Abstract
Enhanced oxidative stress is implicated in the development of atherosclerosis in humans and animal models. F2-isoprostanes are formed in vivo via free radical peroxidation of arachidonic acid, and their quantification has allowed assessment of oxidative stress in vivo. F2-isoprostanes associate with lipids, although their distribution in human plasma lipoproteins is unknown. Our aim was to determine the distribution and levels of F2-isoprostanes in lipoproteins isolated from human plasma by ultracentrifugation and fast protein liquid chromatography (FPLC). F2-isoprostanes were significantly higher in HDL compared with LDL or VLDL after isolation by ultracentrifugation or FPLC. Furthermore, HDL3 particles contained elevated levels of F2-isoprostanes compared with HDL2. Platelet activating factor acetylhydrolase (PAF-AH), which hydrolyses esterified F2-isoprostanes from phospholipids, was predominantly associated with LDL. Reduced F2-isoprostanes in LDL may be related to higher PAF-AH activity in LDL. Paraoxonase 1 (PON-1) activity was associated with HDL2 and may be a contributing factor to the lower F2-isoprostanes in HDL2 compared with HDL3. Further studies are required to establish the implications of these findings on HDL function.
Keywords: HDL oxidation, oxidatively damaged lipid, PAF-AH, PON-1, FPLC
Elevated plasma concentrations of HDL are inversely correlated with atherosclerosis and cardiovascular disease and are, therefore, thought to play an important role in protection from the development of these diseases (1, 2). HDL exerts its protective effect through multiple mechanisms including reverse cholesterol transport, which lowers tissue cholesterol levels (3, 4), inhibition of LDL oxidation (5), and reduced inflammatory responses (6). HDL accumulates high levels of lipid hydroperoxides, suggesting that it may be a carrier for oxidized species and play a role in their detoxification (7–9). Enzymes associated with HDL may be involved in its cardio-protective function. One such enzyme, paraoxonase 1 (PON-1), is a lactonase that requires calcium for activity (10). Low PON-1 activity has been correlated with an increased risk of coronary heart disease (11). Although peroxidase and hydrolase activity are associated with HDL, this is not due to PON-1, but to the esterase platelet activating factor (PAF) acetyl hydrolase (PAF-AH), which coelutes with PON-1 purified from HDL (12). Lipoprotein-associated PAF-AH is a secretory, calcium-independent Group VII phospholipase A2 that degrades PAF, a potent inflammatory mediator (13). Group VII PAF-AHs have also been shown to hydrolyze some oxidized phospholipids (14, 15) as well as F2-isoprostanes (16). Macrophages secrete large quantities of PAF- AH (17). Plasma PAF-AH associates mainly with LDL and a subfraction of HDL (18).
Plasma F2-isoprostanes are formed from the reaction of free radicals with arachidonic acid (19). F2-isoprostane measurement is considered the most reliable assessment of lipid peroxidative stress in vivo (20, 21). In addition, F2-isoprostanes have been shown to associate with atherosclerosis (21) and are independently associated with angiographic evidence of coronary artery disease (22). F2-isoprostanes have been measured in plasma, urine, and tissues. However, it has not yet been determined where circulating F2-isoprostanes reside. Previously, it was shown that approximately 90% of F2-isoprostanes in plasma are present as lipid esters, presumed to be in LDL/HDL particles (23).
Our aim was to measure the distribution of plasma F2-isoprostanes in lipoprotein fractions and determine if certain lipoproteins are selective targets of lipid peroxidation or repositories for oxidized species in vivo. We report that HDL contains the highest levels of F2-isoprostanes in human plasma. Lower levels of F2-isoprostanes in LDL may be related to a higher PAF-AH activity in LDL with consequent release of free F2-isoprostanes from phospholipids (16). It remains to be determined if the high F2-isoprostanes levels in HDL play a role in the antiatherogenic effects of HDL and whether altering the levels of F2-isoprostanes in HDL can modify its functional properties.
MATERIALS AND METHODS
Reagents
2,2′-Azobis(2-methylpropionamidine)dihydrochloride (AAPH) was purchased from Aldrich Chemical Co. (Milwaukee, WI); paraoxon from Supelco (PA); ethylenediamine tetraacetic acid disodium salt dihydrate (Disodium EDTA) from ICN Biomedicals (CA); PBS from Gibco™ Invitrogen (Calsbad, CA); ethyl acetate and hydrochloric acid from Univar (Western Australia); methanol, toluene and acetonitrile from Mallinckrodt (NJ); 15-F2t-isoprostaglandin-d4 from Cayman Chemical Co. (Ann Arbor, MI). Butylated hydroxytoluene (BHT) and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Subjects and sample collection
Plasma and sera for these studies were collected from healthy, nonsmoking men and women recruited from the general population. The study was approved by the Human Ethics Committee of the University of Western Australia and all volunteers provided written informed consent.
Plasma was prepared from venous blood collected into cold tubes containing EDTA (final concentration 1 mg/ml) and reduced glutathione (final concentration 1 mg/ml) and centrifuged immediately at 1,000 g for 10 min at 4°C. The plasma was protected from oxidation by the addition of BHT at a final concentration of 40 μg/ml plasma and used fresh or stored at −80°C. Samples were analyzed within 1 month of collection. Serum samples were collected from blood clotted at 37°C for 30 min.
Measurement of F2-isoprostanes, lipid hydroperoxides, PAF-AH activity, PON-1 activity, fatty acids, cholesterol, and protein
F2-isoprostanes were measured by gas chromatography mass spectrometry using electron capture negative ionization as previously described (24). Lipid hydroperoxides were measured using the ferrous oxidation-xylenol orange assay (25) as described (26). Serum fatty acids were analyzed by gas chromatography using an internal standard to quantify individual fatty acids (27). Total cholesterol was measured using the Chol reagent enzymatic method (Roche Diagnostics GmbH, Germany). Protein content was determined by a modification of the Lowry method (28) using BSA (Sigma, St. Louis, MO) as protein standard.
PAF-AH activity was measured in concentrated (Amicon Ultra-4 centrifugal filter devices, Millipore Australia, North Ryde, NSW) fast protein liquid chromatography (FPLC) fractions with a PAF Acetylhydrolase assay kit (Cayman Chemical Co., Ann Arbor, MI) using 2-thio-PAF as substrate and a Spectra Max 190 Microplate Spectrophotometer (Molecular Devices Corporation, Sunnyvale, CA).
PON-1 activity was measured using the semiautomated microtiter plate method of Charlton-Menys, Liu, and Durrington (29) with paraoxon as substrate. Molar absorptivity of 4-nitrophenol at 405 nm (pH 8.0) was 14220. PON-1 activity was measured in lipoproteins isolated from serum by FPLC and in samples taken during AAPH oxidation, using a Spectra Max 190 Microplate Spectrophotometer (Molecular Devices Corporation, Sunnyvale, CA).
Determination of F2-isoprostanes, PAF-AH activity, and PON-1 activity distribution in lipoproteins
F2-isoprostanes and PAF-AH activity were measured in lipoproteins isolated from plasma and PON-1 activity was measured in serum lipoproteins because of the requirement for calcium to preserve PON-1 activity. Lipoproteins were isolated using ultracentrifugation or FPLC.
Lipoprotein isolation by ultracentrifugation or FPLC for measurement of F2-isoprostanes and PAF-AH activity
For lipoprotein isolation from plasma using density gradient ultracentrifugation (30), plasma was adjusted to density 1.26 g/ml by addition of sodium bromide and gradient solutions (containing 20 μM BHT and 0.27 mM EDTA) (densities 1.21, 1.10, 1.063, 1.04, 1.02 g/ml) were layered on top followed by water. Samples were ultracentrifuged at 250,000 g for 24 h at 20°C using a Beckman L-80 ultracentrifuge (Beckman Instruments, Australia).
Plasma lipoproteins were isolated by aspirating fractions into preweighed tubes and density was measured using a density meter (model DA-110M, Mettler Toledo, Kyoto Electronics Manufacturing, Japan). An aliquot of each fraction was added to 20 μl of BHT in methanol (4 mg/ml) for F2-isoprostane measurement and stored at −80°C.
Arachidonic acid and linoleic acid content were measured in an aliquot stored at −80°C in the presence of 20 μl of BHT in methanol (4 mg/ml).
Lipoproteins were also isolated from plasma using FPLC. Two Superose HR6 10/30 columns (Amersham Biosciences, Uppsala, Sweden) in series were operated at a flow rate of 30 ml/h. Plasma was diluted 1:1 and filtered, and 0.5 ml was injected onto the column, which was eluted with PBS containing 1 mM EDTA pH 7.2. Fractions were collected into tubes containing BHT (20 μl at 4 mg/ml). Protein was monitored continuously by absorbance at 280 nm and total cholesterol was measured in each of the fractions. Fractions corresponding to VLDL, LDL, HDL2, and HDL3 were concentrated. Total cholesterol and PAF-AH activity were measured immediately and the remainder was stored at −80°C for F2-isoprostane measurement.
Lipoprotein isolation for measurement of PON-1 activity
FPLC isolation of lipoproteins from serum used the same procedure as described above, but the columns were eluted with 100 mM Tris pH 8 containing 2 mM CaCl2 to preserve PON-1 activity. Protein was monitored continuously by absorbance at 280 nm, and total cholesterol and PON-1 activity were measured in each of the fractions.
Measurement of F2-Isoprostanes, lipid hydroperoxides, and PON-1 activity during AAPH oxidation of lipoproteins
Serum or plasma was adjusted to density 1.26g/ml with sodium bromide and gradient solutions containing 20 μM BHT, 0.27 mM EDTA, and 2 mM CaCl2 for serum (to preserve PON-1 activity), or without Ca for plasma (for HDL with low PON-1 activity used for in vitro oxidation experiments) were layered and ultracentrifuged as described above. LDL and HDL were obtained by puncturing the tubes and lipoproteins desalted on a PD 10 Sephadex G-25 column (Amersham Biosciences, Uppsala, Sweden) equilibrated with PBS. CaCl2 was added to HDL from serum to a final concentration of 2 mM for the HDL containing active PON-1. Lipoproteins were stored at 4°C in the dark and preparations were used within 2 weeks. LDL and HDL were each diluted to 0.35 mM total cholesterol and incubated with 5 mM AAPH at 37°C. Lipid hydroperoxides, F2-isoprostanes and PON-1 activity were measured in HDL prepared with (in serum) and without (in plasma) added calcium. Aliquots were collected at intervals up to 4 h for measurement of lipid hydroperoxides, F2-isoprostanes and PON-1 activity.
Statistics
Data was analyzed using the Statistical package for the Social Sciences (SPSS version 15, Chicago, IL). When data were not normally distributed analyses were carried out on log transformed data. One way ANOVA was used to determine differences in F2-isoprostanes between lipoproteins. Data are given as mean ± SEM.
RESULTS
Subject characteristics
The study recruited 8 men and 8 women aged 41 ± 3 years. Their fasting serum lipids were: total cholesterol 5.0 ± 0.2 mmol/L, LDL-cholesterol 2.9 ± 0.2 mmol/L, HDL-cholesterol 1.7 ± 0.1 mmol/L, and triglycerides 0.9 ± 0.1 mmol/L. Mean fasting glucose was 4.5 ± 0.1 mmol/L.
F2-Isoprostanes, arachidonic acid, and linoleic acid in lipoprotein fractions isolated by ultracentrifugation
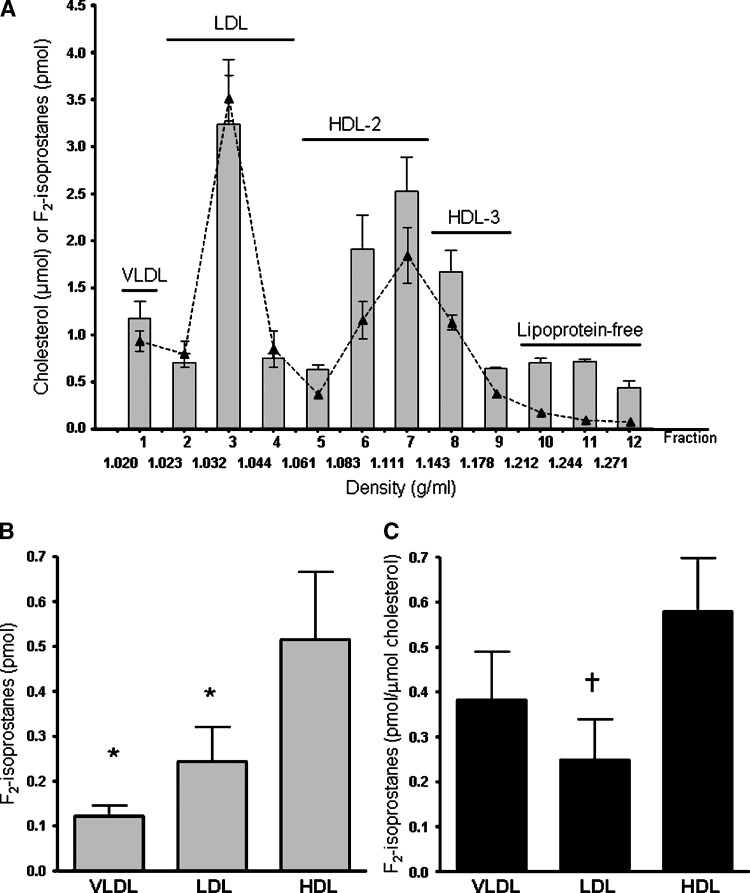
The distribution of F2-isoprostanes and total cholesterol in ultracentrifuged plasma lipoproteins is shown in Fig. 1A. The highest levels of F2-isoprostanes were in fractions 3 (LDL), 6 and 7 (HDL2), and 8 (HDL3). F2-isoprostanes in HDL were significantly higher than in LDL (P = 0.02) and VLDL (P < 0.001) (Fig. 1B). When F2-isoprostanes were normalized for cholesterol, levels in HDL were approximately 2-fold higher than in LDL (P < 0.001) and 50% higher than in VLDL (Fig. 1C). F2-isoprostanes (corrected for cholesterol) in HDL3 (0.76 ± 0.16 pmol/μmol) were 3-fold higher than LDL (P < 0.001) and in HDL2 (0.52 ± 0.12 pmol/μmol) were 2-fold higher than LDL (0.25 ± 0.09 pmol/μmol, P = 0.004). The levels of F2-isoprostanes (corrected for cholesterol) were 50% greater in HDL3 compared with HDL2 fractions (P < 0.02).
Fig. 1.
A: Plasma F2-isoprostanes (solid bars) and total cholesterol (—▴—) in lipoproteins isolated by ultracentrifugation. B: F2-isoprostanes in HDL, LDL, and VLDL isolated by ultracentrifugation. C: F2-isoprostanes (corrected for cholesterol) in HDL, LDL and VLDL isolated by ultracentrifugation. Data ± SEM. * P < 0.05 and † P < 0.01 compared with HDL.
Arachidonic acid was measured as an indicator of available substrate for F2-isoprostane formation. The amount of arachidonic acid (corrected for cholesterol) in LDL (6 ± 3 nmol/μmol cholesterol) was higher than in HDL (1.5 ± 0.2 nmol/μmol). Linoleic acid, one of the main lipid hydroperoxide substrates, was also higher in LDL (30 ± 9 nmol/μmol cholesterol) compared with HDL (12 ± 4 nmol/μmol).
F2-isoprostanes, PAF-AH activity, and PON-1 activity in lipoprotein fractions isolated by FPLC
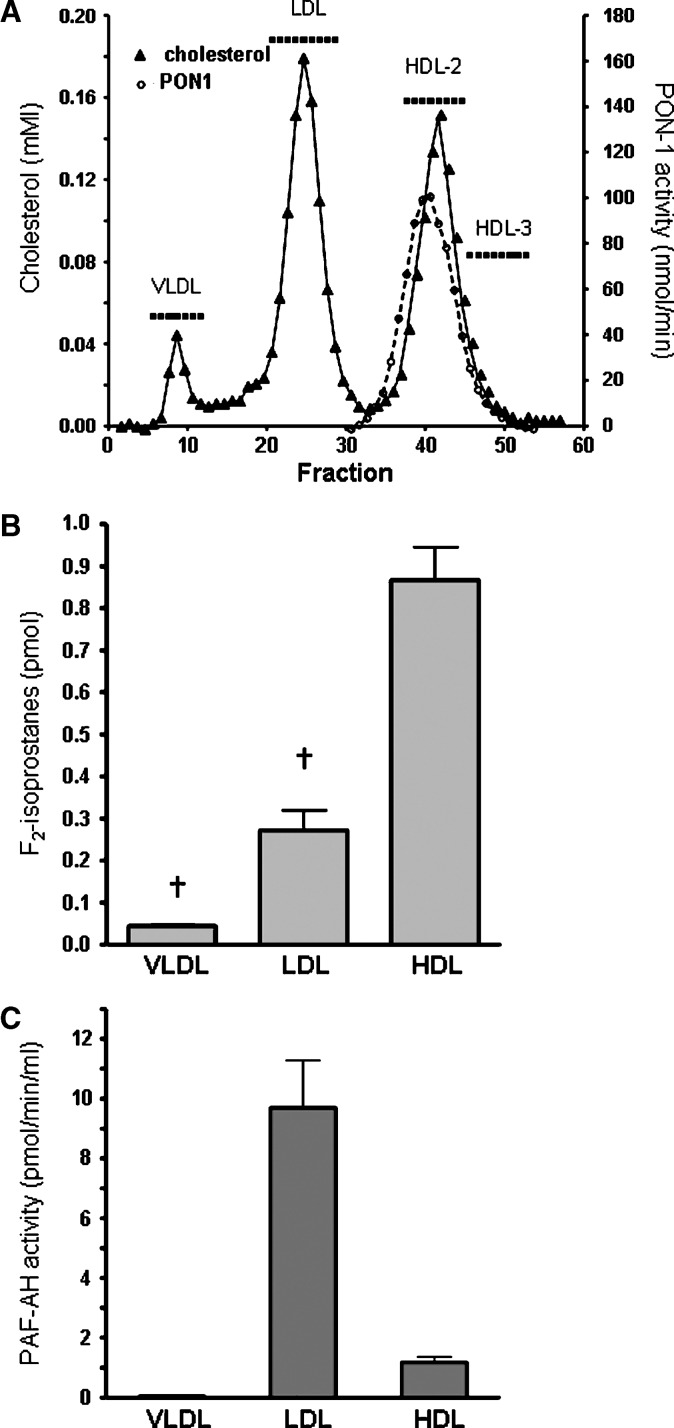
Total cholesterol distribution in fractions from FPLC isolation of plasma lipoproteins is shown in Fig. 2A. Fractions were pooled corresponding to VLDL (fractions 6–12; 14–17.5 ml), LDL (fractions 21–29; 21.5–26 ml), HDL2 (fractions 38–45; 30–34 ml), and HDL3 (fractions 46–53; 34–38 ml) and concentrated.
Fig. 2.
A: Total cholesterol in plasma lipoproteins isolated using fast protein liquid chromatography (FPLC). Paraoxonase 1 (PON-1) activity was measured in fractions isolated from serum (—○—). B: F2-isoprostanes were measured in pooled, concentrated HDL, LDL, and VLDL fractions isolated from plasma by FPLC as indicated (▪▪▪) in A. C: Fractions were pooled as indicated (▪▪▪) in A, and HDL, LDL, and VLDL platelet activating factor acetylhydrolase (PAF-AH) activity was measured. Data ± SEM. † P < 0.01 compared with HDL.
F2-isoprostanes distribution in lipoproteins isolated from plasma using FPLC is shown in Fig. 2B. F2-isoprostanes in HDL were higher than in LDL (P < 0.001) and VLDL (P < 0.001) (Fig. 2B). HDL F2-isoprostanes (corrected for cholesterol) (1.2 ± 0.08 pmol/μmol) remained significantly higher than in LDL (0.27 ± 0.03 pmol/μmol, P < 001). HDL3 F2-isoprostanes (corrected for cholesterol) (1.62 ± 0.20 pmol/μmol) were 6-fold higher than in LDL (P < 0.001), and in HDL2 (1.14 ± 0.07 pmol/μmol) were 4-fold higher than in LDL (P < 0.001). HDL3 contained approximately 50% higher F2-isoprostanes than in HDL2 (P = 0.05), confirming our findings following isolation of lipoproteins by ultracentrifugation.
LDL accounted for 88% of the measured PAF-AH activity with the remainder in the HDL2 fraction (11.6%) (Fig. 2C). Measurement of PON-1 activity showed that it associated with the HDL2 fraction (Fig. 2A), which may account for the reduced levels of F2-isoprostanes in HDL2 compared with HDL3.
Measurement of F2-Isoprostanes, lipid hydroperoxides, and PON-1 activity during AAPH oxidation of lipoproteins
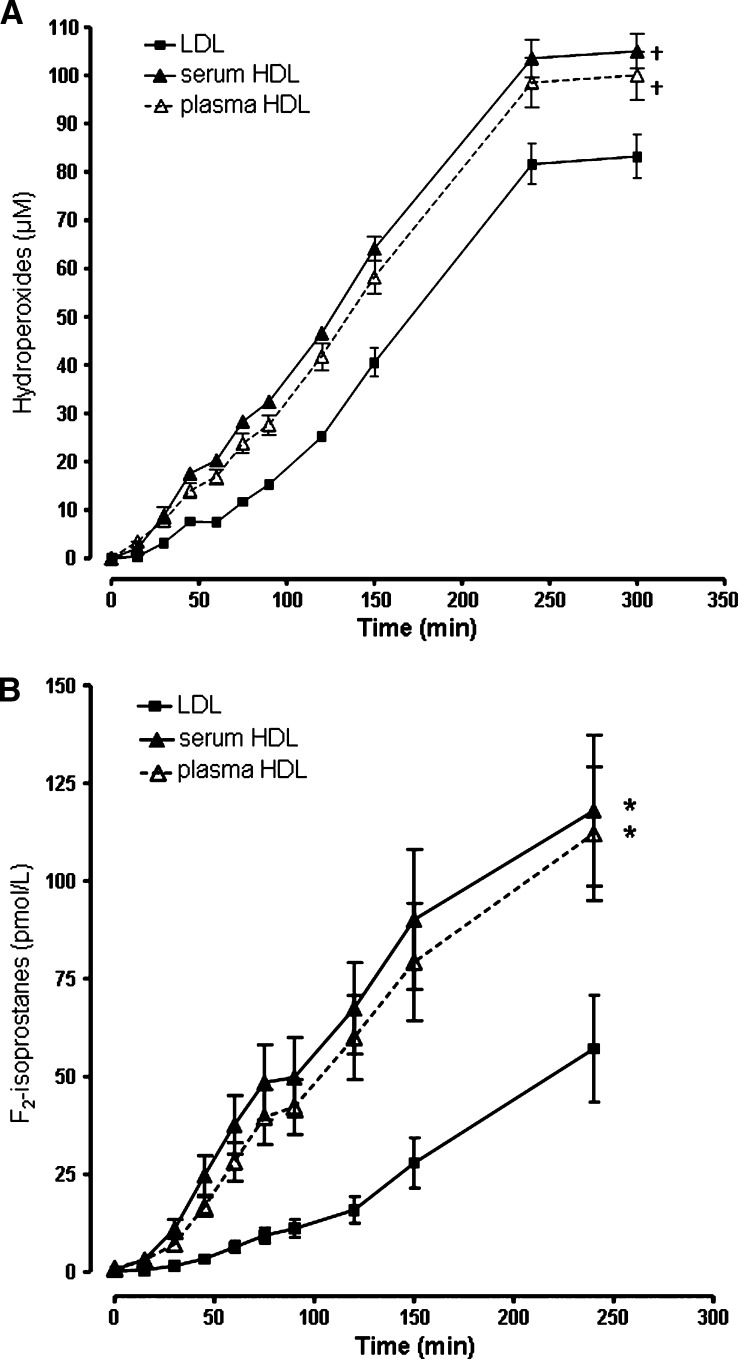
During AAPH oxidation of LDL and HDL at the same cholesterol concentration, hydroperoxides increased in LDL and HDL (Fig. 3A). Relative to LDL [area under the curve 18055 ± 499 (μM)mins, P < 0.001] or plasma [16750 ± 890 (μM)mins, P < 0.006]. F2-isoprostanes increased during oxidation of LDL and HDL with AAPH (Fig. 3B). HDL F2-isoprostanes derived from serum [area under the curve, 15606 ± 2869 (pM)mins, P = 0.02] or plasma [13750 ± 2359 (pM)mins, P = 0.03] were significantly higher than in LDL [5242 ± 1208 (pM)mins]. Preservation of PON-1 activity by the presence of calcium during purification and storage of HDL did not affect hydroperoxide content or F2-isoprostane levels in HDL. However, HDL purified from plasma in the absence of calcium had only 21% of the PON-1 activity of the same HDL purified from serum in the presence of calcium. HDL with active PON-1 had 57% of PON-1 activity remaining after 4 h incubation.
Fig. 3.
A: Lipid hydroperoxides and B F2-isoprostanes, in LDL (—▪—) and HDL isolated from serum (—▴—) or plasma (—△—) during oxidation of LDL (0.35 mM total cholesterol) or HDL (0.35 mM cholesterol) with 5 mM 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AAPH) at 37°C. Data ± SEM. * P < 0.05 and † P < 0.01 compared with LDL (area under the curve).
DISCUSSION
This is the first report that HDL is the major lipoprotein carrier of F2-isoprostanes in human plasma, with levels significantly higher than in LDL or VLDL, after isolation by ultracentrifugation or FPLC. Furthermore, HDL3 particles contained significantly elevated levels of F2-isoprostanes compared with HDL2. Reduced F2-isoprostanes in native LDL may reflect the higher PAF-AH activity associated with LDL, as F2-isoprostanes are released from esterified phospholipids by PAF-AH (16).
F2-isoprostanes are vasoconstrictors (31), increase atherosclerotic plaque formation in ApoE knockout mice (32), and exhibit antiangiogenic effects by inhibiting migration of endothelial cells and formation of vascular tubes (33). F2-isoprostanes in HDL particles could increase as a result of removal of oxidized lipids from cell membranes (8), from macrophages in atherosclerotic lesions (34, 35), or after extraction of oxidized lipids from LDL by HDL (9). The higher concentration of antioxidants in LDL may also result in HDL being a preferential target for oxidation, acting as a “sink” for oxidized lipids, as it is also the major carrier of lipid hydroperoxides in plasma (7). HDL oxidation attributed to the enzyme myeloperoxidase has been observed in patients with cardiovascular disease (36–38).
There is debate whether PAF-AH is pro- or antiatherogenic (13). Plasma PAF-AH levels are positively associated with cardiovascular disease (39). However, it is not known if PAF-AH activity affects atherosclerosis or whether levels rise because of the inflammatory environment. In contrast, overexpression of PAF-AH reduces a number of inflammatory diseases (40). Our data confirmed that PAF-AH activity is mainly associated with LDL isolated by FPLC (41). In contrast, PAF-AH activity redistributes to dense LDL, HDL, and lipoprotein-free fractions during isolation by ultracentrifugation (41). It is possible that the release of F2-isoprostanes from LDL by PAF-AH could be proatherogenic based on the biological properties of free F2-isoprostanes described above. It is likely that PAF-AH activity in LDL may be an important determinant of F2-isoprostanes distribution in lipoproteins and the level of free F2-isoprostanes. However, the role of F2-isoprostanes in lipoproteins, particularly HDL, remains unknown. It is possible that F2-isoprostanes could preferentially associate with HDL in order to facilitate their removal from the circulation via the liver.
PON-1 activity associated with larger HDL2 fractions after FPLC, in agreement with other studies (42). We also showed that FPLC separation of lipoproteins resulted in a qualitatively similar profile of F2-isoprostanes compared with lipoproteins isolated by ultracentrifugation. This is important as it suggests that F2-isoprostanes do not redistribute upon ultracentrifugation. Reduced F2-isoprostanes in HDL2 compared with HDL3 may be related to elevated PON-1 activity in HDL2.
HDL levels of lipid hydroperoxides and F2-isoprostanes increased at a faster rate than those in LDL during in vitro free radical oxidation, despite HDL having less of the fatty acid substrates (linoleic acid and arachidonic acid) than LDL. This finding supports the hypothesis that HDL is more susceptible to oxidation than LDL (43, 44). This may relate to the higher antioxidant content of LDL (7, 45), which delays lipid peroxide formation. The presence of active PON-1 had no effect on lipid peroxides or F2-isoprostanes during free radical in vitro HDL oxidation. This indicates that the reported antiatherogenic effects of PON-1 (11) are not due to protection of HDL from oxidation but are likely due to other mechanisms, such as protection of LDL from oxidation (46, 47).
Our findings show that HDL is the main carrier of F2-isoprostanes in the lipoproteins of human plasma. The relevance of F2-isoprostanes in HDL particles and its relationship to cardiovascular disease has been highlighted in several recent reports. F2-isoprostanes associate with atherosclerosis (21) and angiographic evidence of coronary artery disease (22). Plasma 8-isoprostanes in type 2 diabetic subjects were negatively correlated with antioxidative activity associated with the HDL3 sub-fractions (48).
Future studies should be directed toward determining the levels of F2-isoprostanes in the HDL of subjects at risk of atherosclerosis. It also remains to be determined whether increased F2-isoprostanes in HDL affect its function in cholesterol efflux and reverse cholesterol transport, or its antioxidative and anti-inflammatory properties.
Abbreviations
AAPH, 2,2′-azobis(2-methylpropionamidine)dihydrochloride
BHT, butylated hydroxytoluene
FPLC, fast protein liquid chromatography
PAF, platelet activating factor
PAF-AH, platelet activating factor acetylhydrolase
PON-1, paraoxonase 1
This work was supported by grants from the National Heart Foundation of Australia and the National Health and Medical Research Council of Australia.
Published, JLR Papers in Press, December 2, 2008.
References
- 1.Gordon T., W. P. Castelli, M. C. Hjortland, W. B. Kannel, and T. R. Dawber. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Gordon D. J., and B. M. Rifkind. 1989. High-density lipoprotein-the clinical implications of recent studies. N. Engl. J. Med. 321 1311–1316. [DOI] [PubMed] [Google Scholar]
- 3.Fielding C. J., and P. E. Fielding. 1995. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 36 211–228. [PubMed] [Google Scholar]
- 4.Cuchel M., and D. J. Rader. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113 2548–2555. [DOI] [PubMed] [Google Scholar]
- 5.Navab M., S. Y. Hama, G. M. Anantharamaiah, K. Hassan, G. P. Hough, A. D. Watson, S. T. Reddy, A. Sevanian, G. C. Fonarow, and A. M. Fogelman. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41 1495–1508. [PubMed] [Google Scholar]
- 6.Barter P. J., S. Nicholls, K. A. Rye, G. M. Anantharamaiah, M. Navab, and A. M. Fogelman. 2004. Antiinflammatory properties of HDL. Circ. Res. 95 764–772. [DOI] [PubMed] [Google Scholar]
- 7.Bowry V. W., K. K. Stanley, and R. Stocker. 1992. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA. 89 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimov A. N., K. A. Kozhevnikova, A. A. Kuzmin, A. S. Kuznetsov, and E. V. Belova. 2001. On the ability of high density lipoproteins to remove phospholipid peroxidation products from erythrocyte membranes. Biochemistry. 66 300–304. [DOI] [PubMed] [Google Scholar]
- 9.Navab M., J. A. Berliner, G. Subbanagounder, and S. Y. Hama. 2001. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 21 481–488. [DOI] [PubMed] [Google Scholar]
- 10.Khersonsky O., and D. S. Tawfik. 2005. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry. 44 6371–6382. [DOI] [PubMed] [Google Scholar]
- 11.Mackness B., P. Durrington, P. McElduff, J. Yarnell, N. Azam, and M. M. Mackness. 2003. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 107 2775–2779. [DOI] [PubMed] [Google Scholar]
- 12.Marathe G. K., G. A. Zimmerman, and T. M. McIntyre. 2003. Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J. Biol. Chem. 278 3937–3947. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre, T. M., S. M. Prescott, and D. M. Stafforini. 2008. The emerging roles of PAF acetylhydrolase. J. Lipid Res. In press. [DOI] [PMC free article] [PubMed]
- 14.Stremler K. E., D. M. Stafforini, S. M. Prescott, and T. M. McIntyre. 1991. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J. Biol. Chem. 266 11095–11103. [PubMed] [Google Scholar]
- 15.Kriska T., G. K. Marathe, J. C. Schmidt, T. M. McIntyre, and A. W. Girotti. 2007. Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. J. Biol. Chem. 282 100–108. [DOI] [PubMed] [Google Scholar]
- 16.Stafforini D. M., J. R. Sheller, T. S. Blackwell, A. Sapirstein, F. E. Yull, and T. M. McIntyre. 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281 4616–4623. [DOI] [PubMed] [Google Scholar]
- 17.Elstad M. R., D. M. Stafforini, T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 1989. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J. Biol. Chem. 264 8467–8470. [PubMed] [Google Scholar]
- 18.Prescott S. M., G. A. Zimmerman, D. M. Stafforini, and T. M. McIntyre. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69 419–445. [DOI] [PubMed] [Google Scholar]
- 19.Morrow J. D., K. D. Hill, R. F. Burk, T. M. Nammour, K. F. Badr, and L. J. Roberts. 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA. 87 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore K., and L. J. Roberts. 1998. Measurement of lipid peroxidation. Free Radic. Res. 28 659–671. [DOI] [PubMed] [Google Scholar]
- 21.Morrow J. D. 2005. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 25 279–286. [DOI] [PubMed] [Google Scholar]
- 22.Shishehbor M. H., R. Zhang, H. Medina, M. L. Brennan, D. M. Brennan, S. G. Ellis, E. J. Topol, and S. L. Hazen. 2006. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic. Biol. Med. 41 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow J. D., J. A. Awad, H. J. Boss, I. A. Blair, and L. J. Roberts. 1992. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 89 10721–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori T. A., K. D. Croft, I. B. Puddey, and L. J. Beilin. 1999. An improved method for the measurement of urinary and plasma F2-isoprostanes using gas chromatography-mass spectrometry. Anal. Biochem. 268 117–125. [DOI] [PubMed] [Google Scholar]
- 25.Nourooz-Zadeh J., J. Tajaddini-Sarmadi, and S. P. Wolff. 1994. Measurement of plasma hydroperoxide concentration by ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal. Biochem. 220 403–409. [DOI] [PubMed] [Google Scholar]
- 26.Proudfoot J. M., K. D. Croft, I. B. Puddey, and L. J. Beilin. 1997. The role of copper reduction by alpha-tocopherol in low-density lipoprotein oxidation. Free Radic. Biol. Med. 23 720–728. [DOI] [PubMed] [Google Scholar]
- 27.Woodman R. J., T. A. Mori, V. Burke, I. B. Puddey, G. F. Watts, and L. J. Beilin. 2002. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on glycaemic control, blood pressure and serum lipids in treated-hypertensive Type 2 diabetic patients. Am. J. Clin. Nutr. 76 1007–1015. [DOI] [PubMed] [Google Scholar]
- 28.Markwell M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87 206–210. [DOI] [PubMed] [Google Scholar]
- 29.Charlton-Menys V., Y. Liu, and P. N. Durrington. 2006. Semiautomated method for determination of serum paraoxonase activity using paraoxon as substrate. Clin. Chem. 52 453–457. [DOI] [PubMed] [Google Scholar]
- 30.Croft K. D., J. Proudfoot, C. Moulton, and L. J. Beilin. 1991. The effect of lipoproteins on the release of some eicosanoids by stimulated human leukocytes. A possible role in atherogenesis. Eicosanoids. 4 75–81. [PubMed] [Google Scholar]
- 31.Hou X., L. J. Roberts, F. Gobeil, Jr., D. Taber, K. Kanai, D. Abran, S. Brault, D. Checchin, F. Sennlaub, P. Lachapelle, et al. 2004. Isomer-specific contractile effects of a series of synthetic F2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 36 163–172. [DOI] [PubMed] [Google Scholar]
- 32.Tang M., T. Cyrus, Y. Yao, L. Vocun, and D. Praticò. 2005. Involvement of thromboxane receptor in the proatherogenic effect of isoprostane F2alpha-III: evidence from apolipoprotein E- and LDL receptor-deficient mice. Circulation. 112 2867–2874. [DOI] [PubMed] [Google Scholar]
- 33.Benndorf R. A., E. Schwedhelm, A. Gnann, R. Taheri, G. Kom, M. Didié, A. Steenpass, S. Ergün, and R. H. Böge. 2008. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A (2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ. Res. 103 1037–1046. [DOI] [PubMed] [Google Scholar]
- 34.Nissen S. E., T. Tsunoda, E. M. Tuzcu, P. Schoenhagen, C. J. Cooper, M. Yasin, G. M. Eaton, M. A. Lauer, W. S. Sheldon, C. L. Grines, et al. 2003. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290 2292–2300. [DOI] [PubMed] [Google Scholar]
- 35.Oram J. F., and J. W. Heinecke. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85 1343–1372. [DOI] [PubMed] [Google Scholar]
- 36.Bergt C., S. Pennathur, X. Fu, J. Byun, K. O'Brien, T. O. McDonald, P. Singh, G. M. Anantharamaiah, A. Chait, J. Brunzell, et al. 2004. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 101 13032–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pennathur S., C. Bergt, B. Shao, J. Byun, S. Y. Kassim, P. Singh, P. S. Green, T. O. McDonald, J. Brunzell, A. Chait, et al. 2004. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 279 42977–42983. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L., B. Nukuna, M. L. Brennan, M. Sun, M. Goormastic, M. Settle, D. Schmitt, X. Fu, L. Thomson, P. L. Fox, et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garza C. A., V. M. Montori, J. P. McConnell, V. K. Somers, I. J. Kullo, and F. Lopez-Jimenez. 2007. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin. Proc. 82 159–165. [DOI] [PubMed] [Google Scholar]
- 40.Stafforini D. M. 2009. Biology of Platelet-activating Factor Acetylhydrolase (PAF-AH, Lipoprotein Associated Phospholipase A(2). Cardiovasc. Drugs Ther. 23 73–78. [DOI] [PubMed] [Google Scholar]
- 41.McCall M. R., M. La Belle, T. M. Forte, R. M. Krauss, Y. Takanami, and D. L. Tribble. 1999. Dissociable and nondissociable forms of platelet-activating factor acetylhydrolase in human plasma LDL: implications for LDL oxidative susceptibility. Biochim. Biophys. Acta. 1437 23–36. [DOI] [PubMed] [Google Scholar]
- 42.Blatter M. C., R. W. James, S. Messmer, F. Barja, and D. Pometta. 1993. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur. J. Biochem. 211 871–879. [DOI] [PubMed] [Google Scholar]
- 43.Raveh O., I. Pinchuk, E. Schnitzer, M. Fainaru, Z. Schaffer, and D. Lichtenberg. 2000. Kinetic analysis of copper-induced peroxidation of HDL, autoaccelerated and tocopherol-mediated peroxidation. Free Radic. Biol. Med. 29 131–146. [DOI] [PubMed] [Google Scholar]
- 44.Nagyová A., M. J. Krajcovicová-Kudlácková, and J. Klvanová. 2001. LDL and HDL oxidation and fatty acid composition in vegetarians. Ann. Nutr. Metab. 45 148–151. [DOI] [PubMed] [Google Scholar]
- 45.Goulinet S., and M. J. Chapman. 1997. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 17 786–796. [DOI] [PubMed] [Google Scholar]
- 46.Navab M., S. S. Imes, S. Y. Hama, G. P. Hough, L. A. Ross, and R. W. Bork. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackness B., D. Hine, Y. Liu, M. Mastorikou, and M. Mackness. 2004. Paraoxonase 1 inhibits oxidised LDL-induced MCP-1 production by endothelial cells. Biochem. Biophys. Res. Commun. 318 680–683. [DOI] [PubMed] [Google Scholar]
- 48.Nobécourt E., S. Jacqueminet, B. Hansel, S. Chantepie, A. Grimaldi, M. J. Chapman, and A. Kontush. 2005. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 48 529–538. [DOI] [PubMed] [Google Scholar]