Abstract
The chemical structures of the three major bile acids present in the gallbladder bile of the Red-winged tinamou (Rhynchotus rufescens), an early evolving, ground-living bird related to ratites, were determined. Bile acids were isolated by preparative reversed-phase HPLC. Two of the compounds were identified as the taurine N-acylamidates of (25R)-3α,7α-dihydroxy-5β-cholestan-27-oic acid (constituting 22% of biliary bile acids) and (25R)-3α,7α,12α-trihydroxy-5β-cholestan-27-oic acid (constituting 51%). The remaining compound, constituting 21% of biliary bile acids, was an unknown C27 bile acid. Its structure was elucidated by LC/ESI-MS/MS and NMR and shown to be the taurine conjugate of (25R)-1β,3α,7α-trihydroxy-5β-cholestan-27-oic acid, a C27 trihydroxy bile acid not previously reported. Although C27 bile acids with a 1β-hydroxyl group have been identified as trace bile acids in the alligator, this is the first report of a major biliary C27 bile acid possessing a 1β-hydroxyl group.
Keywords: 1β-hydroxylation; C27 bile acids; 1β,3α,7α-trihydroxy-5β-cholestan-27-oic acid; taurine conjugate; LC-MS; NMR
Bile salts are amphipathic end products of cholesterol metabolism with multiple physiological functions. Most bile salts belong to one of three large classes: C27 bile alcohols, C27 bile acids, and C24 bile acids. For C24 bile acids, which are present in mammals, chenodeoxycholic acid, possessing an α-hydroxyl group at C-3 and at C-7, may be considered the root bile acid (1); in most species, a third hydroxyl group is added to the hydrophilic face of the planar bile acid molecule during bile acid biosynthesis. The most common sites of additional nuclear hydroxylation are at C-12 (cholic acid) or C-16 (2) (for which the name avicholic acid has been proposed) (3). Less-common sites of hydroxylation in major primary bile acids are at C-1 (4, 5), C-6 (6, 7), and C-15 (8). Hydroxylation at C-5 has also been reported to occur in pheasant biliary bile acids (9), and C-19 hydroxy bile acids have been identified in human urinary bile acids (10). Such additional nuclear hydroxylation of chenodeoxycholic acid may have biological utility in that it precludes the formation of lithocholic acid (11). Lithocholic acid is formed when chenodeoxycholic acid undergoes bacterial 7-dehydroxylation in the distal intestine (12). Lithocholic acid is a highly toxic bile acid in many mammalian species (13–16).
This reasoning should be applicable to C27 bile acids, which are the major biliary bile acids in amphibians, some reptiles, and ancient birds (2). The root C27 bile acid would possess an α-hydroxyl group at C-3 and at C-7 and is 3α,7α-dihydroxy-5β-cholestan-27-oic acid (no trivial name has been proposed). The most common site of additional nuclear hydroxylation for C27 bile acids is at C-12 (2). To date, the only additional sites of nuclear hydroxylation that have been identified in C27 bile acids are at C-1 in alligators (17), C-15 in turtles (18), and C-16 in early evolving birds (19). The C-15 hydroxy bile acid is a 7-deoxy bile acid, suggesting that it is a secondary bile acid formed by bacterial 7-dehydroxylation of an unidentified precursor. Hydroxylation at C-15 could occur during primary bile acid biosynthesis or result from C-15 hydroxlation of the 7-deoxy bile acid, as occurs in the wombat (20). Whether such third site nuclear hydroxylation in primary C27 bile acid biosynthesis has biological utility is not known, as the toxicity of the C27 bile acid possessing only a 3α-hydroxyl group has not been examined.
We report here the presence of a new C27 bile acid that is a major biliary bile acid in the Red-winged tinamou, (Rhynchotus rufescens), an ancient bird that is considered to be related to the ratites (rhea, ostrich, emu, cassowary, and kiwi) (21). The new bile acid was found to be (25R)-1β,3α,7α-trihydroxy-5β-cholestan-27-oic acid. This trihydroxy C27 bile acid, occurring in bile as its taurine N-acyl amidate, has not been previously described.
EXPERIMENTAL PROCEDURES
Biological material
Bile was obtained from three adult Red-winged tinamou (Rhynchotus rufescens), donated by the Zoo-botanic Foundation of Belo Horizonte, Brazil. The birds were anesthetized with intravenous injection of sodium pentobarbital (50 mg/Kg BW). Following the induction of anesthesia, the gallbladder was exposed and the bile was collected by aspiration. Bile samples were diluted in 5 volumes of isopropanol and kept at 4°C. Bile samples were then shipped by airmail to the Chemistry Department of Nihon University. Figure 1 is a painting of the Red-winged Tinamou.
Fig. 1.

Red-winged tinamou (Rhynchotus rufescens). The painting is from Ref. 20; permission to use this figure was received from the Oxford University Press.
Principles of research involving animals followed those expressed in the “Princípios éticos para o uso de animais em experimentação,” advocated by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais, Brazil (CETEA-UFMG) http://www.ufmg.br/coep/cetea.html).
Materials and reagents
Authentic taurine conjugates of (25R)- and (25S)-3α,7α-dihydroxy-5β- cholestan-27-oic acids and (25R)- and (25S)-3α,7α,12α-trihydroxy- 5β-cholestan-27-oic acids were previously synthesized in our laboratory (22). The unconjugated forms of these bile acids were kindly donated by M. Une (Department of Pharmaceutical Sciences, Hiroshima International University, Hiroshima, Japan). All other chemicals employed were of analytical reagent grade.
HPLC-evaporative light scattering detection (ELSD) analysis of gallbladder bile of the tinamou
The apparatus used was a Jasco LC-2000plus HPLC system (two PU-2085 high-pressure pumps, an MX-2080-32 solvent mixing module, and a CO-2060 column heater) equipped with a ChromNAV data-processing system (Tokyo, Japan). A Capcell Pak-type MGII column [250 mm × 3.0 mm inner diameter (ID); particle size, 5 μm; Shiseido, Tokyo, Japan] was employed and kept at 37°C. An Alltech 2000ES evaporative light-scattering detector (ELSD) (Deerfield, IL) was used under the following conditions: flow rate of purified compressed air used as a nebulizing gas was 1.6 L/min, and the temperature of the heated drift tube was 82°C. The mobile phases used were 15 mM-ammonium acetate/acetic acid buffer solution (pH 5.4) methanol mixtures. A gradient elution was carried out as follows: initial –10.0 min (70% methanol, constant); 10.1–30.0 min (70→80% methanol, linear gradient); 30.1 min–end (80% methanol, constant). The flow rate was kept at 400 μl/min during the analysis.
Isolation of major bile acids from the bile of the tinamou
Tinamou biles were pooled and diluted with isopropanol (10 ml), filtered, and the filtrate evaporated under a nitrogen stream at below 40°C. The residue was dissolved in methanol/water (1:9, v/v) (5 ml) and then applied to a preconditioned Sep-Pak C18 cartridge (360 mg; Waters, Milford, MA). After the cartridge was washed successively with water (2 ml) and 20% methanol (2 ml), the bile acid fraction was eluted with 90% methanol (3 ml). The 90% methanol eluate was evaporated under a nitrogen stream at 40°C. The residue was then redissolved in 200 μl of methanol, and the major bile acids were isolated by preparative reversed-phase HPLC. The apparatus consisted of a Jasco Gulliver series HPLC system with two PU-980 high-pressure pumps, an HG-980-31 solvent mixing module, and an HG-980-50 degasser. HPLC was carried out by stepwise gradient elution on a Capcell Pak C18-type MGII column (5 μm, 250 mm × 10 mm ID; Shiseido, Tokyo, Japan) using 5 mM-ammonium acetate/methanol mixtures as the mobile phase. The methanol composition was gradually increased at a flow rate of 2 ml/min using the following HPLC conditions: 20% (0–15 min) → 50% (15.1–30 min) → 56% (30.1–90 min) → 62% (90.1–150 min) → 68% (150.1–210 min). The 56%, 62%, and 68% methanol fractions, which contained compounds A, C, and E (see below), respectively, were collected. Each fraction was diluted several times with 5 mM-ammonium acetate solution (pH 6.5) and then freeze-dried under 20 mmHg at room temperature. Each of the isolated components A, C, and E was examined by LC-ESI-MS/MS.
LC-ESI-MS/MS spectra of major components A, C, and E
Negative ion LC-ESI-MS/MS analyses of the tinamou bile components were obtained on an API 5000 LC-MS/MS system (Applied Biosystems, Inc., CA) equipped with a Nanoscope HPLC system (Shiseido, Tokyo, Japan). Chromatographic separation was carried out using a Capcell Pak C18 type MGII column (5 μm, 100 × 2.0 mm ID) using 15 mM-ammonium acetate (pH 6.5)/methanol mixtures as the mobile phase at a flow rate of 178 μl/min. A mixture of 15 mM-ammonium acetate/methanol 6/10 (v/v) was used for the separation of compounds A and C, and 5/10 (v/v) for compound E. The mass detector was set to the following conditions: curtain gas flow, 25 psi; ion source gas 1 flow, 40 psi; ion source gas flow, 60 psi; ion spray voltage, −4500 V; interface temperature, 600°C; Declustering potential, −80 V; entrance potential, −10V; collision energy, −80 V; collision gas pressure, 6.0 × 10−3 mbar.
1H- and 13C-NMR spectra of major components A, C, and E
NMR spectra were recorded at 23°C in CD3OD in a 5 mm tube on a JEOL ECA-600 instrument (600 and 149.4 MHz for 1H and 13C, respectively); 1H and 13C chemical shifts were expressed in δ ppm. 1H and 13C resonance assignments were made using a combination of two-dimensional homonuclear (1H-1H) and heteronuclear (1H-13C) shift-correlated techniques, which include 1H-1H correlation spectroscopy (COSY), 1H-1H nuclear Overhauser effect spectroscopy (NOESY), 1H detected heteronuclear multiple quantum correlation (HMQC), and 1H detected heteronuclear multiple bond correlation (HMBC) experiments. These two-dimensional NMR spectra were recorded using standard pulse sequences and parameters recommended by the manufacturer. The 13C distortionless enhancement by polarization transfer (135°, 90°, and 45°) spectra were also measured to determine the exact 1H signal multiplicity and to differentiate among CH3, CH2, CH, and C based on their proton environments.
RESULTS
Isolation and identification of major bile acids in tinamou bile
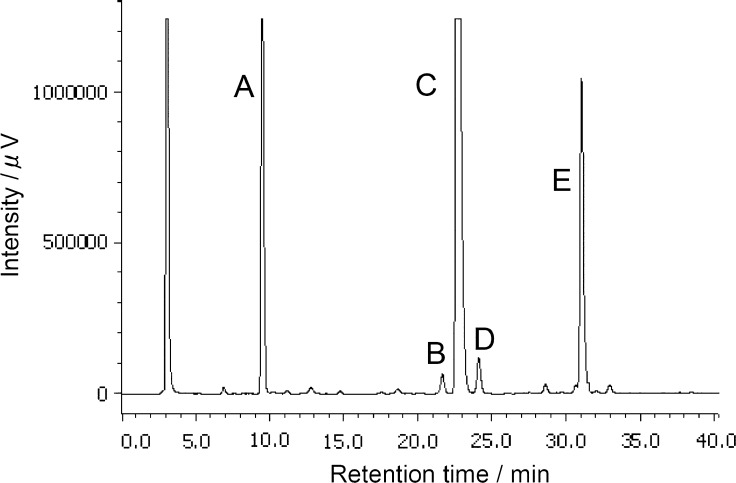
As shown in Fig. 2, HPLC-ELSD analysis of the bile acids present in the gallbladder bile of the tinamou showed three major peaks, which were designated as A (21%), C (51%), and E (22%). Two additional bile acids were present in much lower proportions: B (1.2%) and D (2.3%); because of the limited amount of these bile acids, no attempt was made to elucidate their chemical structure. No C24 bile acids were present.
Fig. 2.
HPLC-evaporative light-scattering detector (ELSD) profile of the bile acids of the tinamou. Peak A, 21% of biliary bile acids was subsequently identified as (25R)-1β,3α,7α-trihydroxy-5β-cholestan-27-oyl taurine. Its retention time (RT) was 9.4 min) peak B, 1.2%, was not identified; its RT was 21.6 min; peak C, 51.4%, was identified as (25R)-3α,7α,12α-trihydroxy- 5β-cholestan-27-oyl taurine, RT 22.9 min; peak D, 2.3%, was not identified, RT 24.1 min; and peak E, 21.9%, was identified as 25R)-3α,7α-dihydroxy-5β-cholestan-27-oyl taurine, RT 31.0 min.
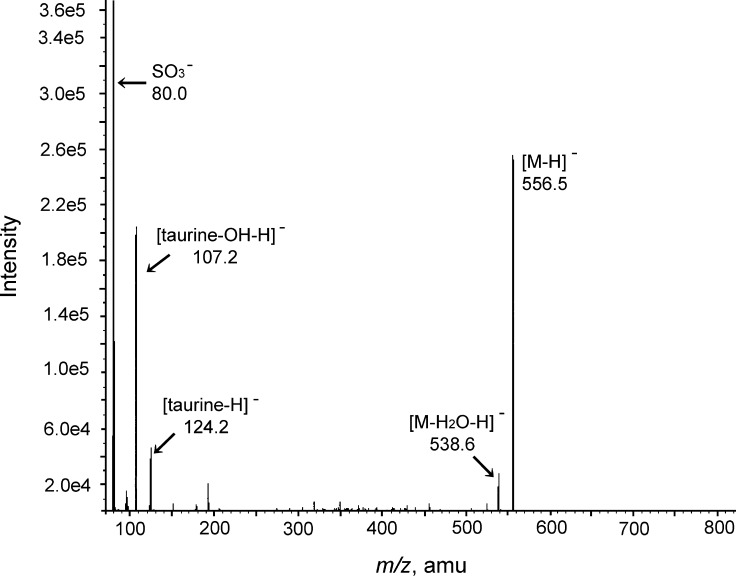
The three major components A, C, and E were isolated by preparative reversed-phase HPLC and then subjected to LC-ESI-MS/MS analyses. In the first ESI-MS spectra, the deprotonated molecules, [M-H]−, were as follows: peaks A and C, m/z 556, taurine-conjugated trihydroxy C27 bile acids; peak E, m/z 540, taurine-conjugated dihydroxy C27 bile acid. In the collision induced dissociation spectra obtained by selecting the deprotonated ions as a precursor ion, the compounds A, C, and E afforded the characteristic fragment ions at m/z 124.2 [taurine-H]−, 107.2 [taurine-OH-H]−, and 80.0 [SO3]−, indicating the presence of an N-acylamide linkage with taurine in the side chain (8, 20). The LC-ESI-MS/MS fragmentation pattern of the compound A is shown in Fig. 3.
Fig. 3.
LC/ESI-MS/MS spectrum of the isolated compound A.
Peaks C and E were readily identified as the well-known C27 bile acid taurine conjugates of (25R)-3α,7α,12α-trihydroxy-5β-cholestan-27-oic acid and (25R)-3α,7α-dihydroxy-5β-cholestan-27-oic acid. Identification was made by a direct comparison of the HPLC-ELSD retention times and the m/z values (556 and 540, respectively) of the deprotonated molecules in the ESI-MS, as well as the fragmentation pattern of the collision induced dissociation spectra, compared with the behavior of authentic standards (22–24).
Peak A, a taurine conjugated trihydroxy C27 bile acid, had a shorter retention time on the reversed-phase HPLC than that of the peak C. In addition, peak A showed no significant LC-MS fragmentation pattern that would provide information on its structure, particularly for the position and stereochemical configuration of the three hydroxyl groups.
To clarify the position of the hydroxyl groups present in compound A, 1H- and 13C-NMR spectra were determined. Table 1 shows the 1H- and 13C-NMR data for the compound A, together with those of compounds C and E. The signal assignments were made on the basis of several two-dimensional NMR techniques, which include HMQC, HMBC, 1H-1H COSY, long-range 1H-1H COSY, and NOESY.
TABLE 1.
1H and 13C–NMR signal assignments of isolated compound A, C, and Ea
| 1β,3α,7α-trihydroxy-5β-cholestan-27-oyl taurine (Peak A) |
3α,7α,12α-trihydroxy-5β-cholestan-27-oyl taurine (Peak C) |
3α,7α-dihydroxy-5β-cholestan-27-oyl taurine (Peak E) |
||||
|---|---|---|---|---|---|---|
| Carbon No. | 13C | 1H | 13C | 1H | 13C | 1H |
| 1 | 74.23 | 3.86 (α-H, brs) | 36.49 | 36.59 | ||
| 2 | 38.12 | 31.20 | 31.38 | |||
| 3 | 67.32 | 3.83 (β-H, brm) | 72.93 | 3.35 (β-H, brm) | 72.91 | 3.35 (β-H, brm) |
| 4 | 40.03 | 40.51 | 40.53 | |||
| 5 | 36.93 | 43.25 | 43.24 | |||
| 6 | 35.49 | 35.77 | 35.87 | |||
| 7 | 68.90 | 3.78 (β-H, brs) | 69.12 | 3.76 (β-H, brs) | 69.10 | 3.78 (β-H, brs) |
| 8 | 41.05 | 41.08 | 40.85 | |||
| 9 | 35.61 | 27.87 | 34.05 | |||
| 10 | 40.69 | 35.92 | 36.24 | |||
| 11 | 21.76 | 28.81 | 21.78 | |||
| 12 | 41.05 | 74.09 | 3.94 (β-H, brs) | 41.08 | ||
| 13 | 43.41 | 47.47 | 43.65 | |||
| 14 | 51.39 | 42.95 | 51.53 | |||
| 15 | 24.67 | 24.23 | 24.63 | |||
| 16 | 29.24 | 29.56 | 29.39 | |||
| 17 | 57.58 | 48.31 | 57.58 | |||
| 18 | 12.19 | 0.68 (s) | 13.00 | 0.70 (s) | 12.17 | 0.68 (s) |
| 19 | 18.03 | 1.01 (s) | 23.15 | 0.91 (s) | 23.39 | 0.92 (s) |
| 20 | 37.07 | 37.10 | 37.08 | |||
| 21 | 19.23 | 0.92 (d, J 6.6) | 18.08 | 0.98 (d, J 6.6) | 19.25 | 0.93 (d, J 6.6) |
| 22 | 37.09 | 37.10 | 37.11 | |||
| 23 | 25.02 | 25.08 | 25.03 | |||
| 24 | 35.73 | 35.77 | 35.74 | |||
| 25 | 42.34 | 42.35 | 42.35 | |||
| 26 | 179.48 | 1.08 (d, J 6.6) | 179.46 | 1.08 (d, J 6.6) | 179.57 | 1.09 (d, J 6.6) |
| 27 | 18.08 | 18.08 | 18.08 | |||
| 28 | 36.47 | 3.59 (m) | 36.49 | 3.59 (m) | 36.47 | 3.59 (m) |
| 29 | 51.58 | 2.95 (t) | 51.57 | 2.96 (t) | 51.53 | 2.96 (t) |
s, singlet; d, doublet; t, triplet; m, multiplet; brs, broad singlet; brm, broad multiplet.
Chemical shifts were expressed as δ ppm, relative to TMS.
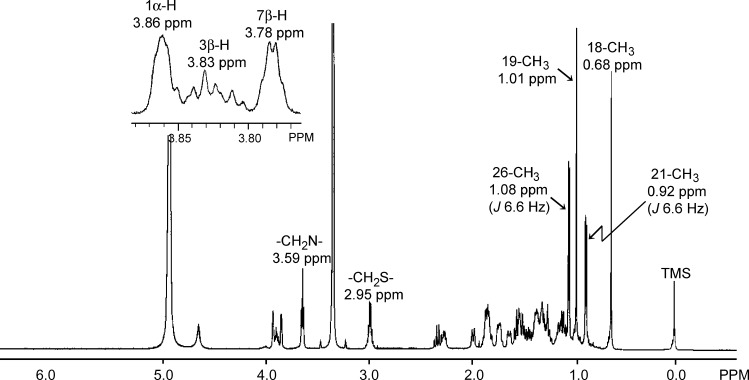
The 1H-NMR spectrum of compound A is illustrated in Fig. 4. Of note was that the 19-CH3 (1.01 ppm) and 3β-H (3.83 ppm) signals caused large down-field shifts, relative to those (0.92 and 3.35 ppm, respectively) of compound E (25R),-3α,7α-dihydroxy-5β-cholestan-27-oyl taurine. The observation strongly suggested that compound A had an additional hydroxyl group in the proximity of both the 19-CH3 and 3β-H (e.g., 1β- or 5β-position).
Fig. 4.
1H NMR spectra of the isolated compound A.
In the HMQC spectrum, correlation peaks arising from the 1J (1H/13C) coupling in the 5β-steroid nucleus were used to confirm the mutual connectivity of protons vicinal to the carbon bearing an oxygen-containing functional group [i.e., the 3β-H (3.83 ppm, brm) with the C-3 (67.32 ppm) and the 7β-H (3.78 ppm, brs) with the C-7 (68.90 ppm)]. An unknown proton peak occurring at 3.86 ppm as a singlet, probably arising from the vicinal proton of a hydroxyl group, was coupled with a carbon signal that resonated at 74.23 ppm.
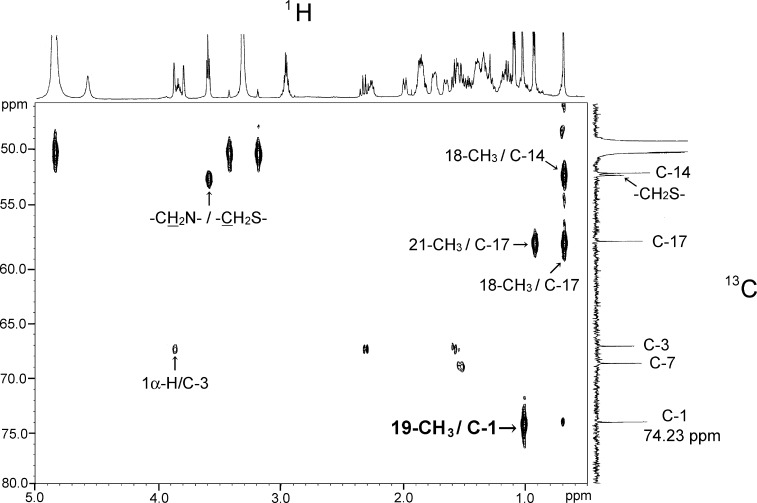
A correlation was observed between the 19-methyl protons (1.01 ppm, singlet) and a carbon peak occurring at 74.23 ppm (3J, 1H/13C) in the HMBC spectrum (Fig. 5). A coupling between the 19-methyl protons and a proton peak that occurred at 3.86 ppm (4J, 1H/1H) also appeared in the long-range 1H-1H COSY spectrum (data not shown). In addition, a distortionless enhancement by polarization transfer experiment indicated that the carbon appearing at 74.23 ppm was a methine carbon having a hydrogen atom. Taken together, the findings implied that the third hydroxylation position in compound A was C-1, but not C-5.
Fig. 5.
Partial heteronuclear multiple bond correlation (HMBC) spectra of the isolated compound A.
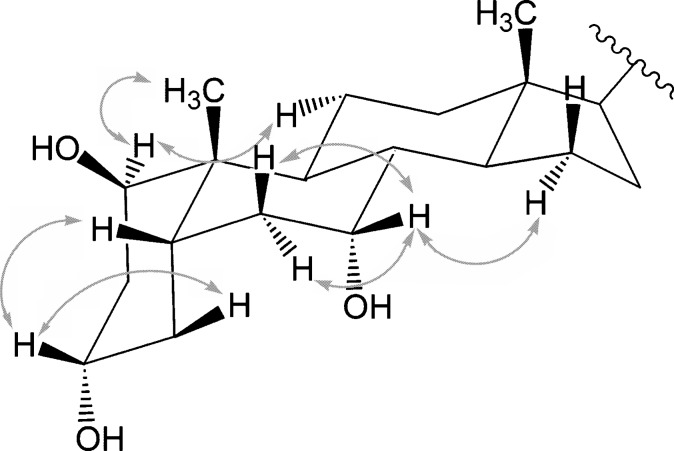
The NOESY spectrum in compound A was then measured to determine the stereochemical configuration of the hydroxyl group at C-1. Several specific NOE correlations were observed, as illustrated in Fig. 6. The quasi-1,3-diaxial correlation between the 1β-H (ax.) and 3β-H (ax.) coupling was not present, indicating that the hydroxyl group at C-1 was in a β-configuration.
Fig. 6.
Nuclear Overhauser effect (NOE) correlations observed for the isolated compound A.
In addition to the 1H- and 13C-NMR characteristics of compound A mentioned above, characteristic 13C signals were observed at 36.47 and 51.58 ppm. These were assigned to CH2N and CH2S, respectively; adjacent proton signals were also observed at 3.57 (CH2N) and 2.89 ppm (CH2S). These data provided the confirmatory evidence for the presence of the N-acylamide linkage with taurine in the side chain (8, 20).
DISCUSSION
The combination of NMR and MS data established the chemical structure of the unknown bile acid A in the bile of the Red-winged tinamou as the taurine conjugate of (25R)-1β,3α,7α-trihydroxy-5β-cholestan-27-oic acid. This is the first identification of a C27 bile acid carrying a 1β-hydroxyl group as a major biliary bile acid in any vertebrate. The synthesis of this compound has not been reported. Which cytochrome P450 hydroxylase(s) mediate hydroxylation of C27 bile acids or a precursor or both is not known.
The 1H chemical shifts and the signal multiplicity of the 1α-H, 3β-H, 7β-H, and 19-CH3 in compound A were in good agreement with those reported previously for the C24 homolog, 1β,3α,7α-trihydroxy-5β-cholan-24-oic acid (5). Almost all of the 13C chemical shifts of the individual carbon atoms in 5β-steroid skeleton agreed well with those reported for 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid (25) [but with some exceptions (C-9, C-11, C-12, C-13, C-14, C-17, and C-18)]. The 13C chemical shifts for the relevant carbon atoms in the A and B rings differed markedly from those reported for the 1α-hydroxy epimer, 1α,3α,7α-trihydroxy-5β-cholan-24-oic acid (4).
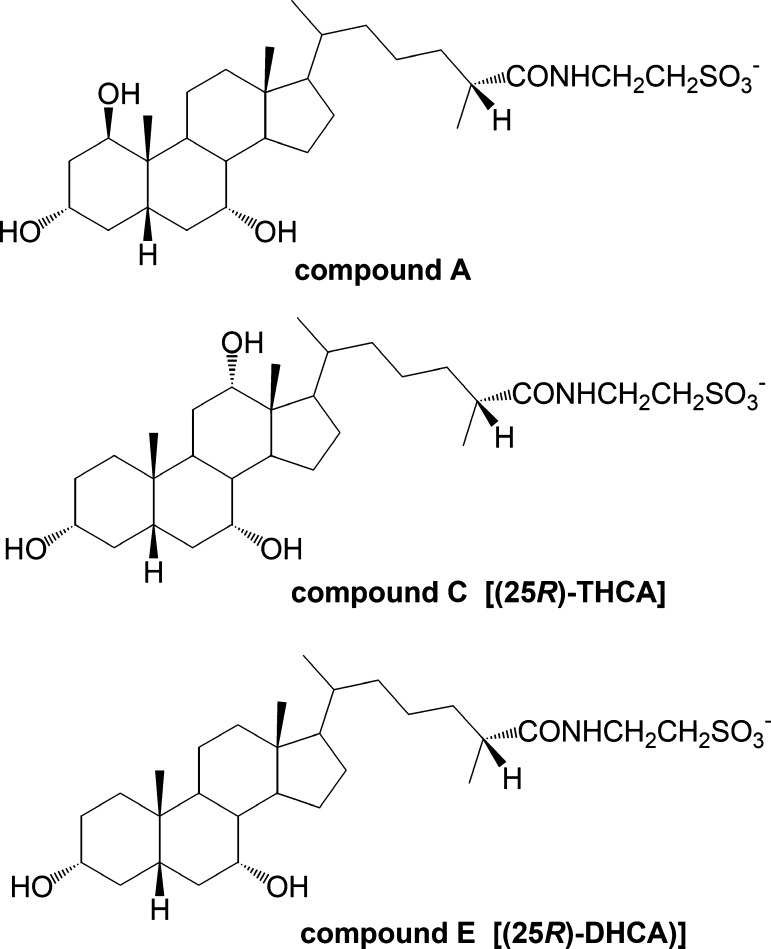
Although the absolute configuration of the chiral center at C-25 in compound A could not be determined directly from the LC/MS and NMR data, there is strong evidence that it is in the (25R)-configuration. One reason is the concurrent presence in tinamou bile of the two 25 diastereoisomers (25R)-3α,7α-dihydroxy- 5β-cholestan-27-oic acid (comprising 22% of biliary bile acids) and (25R)-3α,7α,12α-trihydroxy-5β-cholestan-27-oic acid (51%). Additional supporting evidence is that C24 bile acids were completely absent from tinamou bile. In the biosynthesis of C24 bile acids from cholesterol (C27), a key step is racemization of the 25R epimer of the C27 cholestanoic acid precursors to their corresponding 25S epimers by a peroxisomal racemase. Only the 25S epimers are substrates for the peroxisomal oxidases mediating subsequent oxidative cleavage of the C8 side chain (26–30). Thus, we conclude that compound A is the taurine conjugate of (25R)-1β,3α,7α-trihydroxy-5β-cholestan-27-oic acid. (Fig. 7).
Fig. 7.
Structure of the C27 bile acids isolated from tinamou (25R): -1β,3α,7α-trihydroxy-5β-cholestan-27-oyl taurine (compound A) (25R); -3α,7α,12α-trihydroxy-5β-cholestan-27-oyl taurine (compound C); and (25R)-3α,7α-dihydroxy-5β-cholestan-27-oyl taurine (compound E).
The 12α-hydroxy derivative of the compound identified in tinamou bile (1β,3α,7α,12α-tetrahydroxy-5β-cholestan-27-oic acid) was shown to be present in the bile of the alligator, Alligator mississippiensis (17), as a trace constituent. This 1β-hydroxylated C27 bile acid was also found in the urine of patients with Zellweger syndrome (31–34), who have impaired peroxisomal function.
The C24 bile acid, 1β,3α,7α-trihydroxy-5β-cholan-24-oic acid is the C24 homolog of compound A, and has been isolated from the biliary bile acids of fruit pigeons and doves (Columbiformes) in whom it occurs as its glycine or taurine amidate (5). Bile acids (C24) with a 1β-hydroxyl group have also been detected in the urine of neonates (35, 36), in the feces of young children (37), and in the urine of patients with cholestatic liver disease (38). The 1β-hydroxy derivative of ursodeoxycholic acid has been identified in the urine of patients ingesting ursodeoxycholic acid for cholestatic liver disease (39, 40). The C-1α epimer, 1α,3α,7α-trihydroxy-5β-cholan-24-oic acid (vulpecholic acid), has been identified as a major bile acid in the bile of the Australian opossum (Trichosurus vulpecula) (4, 41); this is the only bile acid identified to date that is present in mammalian bile in considerable proportion in unconjugated form.
The Red-winged tinamou is a ground-living bird averaging 40 cm in length, and is found in the Neotropics, mainly in the Pampas and Cerrado of Argentina, Brazil, and Bolivia. The tinamou belongs to the order Tinamiformes, and the family Tinamidae. In this family, there are 9 genera and 47 species. Based on the fossil record, the tinamou is considered to have evolved at least 10 million years ago, during the Miocene epoch (42). The tinamou is considered to be related to ratite birds (rhea, ostrich, emu, cassowary, and kiwi) based on morphological, molecular, and genetic criteria with the fossil record for these species dating back to the late Paleocene, 55 million years ago (21). The biliary bile acids of other ratites have been examined by HPLC and shown to be mixtures of C27 bile acids, just as in the tinamou (L. R. Hagey and A. F. Hofmann, unpublished observations).
Acknowledgments
We thank Dr. Joseph H. Steinbach for aid in figure preparation.
Published, JLR Papers in Press, November 14, 2008.
Footnotes
This work was supported by National Institutes of Health Grant AM 64891 (to A.F.H.) and by a Grant-in-Aid for Young Scientists (B) (to G.K., Grant 20,750,141) for 2008–2009; a Grant-in-Aid for Scientific Research (C) (to T.I., Grant 19,510,223) for 2007–2008 from the Ministry of Education, Sciences, Sports, and Culture of Japan; and Nihon University Multidisciplinary Research Grant (to T.I.) for 2008.
References
- 1.Hofmann A. F., and L. R. Hagey. 2008. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 65 2461–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moschetta A., F. Xu, L. R. Hagey, G. P. van Berge-Henegouwen, K. J. van Erpecum, J. F. Brouwers, J. C. Cohen, M. Bierman, H. H. Hobbs, J. H. Steinbach, et al. 2005. A phylogenetic survey of biliary lipids in vertebrates. J. Lipid Res. 46 2221–2232. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S., and U. Maitra. 2004. Facile synthesis, aggregation behavior, and cholesterol solubilization ability of avicholic acid. Org. Lett. 6 31–34. [DOI] [PubMed] [Google Scholar]
- 4.Lee S. P., R. Lester, and J. S. Pyrek. 1987. Vulpecholic acid (1α,3α,7α-trihydroxy-5β-cholan-24-oic acid): a novel bile acid of a marsupial, Trichosurus vulpecula (Lesson). J. Lipid Res. 28 19–31. [PubMed] [Google Scholar]
- 5.Hagey L. R., C. D. Schteingart, H-T. Ton-Nu, and A. F. Hofmann. 1994. Biliary bile acids of fruit pigeons and doves (Columbiformes): presence of 1β-hydroxychenodeoxycholic acid and conjugation with glycine as well as taurine. J. Lipid Res. 35 2041–2048. [PubMed] [Google Scholar]
- 6.Hsia, S. L. 1971. Hyocholic and muricholic acids. In The Bile Acids: Chemistry, Physiology, and Metabolism. P.P. Nair and D. Kritchevsky, editors. Plenum Press, New York. 95–120.
- 7.Iida T., S. Nishida, F. C. Chang, T. Niwa, J. Goto, and T. Nambara. 1993. Potential bile acid metabolites. 19. The epimeric 3α,6,7β-trihydroxy- and 3α,6,7β,12α-tetrahydroxy-5α-cholanoic acids. Steroids. 58 148–152. [DOI] [PubMed] [Google Scholar]
- 8.Kakiyama G., T. Iida, T. Goto, N. Mano, J. Goto, T. Nambara, L. R. Hagey, C. D. Schteingart, and A. F. Hofmann. 2006. Identification of a novel bile acid in swans, tree ducks, and geese: 3α,7α,15α-trihydroxy-5β-cholan-24-oic acid. J. Lipid Res. 47 1551–1558. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann, A. F., C. D. Schteingart, and L. R. Hagey. 1995. Species differences in bile acid metabolism. In Bile Acids and Liver Diseases (International Falk Workshop), G. Paumgartner and U. Beuers, editors. Kluwer Academic Publishers, Boston. 3–30.
- 10.Kurosawa T., Y. Nomura, R. Mahara, T. Yoshimura, A. Kimura, S. Ikegawa, and M. Tohma. 1995. Synthesis of 19-hydroxylated bile acids and identification of 3α,7α,12α,19-tetrahydroxy-5β-cholan-24-oic acid in human neonatal urine. Chem. Pharm. Bull. (Tokyo). 43 1551–1557. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann, A. F., and L. R. Hagey. 1998. Bile acids and biliary disease: Peaceful coexistence versus deadly warfare. In Gut and Liver. H.E. Blum, C.E. Bode, J.C. Bode, and R.B. Sartor, editors. Kluwer Academic Publishers, Lancaster, UK. 85–103.
- 12.Ridlon J. M., D. J. Kang, and P. B. Hylemon. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47 241–259. [DOI] [PubMed] [Google Scholar]
- 13.Palmer R. H. 1972. Bile acids, liver injury, and liver disease. Arch. Intern. Med. 130 606–617. [PubMed] [Google Scholar]
- 14.Palmer, R. H. 1976. Toxic effects of lithocholate on the liver and biliary tree. In The Hepatobiliary System. Fundamental and Pathological Mechanisms. W. Taylor, editor. Plenum Press, New York. 227–240.
- 15.Hofmann A. F. 2004. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug Hepatotoxicity. Drug Metab. Rev. 36 703–722. [DOI] [PubMed] [Google Scholar]
- 16.Fickert P., A. Fuchsbichler, H-U. Marschall, M. Wagner, G. Zollner, R. Krause, K. Zatloukal, H. Jaeschke, H. Denk, and M. Trauner. 2006. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am. J. Pathol. 168 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kihira K., A. Okamoto, and T. Hoshita. 1987. Identification of new C27 and C24 bile acids in the bile of Alligator Mississippiensis. J. Biochem. 101 1377–1384. [DOI] [PubMed] [Google Scholar]
- 18.Kuramoto T., Y. Kameyama, M. Kaneda, M. Shiro, T. Hoshita, and M. Une. 2000. Structure and stereochemistry of the higher bile acid isolated from turtle bile: (22S,25R)-3α,12α,15α,22-Tetrahydroxy-5β-cholestan-26-oic acid. Chem. Pharm. Bull. (Tokyo). 48 53–55. [DOI] [PubMed] [Google Scholar]
- 19.Hagey, L. R. 1992. Bile Acid Biodiversity in Vertebrates: Chemistry and Evolutionary Implication. PhD Dissertation. University of California, San Diego.
- 20.Kakiyama G., H. Tamegai, T. Iida, K. Mitamura, S. Ikegawa, T. Goto, N. Mano, J. Goto, P. Holz, L. R. Hagey, et al. 2007. Isolation and chemical synthesis of a major novel biliary bile acid in the common wombat (Vombatus urinus): 15α-hydroxylithocholic acid. J. Lipid Res. 48 2682–2692. [DOI] [PubMed] [Google Scholar]
- 21.Davies, S. J. J. F. 2002. Ratites and tinamous (Bird families of the world). Oxford University Press, Oxford, England. 1–360.
- 22.Goto J., G. Shao, H. Miura, and T. Nambara. 1989. Separation of C-25 epimers of 5β-cholanoic acids by high performance liquid chromatography with precolumn fluorescence labeling. Anal. Sci. 5 19–22. [Google Scholar]
- 23.Kihira K., A. K. Batta, E. H. Mosbach, and G. Salen. 1979. Reverse cross-coupling in the synthesis of 3α,7α-dihydroxy-5β-cholestanoic acid. J. Lipid Res. 20 421–427. [PubMed] [Google Scholar]
- 24.Une M., N. Matsumoto, K. Kihira, M. Yasuhara, T. Kuramoto, and T. Hoshita. 1980. Bile salts of frogs: a new higher bile acid, 3α,7α,12α,26-tetrahydroxy-5β- cholestanoic acid from the bile of Rana plancyi. J. Lipid Res. 21 269–276. [PubMed] [Google Scholar]
- 25.Back P., H. Fritz, and C. Populoh. 1984. The isolation of tetrahydroxy bile acids as methyl esters from human urine and their characterization by 1H- and 13C-nuclear magnetic resonance spectroscopy. Hoppe Seylers Z. Physiol. Chem. 365 479–484. [DOI] [PubMed] [Google Scholar]
- 26.Ikegawa S., T. Goto, N. Mano, and J. Goto. 1998. Substrate specificity of THCA-CoA oxidases from rat liver light mitochondrial fractions on dehydrogenation of 3α,7α,12α-trihydroxy-5β-cholestanoic acid CoA thioester. Steroids. 63 603–607. [DOI] [PubMed] [Google Scholar]
- 27.Ikegawa S., T. Goto, H. Watanabe, and J. Goto. 1995. Stereoisomeric inversion of (25R) and (25S)-3α,7α,12α-trihydroxy-5β-cholestanoic acids in rat liver peroxisome. Biol. Pharm. Bull. 18 1027–1029. [DOI] [PubMed] [Google Scholar]
- 28.Ikegawa S., T. Goto, H. Watanabe, and J. Goto. 1997. Stereoisomeric bio-inversion of (25R)- and (25S)-3α,7α,12α-trihydroxy-5β-cholestanoic acid CoA thioesters in rat liver peroxisome. Enantiomer. 2 333–342. [Google Scholar]
- 29.Ikegawa S., H. Watanabe, T. Goto, N. Mano, J. Goto, and T. Nambara. 1995. Stereospecific dehydrogenation of (25R)- and (25S)-3α,7α,12α-trihydroxy- 5β-cholestanoic acids by acyl-CoA oxidase in rat liver light mitochondrial fraction. Biol. Pharm. Bull. 18 1041–1044. [DOI] [PubMed] [Google Scholar]
- 30.Setchell K. D., J. E. Heubi, K. E. Bove, N. C. O'Connell, T. Brewsaugh, S. K. Steinberg, A. Moser, and R. H. Squires, Jr. 2003. Liver disease caused by failure to racemize trihydroxycholestanoic acid; gene mutation and effect of bile acid therapy. Gastroenterology. 124 217–232. [DOI] [PubMed] [Google Scholar]
- 31.Une M., Y. Tazawa, K. Tada, and T. Hoshita. 1987. Occurrence of both (25R)- and (25S)-3α,7α,12α-trihydroxy-5β-cholestanoic acids in urine from an infant with Zellweger's syndrome. J. Biochem. 102 1525–1530. [DOI] [PubMed] [Google Scholar]
- 32.Lawson A. M., M. J. Madigan, D. Shortland, and P. T. Clayton. 1986. Rapid diagnosis of Zellweger syndrome and infantile Refsum's disease by fast atom bombardment-mass spectrometry of urine bile salts. Clin. Chim. Acta. 161 221–231. [DOI] [PubMed] [Google Scholar]
- 33.Deleze G., I. Björkhem, and G. Karlaganis. 1986. Bile acids and bile alcohols in two patients with Zellweger (cerebro-hepato-renal) syndrome. J. Pediatr. Gastroenterol. Nutr. 5 701–710. [DOI] [PubMed] [Google Scholar]
- 34.Kurosawa T., M. Sato, F. Kikuchi, Y. Tazawa, and M. Tohma. 1996. Capillary gas chromatographic determination of C27-bile acids in biological samples and its application to the urine of a patient with Zellweger syndrome. Anal. Sci. 12 839–846. [Google Scholar]
- 35.Obinata K., H. Nittono, K. Yabuta, R. Mahara, and M. Tohma. 1992. 1β-hydroxylated bile acids in the urine of healthy neonates. J. Pediatr. Gastroenterol. Nutr. 15 1–5. [DOI] [PubMed] [Google Scholar]
- 36.Tohma M., R. Mahara, H. Takeshita, T. Kurosawa, and S. Ikegawa. 1986. Synthesis of the 1β-hydroxylated bile acids, unusual bile acids in human biological fluids. Chem. Pharm. Bull. (Tokyo). 34 2890–2899. [DOI] [PubMed] [Google Scholar]
- 37.Jönsson G., A. C. Midtvedt, A. Norman, and T. Midtvedt. 1995. Intestinal microbial bile acid transformation in healthy infants. J. Pediatr. Gastroenterol. Nutr. 20 394–402. [DOI] [PubMed] [Google Scholar]
- 38.Shoda J., T. Osuga, R. Mahara, M. Tohma, K. Matsuura, N. Tanaka, Y. Matsuzaki, and H. Miyazaki. 1989. Altered metabolism of bile acids in cholestasis: determination of 1β and 6α-hydroxylated metabolites. J. Chromatogr. 488 315–328. [DOI] [PubMed] [Google Scholar]
- 39.Fischer S., M. Neubrand, and G. Paumgartner. 1993. Biotransformation of orally administered ursodeoxycholic acid in man as observed in gallbladder bile, serum, and urine. Eur. J. Clin. Invest. 23 28–36. [DOI] [PubMed] [Google Scholar]
- 40.Yamakawa R., A. Kimura, K. Aoki, M. Suzuki, R. Mahara, and M. Tohma. 1996. Urinary 1β-hydroxyursodeoxycholic acid during ursodeoxycholic acid therapy. J. Gastroenterol. 31 917–918. [DOI] [PubMed] [Google Scholar]
- 41.St. Pyrek J., S. P. Lee, L. Thomsen, C. Tasman-Jones, and B. Leydon. 1991. Bile acids of marsupials. 2. Hepatic formation of vulpecholic acid (1α,3α,7α-trihydroxy-5β-cholan-24-oic acid) from chenodeoxycholic acid in a marsupial, Trichosurus vulpecula (Lesson). J. Lipid Res. 32 1417–1427. [PubMed] [Google Scholar]
- 42.Bartelli S., and L.M. Chiappe. 2005. Earliest Tinamous (Aves: Palaeognathae) from the Miocene of Argentina and their phylogenetic position. Serial Publications of the Natural History Museum of Los Angeles County. 502 1–14. [Google Scholar]