shield (Vector Laboratories, Peterborough, UK) for analysis. Control sections were processed as above omitting the primary antisera.
Image analysis and quantification
All analysis was performed in a ‘blind’ manner Fluorescent images were visualized using a Leica DMR fluorescence microscope (Leica, Milton Keynes, UK) and analysed using Leica QWin software (Leica) as described previously (Wallace et al., 2007). For DRG immunohistochemistry, analysis was performed at ×20 on 6-8 randomly selected sections of DRG (separation of 180 μm) from each of four rats per group; only neurons with clear nuclei were counted. In each case, the analysis was performed in an automated manner using constant detection threshold levels. The total number of neurons was determined by counting both NF-200 labelled and non-labelled neuronal cells bodies and the number of DRG neurons expressing immunoreactivity (IR) for the protein of interest and reported as percent of total number of neurons/section. To measure alterations in DRG satellite cell populations the number of neuronal cell bodies associated with GFAP-IR satellite cells were counted and expressed as a percentage of total DRG neurons per section. To quantify the activated or infiltrating macrophages in DRG, CD68-IR cellular profiles were counted in a defined area (450 × 450 μm) and expressed as the total number/area. For CD68-IR in the sciatic nerve, six sections from each of four rats per group were captured at the site of HIV-gp120 or RSA application and the number of CD68-IR cellular profiles expressed as total number per unit area (450 × 250 μm). To quantify epidermal nerve fibre density (McArthur et al., 1998), PGP 9.5-IR nerves were counted in the epidermis of four footpad sections from each of four rats per group at ×20 using previously described methods (Hsieh et al., 2000; Lin et al., 2001; Keswani et al., 2006; Wallace et al., 2007). The total length of the epidermis along the upper margin of the stratum corneum in each footpad was measured and epidermal nerve density was expressed as the number of fibres per millimetre of epidermal length.
Analysis of spinal cord immunoreactivity was performed on a minimum of five randomly selected L5 coronal spinal cord sections from each of four rats per group. The percentage area of immunoreactivity (determined by the number of positive pixels) for GFAP or OX-42 staining in the ipsilateral and contralateral dorsal horn (laminae I-IV) was determined in a defined grid area (860 um × 580 um) using an automated grey-scale detection system using Leica QWin software version 3.0 (Leica) and expressed as mean% area ± SEM (Blackbeard et al., 2007).
Pharmacological manipulation
For all experiments, animals were randomized into treatment group and the experimenter ‘blinded’ to drug treatments received. Hind-paw withdrawal thresholds to punctuate mechanical stimuli were measured as described previously using an electronic von Frey device (Moller et al., 1998; Hasnie et al., 2007; Wallace et al., 2007). Baseline measurements were obtained at least twice prior to surgery. For investigations into the effect of minocycline (Sigma, 40 mg/kg in saline), ddC rats received a single dose 1 h prior to the first dose of ddC and once daily thereafter for 21 days. gp120+ddC rats received a single dose of minocyline 1 h prior to perineural HIV-gp120 surgery and once daily thereafter for 14 days. Hind-paw withdrawal thresholds were tested at regular intervals during minocycline treatment. For all other pharmacological investigations, reflex thresholds were determined on days 11 and 14, only rats that developed mechanical hypersensitivity of at least 30% change were included. On days 15-18, animals were administered the drug or vehicle twice daily (b.d.), and behavioural tests performed once each day 1.5-2 h following the first injection. The following drugs were tested: morphine sulphate (Sigma; 2.5 mg/kg in 0.5 ml saline i.p. b.d.); the CB1/CB2 cannabinoid receptor agonist WIN 55,212-2 [(mesylate (R)-(+)-(2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo(1,2,3-de)-1,4-benzoxazin-6-yl)-1-naphthalenylmethanone) Sigma, 2.5 mg/kg in 0.75 ml saline with 40% dimethylsulfoxide (DMSO) i.p. b.d.]; gabapentin (Pfizer Ltd 30 mg/kg in 0.5 ml saline i.p. b.d.); and amitriptyline (Sigma 10 mg/kg in 0.5 ml saline i.p. b.d.). Doses were based on previous studies (Hasnie et al., 2007; Wallace et al., 2007).
Statistical analysis
Sigmastat version 2.03 (SPSS Inc., Surrey, UK) was used to calculate statistical significance (P < 0.05) throughout the study. For behavioural measures all groups were compared using a one-way Analysis of Variance (ANOVA) with Dunn’s all pairwise multiple comparisons post hoc analysis. For immunohistochemical and pharmacological studies, experimental groups were compared using a Kruskal-Wallis one-way ANOVA on ranks with an all pairwise multiple comparisons procedure (Dunn’s method).
Results
Systemic ddC treatment is associated with hypersensitivity to mechanical, but not to heat or cool stimuli
Rats treated with 50 mg/kg ddC developed hind-paw mechanical hypersensitivity as measured by (a) graded von Frey filaments (gvF) (Fig. 1A) and (b) the electronic von Frey device (evF) (Fig. 1B). No such behaviour was observed in vehicle-treated control rats. This change peaked between day 14 and day 32 post initial injection and persisted until day 43. At 5 mg/kg, ddC produced no development of sensitivity, whereas at 25 mg/kg, ddC was associated with a small but significant mechanical sensitivity that peaked between days 14 and 21 (Fig. 1A and B) and recovered quickly. Therefore, we used 50 mg/kg as for subsequent experiments. At all time points, there was no evidence of hypersensitivity to thermal (Fig. 1C) or cold stimuli (Fig. 1D). We observed no overt motor deficits during or after ddC treatment which was further confirmed by measuring spontaneous locomotor activity in the open field area (see later).
Fig. 1.
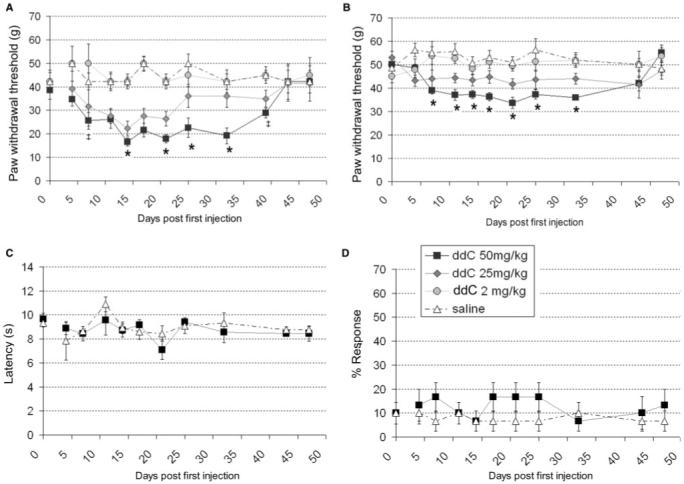
The development of hind paw reflex sensitivity to punctate mechanical stimuli, but not to thermal or cold stimuli, in rats treated with ddC. (A-D) Hind-paw withdrawal thresholds to (A) graded von Frey filaments, (B) an electronic von Frey device, (C) a noxious thermal stimulus and (D) an acetone drop, measured following treatment with (filled circle) 5 mg/kg ddC, (filled diamond) 25 mg/kg ddC, (filled square) 50 mg/kg ddC or (open triangle) saline (all groups n = 6; ddC administered i.p. 3×/week for 21 days). Statistical significance of differences between groups (P < 0.05) was determined by an ANOVA with Dunn’s all pairwise multiple comparisons, where asterisk represents significant difference from all other group and ‡ represents significance from the saline control group. Each value is the mean ± SEM.
Systemic ddC treatment exacerbates mechanical hypersensitivity induced by perineural HIV-gp120
Perineural administration of HIV-gp120 results in hypersensitivity to punctuate static mechanical stimuli (Wallace et al., 2007). We therefore investigated the effect of systemic ddC treatment on this behaviour. gp120+ddC-treated rats developed an increased mechanical hypersensitivity as compared to either treatment alone (Fig. 2A and B) without sensitivity to either thermal (Fig. 2C) or cold stimuli (Fig. 2D). Again we observed no overt motor deficit. In all cases, sham controls, showed no such effect.
Fig. 2.
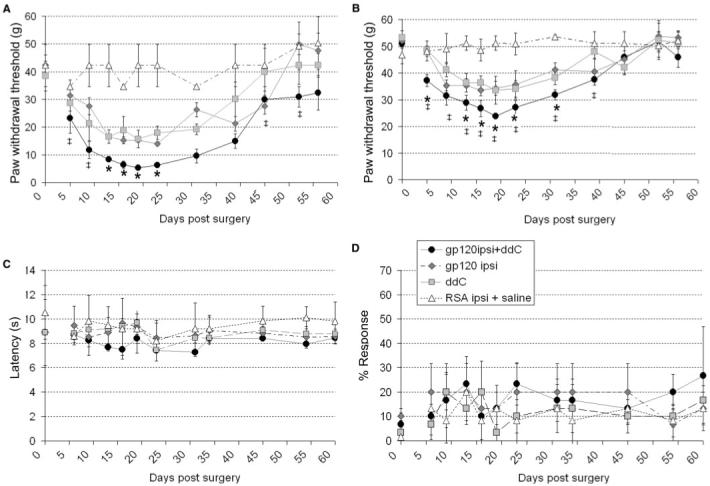
The development of reflex sensitivity to punctate mechanical stimuli, but not to thermal or cold stimuli, in rats treated with gp120+ddC as compared to each treatment alone. (A-D) Hind-paw withdrawal thresholds to (A) graded von Frey filaments (B) an electronic von Frey device, (C) a noxious thermal stimulus and (D) an acetone drop, measured following treatment with (filled circle) gp120+ddC (50 mg/kg), (filled square) 50 mg/kg ddC, (filled diamond) perineural HIV-gp120 or (open triangle) perineural RSA and systemic saline (all groups n = 6). Statistical significance of differences between groups (P < 0.05) was determined by an ANOVA with Dunn’s all pairwise multiple comparisons, where asterisk represents significant difference from all other group and ‡ represents significance from the sham-control group. Each value is the mean ± SEM.
We have shown repeatedly that the presence of mechanical hypersensitivity is detectable using either graded von Frey filaments or the electronic von Frey device (Bridges et al., 2001; Wallace et al., 2007; Hasnie et al., 2007), therefore for the remainder of experiments, we used only the electronic von Frey device to measure mechanical hypersensitivity.
Systemic ddC treatment with or without concomitant HIV-gp120 delivery is associated with altered spontaneous locomotor activity and thigmotaxis
We have previously shown spontaneous exploratory activity to be pathologically altered in rats following perineural delivery of gp120 (Wallace et al., 2007). Therefore, we assessed whether this behaviour was apparent in ddC and gp120+ddC-treated rats. As an indicator of normal locomotion, there was no significant difference in the total distance moved by ddC-treated or gp120+ddC-treated rats versus sham controls (Fig. 3A), suggesting a lack of overt motor deficit in these models. However, at the time of peak mechanical hypersensitivity (day 21), exploration of the centre of the arena (as calculated by the number of entries into a defined inner zone and the time spent in the inner zone) was significantly decreased in ddC-treated rats as compared to vehicle controls [Fig. 3B and D(ii)] indicative of thigmotaxis (anxiety-like behaviour). This abnormal pattern of behaviour was also a feature of gp120+ddC rats (Fig. 3Diii) when tested within the period of peak mechanical hypersensitivity (14-18 days post surgery), as compared to sham controls [Fig. 3C and D(i)]. These findings concur with those previously published for rats treated with perineural HIV-gp120 alone (Wallace et al., 2007) as well as models of traumatic neuropathy and VZV infection (Hasnie et al., 2007), indicating that thigmotaxis is a consistent feature of rat models of neuropathic pain in rats.
Fig. 3.
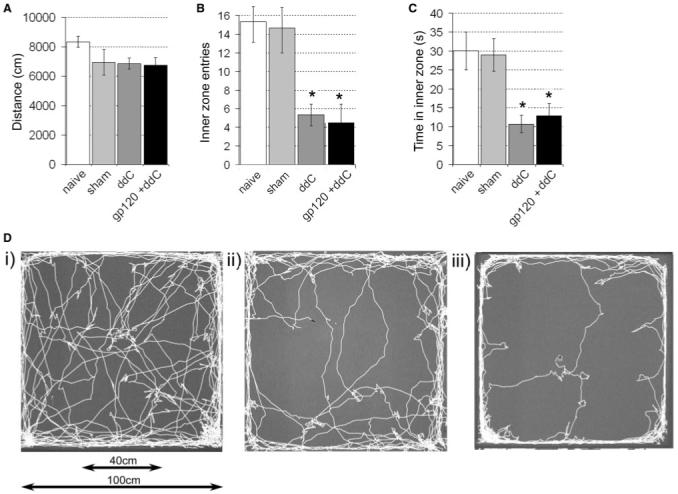
Alterations in spontaneous exploratory activity as measured in an open field arena in ddC-treated rats at day 21 and in gp120+ddC-treated rats at day 14. (A) The total distance moved; (B) the number of entries into the inner zone (40 × 40 cm2) and (C) the time spent in the inner zone of the open field were assessed in sham rats treated perineural RSA and saline, ddC or gp120+ddC. The statistical significance of differences between treatment groups and their relevant control (*P < 0.05) was determined using a one-way ANOVA with Dunn’s all pairwise multiple comparisons. Each value is the mean ± SEM. (D) Example traces of (i) sham, (ii) ddC and (iii) gp120+ddC-treated rats over 15 min in the open field arena.
Systemic ddC affects satellite cells in the DRG but does not alter neuronal protein expression changes associated with perineural HIV-gp120
We have previously demonstrated that following perineural HIV-gp120 there is an up-regulation of the stress-related transcription factor ATF3 and the neuropeptides, galanin and NPY in sensory neurons, and of the intermediate filament, GFAP, in satellite cells (Wallace et al., 2007). Therefore, we assessed the presence of such changes following systemic ddC treatment. We found no significant expression of ATF3, galanin or NPY in the DRG of ddC-treated rats at day 21. There was, however, a small but significant increase in GFAP-IR satellite cells (Fig. 4A). In contrast, at day 14, DRG ipsilateral to perineural HIV-gp120 from gp120+ddC rats, expressed ATF3, galanin and, NPY in a significant number of neurones (Table 1) as well as GFAP in satellite cells (Fig. 4A). This expression was apparent in a similar proportion of cells as has been previously reported for perineural HIV-gp120 alone (Wallace et al., 2007) (Table 1). This suggests that there is no additive effect of ddC on such mechanisms and that ddC-induced neuropathic pain involves processes distinct from other models of neuropathy.
Fig. 4.
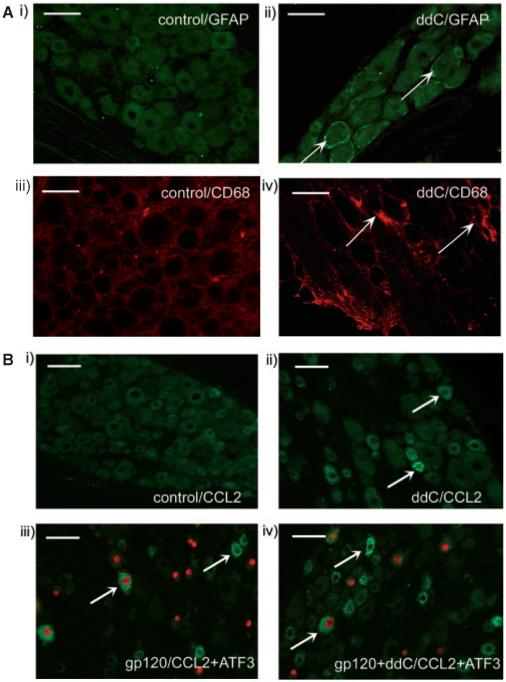
Immunohistochemical characterization of the L4/L5 DRG in ddC and gp120+ddC-treated rats. (A) Examples of the non-neuronal cell expression of the satellite cell marker GFAP and of the macrophage marker CD68 in the DRG of ddC-treated rats at day 21. Images show (i) GFAP in control DRG; (ii) GFAP in ddC DRG; (iii) CD68 in control DRG and (iv) CD68 in ddC DRG; scale bar = 50 μm. Arrows indicate examples of cells immunoreactive for the protein of interest. (B) Examples of the neuronal expression of CCL2 (green) co-stained for ATF3 (red); scale bar = 50 μm. Arrows indicate examples of cells immunoreactive for the protein of interest. Images are examples of L4/5 DRG (i) ipsilateral to perineural RSA and systemic saline as a control for all treatments; (ii) from ddC-treated rats at day 21; (iii) ipsilateral to perineural HIV-gp120 at day 14 and (iv) ipsilateral to perineural gp120 in gp120+ddC rats at day 14.
Table 1.
Immunohistochemical assessment of neuronal and non neuronal markers in DRG cells following perineural gp120 treatment with or without systemic ddC
| Saline day 21 (control) | ddC day 21 (50mg/kg) | gp120 day 14 (ipsilateral) | gp120+ddC day 14 (ipsilateral) | RSA sham day 14 (ipsilateral) | |
|---|---|---|---|---|---|
| (i) ATF3 | 0 | 0 | 26.9 ± 2.9%* | 31.9 ± 3.6%* | 4.6 ± 2.4% |
| (ii) Galanin | 0 | 0 | 13.9 ± 1.3%* | 15.2 ± 2.3%* | 3.2 ± 1.5% |
| (iii) NPY | 0 | 0 | 12.2 ± 1.9%* | 11.2 ± 3.9%* | 1.6 ± 0.3% |
| (iv) GFAP | 0 | 6.2 ± 1.1%‡ | 11.6 ± 4.3%* | 13.1 ± 3.6%* | 3.6 ± 0.6% |
| (v) CD68 | 22.1 ±3.4 | 48.4 ± 3.4‡ | 88.4 ± 7.4* | 92.6 ± 8.4* | 32.3 ± 2.7 |
| (vi) CCL2 | 0 | 12.5 ± 1.1%‡ | 13.3 ± 1.9%* | 19.3 ± 2.1%** | 2.2 ± 1.2% |
The expression of (i) ATF3, (ii) galanin and (iii) NPYgiven as a percentage of the total number of neurones; (iv) the expression of GFAP as a percentage of total number of neurones associated with GFAP-IR satellite cells and (v) the average number of CD-68-IR cells (macrophages) in DRG from; ddC treated animals (50 mg/kg) at day 21 versus DRG ipsilateral to perineural HIV-gp120 treatment of the sciatic nerve (Wallace et al., 2007) with or without the combined treatment with ddC; versus DRG ipsilateral to perineural RSA (sham) on post-treatment day 14 (n = 6 in each case).
(P < 0.05) represents statistical significance of difference between gp120, gp120+ddC and sham DRG as determined by a Kruskal-Wallis one-way ANOVA on ranks with Dunn’s post-hoc analysis.
(P < 0.05) represents statistical significance of difference between ddC-treated and saline-control-treated DRG as determined by a Student’s t-test.
(P < 0.05) represents statistical significance of gp120+ddC versus all other groups as determined by a Kruskal-Wallis one-way ANOVA on ranks with Dunn’s post-hoc analysis.
Systemic ddC and perineural HIV-gp120 induce CCL2 expression in the DRG in an additive manner
As a novel mechanistic correlate of perineural HIV-gp120 and systemic ddC treatment we assessed the induction of CCL2 expression in the DRG. CCL2 was significantly expressed in DRG from ddC rats and DRG ipsilateral to perineural HIV-gp120 treatment with no expression in sham-treated DRGs (Table 1 and Fig. 4B). CCL2 expression was greater in ipsilateral DRG from gp120+ddC rats as compared to either treatment alone (Table 1 and Fig. 4B). In gp120-treated rats (+/- ddC); a small proportion of CCL2 positive neurons were ATF-3 positive. Whereas, no ATF3-IR neurons were observed in DRG from ddC-treated rats. This highlights CCL2 as a novel mechanistic target for all three models that is not necessarily associated with cell damage/stress.
ddC-associated macrophage infiltration
Perineural HIV-gp120 is associated with macrophage infiltration into the peripheral nerve and the DRG (Wallace et al., 2007). No increased macrophage population was observed in the peripheral nerve of ddC-treated rats (data not shown). However there was a small increase in the average number of CD-68-IR macrophages present in the DRG from ddC-treated rats at day 21 as compared to control DRG. This expression was of a similar magnitude to that seen in rats treated with perineural HIV-gp120 alone and gp120+ddC-treated rats again suggesting an overlap of possible mechanisms (Table 1).
Systemic ddC treatment with or without concomitant HIV-gp120 delivery is associated decreased intraepidermal nerve fibre density
Immunostaining of skin biopsies for the neuronal marker protein gene product 9.5 (PGP 9.5), (McCarthy et al., 1995; Kennedy et al., 1996) is used to quantify intraepidermal nerve fibre (IENF) density (Smith et al., 2005) in humans and rodents (Lauria et al., 2007; McCarthy et al., 1995; Herrmann et al., 1999; Lin et al., 2001; Keswani et al., 2006). We quantified IENF densities in the lateral plantar surface of the rat hind paw [i.e. the innervation of the sciatic nerve (Decosterd and Woolf, 2000)] treated with systemic ddC or with gp120+ddC. In ddC-treated rats, there were significantly fewer nerve fibres as compared to controls (Fig. 5). Similarly, in gp120+ddC rats, there were significantly fewer nerve fibres ipsilateral to perineural HIV-gp120 as compared to sham controls (Fig. 5). As a comparator to another non-nerve injury associated model that is characterized by mechanical hypersensitivity, we assessed the IENFD in rats 21 days following injection of VZV into the hind paw (Hasnie et al., 2007). Similarly, this model was associated with a significantly decreased IENFD as compared to controls (Fig. 5).
Fig. 5.
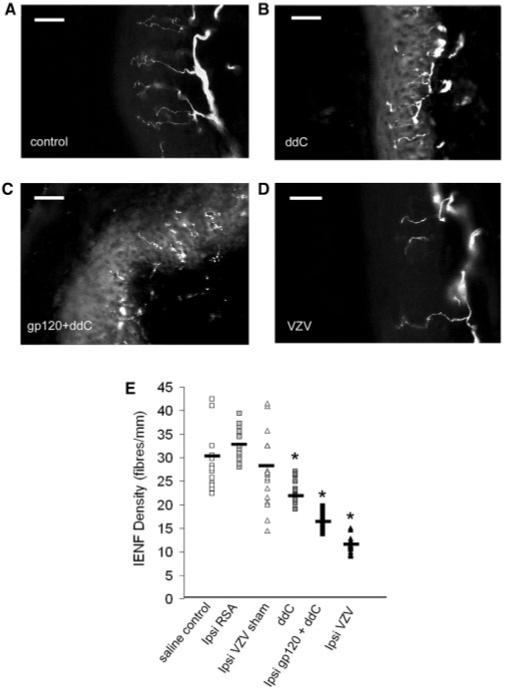
Decreased intraepidermal nerve fibre density in three models of mechanical hypersensitivity. (A-C) Examples of PGP-9.5-immunoreactive profiles in the hind-paw skin from (A) control rats, (B) ddC-treated rats at day 21; (C) ipsilateral to perineural gp120 in gp120+ddC rats at day 14 and (D) ipsilateral to injection of VZV-infected fibroblasts at day 14. Scale bar = 30 μm. (E) Immunohistochemical analysis of the glabrous skin of the hind paw (n = 4 rats per group with four sections per rat) displayed as a scatter plot of individual values with mean value indicated). Statistical significance of differences between treatment and control groups (*P < 0.05) was determined by an ANOVA with Dunn’s all pairwise multiple comparisons.
Systemic ddC treatment augments neuropathy-associated microgliosis but not astrocytosis following perineural HIV-gp120
Spinal gliosis occurs following perineural HIV-gp120 administration (Wallace et al., 2007). We therefore assessed microgliosis and astrocytosis (by measurement of OX-42-IR and GFAP-IR, respectively) in ddC and gp120+ddC-treated rats. In ddC rats at day 21, there was a small increase in OX-42-IR in the spinal dorsal horn as compared to vehicle controls (Fig. 6A). In gp120+ddC rats at day 14 there was a greater increase in OX-42-IR ipsilateral to perineural gp120 treatment, as compared to either treatment alone, or to contralateral values or ipsilateral to sham (Fig. 6A). In ddC rats at day 21, there was a small increase in GFAP-IR as compared to vehicle control rats (Fig. 6B). GFAP-IR was also increased ipsilateral to perineural HIV-gp120 in both the gp120 and gp120+ddC models as compared to controls (Fig. 6B). However this expression was not different between the two models suggesting that unlike the effect on microgliosis, ddC did not exacerbate astrocytosis.
Fig. 6.
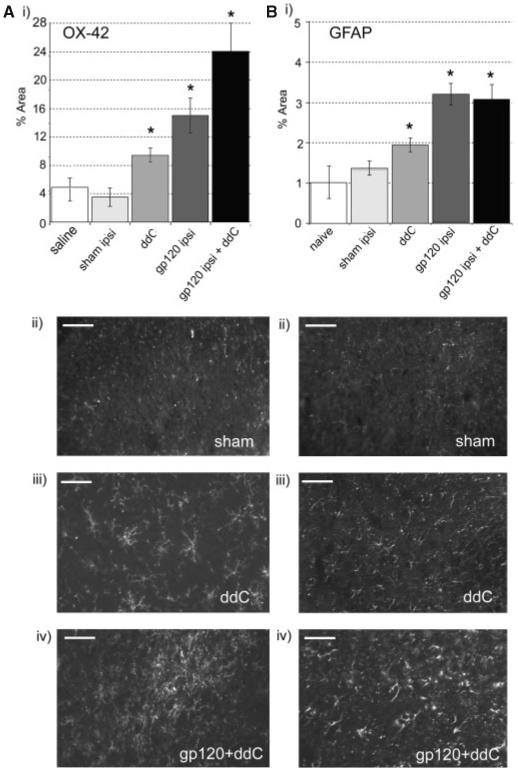
Immunohistochemical analysis of microglia and astrocytes in the dorsal horn of the L5 spinal cord in ddC, gp120 and gp120+ddC-treated rats. (i) The% area of the spinal cord immunoreactive for (A) OX-42 and (B) GFAP was measured in the dorsal horn of the L5 spinal cord of rats 21 days after treatment with ddC (n = 5); 14 days after perineural gp120 (n = 5) (Wallace et al., 2007); 14 days after perineural gp120+ddC treatment (n = 5) and in control rats (n = 5). Statistical significance of differences between groups (*P < 0.05) was determined by an ANOVA with Dunn’s all pairwise multiple comparisons. Each value is the mean ± SEM. (ii-iv) Example images of the ipsilateral dorsal horn of rats treated with (i) perineural RSA (sham) + saline (day 14); (ii) ddC (day 21) or (iii) perineual gp120+ddC (day 14). Scale bar = 30 μm.
The sensitivity of ddC-induced pain behaviour and gp120+ddC-induced pain behaviour to minocycline
To assess the contribution of microglial-related processes to mechanical hypersensitivity and thigmotactic behaviour, we assessed the effect of the microglial activation inhibitor, minocycline, on behavioural responses in our models. When administered daily, commencing 1 h prior to the first ddC administration, minocycline (40 mg/kg i.p.) was not associated with any effect on the development of the mechanical hypersensitivity associated with ddC treatment (Fig. 7A). Nor did it affect measures of thigmotaxis in the open field. In contrast, when administered to gp120+ddC-treated rats, minocycline (40 mg/kg i.p.) significantly delayed the onset of mechanical sensitivity ipsilateral to perineural gp120 treatment as compared to vehicle controls (Fig. 7B). However, by day 14 post surgery, the minocycline-treated rats had developed mechanical hypersensitivity of a similar magnitude to that of vehicle-treated controls. Minocycline also decreased thigmotaxis in gp120+ddC rats as measured by time spent in the central zone of the open field arena, at day 14 (Fig. 7C).
Fig. 7.
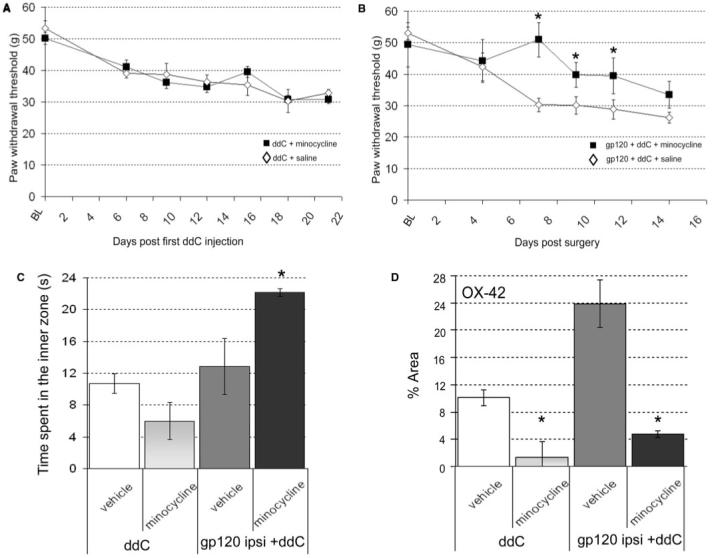
The effect of minocycline in ddC or gp120+ddC-treated rats. The effect of minocycline (40 mg/kg i.p. daily) on (A) mechanical hypersensitivity in ddC-treated rats (n = 8); (B) mechanical hypersensitivity in gp120+ddC-treated rats and (C) the time spent in the inner zone of the open filed arena. (D) Analysis of the effect of minocycline on immunohistochemical staining for OX-42-IR in both ddC (day 21) (n = 6) and gp120+ddC (day 14) (n = 6) treated rats. Statistical significance of differences between each treatment group and its relevant control (*P < 0.05) was determined by a one-way ANOVA with Dunn’s all pairwise multiple comparisons. Each value is the mean ± SEM.
The effect of minocycline on microglial activation
Analysis of spinal OX-42-IR at the end of minocycline treatment confirmed that minocycline attenuated microgliosis in the dorsal horn of ddC-treated rats at day 21 (Fig. 7D) and of gp120+ddC rats at day 14 (Fig. 7D).
The sensitivity of HIV-gp120-treated rats to analgesic drugs
Amitriptyline (10 mg/kg i.p. b.d.) was not associated with any reversal of the heightened sensitivity to punctuate mechanical stimuli in ddC-treated rats [Fig. 8A(i)] or gp120+ddC rats [Fig. 8A(ii)]. In contrast, gabapentin (30 mg/kg, i.p. b.d. on d15-d18), was associated with a complete attenuation of the mechanical hypersensitivity observed with ddC treatment [Fig. 8B(i)] and a partial reversal of mechanical hypersensitivity in gp120+ddC rats [Fig. 8B(ii)]. Morphine (2.5 mg/kg i.p b.d.) reversed mechanical hypersensitivity displayed by both ddC and gp120+ddC-treated rats (Fig. 8C). In all cases, vehicle administration had no significant effect. Finally, systemic administration of the mixed CB1/CB2 cannabinoid receptor agonist WIN 55,212-2 (2 mg/kg, i.p. b.d.) completely reversed the mechanical hypersensitivity associated with ddC treatment [Fig. 8D(i)] and partially reversed mechanical hypersensitivity in gp120+ddC rats [Fig. 8D(ii)]. In both models, the effect of WIN 55,212-2 was significantly greater than the effect of the vehicle (40% DMSO). For all drugs tested, mechanical hypersensitivity was re-established by d28, indicating reversal of the drug effect and re-establishment of the neuropathic state.
Fig. 8.
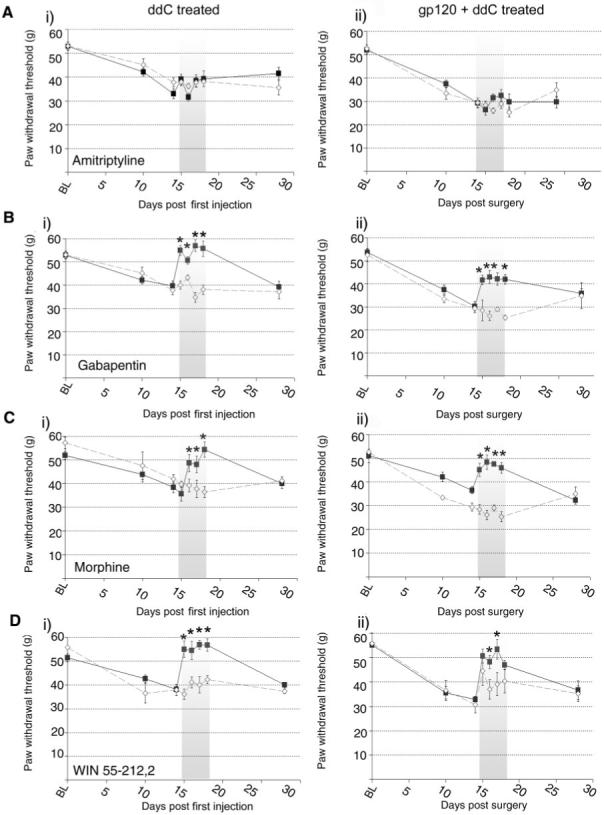
The effect of systemic administration of analgesic compounds on hind-paw mechanical hypersensitivity in ddC and gp120+ddC rats. (A-D) The effect of drugs delivered at the time of peak behavioural change on paw withdrawal thresholds in response cutaneous punctate mechanical stimulation in (i) ddC-treated rats and (ii) gp120+ddC-treated rats. Ipsilateral values are displayed before, during and after i.p. administration of each pharmacological agent (filled square) versus the appropriate vehicle (open diamond) as paw withdrawal threshold (g). Drug administration period (d15-18) represented by shaded area. For all measurements, each value is the mean ± SEM and statistical significance (*P < 0.05) of any differences between drug and vehicle threshold values was determined by a Kruskal-Wallis one-way ANOVA with Dunn’s all pairwise multiple comparisons group post hoc analyses.
Discussion
Whilst the exact pathogenesis of HIV-DSP is unclear, it is likely that in those receiving HAART therapy, a combination of HIV-associated pathology and anti-retroviral neurotoxicity occurs (Pardo et al., 2001). Therefore, to investigate the mechanisms of HIV-DSP, we have pharmacologically and mechanistically characterized two rodent models: the first models the effects of systemic treatment with the NRTI, ddC, and the second investigates, for the first time, the combined effect of systemic ddC and perineural exposure to HIV-gp120 in the rat. In both models behavioural and mechanistic correlates of neuropathic pain are evident, a number of which are enhanced by the combination of anti-retroviral treatment and perineural HIV-gp120.
As with other drug-induced neuropathy models (Aley et al., 1996; Authier et al., 2000; Polomano et al., 2001; Authier et al., 2003a, b; Joseph et al., 2004), systemic ddC treatment caused a dose-dependant mechanical hypersensitivity. Furthermore, when combined with perineural HIV-gp120, this mechanical hypersensitivity was increased suggesting that an additive effect of gp120 and ddC-related mechanisms occurs. Additionally, in both models, and in contrast to many neuropathic pain models, no hypersensitivity to heat or cold stimuli developed. This is consistent with clinical data showing that patients with HIV-related painful neuropathy do not usually present with thermal hypersensitivity (Martin et al., 2003). At the time of peak mechanical hypersensitivity, both models display decreased spontaneous exploratory behaviour and thigmotaxis in the open field, a behaviour thought to reflect anxiety (Cryan and Holmes, 2005). This suggests the presence of pain-driven alterations in affect, which may be representative of ongoing pain and/or pain-related affective co-morbidities which are known to be a feature of neuropathic pain in humans (Meyer-Rosberg et al., 2001; Nicholson and Verma, 2004; Gore et al., 2005). This supports the use of this paradigm in the future to test the efficacy of novel drugs. A lack of overt motor deficit in both models was indicated by this test which may be further confirmed by additional tests of motor function.
Perineural HIV-gp120 is associated with signs of neuronal insult, as indicated by expression of ATF3 in the DRG (Wallace et al., 2007) and the presence of macrophages in the nerve, a change which correlates well with the clinical scenario (de la Monte et al., 1988; Yoshioka et al., 1994; Shapshak et al., 1995; Nagano et al., 1996; Pardo et al., 2001). This implies that HIV-gp120 may interact directly with axons, or indirectly via stimulation of macrophages to precipitate damage and that these factors may underlie the axonal ‘dying back’ observed in the skin of HIV patients. In line with a model of diabetic neuropathy (Wright et al., 2004), gp120+ddC-associated ATF3 expression indicates the presence of axonal stress in models of disease states, a phenomenon that merits further investigation. In contrast, we found no nerve macrophage infiltration or DRG expression of ATF3 in ddC-treated rats suggesting that such mechanisms may be associated with viral effects on the nerve alone. However, we did find a reduced numbers of C-fibre axon terminals in the plantar hind paw of ddC and gp120+ddC rats at times of peak mechanical hypersensitivity. IENF density, representing the number of terminals of C (nociceptive) fibres, is commonly used for diagnosis of sensory neuropathy (McCarthy et al., 1995; Kennedy et al., 1996; Lin et al., 2001; Lauria et al., 2007). Intraepidermal nerve fibres, are known to degenerate in patients with HIV-DSP and their density is inversely correlated with the presence of neuropathic pain in HIV patients (Polydefkis et al., 2002). Therefore, our results reflect the clinical scenario of a small fibre neuropathy affecting the distal nerve (Martin et al., 2003; Verma et al., 2005). The cause for IENF density decrease is uncertain. The lack of ATF3 in the ddC model indicates that neuronal injury or death is not necessarily required for reduced peripheral innervation density suggesting this may be due to a local effect on axons. Nevertheless, these findings suggests that even though ddC is associated with neuropathic changes distinct to those of gp120, the presentation of axonal ‘dying back’ in patients may be associated with both the presence of the virus and of the use of anti-retroviral drugs. This supports the possibility that NRTI drugs are exacerbating a virally mediated neuropathy and support the use of these models in the investigation of such mechanisms. These data are in contrast to a recent study in which the effects of gp120+ didanosine were assessed in mice (Keswani et al., 2006). However, this study investigated a gp120 expressing transgenic mouse treated with an alternative anti-retroviral drug, didanosine. Therefore, the differences between the models in question suggest that little comparison can be made between these studies.
A number of changes in DRG cell phenotypic occur following perineural HIV-gp120 (Wallace et al., 2007) which were not associated with systemic ddC treatment, again suggesting a limited effect of the drugs on the primary sensory neurons themselves. There was, however, evidence for changes in satellite cells and for the presence of macrophages in the DRG, both of which are changes associated with perineural HIV-gp120 and are features of models of nerve-lesion-associated pain (Ma and Bisby, 1998; Wynick et al., 2001). Furthermore, as a novel target for HIV-related neuropathies, there was significant up-regulation of the chemokine CCL2 (MCP-1) in the DRG of ddC and/or perineural HIV-gp120-treated rats. CCL2 is expressed in the DRG following nerve injury (Tanaka et al., 2004) and has been linked to the pathogenesis of pain both in the PNS and CNS (Zhang and Koninck, 2006; Sun et al., 2006). We found that the majority of CCL2 expressing neurons were not positive for ATF3 highlighting CCL2 as a novel mechanistic target for all three models that is not necessarily associated with cell damage/stress. Furthermore, the apparent synergism of ddC and gp120 in the up-regulation of CCL2, as well as the presence of macrophages and activated satellite cells in the DRG, suggests that such inflammatory-linked pathways may provide drug targets for treatment of HIV and drug-related neuropathies. Importantly, this study has highlighted changes that may represent mechanisms by which the anti-retroviral drugs can indirectly influence the function of the neurons and promote the generation of pain.
Spinal astrocytes and microglia can produce and/or respond to a number of pronociceptive substances (Tsuda et al., 2005). Although a direct causal relationship is controversial (Colburn et al., 1997), their activation has been demonstrated in various models of neuropathic pain (Colburn et al., 1997; Milligan et al., 2003; Tanga et al., 2004) making them potential targets for therapeutic intervention. Here we have shown for the first time that rats treated with ddC displayed a modest level of microgliosis whereas; gp120+ddC rats (which display a greater magnitude of mechanical hypersensitivity) display significantly greater levels of microglial activation. This suggests that microgliosis may be important, but not necessary, for the development of behavioural indices of pain in these different rodent models. In support of this hypothesis, inhibition of microglial activation had no effect on the development of ddC-associated hypersensitivity or thigmotaxis. In contrast, the onset of gp120+ddC-associated hypersensitivity was significantly affected by microglial inhibition which also attenuated thigmotaxis. These data suggest that microglial activation may not be necessary for the eventual development and maintenance of a persistent pain state but plays an important role in the onset of mechanical hypersensitivity as well as non-reflex-based measures of pain-associated behaviour and therefore merits further investigation for possible mechanistic targets. Of course, minocycline may also be targeting the immune system systemically (Zanjani et al., 2006) which may also play a role in the observed effects. Nevertheless, these data suggest a differential role for microglia in neuropathic pain states.
To assess the suitability of these models for the investigation of neuropathic pain mechanisms, we have tested the effects of compounds known to be analgesic in rodent models of pain and in humans. Amitriptyline has known analgesic efficacy in neuropathic pain in experimental models (Abdi et al., 1998; De Vry et al., 2004) and in the clinic (Hempenstall et al., 2005; Finnerup et al., 2005). However, clinical trial data, which are largely based on patients receiving HAART and therefore who are exposed to both HIV and anti-retrovirals, suggests that amitriptyline is not effective for the treatment of HIV-related neuropathic pain (Kieburtz et al., 1998; Shlay et al., 1998). In concordance with these clinical data amitriptyline had no effect on mechanical hypersensitivity in either of our models. In contrast, gabapentin and morphine were both effective. Gabapentin is analgesic in rodent (Abdi et al., 1998; De Vry et al., 2004; Donovan-Rodriguez et al., 2005) and human neuropathic pain conditions (Hempenstall et al., 2005; Finnerup et al., 2005), including HIV neuropathy (Hahn et al., 2004). Morphine attenuates hypersensitivity in rodent neuropathy models (Lee et al., 1994; Backonja et al., 1995) and evidence is accumulating to suggest that opioids relieve symptoms of human neuropathic pain of peripheral origin (Hempenstall et al., 2005; Finnerup et al., 2005). The effects of these analgesics in our models correlate well with their clinical efficacy further supporting the use of this model for the assessment of novel therapeutic agents. For example, evidence indicates that cannabinoids may exert beneficial effects including analgesia in human neuropathic pain (Rice, 2005; Finnerup et al., 2005; Rice et al., in press) including HIV-DSP (Abrams et al., 2007). Here we have shown that in line with previous rodent neuropathic pain studies (Bridges et al., 2001; Wallace et al., 2003; Hasnie et al., 2007), the CB1/CB2 receptor agonist, WIN 55,212-2 effectively attenuated mechanical hypersensitivity in both models further supporting the potential therapeutic benefit of cannabinoids.
In conclusion, we have characterized two neuropathic pain models that mimic many features of HIV-DSP and anti-retroviral-induced neuropathy. The associated characteristics, especially the robust but specific behavioural changes, highlight the utility of these models for many further mechanistic studies and potentially the discovery of new therapeutic targets for the treatment of neuropathic pain in general.
Acknowledgements
Wellcome Trust (London Pain Consortium), Pfizer for donation of gabapentin and Roche for donation of ddC. Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- ANOVA
Analysis of Variance
- gp120
Glycoprotein 120
- DRG
dorsal root ganglion
- DSP
distal symmetrical polyneuropathy
- CB
cannabinoid
- HIV-1
Human Immunodeficiency Virus Type 1
- NPY
neuropeptideY
- ATF3
activating transcription factor 3
- NF-200
neurofilament 200 kDa
- GFAP
Glial fibrillary acidic protein
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- OCT
optimum cryostat temperature
- RSA
rat serum albumin
- gvF
graded von Frey filaments
- evF
electronic von Frey device
- VZV
Varicella Zoster Virus
References
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998;87:1360–6. [PubMed] [Google Scholar]
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–21. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Aley KO, Reichling DB, Levine JD. Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience. 1996;73:259–65. doi: 10.1016/0306-4522(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Apostolski S, McAlarney T, Quattrini A, Levison SW, Rosoklija G, Lugaressi A, et al. The gp120 glycoprotein of human immunodeficiency virus type 1 binds to sensory ganglion neurons. Ann Neurol. 1993;34:855–63. doi: 10.1002/ana.410340616. [DOI] [PubMed] [Google Scholar]
- Authier N, Fialip J, Eschalier A, Coudore F. Assessment of allodynia and hyperalgesia after cisplatin administration to rats. Neurosci Lett. 2000;291:73–6. doi: 10.1016/s0304-3940(00)01373-2. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology. 2003a;24:797–805. doi: 10.1016/S0161-813X(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003b;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Miletic G, Miletic V. The effect of continuous morphine analgesia on chronic thermal hyperalgesia due to sciatic constriction injury in rats. Neurosci Lett. 1995;196:61–4. doi: 10.1016/0304-3940(95)11844-m. [DOI] [PubMed] [Google Scholar]
- Beck EJ, Mandalia S, Williams I, Power A, Newson R, Molesworth A, et al. National Prospective Monitoring System Steering Group Decreased morbidity and use of hospital services in English HIV-infected individuals with increased uptake of anti-retroviral therapy 1996-1997. AIDS. 1999;13:2157–64. doi: 10.1097/00002030-199910220-00020. [DOI] [PubMed] [Google Scholar]
- Berger AR, Arezzo JC, Schaumburg HH, Skowron G, Merigan T, Bozzette S, et al. 2′,3′-dideoxycytidine (ddC) toxic neuropathy: a study of 52 patients. Neurology. 1993;43:358–62. doi: 10.1212/wnl.43.2.358. [DOI] [PubMed] [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackbeard J, O’Dea KP, Wallace VC, Segerdahl A, Pheby T, Takata M, et al. Quantification of the rat spinal microglial response to peripheral nerve injury as revealed by immunohistochemical image analysis and flow cytometry. J Neurosci Methods. 2007;164:207–17. doi: 10.1016/j.jneumeth.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice AS. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–94. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–23. [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Pezeshkpour GH. Neuromuscular diseases associated with human immunodeficiency virus infection. Ann Neurol. 1988;23(Suppl):S38–48. doi: 10.1002/ana.410230713. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC) Lab Invest. 2001;81:1537–44. doi: 10.1038/labinvest.3780367. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Gabuzda DH, Ho DD, Brown RH, Jr, Hedley-Whyte ET, Schooley RT, et al. Peripheral neuropathy in the acquired immunodeficiency syndrome. Ann Neurol. 1988;23:485–92. doi: 10.1002/ana.410230510. [DOI] [PubMed] [Google Scholar]
- De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2004;491:137–48. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistant peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–40. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- Fiala M, Murphy T, MacDougall J, Yang W, Luque A, Iruela-Arispe L, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol. 2004;4:327–37. doi: 10.1385/ct:4:4:327. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gazzard B, Bernard AJ, Boffito M, Churchill D, Edwards S, Fisher N, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. HIV Med. 2006;7:487–503. doi: 10.1111/j.1468-1293.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- Hahn K, Arendt G, Braun JS, von Giesen HJ, Husstedt IW, Maschke M, et al. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. J Neurol. 2004;251:1260–6. doi: 10.1007/s00415-004-0529-6. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown FFC, Jovis J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–9. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–40. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin. J Neuropathol Exp Neurol. 2000;59:297–307. doi: 10.1093/jnen/59.4.297. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Exp Neurol. 2003;184:590–605. doi: 10.1016/S0014-4886(03)00307-8. [DOI] [PubMed] [Google Scholar]
- Jones G, Zhu Y, Silva C, Tsutsui S, Pardo CA, Keppler OT, et al. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334:178–93. doi: 10.1016/j.virol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–58. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22:685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–8. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol. 2003a;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003b;54:287–96. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Simpson D, Yiannoutsos C, Max MB, Hall CD, Ellis RJ, et al. AIDS Clinical Trial Group 242 Protocol Team A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. Neurology. 1998;51:1682–8. doi: 10.1212/wnl.51.6.1682. [DOI] [PubMed] [Google Scholar]
- Lauria G, Lombardi R. Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ. 2007;334:1159–64. doi: 10.1136/bmj.39192.488125.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, et al. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–42. [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kayser V, Desmeules J, Guilbaud G. Differential action of morphine and various opioid agonists on thermal allodynia and hyperalgesia in mononeuropathic rats. Pain. 1994;57:233–40. doi: 10.1016/0304-3959(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Lin YW, Tseng TJ, Lin WM, Hsieh ST. Cutaneous nerve terminal degeneration in painful mononeuropathy. Exp Neurol. 2001;170:290–6. doi: 10.1006/exnr.2001.7704. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–77. doi: 10.1146/annurev.pharmtox.38.1.159. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience. 1998;86:1217–34. doi: 10.1016/s0306-4522(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Marshall S, Coyle S, Rice AS. Pain in Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome. In: Ballentyne JC, editor. Massachusetts General Hospital handbook of pain management. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 446–60. [Google Scholar]
- Martin C, Pehrsson P, Osterberg A, Sonnerborg A, Hansson P. Pain in ambulatory HIV-infected patients with and without intravenous drug use. Eur J Pain. 1999;3:157–64. doi: 10.1053/eujp.1999.0111. [DOI] [PubMed] [Google Scholar]
- Martin C, Solders G, Sonnerborg A, Hansson P. Painful and non-painful neuropathy in HIV-infected patients: an analysis of somatosensory nerve function. Eur J Pain. 2003;7:23–31. doi: 10.1016/s1090-3801(02)00053-8. [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–55. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006 doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1:71–104. [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–40. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller KA, Johansson B, Berge O. Assessing mechanical allodynia in the rat paw with a new electronic algometer. J Neurosci Methods. 1998;84:41–7. doi: 10.1016/s0165-0270(98)00083-1. [DOI] [PubMed] [Google Scholar]
- Nagano I, Shapshak P, Yoshioka M, Xin K, Nakamura S, Bradley WG. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. J Neurol Sci. 1996;136:117–28. doi: 10.1016/0022-510x(95)00317-u. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5:9–27. doi: 10.1111/j.1526-4637.2004.04019.x. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–35. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–7. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, et al. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–9. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- Rice ASC. Melzack and Wall: textbook of pain. London: Elsevier; 2005. Cannabinoids; pp. 521–40. [Google Scholar]
- Rice ASC, Lever I, Zarnegar R. Cannabinoids and analgesia with special reference to neuropathic pain. In: McQuay HJ, Kalso E, Moore RA, editors. Systematic reviews and meta-analyses in pain. Seattle: IASP Press; 2007. in press. [Google Scholar]
- Rizzuto N, Cavallaro T, Monaco S, Morbin M, Bonetti B, Ferrari S, et al. Role of HIV in the pathogenesis of distal symmetrical peripheral neuropathy. Acta Neuropathol (Berl) 1995;90:244–50. doi: 10.1007/BF00296507. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson CM, Andrews A, Sandberg JA, Nickols J, Slikker W., Jr. Evaluation of brain and nerve pathology in rats chronically dosed with ddI or isoniazid. Neurotoxicol Teratol. 1996;18:555–63. doi: 10.1016/0892-0362(96)00088-8. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Nagano I, Xin K, Bradley W, McCoy CB, Sun NC, et al. HIV-1 heterogeneity and cytokines. Neuropathogenesis. Adv Exp Med Biol. 1995;373:225–38. doi: 10.1007/978-1-4615-1951-5_31. [DOI] [PubMed] [Google Scholar]
- Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA. 1998;280:1590–5. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228:65–9. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, Lamotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006 doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–9. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Simpson D. HIV-associated neuropathic pain: epidemiology, pathophysiology and management. CNS Drugs. 2005;19:325–34. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Segerdahl A, Hasnie FS, Pheby T, McMahon SB, et al. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007 doi: 10.1016/j.pain.2007.02.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–33. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005;4:834–44. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Ryals JM, McCarson KE, Christianson JA. Diabetes-induced expression of activating transcription factor 3 in mouse primary sensory neurons. J Peripher Nerv Syst. 2004;9:242–54. doi: 10.1111/j.1085-9489.2004.09404.x. [DOI] [PubMed] [Google Scholar]
- Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–60. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- Wynick D, Thompson SW, McMahon SB. The role of galanin as a multi-functional neuropeptide in the nervous system. Curr Opin Pharmacol. 2001;1:73–7. doi: 10.1016/s1471-4892(01)00006-6. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Shapshak P, Srivastava AK, Stewart RV, Nelson SJ, Bradley WG, et al. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology. 1994;44:1120–30. doi: 10.1212/wnl.44.6.1120. [DOI] [PubMed] [Google Scholar]
- Zanjani TM, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain 1. Eur J Pharmacol. 2006;538:66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]
- Zhang J, Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–83. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


