Abstract
A facile synthetic route to prepare monofunctional carbocyanine dyes for biological application is developed. Three pentamethine carbocyanine dyes have been successfully modified with a variety of functional groups such as: carboxylic acids, azides, or alkynes. The new dyes are characterized by strong NIR fluorescence emission, high extinction coefficients and good quantum yields. The azide and alkyne dyes have potential utility as components in bioorthogonal labeling schemes via [2+3] dipolar cycloaddition “click” reactions. The application of one derivative, CyAM-5 alkyne, for bioorthogonal labeling is demonstrated. Fluorescence microscopy shows coupling of CyAM-5 alkyne to Chinese hamster ovary (CHO) cells preincubated with azide modified glycans.
INTRODUCTION
Near infrared (NIR) fluorescence-based imaging is becoming a key tool for early disease diagnosis, therapeutic applications and for biochemical analysis, due to the high sensitivity, excellent temporospatial resolution, and potential for multi-channel imaging (1, 2). There is currently a need for improved, bright near infrared, water-soluble dyes containing diverse functional groups for conjugation to biomolecules (3, 4). Asymmetric monofunctional carbocyanine dyes containing carboxylic acids, were first reported in the early 1990’s (5, 6). Due to their bright fluorescence and NIR emission, these fluorophores are receiving considerable interest as components of new NIR optical imaging agents (5-7). For example, the Cy5.5 and Cy7 dyes have been used for labeling a variety biological/biomedical macromolecules and nanoparticles (8, 9). Two major synthetic strategies are currently employed in the preparation of functionalized carbocyanine dyes. One is a stepwise synthetic route to prepare asymmetric cyanine dyes, in which different indoleninium or benzindoleninium moieties are condensed to a polymethine chain (5, 6). Although many commonly used monofunctional cyanine dyes are prepared through this route, synthetic yields are often less than 10% and significant amounts of the undesired symmetric dyes are formed (10). A second synthetic route for incorporating functional groups into symmetric heptamethine cyanine dyes also has been developed (11). This route proceeds via reaction of nucleophiles with commercially available chloro-substituted cyanine dyes to give a variety of aryl-ether, thiol-ether and aryl-thioether modified tricarbocyanine fluorophores often in greater than 90% yield (12-14). More recently, a modified Suzuki-Miyaura coupling was proposed to prepare meso-aryl, arylether and arylamino tricarbocyanine dyes in good yields (15). Unfortunately, most of these compounds have lower quantum yields (often Φ< 0.1) than canonical Cy7 (Φ = 0.27), or are chemically unstable (16). Furthermore, the physical behavior of these carbocyanine derivatives can be altered significantly by their functional substituents. For example, aryl-ether modified dyes can show an increased tendency to aggregate in aqueous solution (12). There are several criteria, which need to be met, for design of dyes that are useful for bioconjugation and bioimaging applications. These include universal requirements, such bright fluorescence emission, water solubility, chemical stability, as well as far-red/NIR absorption and emission. In addition to these parameters, a flexible high yielding synthetic methodology is desirable. In order to achieve these requirements and circumvent the shortcomings of existing synthetic routes, we present in this paper a new procedure based on modification of the malonaldehyde dianil dye precursors for preparation of monofunctional far-red/NIR pentamethine carbocyanine dye derivatives. This synthetic paradigm can be extended to incorporate a variety of functional groups, such as carboxyl, azido and alkynyl groups, into the cyanine fluorochromes. Azido and alkynyl groups are two essential reagents in the click reaction. In the presence of Cu(I) catalyst, azide and alkyne containing molecules combine in a [3+2] dipolar cycloadditon to form a stable triazole moiety (17, 18). Since there are few, if any, azides or alkynes that occur naturally in biology, the click reaction is receiving increased attention as a potential bioorthogonal conjugation scheme (19-21). However, to date there has been little effort to develop NIR fluorophores modified with azides or alkynes that are suitable for biological and in vivo imaging applications.
EXPERIMENTAL PROCEDURES
General materials and methods
All chemicals were purchased from Aldrich or TCI and were used as received. All of the solvents were at least of reagent grade and were used without further purification. All of the dyes for characterization were purified by high-performance liquid chromatography (HPLC) on a Varian 210 instrument equipped with a 335 diode array detector. If not otherwise noted, HPLC buffer A is 0.1% trifluoroacetic acid (TFA) in water and buffer B is acetonitrile with 10% water and 0.1% TFA. 1H (400 MHz) and 13C NMR (100 MHz) spectra were collected on a Bruker Advance-400 NMR spectrometer at ambient temperature. The chemical shifts were measured versus tetramethylsilane (TMS) as an internal standard. High-resolution electrospray ionization (ESI) mass spectra were obtained on a Bruker Daltonics APEXIV 4.7 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer (FT-ICR-MS) in the Department of Chemistry Instrumentation Facility at the Massachusetts Institute of Technology.
Synthesis of 5-(phenylamino)-4-(phenylimino)methyl)-4-pentenoic acid derivatives (Intermediates 3a-c)
General synthetic route
Anhydrous dimethylformamide (1.01mL, 13 mmole) and oxalyl chloride (1.26g, 10 mmole) were added sequentially into 30 mL anhydrous dichloromethane (DCM) in an acetone/dry ice bath. The mixture was allowed to warm to room temperature over 15 minutes. Methyl 5,5-dimethoxyvalerate (881mg, 5 mmole) was then added dropwise to the reaction solution followed by heating at 70 °C for 2 h, allowing the DCM to evaporate. The resulting yellow oil was dissolved in 5 mL of 4 M NaOH and was heated at 70 °C for 1 hour. Before their use, the aniline derivatives (4-aminobenzoic acid, 4-azidoaniline, 3-ethynylaniline) were converted to their corresponding chloride salts on a 10 mmol scale by dissolving the aniline in acetone and precipitating its salt by addition of excess concentrated aqueous HCl. After removing the solvent under vacuum, the appropriate aniline chloride salt (10 mmol), dissolved in 5 mL water, was added to the basic, crude reaction solution and was allowed to stir at room temperature for 1 or more hours, until the reaction was complete. The final malonaldehyde dianil hydrochloride salts were precipitated as light yellow solids after the addition of 5 mL of 10 % aqueous HCl and were collected by filtration. The products were above 95% pure and used directly in the dye synthesis without further purification if not mentioned specifically.
Carboxylic acid intermediate (3a)
Yield 65%, 1.22 g. 1H NMR (400 MHz, DMSO-d6): δ 11.65 (b, 2H), 8.80 (s, 2H), 8.08 (d, 4H, J = 8.8 Hz), 7.63 (d, 4H, J = 8.8 Hz), 2.90 (t, 2H, J = 7.6 Hz), 2.48 (t, 2H, J = 7.9 Hz). 13C NMR (400 MHz, DMSO-d6): δ 174.78, 167.50, 160.44, 143.41, 131.97, 128.74, 119.31 112.49, 32.75, 18.35. HRMS-ESI [M+H]+ m/z calcd. for [C20H18N2O6]+ 383.1238, found 383.1231.
Azide intermediate (3b)
4-Azidoaniline hydrochloride was used as received. Yield 61%, 1.24 g. 1H NMR (400 MHz, DMSO-d6): δ 8.85 (s, 2H), 7.65 (d, 4H, J = 8.8 Hz), 7.22 (d, 4H, J = 8.7 Hz), 6.82 (d, 1H, J = 8.4 Hz), 6.68 (d, 1H, J = 8.5 Hz), 2.91 (t, 2H, J = 7.3 Hz), 2.45 (t, 2H, J = 7.8 Hz). 13C NMR (400 MHz, DMSO-d6): δ 173.84, 158.22, 145.09, 136.89, 136.44, 127.16, 120.35, 119.93, 115.91, 109.97, 32.72, 19.10. HRMS-ESI [M+H]+ m/z calcd. for [C18H16N8O2]+ 377.1469, found 377.1473.
Alkyne intermediate (3c)
Crude precipitates were purified on a silica column starting from 5% MeOH in DCM. The pure product was eluted at 15% MeOH in DCM. Yield 24%, 440mg. 1H NMR (400 MHz, DMSO-d6): δ 8.18 (s, 2H), 7.39 (s, 2H), 7.35 (d, 2H, J = 7.2 Hz), 7.32 (d, 2H, J = 1.5Hz), 7.18 (d, 2H, J = 7.0), 4.24 (s, 2H), 2.79 (t, 2H, J = 7.5 Hz), 2.44 (t, 2H, J = 7.8 Hz). 13C NMR (400 MHz, DMSO-d6): δ 175.02, 155.59, 145.80, 130.38, 127.38, 123.30, 121.25, 119.73, 113.94, 83.81, 81.57, 32.72, 19.10. HRMS-ESI [M+H]+ m/z calcd. for [C22H18N2O2]+ 343.1441, found 343.1443.
Dye Synthesis (CyAM-5 acid, CyAM-5 azide and CyAM-5 alkyne)
General synthetic route
1-Ethyl-2,3,3-trimethylindoleninium-5-sulfonate (4) was prepared according to literature procedures (5). Indoleninium 4 (4 equiv.) and intermediate 3a,b, or c (1 equiv.) were dissolved in 5 mL acetic anhydride with 0.5% v/v triethylamine. The reaction solution was heated at 115 °C for 1 hour in a sealed flask. The crude product was then precipitated by the addition of diethyl ether and was washed with additional diethyl ether. Preparative HPLC (Varian Pursuit XRs 10 C18 250×21.2 mm column, 0 - 25% buffer B over 30 minutes at a flow rate of 21 mL/min) was applied to purify CyAM-5-acid giving the blue dye as the free acid. The other dyes (CyAM-5 azide and CyAM-5 alkyne) were isolated as their ammonium salts by preparative HPLC over the same gradient but with buffer A as 50 mM NH4OAc in water and buffer B as 100% acetonitrile.
CyAM-5 acid (5a)
Yield 66%, 252 mg starting with 0.5 mmole of 3a. 1H NMR (400 MHz, DMSO-d6): δ 10.30 (s, 1H), 8.21 (d, 2H, J = 14.1 Hz), 7.86 (d, 2H, J = 8.7 Hz), 7.84 (s, 2H), 7.72 (d, 2H, J = 8.8 Hz), 7.66 (d, 2H, J = 8.2 Hz), 7.35 (d, 2H, J = 8.4 Hz), 6.33 (d, 2H, J = 14.2 Hz), 4.21 (m, 4H), 2.99 (t, 2H, J = 6.7 Hz), 2.60 (t, 2H, J = 7.1 Hz), 1.71 (s, 12H), 1.30 (t, 6H, J = 7.1 Hz). 13C NMR (400 MHz, DMSO-d6): δ 173.43, 172.03, 167.51, 154.11, 145.75, 143.81, 142.19, 141.40, 133.99, 130.99, 126.73, 125.62, 120.61, 118.98, 110.63, 100.36, 49.61, 39.67, 36.10, 27.46, 20.58, 12.69. HRMS-ESI [M]+ m/z calcd. for [C39H44N3O9S2]+ 762.2513, found 762.2539.
CyAM-5 azide (5b)
Yield: 64%, 116 mg starting with 0.24 mmole of 3b. 1H NMR (400 MHz, DMSO-d6): δ 10.08 (s, 1H), 8.20 (d, 2H, J = 14.2 Hz), 7.83 (s, 2H), 7.66 (d, 2H, J = 8.9 Hz), 7.64 (d, 2H, J = 8.7 Hz), 7.34 (d, 2H, J = 8.4 Hz), 7.03 (d, 2H, J = 8.9 Hz), 6.32 (d, 2H, J = 14.2 Hz), 4.20 (m, 4H), 2.98 (t, 2H, J = 6.7 Hz), 2.54 (t, 2H, J = 6.8 Hz), 1.70 (s, 12H), 1.29 (t, 6H, J = 7.1 Hz). 13C NMR (400 MHz, DMSO-d6): δ 173.73, 171.74, 154.46, 146.15, 142.49, 141.71, 137.42, 134.65, 133.61, 17.05, 121.55, 120.94, 120.31, 110.93, 100.73, 49.91, 39.63, 36.23, 27.79, 21.04, 13.02. HRMS-ESI [M]+ m/z calcd. for [C38H43N6O7S2]+ 759.2629, found 759.2630.
CyAM-5 alkyne (5c)
Yield 65%, 114 mg starting with 0.2 mmole of 3c. 1H NMR (400 MHz, DMSO-d6): δ 10.14 (s, 1H), 8.25 (d, 2H, J = 14.2 Hz), 7.88 (s, 2H), 7.85 (s, 1H), 7.71 (d, 2H, J = 8.1 Hz), 7.54 (d, 1H, J = 8.8 Hz), 7.40 (d, 2H, J = 8.4 Hz), 7.31 (t, 1H, J = 7.9 Hz), 6.38 (d, 2H, J = 14.2 Hz), 4.26 (m, 4H), 4.12 (s, 1H), 3.05 (t, 2H, J = 6.8 Hz), 2.61 (t, 2H, J = 6.8 Hz), 1.74 (s, 12H), 1.23 (t, 6H, J = 7.3 Hz). 13C NMR (400 MHz, DMSO-d6): δ 172.81, 171.24, 153.59, 145.20, 141.57, 140.79, 139.28, 132.49, 129.14, 126.39, 126.13, 122.19, 121.91, 119.99, 119.77, 110.03, 99.83, 83.33, 80.47, 48.98, 38.70, 35.49, 26.84, 20.30, 12.11. HRMS-ESI [M]+ m/z calcd. for [C40H44N3O7S2]+ 742.26-5, found 742.2612.
Optical properties
Absorption spectra and extinction coefficients of the fluorophores were obtained on a Varian Cary 50-Bio UV-visible spectrophotometer. Extinction coefficients were measured in phosphate-buffered saline (PBS), 10 mM phosphate buffer pH 7.0, 27 mM potassium chloride and 137 mM sodium chloride and were averaged over at least three sets of parallel experiments. For each trail, 213 mg of the HPLC-purified dyes were weighed on a Mettler AT201 analytical balance with an error of ±0.01 mg and were dissolved in deionized water using a 10 mL volumetric flask to prepare the stock solutions. Emission spectra were collected on a Varian Cary Eclipse fluorescence spectrophotometer. Quantum yield measurements were performed on at least eight trails for each dye derivative with the maximum absorption for each sample less than 0.1,using Cy5 as a standard (Φ = 0.27) (5). The standard deviation for both the extinction coefficient and quantum yield measurements is less than 5%. Photostability data were collected on a Horiba Jobin Yvon Fluorolog13 spectrofluorometer (Edison, NY) equipped with a 450W xenon lamp (Ushio inc. Japan). Dye photobleaching was observed by monitoring the fluorescence emission of absorbance matched dye samples (absorbance < 0.1) at an excitation wavelength 620 nm for 60 minutes.
Fluorescence labeling and cell imaging
Chinese hamster ovary (CHO) cells were maintained in a 5% CO2, water-saturated atmosphere and grown in F12 HAMS medium supplemented with 10% FBS, 1% penstrep and 1% L-glutamine. For biolabeling experiments, the cells were incubated for 2∼3 days in the regular medium or medium supplemented with 100 μM Ac4ManNAc (Molecular probes) in an eight-well LabTek II chamber slide (Nunc). The medium was gently aspirated, and the cells were fixed at -20 °C with methanol for 10 min and then acetone for 1 min. The cells then were washed with 500 μL of PBS (pH 7.0) three times and were treated with a bioorthogonal reaction solution containing 20 μM CyAM-5 alkyne, 100 μM CuSO4/100 μM Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) if mentioned, and 1 mM sodium ascorbate in PBS (pH 7.0) at room temperature for 1∼2 hours. Immediately prior to imaging, Vectra Shield mounting medium containing DAPI (Vector Labs) was applied to the cells. Fluorescent images were captured on a Nikon Eclipse TE2000-S fluorescence microscope with 40X objective equipped with a Photometrics Cascade 512B CCD camera using excitation and emission filters from Chroma Technology. Exposure times of 200 and 1000 ms, for the nuclear stain and carbocyanine dye channels, respectively, were used for image collection. All images from the carbocyanine dye channel were processed with identical leveling.
RESULTS AND DISCUSSION
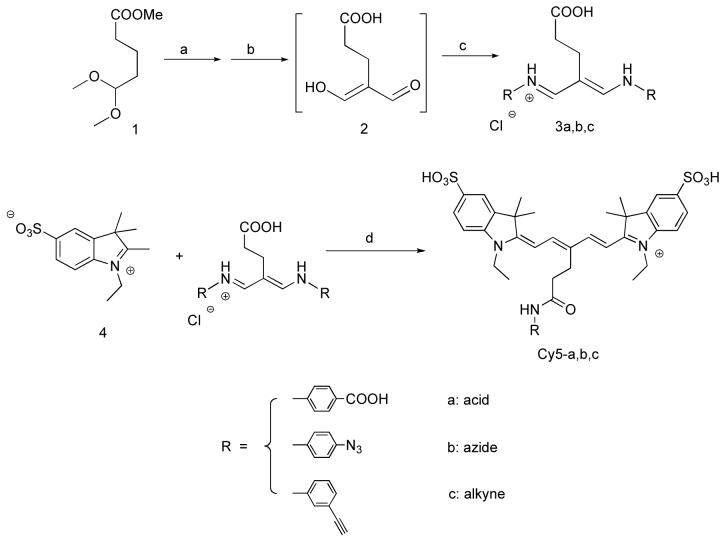
Our design of monofunctional dyes focuses on symmetric carbocyanine scaffolds, as shown in Scheme 1. These dyes have two identical monosulfonated-indoleninium moieties (4), and are prepared in simple one-stage dye condensation reactions, which typically have significantly higher yields than reactions reported for asymmetric cyanine dye synthesis (5, 6). The two sulfonate groups on the aromatic rings confer excellent water solubility to the final dyes. The acid, azide, and alkyne handles are introduced onto the central carbon atom of the polymethine chain through an alkyl chain followed by an arylamide linkage that is formed during the dye synthesis.
Scheme 1.
Synthetic route for preparation of monofunctional dye derivatives. A. (Upper) Synthesis of malonaldehyde dianil intermediates. B. (Lower) Synthetic scheme of monofunctional carbocyanine dyes.
a. C2O2Cl/DMF/CH2Cl2, 70 °C; b. 4 M NaOH; c. R-NH2Cl/10 % HCl; d. Acetic acid/5 % TEA, 115 °C.
The desired functional groups are incorporated into the malonaldehyde dianil intermediates (3a-c) (Scheme 1a). These malonaldehyde dianil derivatives are prepared via carboxylic acid intermediate, (2), which is synthesized from methyl 5,5-dimethoxyvalerate (1) via a Vilsmeier-Haack-Arnold aminoformylation followed by basic hydrolysis. An aniline moiety containing a carboxylic acid, alkyne, or azide in either the para- or meta- position is then added to reaction solution to yield the modified malonaldehyde dianil derivatives. Compounds 3a-c, which are isolated in 24-65% yield as their chloride salts after acidic workup, are of sufficient purity for subsequent use in the dye condensation reactions. The unique pathway for preparation of the malonaldehyde dianil intermediates in this report allows for facile introduction of a variety of functional groups onto the final cyanine dyes. By using different indoleninium and benzindolinium derivatives, these malonaldehyde dianil derivatives can be applied to prepare not only Cy5 dyes but also their monofunctional Cy5.5 analogs.
Synthesis of the monofuctional carbocyanine fluorophores is achieved by combining 1 equivalent of intermediate 3 and 4 equivalents of 1-ethyl-2,3,3-trimethylindoleninium-5-sulfonate (4) in acetic anhydride with 0.5% triethylamine. During the dye condensation, one of the two aniline groups on the malonaldehyde derivatives undergoes an intramolecular nucleophilic attack, reacting with the carboxylic acid of the malonaldehyde derivative forming an amide bond, as shown in Scheme 1b. This unique intramolecular rearrangement occurs during the synthesis of all the three dyes. The intramolecular rearrangement allows for facile installation of diverse functional groups onto the polymethine chain of the cyanine dye scaffold in a one-stage reaction to give a variety of cyanine dyes suitable for conjugation reactions. Combined with the simple isolation of the dyes by precipitation from diethyl ether, the overall yields for the dye condensations are all near 65% even after HPLC purification.
Regardless of the functional groups attached to the polymethine scaffold, the spectroscopic profiles of the three dye derivatives, CyAM-5 acid (5a), CyAM-5 azide (5b), and CyAM-5 alkyne (5c) are nearly identical. Absorption and emission spectra of CyAM-5 acid are shown in Figure 1. CyAM-5 acid has optical features of a typical pentamethine cyanine dye with absorption and emission maxima at 642 and 658 nm, respectively. The absorption and emission maxima of all three dyes are blue-shifted by approximately 5 nm in comparison to those of Cy5, whereas the stoke shifts are nearly identical to Cy5, ∼ 16 nm. Table 1 summarizes the optical properties of the monofuctional dyes. All of the three new dyes are extremely bright with extinction coefficients greater than 215,000 M-1cm-1 and quantum yields of 0.17 or higher. Furthermore, the new dyes have better photostability than typical cyanine derivatives that have unsubstituted polymethine chains. As shown in figure S2, after an hour irradiation, CyAM-5 acid shows 28% less photobleaching when compared to Cy5.
Figure 1.
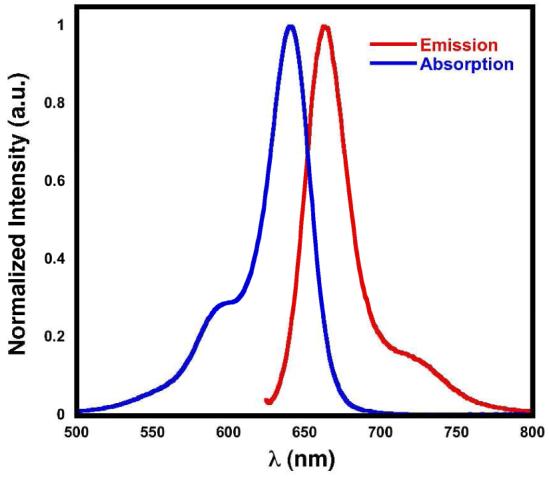
Normalized absorption and emission spectra of CyAM-5 acid in PBS, pH 7.0.
Table 1.
Optical properties of pentamethine carbocyanine dyes
| λabs (nm)a | λem (nm) | ε (M-1cm-1)b | Φc | |
|---|---|---|---|---|
| CyAM-5 acid | 642 | 656 | 215000 | 0.17 |
| CyAM-5 azide | 641 | 658 | 222000 | 0.20 |
| CyAM-5 alkyne | 641 | 657 | 240000 | 0.20 |
Spectra were obtained in phosphate buffered solution, pH 7.0. Details are listed in experimental procedures.
Extinction coefficients at 640 nm are averaged over at least three sets of equivalent experiments. Details are listed in experimental procedures.
Emission spectra were excited at 620 nm. Details are listed in experimental procedures.
Due to their bright fluorescence, long wavelength absorption and emission as well as the ability to accommodate a variety of functional groups for conjugation reactions, this new class of cyanine dyes should find use in many biological labeling and imaging applications. For example, CyAM-5 acid can be conveniently converted to its corresponding activated succinimidyl ester (22) for conjugation to amine containing molecules. CyAM-5 azide and alkyne have a variety of potential uses in which they can be employed as a component in the click reaction. In addition, the aryl azide of CyAM-5 azide may find use in photocrosslinking experiments.
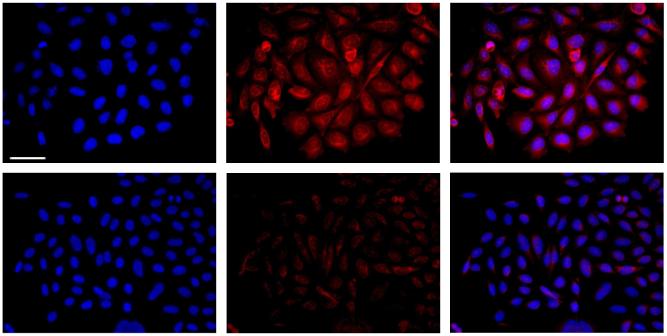
Recently fluorescence labeling methods based upon click chemistry have been developed for visualization of glycoproteins in cells incubated with azide containing sugars (19-21). We have adapted this sugar labeling system to demonstrate the ability of our monofunctional dyes to undergo efficient bioorthogonal coupling in a bio-relevant system. Azide sugar modified Chinese hamster ovary (CHO) cells were first prepared by incubation of the cells with azidoacetylmannosamine containing media for 3 days. These cells then are fixed with methanol/acetone and are labeled with CyAM-5 alkyne in the presence of copper (I) as a catalyst. The cells bearing azido sialic acid in their glycoprotein show increased fluorescence signal when compared to control cells that were not incubated with the azido-mannosamine, as shown in Figure 2, indicating the labeling of the cells with CyAM-5 alkyne via formation of a triazole linkage. The enhancement of fluorescence signal is only observed in the presence of both catalyst Cu(I) and azido glycans (Figure S3). The Cu(I) chelating ligand, trisbenzyltriazolylmethylamine (TBTA), facilitates the reaction, but is not necessary for efficient labeling of the cells. The requirement of both Cu(I) and azido-sugar treatment for fluorescent labeling of CHO cells with CyAM-5 alkyne indicates that the resulting click reaction is a selective, bioorthonogal method for cell labeling.
Figure 2.
Fluorescene microscopy of CHO cells labeled with CyAM-5 alkyne. Cells incubated with azido-sugar supplimented media (top) or unsupplimented media (bottom) after treatment with CyAM-5 alkyne in the presence of Cu(I)/TBTA as catalyst. Experimental details and additional controls are shown in the supporting material. Fluorescence signal from the nuclear stain, DAPI, colored blue (left), CyAM-5 alkyne colored red (center) and the merged images (right) are shown in the figure. The scale bar represents 50 μm.
In conclusion, a unique, general strategy for synthesizing monofunctional carbocyanine derivatives has been developed that is amenable to the preparation of far-red/NIR cyanine dyes with high yields containing acid, alkyne, or azide synthetic handles. These dyes are expected to have a variety of potential applications in bio- and bioorthogonal labeling schemes. Furthermore, using this synthetic approach, installation of additional useful functional groups such as amines, thiols, and maleimides should be feasible.
Supplementary Material
ACKNOWLEGEMENT
This work is supported by NIH grant 1-U01-HL080731. We thank Dr. Neal Devaraj for helpful discussion on click chemistry.
LITERATURE CITED
- (1).Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17:375–8. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- (2).Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat. Med. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- (3).Ballou B, Ernst LA, Waggoner AS. Fluorescence imaging of tumors in vivo. Curr. Med. Chem. 2005;12:795–805. doi: 10.2174/0929867053507324. [DOI] [PubMed] [Google Scholar]
- (4).Lin Y, Weissleder R, Tung CH. Novel near-infrared cyanine fluorochromes: synthesis, properties, and bioconjugation. Bioconjug. Chem. 2002;13:605–10. doi: 10.1021/bc0155723. [DOI] [PubMed] [Google Scholar]
- (5).Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS. Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 1993;4:105–11. doi: 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
- (6).Mujumdar SR, Mujumdar RB, Grant CM, Waggoner AS. Cyanine-labeling reagents: sulfobenzindocyanine succinimidyl esters. Bioconjug. Chem. 1996;7:356–62. doi: 10.1021/bc960021b. [DOI] [PubMed] [Google Scholar]
- (7).Narayana N, Patonay G. A new method for the synthesis of heptamethine cyanine dyes: synthesis of new near-infrared fluorescent labels. J. Org. Chem. 1995;60:2391–2395. [Google Scholar]
- (8).Pham W, Medarova Z, Moore A. Synthesis and application of a water-soluble near-infrared dye for cancer detection using optical imaging. Bioconjug. Chem. 2005;16:735–40. doi: 10.1021/bc049700+. [DOI] [PubMed] [Google Scholar]
- (9).McCarthy JR, Kelly KA, Sun EY, Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomed. 2007;2:153–67. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- (10).Tung CH, Lin Y, Moon WK, Weissleder R. A receptor-targeted near-infrared fluorescence probe for in vivo tumor imaging. Chembiochem. 2002;3:784–6. doi: 10.1002/1439-7633(20020802)3:8<784::AID-CBIC784>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- (11).Strekowski L, Lipowska M, Patonay G. Substitution reactions of a nucleofugal group in heptamethine cyanine dyes. Synthesis of an isothiocyanato derivative for labeling proteins with a near-infrared chromophore. J. Org. Chem. 1992;57:4578–4580. [Google Scholar]
- (12).Flanagan JH, Jr., Khan SH, Menchen S, Soper SA, Hammer RP. Functionalized tricarbocyanine dyes as near-infrared fluorescent probes for biomolecules. Bioconjug. Chem. 1997;8:751–6. doi: 10.1021/bc970113g. [DOI] [PubMed] [Google Scholar]
- (13).Kiyose K, Kojima H, Urano Y, Nagano T. Development of a ratiometric fluorescent zinc ion probe in near-infrared region, based on tricabocyanine chromophore. j. Am. Chem. Soc. 2006;128:6548–6549. doi: 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]
- (14).Hilderbrand SA, Kelly KA, Weissleder R, Tung CH. Monofunctional near-infrared fluorochromes for imaging applications. Bioconjug. Chem. 2005;16:1275–1281. doi: 10.1021/bc0501799. [DOI] [PubMed] [Google Scholar]
- (15).Lee H, CMason JC, Achilefu S. Synthesis and spectral properties of near-infrared aminophenyl-, hydroxyphenyl-, and phenyl-substituted heptamethine cyanines. J. Org. Chem. 2008;73:723–725. doi: 10.1021/jo701793h. [DOI] [PubMed] [Google Scholar]
- (16).Zaheer A, Wheat TE, Frangioni JV. IRDye78 conjugates for near-infrared fluorescence imaging. Mol. Imaging. 2002;1:354–64. doi: 10.1162/15353500200221302. [DOI] [PubMed] [Google Scholar]
- (17).Fokin VV. Click imaging of biochemical processes in living systems. ACS Chem. Biol. 2007;2:775–8. doi: 10.1021/cb700254v. [DOI] [PubMed] [Google Scholar]
- (18).Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemicl function from a few good reaction. Angew. Chem. Intl. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- (19).Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA. 2007;104:16793–7. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:12371–6. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc. Natl. Acad. Sci. USA. 2007;104:2614–9. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Unpublished data. Reaction is complete within one hour at room temperature in the presence of 4 equiv. disuccimidyl carbonate and 4 equiv. triethylamine in anhydrous DMF.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.