Abstract
We measured plasma markers of cholesterol synthesis (lathosterol) and absorption (campesterol, sitosterol, and cholestanol) in order to compare the effects of maximal doses of rosuvastatin with atorvastatin and investigate the basis for the significant individual variation in lipid lowering response to statin therapy. Measurements were performed in participants (n = 135) at baseline and after 6 weeks on either rosuvastatin (40 mg/day) or atorvastatin (80 mg/day) therapy. Plasma sterols were measured using gas-liquid chromatography. Rosuvastatin and atorvastatin significantly (P < 0.001) altered plasma total cholesterol (C) levels by −40%, and the ratios of lathosterol/C by −64% and −68%, and campesterol/C by +52% and +72%, respectively, with significant differences (P < 0.001) between the treatment groups for the latter parameter. When using absolute values of these markers, subjects with the greatest reductions in both synthesis (lathosterol) and absorption (campesterol) had significantly greater reductions in total C than subjects in whom the converse was true (−46% versus −34%, P = 0.001), with similar effects for LDL-C. Rosuvastatin and atorvastatin decreased markers of cholesterol synthesis and increased markers of fractional cholesterol absorption, with rosuvastatin having significantly less effect on the latter parameter than atorvastatin. In addition, alterations in absolute values of plasma sterols correlated with the cholesterol lowering response.
Keywords: plasma sterols, lathosterol, campesterol, sitosterol, STELLAR study
There is extensive evidence that HMG-CoA reductase inhibitors (statins) significantly lower total cholesterol and LDL cholesterol (C) levels and reduce coronary heart disease (CHD) risk (1). Although reductions of up to 60% in LDL-C levels have been reported (2), there is large variation in the lipid-lowering response between individuals (3). These variations have been attributed to intrinsic factors, such as genetic variation, as well as extrinsic factors, such as compliance, time of administration, concomitant drug therapy, and dietary intake (4).
Total body cholesterol pools represent a balance between endogenous synthesis and dietary absorption (5). It has been documented using formal cholesterol balance studies, which measure cholesterol synthesis and intestinal cholesterol absorption, that the plasma sterols lathosterol and desmosterol serve as markers of cholesterol synthesis, while campesterol, sitosterol, and cholestanol are markers of fractional cholesterol absorption (6). The benefit of statins has largely been linked to total cholesterol and LDL-C lowering, and it has been suggested that the degree of lowering may relate to reductions in synthesis markers, which may be offset by increases in markers of absorption. In small-scale cholesterol balance studies, Miettinen et al. (7–9) have documented that statins markedly lower cholesterol synthesis and bile acid production, but also significantly increase fractional intestinal cholesterol absorption. Duane (10) reported similar observations. Miettinen, Strandberg, and Gylling (11) have also reported in a subset of CHD patients participating in the Scandinavian Simvastatin Survival Study (4S) that those in the highest quartile of the cholestanol/C ratio (indicative of high cholesterol absorption), while on simvastatin had no reduction in CHD events as compared with the placebo-treated group, with the converse also being the case. Moreover Miettinen, Strandberg, and Gylling (11) have also reported that plasma sterols serve as markers of LDL-C lowering response to statins. Despite these data, there has been some controversy in the field as to whether statins actually increase intestinal cholesterol absorption. An alternative explanation to account for the relative increase in campesterol and beta sitosterol, putative markers of fractional cholesterol absorption, may be that statins are more active in reducing the biliary excretion of cholesterol and sterols. In addition, there is limited epidemiological data available on head-to-head comparisons between different statins and their effects on overall cholesterol homeostasis.
The current study is a posthoc analysis of a subset of patients who participated in the Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin (STELLAR) trial, which compared the effects of rosuvastatin in the reduction of LDL-C with other statins (12, 13). The aim of the current study was to investigate the effects of maximal dose rosuvastatin and atorvastatin treatment on markers of cholesterol synthesis and absorption. In addition, we investigated whether changes in synthesis and absorption markers correlated with changes in total cholesterol, LDL-C, HDL-C, triglycerides, and small dense (sd) LDL-C. The novel aspect of the current study is that it compares the effects of the most potent statin treatments available, 40 mg/day of rosuvastatin and 80 mg/day of atorvastatin, on plasma lipid and sterol changes from baseline.
METHODS
Study design and patients
The current investigation was performed in a subset of 135 patients participating in the STELLAR study; the inclusion criteria was the availability of both a baseline- and a 6-week plasma sample for the measurement of the synthesis and absorption markers. The details of the design and conduct of the STELLAR study and of the patient population have been previously published (12, 13). Briefly, the STELLAR study was an open-label, randomized, parallel group study in hypercholesterolemic patients conducted in 182 US centers. The primary objective was to compare the efficacy of rosuvastatin in the reduction of LDL-C with other statins across dose ranges. Secondary objectives included a comparison of the effects of the statins on other lipoprotein parameters such as HDL-C, apolipoprotein (apo) A-I and B, and lipid ratios (12, 13). Men and nonpregnant women (adults aged 18 or more) with hypercholesterolemia (LDL-C > 160 mg/dl) were asked to follow a National Cholesterol Education Program step 1 diet for 6 weeks. Those who were compliant with the diet and had fasting calculated LDL-C levels between 160 mg/dl and 250 mg/dl and triglycerides (TG) < 400 mg/dl were randomized to the different statin doses as described (12, 13). The relevant institutional review boards approved the STELLAR study protocol, and all participants gave informed consent.
Measurement of lipids and lipoproteins
Blood samples were collected on at least three occasions before randomization and after 4 and 6 weeks of treatment and sent to a central lab (Medical Research International, Highland Heights, KY) for the measurement of lipid and lipoprotein parameters that included LDL-C, HDL-C, and TG as described (12, 13). Plasma samples were stored at −80°C at Medical Research International. For the current study, available serum samples were sent on dry ice to the Cardiovascular Research Laboratory, Tufts University, in Boston, MA. In the rosuvastatin 40 mg and atorvastatin 80 mg arms of the STELLAR study, 66 and 69 patients, respectively, were randomized and had data recorded at baseline and after 6 weeks treatment. In our laboratory we have previously measured sdLDL-C as previously described (14). Coefficients of variations within and between runs for all assays were less than 5%.
Measurements of glycated albumin
Glycated albumin was measured according to the method described by Kouzuma et al. (15).
Measurements of plasma sterols
Plasma concentrations of lathosterol, campesterol, sitosterol, and cholestanol were assessed in all 135 subjects using gas-liquid chromatography according to methods previously described (16). Because these plasma sterols are mainly carried in the LDL fraction (7), it is common practice to adjust them for the total plasma cholesterol level by expressing them as a ratio to cholesterol. In the current study, plasma sterols were expressed corrected for plasma cholesterol levels as well as in absolute terms. Routine quality control assays show no significant differences when the results from fresh plasma samples were compared with values obtained after prior freezing at −80°C and subsequent thawing. The coefficients of variations between runs for plasma sterols were derived from routine quality control assays and were less than 5%.
Statistical analyses
All continuous variables were checked for their distribution and expressed as means ± standard deviation if they were normally distributed, or, in case of nonlinear distributions, as medians and interquartile ranges. Correlation coefficients were based on pairwise comparisons. For the correlation and the regression models, nonlinear variables were log transformed. Comparisons between baseline plasma lipid and sterol levels and their changes after 6 weeks of treatment were based on a paired t-test or a Wilcoxon signed rank test for nonlinear variables. Comparisons between the statin treatments were based on an independent t-test or a Wilcoxon-Mann-Whitney test for nonlinear variables. Study subjects were divided into four groups based on having above or below median changes with statin treatment in the absolute values of lathosterol and either an increase or a decrease in absolute campesterol levels (high-increase, high-decrease, low-increase, and low-decrease synthesis-absorption changes). Total cholesterol changes were investigated among high/low synthesis and increase/decrease absorption change subgroups using a one-way ANOVA with Bonferroni corrections for post hoc testing.
Linear regression analyses were used to characterize the variables associated with changes in lathosterol, campesterol, and cholestanol during treatment. In turn, changes in lathosterol, campesterol, and cholestanol, served as dependent variables while age, gender, statin treatment, baseline total cholesterol, and changes in total cholesterol served as independent variable. In addition, depending on the model, baseline levels of lathosterol, campesterol, and cholestanol were added as independent variables. Linear regression analyses were also used to investigate the association of baseline levels of plasma sterols with changes in total cholesterol and LDL-C levels and changes in lathosterol and campesterol with changes in total cholesterol and LDL-C. In these models, changes in total cholesterol and LDL-C respectively served as dependent variables, while age, gender, statin treatment, and alternatively baseline levels of plasma sterols and changes in plasma sterol served as independent variable. A P value smaller than 0.05 was considered statistical significant and all analyses were performed using STATA version 10.0.
RESULTS
Statin treatment and changes in lipid and lipoprotein levels
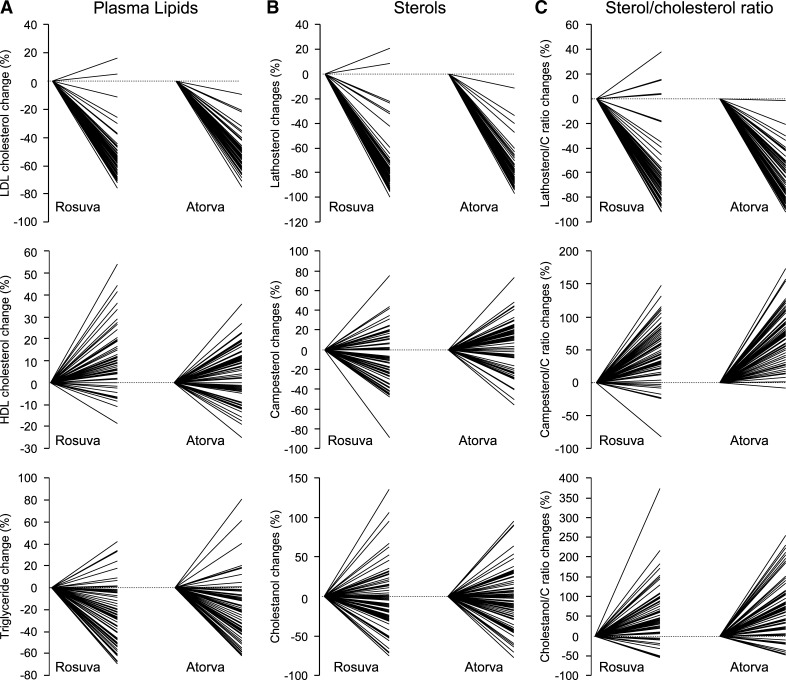
Gender distributions were similar among the treatment groups (rosuvastatin: 33 males, 33 females and atorvastatin: 33 males, 36 females, P = 0.80). The average age was somewhat higher in the atorvastatin group; however the difference did not reach statistical significance (56 ± 13 versus 60 ± 11 years, P = 0.08). Data on lipid and plasma sterol levels at baseline and after 6 weeks of treatment with maximal doses of either rosuvastatin or atorvastatin are presented in Table 1. Both therapies significantly decreased the levels of total cholesterol, LDL-C and triglycerides (P change < 0.001 for both treatments). These differences, however, were not significant among the statin treatment groups. On the other hand, a significant 9% increase in HDL-C was observed in the rosuvastatin treatment group (P change < 0.001), while a nonsignificant increase of 2% was seen for the atorvastatin-treated patients. In both groups, sdLDL-C levels decreased significantly (P change < 0.001 for both treatments), but the decrease was more profound in the rosuvastatin when compared with the atorvastatin-treated patients (−61% vs. −50%, P = 0.003). There was a wide individual response to therapy for LDL-C, HDL-C, and triglycerides (Fig. 1A).
TABLE 1.
Lipid levels and levels of plasma sterols before and after treatment with rosuvastatin or atorvastatin
| Rosuvastatin (n = 66) |
Atorvastatin (n = 69) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | Mean % change | P change | Baseline | 6 weeks | Mean % change | P change | P treatment | |
| Total cholesterol, mg/dl | 287 (29) | 173 (36) | −40 | <0.001 | 281 (31) | 169 (24) | −40 | <0.001 | 0.684 |
| LDL-C, mg/dl | 197 (28) | 89 (32) | −55 | <0.001 | 193 (26) | 91 (21) | −53 | <0.001 | 0.333 |
| HDL-C, mg/dl | 54.5 (11.5) | 59.5 (11.9) | 9 | <0.001 | 53.5 (13.0) | 54.6 (12.7) | 2 | 0.228 | 0.001 |
| Triglycerides, mg/dl | 171 [122–219] | 110 [92-138] | −36 | <0.001 | 169 [123–218] | 114 [94–144] | −33 | <0.001 | 0.738 |
| sdLDL-C, mg/dl | 69 (30) | 27 (16) | −61 | <0.001 | 62 (24) | 31 (15) | −50 | <0.001 | 0.003 |
| Lathosterol, μmol/L | 7.7 (3.1) | 1.7 (1.8) | −78 | <0.001 | 8.4 (3.0) | 1.6 (1.6) | −81 | <0.001 | 0.102 |
| Campesterol, μmol/L | 14.0 [10.3–20.0] | 13.7 [9.2–17.2] | −2 | 0.002 | 13.7 [11.3–19.4] | 14.4 [10.8–19.9] | 5 | 0.477 | 0.001 |
| Sitosterol, μmol/L | 6.0 [4.5–8.6] | 5.9 [4.3–8.2] | −2 | 0.013 | 6.4 [5.0–8.5] | 7.1 [5.6–8.7] | 11 | 0.042 | 0.001 |
| Cholestanol, μmol/L | 7.3 (2.9) | 6.5 (2.6) | −11 | 0.025 | 7.7 (2.4) | 7.1 (2.7) | −8 | 0.077 | 0.706 |
| Lathosterol/Ca | 101 (38) | 36 (32) | −64 | <0.001 | 107 (39) | 34 (27) | −68 | <0.001 | 0.253 |
| Campesterol/Ca | 178 [145–254] | 270 [203-405] | 52 | <0.001 | 192 [146–254] | 331 [236–430] | 72 | <0.001 | <0.001 |
| Sitosterol/Ca | 76 [59–109] | 127 [90-171] | 67 | <0.001 | 82 [69–113] | 161 [122–204] | 96 | <0.001 | <0.001 |
| Cholestanol/Ca | 95 (32) | 140 (56) | 47 | <0.001 | 100 (30) | 156 (56) | 56 | <0.001 | 0.263 |
| Lathosterol/campesterol ratio | 0.53 [0.32–0.82] | 0.09 [0.05–0.18] | −83 | <0.001 | 0.54 [0.36–0.84] | 0.10 [0.05–0.12] | −81 | <0.001 | 0.218 |
C, cholesterol. Values are expressed as mean (SD) or median [interquartile range].
Ratio of the plasma sterols to 102 umol/mmol of cholesterol.
Fig. 1.
The individual percentage responses of LDL cholesterol (C), HDL-C, and triglycerides (A) and the plasma sterols lathosterol, campesterol and cholestanol, in absolute terms (B) and relative to total cholesterol (C) among the statin treatment groups.
Statin treatment and changes in cholesterol synthesis and absorption markers
Treatment with both statins decreased lathosterol, the marker of cholesterol synthesis, in both absolute and relative terms (ratio lathosterol/C). The absolute values of the absorption markers, campesterol and cholestanol, did not change significantly in the atorvastatin-treated group, while a significant decrease was observed in the rosuvastatin group (campesterol: −2%, P change = 0.002 and cholestanol: −11%, P change = 0.025). The absolute concentration of the absorption marker sitosterol changed significantly in both groups (rosuvastatin −2%, P = 0.013 and atorvastatin +11%, P = 0.042). The treatment effects were significant for campesterol and sitosterol (P treatment = 0.001 for both observations), but not for cholestanol (P treatment = 0.706).
When considering the relative effects (i.e., the ratio to cholesterol) of the statin therapies on campesterol, sitosterol, and cholestanol, all the absorption markers increased significantly within both treatment groups (P < 0.001); however, there was a greater increase observed for the ratios of campesterol and sitosterol to cholesterol in the atorvastatin-treated patients when compared with the rosuvastatin group (P treatment < 0.001 for both observations). The changes in the cholestanol/C ratio tended to be higher in the atorvastatin-treated group; however this difference did not reach statistical significance between treatment groups. Both statins had a significant impact on the lathosterol/campesterol ratio, showing a decrease of more than 80% (P change < 0.001 for both observations).
There was a wide individual response for the plasma sterols lathosterol, campesterol, and cholestanol in absolute terms (Fig. 1B) as well as relative to total cholesterol (Fig. 1C) among the statin-treatment subgroups. The response of (absolute and relative) sitosterol to therapy showed the same typical pattern as the other absorption markers, campesterol and cholestanol (data not shown).
Lipids, lipoproteins, and cholesterol synthesis and absorption markers at baseline
The baseline correlations of lipids and lipoproteins with the plasma sterols are presented in Table 2. The marker of cholesterol synthesis, lathosterol, correlated with total cholesterol levels (r = 0.233, P < 0.01), LDL-C (r = 0.172, P < 0.05), triglycerides (r = 0.257, P < 0.01), and sdLDL-C (r = 0.310, P < 0.001). While the lathosterol/C ratio did not correlate with total cholesterol levels (r = −0.053), a negative correlation with HDL-C was observed (r = −0.207, P < 0.05) and the correlation with triglycerides and sdLDL-C remained significant (r = 0.195, P < 0.05 and r = 0.234, P < 0.01, respectively). The concentrations of campesterol and sitosterol correlated significantly with total cholesterol and LDL-C (campesterol: r = 0.174, P < 0.05 and r = 0.198, P < 0.05; and sitosterol: r = 0.261, P < 0.01 and r = 0.247, P < 0.01, respectively). In addition, concentrations of sitosterol also correlated significantly with HDL-C (r = 0.244, P < 0.01), while the sitosterol/C ratio correlated negatively with triglycerides and sdLDL-C (r = −0.206, P < 0.05 and r = −0.204, P < 0.05, respectively). Concentrations of cholestanol correlated with HDL-C (r = 0.284, P < 0.001), and there was a negative correlation with triglycerides and sdLDL-C (r = −0.187, P < 0.05 and r = −0.226, P < 0.01). The cholestanol/C ratio correlated negatively with total cholesterol, LDL-C, triglycerides, and sdLDL-C (r = −0.278, P < 0.01; r = −0.239, P < 0.01; r = −0.263, P < 0.01; and r = −0.273, P < 0.01). The lathosterol/C ratio did not correlate with total cholesterol, LDL-C, or HDL-C levels; however, there was a significant correlation with sdLDL-C (r = 0.237, P < 0.01).
TABLE 2.
Baseline (n = 135) correlations of plasma lipids and lipoproteins with plasma sterols
| Total cholesterol | LDL-C | HDL-C | Triglycerides | sdLDL-C | |
|---|---|---|---|---|---|
| Lathosterol | 0.233b | 0.172c | −0.068 | 0.251b | 0.310d |
| Campesterol | 0.174c | 0.190c | 0.133 | −0.105 | −0.105 |
| Sitosterol | 0.261b | 0.247b | 0.244b | −0.137 | −0.138 |
| Cholestanol | 0.017 | −0.020 | 0.284d | −0.187c | −0.226b |
| Lathosterol/Ca | −0.053 | −0.059 | −0.207c | 0.195c | 0.234b |
| Campesterol/Ca | −0.010 | 0.059 | 0.027 | −0.159 | −0.152 |
| Sitosterol/Ca | 0.094 | 0.132 | 0.160 | −0.206c | −0.204c |
| Cholestanol/Ca | −0.278b | −0.239b | 0.122 | −0.263b | −0.273b |
| Lathosterol/campesterol ratio | 0.010 | −0.046 | −0.157 | 0.255b | 0.273b |
Ratio of the plasma sterols to 102 umol/mmol of cholesterol.
P < 0.01.
P < 0.05.
P < 0.001. The non linear variables were log transformed for the correlations.
Efficacy of statin therapy on lipid lowering in relationship to cholesterol synthesis and absorption markers
Table 3 shows the correlations between changes in plasma lipids and sterols per treatment group. Changes in lathosterol levels significantly correlated with changes in total cholesterol, LDL-C, and sdLDL-C in both treatment groups (rosuvastatin: r = 0.406, P < 0.001; r = 0.346, P < 0.01; and r = 0.337, P < 0.01, respectively, and atorvastatin: r = 0.390, P < 0.001; r = 0.331, P < 0.01; and r = 0.274 and P < 0.05, respectively). Changes in campesterol correlated with changes in total cholesterol and LDL-C in both treatment groups, while only reaching significance in the atorvastatin group (r = 0.311, P < 0.01 and r = 0.298, P < 0.05 respectively). Interestingly, changes of cholestanol correlated positively with LDL-C (r = 0.258, P < 0.05) in the rosuvastatin-treated patients, while a nonsignificant negative correlation was observed in the atorvastatin-treated patients. When the changes in cholestanol were adjusted for total cholesterol levels, the correlation with LDL-C shifted to a negative correlation in the rosuvastatin-treated patients, while the negative correlation with total and LDL-C became stronger and statistical significant in the atorvastatin-treated patients (r = −0.376, P < 0.01 and r = −0.317, P < 0.01).
TABLE 3.
Correlations between changes in plasma lipids and lipoproteins and plasma sterols per statin treatment
| Rosuvastatin (n = 66) |
Atorvastatin (N = 69) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ TC | Δ LDL-C | Δ HDL-C | Δ TG | Δ sdLDL-C | Δ TC | Δ LDL-C | Δ HDL-C | Δ TG | Δ sdLDL-C | |
| Δ Lathosterol | 0.406b | 0.346d | 0.200 | 0.049 | 0.337d | 0.390b | 0.331d | 0.042 | 0.216 | 0.274c |
| Δ Campesterol | 0.233 | 0.231 | 0.022 | −0.026 | −0.082 | 0.311d | 0.298c | 0.071 | 0.164 | 0.188 |
| Δ Sitosterol | 0.229 | 0.214 | 0.170 | 0.012 | −0.104 | 0.252c | 0.186 | 0.149 | 0.162 | 0.268c |
| Δ Cholestanol | 0.240 | 0.258c | 0.047 | −0.140 | −0.087 | −0.168 | −0.128 | 0.080 | −0.112 | 0.008 |
| Δ Lathosterol/Ca | 0.301c | 0.238 | 0.127 | 0.0.69 | 0.285c | 0.144 | 0.139 | 0.044 | −0.011 | 0.126 |
| Δ Campesterol/Ca | −0.124 | −0.104 | −0.239 | 0.106 | −0.118 | 0.055 | −0.028 | −0.012 | 0.170 | 0.168 |
| Δ Sitosterol/Ca | −0.216 | −0.242 | −0.065 | 0.205 | −0.195 | −0.013 | −0.119 | 0.057 | 0.134 | 0.201 |
| Δ Cholestanol/Ca | −0.165 | −0.135 | −0.193 | −0.001 | −0.052 | −0.376d | −0.317d | 0.078 | −0.135 | −0.076 |
| Δ Lathosterol/campesterol ratio | 0.231 | 0.211 | 0.072 | 0.055 | 0.264c | 0.084 | −0.001 | −0.021 | 0.191 | 0.230 |
Ratio of the plasma sterols to 102 umol/mmol of cholesterol. TC, total cholesterol; TG, triglycerides.
P < 0.001.
P < 0.05.
P < 0.01.
Factors related to changes in cholesterol synthesis and absorption markers during statin treatment
Regression models were developed using lathosterol, campesterol, and cholestanol, respectively, as outcome variables and age, gender, statin treatment, baseline levels of plasma sterols, and total cholesterol and changes in total cholesterol as independent variables (see Table 4). For the changes in lathosterol, campesterol, and cholestanol, the baseline values of these sterols were the strongest predictors for the changes in these same sterols during treatment (standardized β coefficient −0.838, −0.543, and −0.561, respectively; P < 0.001 for all). Absolute levels of campesterol and cholestanol decreased significantly when both treatment arms were combined (data not shown), explaining the negative β coefficient. Change in total cholesterol was the next most important predictor of changes in lathosterol and campesterol (standardized β coefficient 0.315 and 0.264, P < 0.001 and P = 0.004) but not for the changes in cholestanol (standardized β coefficient 0.150, P = 0.121; see Table 4). There were no data available in our study on dietary intake of plant sterols. However, the estimated impact of variations in dietary plant sterol on circulating levels of plant sterols is less than 4% (17); thus, variations in dietary sterols most likely have a marginal effect on changes in campesterol and cholestanol in our study.
TABLE 4.
Multiple regression models for variables associated with changes in lathosterol, campesterol, and cholestanol levels during statin treatment
| Standardized Beta Coefficient | P value | |
|---|---|---|
| Δ Lathosterol modela | ||
| Age, y | −0.099 | 0.034 |
| Gender, m/f | 0.001 | 0.977 |
| Treatment, rosuvastatin / atorvastatin | −0.013 | 0.766 |
| Baseline lathosterol, μmol/L | −0.838 | <0.001 |
| Baseline total cholesterol, mg/dl | 0.128 | 0.030 |
| Changes in total cholesterol, mg/dl | 0.315 | <0.001 |
| Δ Campesterol modelb | ||
| Age, y | 0.093 | 0.215 |
| Gender, m/f | 0.142 | 0.067 |
| Treatment, rosuvastatin / atorvastatin | 0.236 | 0.001 |
| Baseline campesterol, μmol/L | −0.543 | <0.001 |
| Baseline total cholesterol, mg/dl | 0.011 | 0.908 |
| Changes in total cholesterol, mg/dl | 0.264 | 0.004 |
| Δ Cholestanol modelc | ||
| Age, y | 0.008 | 0.925 |
| Gender, m/f | 0.191 | 0.022 |
| Treatment, rosuvastatin / atorvastatin | 0.173 | 0.026 |
| Baseline cholestanol, μmol/L | −0.561 | <0.001 |
| Baseline total cholesterol, mg/dl | 0.065 | 0.513 |
| Changes in total cholesterol, mg/dl | 0.150 | 0.121 |
In the linear regression models, changes in total lathosterol, campesterol, and cholestanol (in mg/dl) respectively served as outcome variables, while age, gender, statin treatment, baseline total cholesterol, total cholesterol changes, and depending on the model, baseline lathosterol, campesterol, and cholestanol served as independent variables.
Adjusted R squared = 0.781.
Adjusted R squared = 0.417.
Adjusted R squared = 0.321.
Diabetic status and markers of cholesterol synthesis and absorption
In the original STELLAR cohort, 7.5% of the participants reported a history of diabetes (9). In the current study, glycated albumin was used as a marker for glycemic control. Glycated albumin correlated with lathosterol levels and lathosterol/C at baseline (r = −0.183, P = 0.035 and r = −0.205 and P = 0.018) but not with the other sterols. Furthermore, glycated albumin was not a significant predictor of lathosterol changes as a result of statin treatment (data not shown).
Lipid changes with statin treatment among subgroups with variable cholesterol synthesis and absorption markers
In order to further explore the relationships between changes in synthesis and absorption markers and cholesterol, treatment groups were combined and changes in total lathosterol and campesterol were divided into two groups (reflecting high and low changes in the synthesis marker, lathosterol, and the increase or decrease in the absorption marker, campesterol, respectively), (Table 5). The greatest reduction of total cholesterol was observed in the high change in synthesis/decreased absorption subgroup, while the lowest reductions of total cholesterol was seen in the low change in synthesis/increased absorption subgroup [−132 ± 30 mg/dl (−46%) vs. −97 ± 40 mg/dl (−34%), P difference = 0.001]. Similar effects were observed for LDL-C changes (Table 5), but not for changes in HDL-C or triglycerides (data not shown). In addition, multivariate modeling of the synthesis and absorption markers clearly indicated that alterations in both types of parameters (i.e., change in absolute levels of lathosterol and change in absolute levels of campesterol) were significantly associated with change in total cholesterol and LDL-C levels (see supplementary Table I). Baseline values of plasma sterol, however, were not predictive of total cholesterol or LDL-C changes in regression models adjusted for age, gender, statin treatment, and baseline value of total cholesterol or LDL-C, respectively (see supplementary Table II).
TABLE 5.
Subgroup combinations of lathosterol and campesterol changes in relation to changes in total cholesterol and LDL cholesterol during statin treatment
| Lathosterol decrease | Campesterol change | N | Changes in total cholesterol, mg/dl | Changes in LDL-C, mg/dl |
|---|---|---|---|---|
| High | Increase | 32 | −107 (38)a | −99 (33)c |
| High | Decrease | 37 | −132 (30)a,b | −124 (32)c,d |
| Low | Increase | 30 | −97 (40)b | −94 (38)c |
| Low | Decrease | 36 | −112 (32) | −107 (31) |
Values are means (SD). A high decrease in lathosterol is defined as a decrease greater than 6.3 μmol/L. The symbols indicate significant pairs derived from post hoc testing. Tests for significance were adjusted using the Bonferroni method for multiple comparisons.
P = 0.027.
P = 0.001.
P = 0.015.
P = 0.003.
DISCUSSION
In order to gain more insight into the effects of intensive statin therapy on changes in markers of cholesterol synthesis and absorption, we measured plasma sterols in a subset of 135 participants of the STELLAR study.
It is well known that the primary effect of HMG-CoA reductase inhibitors, or statins, is to inhibit cholesterol synthesis causing a decrease in intracellular cholesterol content. As a result, there is an up-regulation of LDL receptor activity and enhanced fractional clearance of all apoB-containing lipoproteins from the plasma space. This effect not only leads to marked reduction in VLDL-C and LDL-C concentrations in plasma, but also in the levels of plasma sterols carried on these lipoproteins. Because plasma sterols are carried in lipoproteins, Miettinen and Gylling (7) advocate expressing plasma sterols as a ratio with total cholesterol. The view among the majority of researchers is that plasma sterols should be adjusted for total cholesterol concentration in plasma by expressing their levels as a ratio to cholesterol. It has been postulated on the other hand, that using adjusted values is not correct in statin studies, as the decrease in total cholesterol can increase the sterol to cholesterol ratio even though there is no change in the sterol concentration (17). Indeed, the main disadvantage of using ratios is that they are difficult to interpret when used in correlation and regression models, and can lead to incorrect or misleading conclusions (18). This was also the case for the correlations in our data analysis. We observed only the expected correlation with total cholesterol at baseline when the synthesis and absorption markers were expressed in absolute terms, and not in relative terms. Such findings raise the questions whether synthesis and absorption markers should be expressed in absolute terms or relative terms.
In our opinion, the manner in which plasma sterols are expressed should depend on the study question being asked. When investigating synthesis and absorption differences among two groups, one would like to be certain that these effects are independent of the cholesterol concentration and therefore values should be expressed relative to cholesterol (i.e., sterol/total cholesterol ratio). However, when investigating the relationship between absorption markers, synthesis markers, and cholesterol levels, the absolute sterol concentrations should be used due to the fact that normalizing or adjusting for total cholesterol would mean that you are masking the outcome variable you are interested in assessing.
As expected, lathosterol, the synthesis marker, declined greatly after treatment with statins whether expressed in absolute or relative terms. Moreover, both statins decreased this parameter to a similar degree. The effect of atorvastatin treatment on synthesis markers has been well established (7, 19–23). It should be noted that Naoumova et al. (24) were the first to document in human subjects (n = 35) that statins (atorvastatin, pravastatin, and atorvastatin) decreased markers of cholesterol synthesis. In their study, they measured plasma mevalonate levels and reported significant reductions in absolute levels, especially for atorvastatin (24). We have recently documented in a kinetic study with nine hypercholesterolemic individuals that atorvastatin at a dose of 80 mg/day reduced the relative amount of lathosterol by 76% (20). In agreement, the current study shows that patients receiving 80 mg/day of atorvastatin decreased their lathosterol levels in a similar fashion versus baseline (68% in relative terms and 81% in absolute terms). Recently Ooi et al. (25) have reported in kinetic studies of 12 obese dyslipidemic subjects that rosuvastatin at a dose of 40 mg/day decreased the relative amount or levels of lathosterol by 75% and the absolute by 86% as compared with placebo. In our study, with a larger number of subjects, 40 mg/day rosuvastatin reduced lathosterol by 64% in relative terms and by 78% in absolute terms. We did not observe any significant treatment differences between the two statins with regard to the reductions in the synthesis marker lathosterol. We also did not observe any significant differences between statins with regard to reductions in the other synthesis marker, desmosterol (data not shown).
With regard to markers of fractional cholesterol absorption, we noted significant differences between rosuvastatin and atorvastatin. Rosuvastatin modestly decreased campesterol and sitosterol in absolute terms (−2% for both), while atorvastatin modestly increased the absolute levels of these markers (+5% and +11%, respectively). When these markers were expressed in relative terms, both statins significantly increased the ratios of campesterol/C (+52% and +72%) and sitosterol/C (+67% and +96%) relative to baseline. We noted a significant difference (P < 0.001) between these two statins with rosuvastatin not raising the relative amounts of campesterol or sitosterol as much as atorvastatin. Therefore rosuvastatin caused less of an up-regulation in markers of fractional cholesterol absorption than atorvastatin, indicating that this statin may have less of an effect on the intestine than atorvastatin. An alternative explanation may be that in contrast to rosuvastatin, atorvastatin is more active in reducing the biliary excretion of cholesterol and sterols. This would fit with the reported effects that fibrates have (i.e., to increase biliary cholesterol excretion and raise HDL cholesterol). As we now know ABCG5/G8 transporters work in the intestines and liver with their major role being to pump cholesterol and other sterols back into either the intestinal lumen or into bile (26) and are probably responsible for both processes occurring simultaneously during the treatment with statins. To our knowledge, there are no reports on the interaction of rosuvastatin with ABCG5/8 transporters; however, an interaction with atorvastatin has been reported (27).
Overall, it is difficult to speculate on the underlying mechanism causing the different responses. In contrast to atorvastatin, rosuvastatin is known to be a more hydrophilic statin that is not metabolized through the cytochrome P450 system (2). In addition, the bioavailability of atorvastatin is affected by dietary intake in contrast to rosuvastatin (2). Thus with regard to the absorption markers, both statins have different pharmacokinetic properties, which may account for the somewhat greater efficacy in LDL-C lowering and HDL-C increasing for rosuvastatin than atorvastatin.
We were also interested in how alterations in plasma sterol levels affected responsiveness to statins in terms of variability in reductions in total cholesterol, triglyceride, and LDL-C, and increases in HDL-C (see Fig. 1A). Even though the observed differences in campesterol alterations among the statin treatment groups were statistically significant, these differences had only marginal effects on the changes in lipid parameters. Alterations in the absolute levels of campesterol significantly correlated with changes in total cholesterol and LDL-C in the atorvastatin group, with similar correlations in the rosuvastatin group (although in this case they were not significant). These findings justified pooling the treatment groups in order to investigate effects of alterations in synthesis and absorption markers on plasma lipid changes. Our data indicate that the greatest total cholesterol and LDL-C reductions were achieved in subjects with the greatest reduction in lathosterol, and no increase in cholesterol absorption markers, as compared with subjects in whom the converse was true.
Thus, in line with the general hypothesis that high synthesizers respond better to treatment with statins, our findings again underline that statin treatment is especially effective if the individual was also a low absorber (i.e., not capable of up-regulating cholesterol absorption). The converse is also the case; individuals who are poor synthesizes, but are able to up-regulate absorption, do considerably less well on statin treatment in terms of total cholesterol lowering.
The current study suggests that patients who are high absorbers may actually benefit from additional therapy with cholesterol absorption inhibiters. In humans it has been shown that the absorption inhibiter, ezetimibe, reduces the absorption of cholesterol and in line, the absorption markers are also significantly lower (28). However, a result of ezetimibe treatment is a compensatory increase in cholesterol synthesis (28, 29), emphasizing that patients receiving a combination of statins and ezetimibe should have the greatest lipid-lowering effects. Indeed, in large clinical trials like the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) study and the EXamination of Potential Lipid-modifying effects Of Rosuvastatin in combination with Ezetimibe versus Rosuvastatin (EXPLORER) study, the coadministration of ezetimibe with a statin, significantly showed a greater reduction of total cholesterol levels when compared with patients treated with statins alone (30, 31). To date, however, the effect of ezetimibe in combination with statins on markers of cholesterol synthesis and absorption has only been marginally investigated, and only one human study showed a significant effect of the coadministration of ezetimibe and statins on the reduction of these markers (19). Thus, further investigations are needed to understand the effects these drug combinations have on the synthesis and absorption of cholesterol.
The total cholesterol lowering response to statins among the subgroups of high/low synthesizers and absorbers was markedly different, thus raising the question whether the changes in the synthesis and absorption status also correlate with a higher risk of developing cardiovascular disease. Our study, however, was not designed to address this question. Although a number of studies have found an association between increased levels of plasma sterols and cardiovascular disease (19, 32–34), a recent large prospective investigation did not confirm this relationship (35). Our data are consistent with the concept that cholesterol synthesis and absorption seem to be inversely linked in maintaining a constant cholesterol balance (i.e., when absorption increases, synthesis decreases, and visa versa). We observed a positive correlation for both synthesis and absorption markers (lathosterol and campesterol, respectively) with total cholesterol at baseline, however, baseline total cholesterol did not correlate with the ratio lathosterol/campesterol, which is the marker for the overall cholesterol homeostasis. This suggests that in a general population the effects of synthesis and absorption are balanced and therefore not pronounced enough to have an actual clinically relevant effect. For this reason it would be better to study this relationship in populations where the cholesterol balance is being disrupted by either diet or drug treatment, and indeed, Miettinen et al. (36) found that a low baseline cholestanol/C ratio was associated with significant risk reduction of cardiovascular events in patients treated with simvastatin in contrast to treated patients with a high ratio at baseline.
We have reviewed all the studies in the literature that we have identified on the topic under discussion (see Table 6). There is clear trend for statins to decrease cholesterol synthesis markers in absolute and relative terms, and to increase markers of cholesterol absorption in relative terms. In an analysis of all the data shown in Table 6, the correlations between changes in total cholesterol and absolute reductions in synthesis were highly significant (r = 0.959, P = 0.001). These data could be fit with the following regression equation: percentage change in total C = −2.5 + 0.48 (% change in lathosterol). We did not observe a significant correlation between changes in plasma campesterol in absolute terms. This analysis of group data is somewhat different than our analysis of individual data, whereby markers of synthesis and absorption both contributed to variability in total cholesterol response to statin therapy. Moreover as can be seen in Fig. 1B, there are some subjects whose lathosterol levels do not decrease in either absolute or relative terms. Such subjects in our view are probably noncompliers.
TABLE 6.
Overview of the majora studies investigating the effects of statin treatment on changes of lathosterol and campesterol
| Author | Year | Statin | N | Duration | Total cholesterol Changes, % | Lathosterol Changes, % | Lathosterol/C Changes, % | Campesterol Change, % | Campesterol/C Change, % |
|---|---|---|---|---|---|---|---|---|---|
| Uusitupa et al. (37) | 1992 | Lovastatin (80 mg/day) | 62 | 18 weeks | −34% | −63% | −43% | −14% | +33% |
| Miettinen et al. (11) | 2000 | Simvastatin (20 mg/day) | 434 | 6 weeks | NR | NR | −34% | NR | NR |
| Miettinen et al. (38) | 2002 | Simvastatin (20m//day) | 319 | 1 year | −30% | −55% | −36% | +4% | +48% |
| Simvastatin (40 mg/day) | 115 | 1 year | −27% | −52% | −35% | +4% | +46% | ||
| Miettinen et al. (21) | 2003 | Atorvastatin (29 mg/dayb) | 102 | 1 year | −36% | NR | −52% | NR | +74% |
| Simvastatin (34 mg/dayb) | 105 | 1 year | −33% | NR | −43% | NR | +33% | ||
| Assmann, et al. (39) | 2008 | Atorvastatin (10–80 mg/day) | 160 | 12 weeks | −33% | −69% | −59% | +10% | +58% |
| Simvastatin (10–80 mg/day) | 232 | 12 weeks | −27% | −54% | −42% | −2% | +35% | ||
| Van Himbergen et al. | Current study | Rosuvastatin (40 mg/day) | 66 | 6 weeks | −40% | −78% | −64% | −2% | +52% |
| Atorvastatin (80 mg/day) | 69 | 6 weeks | −40% | −81% | −68% | +5% | +72% |
NR, not reported.
Inclusion criteria: statin studies with more than 50 participants, including lathosterol and campesterol measurements as markers for synthesis and absorption, respectively.
Average dose over a 1-year treatment period.
Our data are consistent with the observations of other investigators on the effects of statins on markers of cholesterol synthesis and absorption, but no data have previously been published on rosuvastatin, and little effort has been made to attempt to integrate these alterations and lipid-lowering response. It is clearly recognized that the major effect of statins is to reduce cellular cholesterol synthesis, resulting in an up-regulation of LDL receptor activity, enhanced fractional clearance of LDL from plasma, and reduction in plasma LDL cholesterol levels. However these effects may be offset by an up-regulation in cholesterol absorption. Our data indicate that effects on both of these types of markers are important to account for efficacy of the statins tested in determining the total cholesterol-lowering response. In summary, both statins significantly decreased cholesterol synthesis and increased markers of fractional cholesterol absorption. This study strengthens the hypothesis that successful lipid-lowering depends on the synthesis/absorption status of the patient. In addition, the current study emphasizes that changes in these markers are more informative than baseline levels. We noted that the most effective lipid-lowering was observed in patients with the greatest reductions in synthesis and no increases in absorption, with the converse also being the case. Because ezetimibe very significantly reduces intestinal cholesterol absorption, but increases synthesis, and because statins have the opposite effect, it would appear that combination therapy would be ideal. In addition, because statin therapy is often long term, measuring sterols may prove to be a useful tool for optimizing therapy and reducing CHD risk.
Supplementary Material
Presented in part at the annual Scientific Sessions of the American Heart Association and the Young Investigator Scientific Sessions of the Council on Arteriosclerosis, Thrombosis, and Vascular Biology, November 2008.
Published, JLR Papers in Press, November 30, 2008.
Footnotes
T.M. van Himbergen was supported by the Ruth L. Kirschstein National Research Service Award, training grant DK07651. S. Otokozawa and M. Ai were supported by research fellowships from Kyowa Medex Co, Tokyo Japan and Denka Seiken Co, Tokyo Japan, respectively. E.J. Schaefer was supported by grants R01 HL-60935, HL 74753 and PO50HL083813 from the National Institutes of Health and contract 53-3K-06 from the United Department of Agriculture Research Service. The original Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin (STELLAR) study was supported by contracts to Drs. Stein and Jones from the AstraZeneca Company, Wilmington, DE; however no research support for this investigation was received from AstraZeneca.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
References
- 1.Baigent C., A. Keech, P. M. Kearney, L. Blackwell, G. Buck, C. Pollicino, A. Kirby, T. Sourjina, R. Peto, R. Collins, et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366 1267–1278. [DOI] [PubMed] [Google Scholar]
- 2.Schachter M. 2005. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam. Clin. Pharmacol. 19 117–125. [DOI] [PubMed] [Google Scholar]
- 3.Pedro-Botet J., E. J. Schaefer, R. G. Bakker-Arkema, D. M. Black, E. M. Stein, D. Corella, and J. M. Ordovas. 2001. Apolipoprotein E genotype affects plasma lipid response to atorvastatin in a gender specific manner. Atherosclerosis. 158 183–193. [DOI] [PubMed] [Google Scholar]
- 4.Thompson G. R., F. O'Neill, and M. Seed. 2002. Why some patients respond poorly to statins and how this might be remedied. Eur. Heart J. 23 200–206. [DOI] [PubMed] [Google Scholar]
- 5.Matthan N. R., and A. H. Lichtenstein. 2004. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 174 197–205. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen T. A., R. S. Tilvis, and Y. A. Kesaniemi. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131 20–31. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen T. A., and H. Gylling. 2003. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur. J. Clin. Invest. 33 976–982. [DOI] [PubMed] [Google Scholar]
- 8.Vanhanen H., Y. A. Kesaniemi, and T. A. Miettinen. 1992. Pravastatin lowers serum cholesterol, cholesterol-precursor sterols, fecal steroids, and cholesterol absorption in man. Metabolism. 41 588–595. [DOI] [PubMed] [Google Scholar]
- 9.Vanhanen H. T., and T. A. Miettinen. 1995. Cholesterol absorption and synthesis during pravastatin, gemfibrozil and their combination. Atherosclerosis. 115 135–146. [DOI] [PubMed] [Google Scholar]
- 10.Duane W. C. 1993. Effects of lovastatin and dietary cholesterol on sterol homeostasis in healthy human subjects. J. Clin. Invest. 92 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen T. A., T. E. Strandberg, and H. Gylling. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 20 1340–1346. [DOI] [PubMed] [Google Scholar]
- 12.Jones P. H., D. B. Hunninghake, K. C. Ferdinand, E. A. Stein, A. Gold, R. J. Caplan, and J. W. Blasetto. 2004. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin. Ther. 26 1388–1399. [DOI] [PubMed] [Google Scholar]
- 13.Jones P. H., M. H. Davidson, E. A. Stein, H. E. Bays, J. M. McKenney, E. Miller, V. A. Cain, and J. W. Blasetto. 2003. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am. J. Cardiol. 92 152–160. [DOI] [PubMed] [Google Scholar]
- 14.Ai M., S. Otokozawa, B. F. Asztalos, K. Nakajima, E. Stein, P. H. Jones, and E. J. Schaefer. 2008. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am. J. Cardiol. 101 315–318. [DOI] [PubMed] [Google Scholar]
- 15.Kouzuma T., Y. Uemastu, T. Usami, and S. Imamura. 2004. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin. Chim. Acta. 346 135–143. [DOI] [PubMed] [Google Scholar]
- 16.Matthan N. R., A. Giovanni, E. J. Schaefer, B. G. Brown, and A. H. Lichtenstein. 2003. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J. Lipid Res. 44 800–806. [DOI] [PubMed] [Google Scholar]
- 17.Chan Y. M., K. A. Varady, Y. Lin, E. Trautwein, R. P. Mensink, J. Plat, and P. J. Jones. 2006. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr. Rev. 64 385–402. [DOI] [PubMed] [Google Scholar]
- 18.Kronmal R. A. 1993. Spurious correlation and the fallacy of the ratio standard revisited. J. R. Stat. Soc. Ser. A Stat. Soc. 156 379–392. [Google Scholar]
- 19.Assmann G., P. Cullen, J. Erbey, D. R. Ramey, F. Kannenberg, and H. Schulte. 2006. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 16 13–21. [DOI] [PubMed] [Google Scholar]
- 20.Lamon-Fava S., M. R. Diffenderfer, P. H. Barrett, A. Buchsbaum, N. R. Matthan, A. H. Lichtenstein, G. G. Dolnikowski, K. Horvath, B. F. Asztalos, V. Zago, et al. 2007. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J. Lipid Res. 48 1746–1753. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen T. A., H. Gylling, N. Lindbohm, T. E. Miettinen, R. A. Rajaratnam, and H. Relas. 2003. Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J. Lab. Clin. Med. 141 131–137. [DOI] [PubMed] [Google Scholar]
- 22.Smahelova A., R. Hyspler, T. Haas, A. Ticha, V. Blaha, and Z. Zadak. 2005. Effect of atorvastatin on non-cholesterol sterols in patients with type 2 diabetes mellitus and cardiovascular disease. Pharmacol. Res. 51 31–36. [DOI] [PubMed] [Google Scholar]
- 23.Watts G. F., D. C. Chan, P. H. Barrett, F. H. O'Neill, and G. R. Thompson. 2003. Effect of a statin on hepatic apolipoprotein B-100 secretion and plasma campesterol levels in the metabolic syndrome. Int. J. Obes. Relat. Metab. Disord. 27 862–865. [DOI] [PubMed] [Google Scholar]
- 24.Naoumova R. P., A. D. Marais, J. Mountney, J. C. Firth, N. B. Rendell, G. W. Taylor, and G. R. Thompson. 1996. Plasma mevalonic acid, an index of cholesterol synthesis in vivo, and responsiveness to HMG-CoA reductase inhibitors in familial hypercholesterolaemia. Atherosclerosis. 119 203–213. [DOI] [PubMed] [Google Scholar]
- 25.Ooi E. M., P. H. Barrett, D. C. Chan, P. J. Nestel, and G. F. Watts. 2008. Dose-dependent effect of rosuvastatin on apolipoprotein B-100 kinetics in the metabolic syndrome. Atherosclerosis. 197 139–146. [DOI] [PubMed] [Google Scholar]
- 26.Yu L., J. Li-Hawkins, R. E. Hammer, K. E. Berge, J. D. Horton, J. C. Cohen, and H. H. Hobbs. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kajinami K., M. E. Brousseau, C. Nartsupha, J. M. Ordovas, and E. J. Schaefer. 2004. ATP binding cassette transporter G5 and G8 genotypes and plasma lipoprotein levels before and after treatment with atorvastatin. J. Lipid Res. 45 653–656. [DOI] [PubMed] [Google Scholar]
- 28.Sudhop T., D. Lutjohann, A. Kodal, M. Igel, D. L. Tribble, S. Shah, I. Perevozskaya, and K. von Bergmann. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 106 1943–1948. [DOI] [PubMed] [Google Scholar]
- 29.Jakulj L., M. D. Trip, T. Sudhop, K. von Bergmann, J. J. Kastelein, and M. N. Vissers. 2005. Inhibition of cholesterol absorption by the combination of dietary plant sterols and ezetimibe: effects on plasma lipid levels. J. Lipid Res. 46 2692–2698. [DOI] [PubMed] [Google Scholar]
- 30.Kastelein J. J., F. Akdim, E. S. Stroes, A. H. Zwinderman, M. L. Bots, A. F. Stalenhoef, F. L. Visseren, E. J. Sijbrands, M. D. Trip, E. A. Stein, et al. 2008. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358 1431–1443. [DOI] [PubMed] [Google Scholar]
- 31.Ballantyne C. M., R. Weiss, T. Moccetti, A. Vogt, B. Eber, F. Sosef, and E. Duffield. 2007. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am. J. Cardiol. 99 673–680. [DOI] [PubMed] [Google Scholar]
- 32.Glueck C. J., J. Speirs, T. Tracy, P. Streicher, E. Illig, and J. Vandegrift. 1991. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism. 40 842–848. [DOI] [PubMed] [Google Scholar]
- 33.Sudhop T., B. M. Gottwald, and K. von Bergmann. 2002. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism. 51 1519–1521. [DOI] [PubMed] [Google Scholar]
- 34.Rajaratnam R. A., H. Gylling, and T. A. Miettinen. 2000. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35 1185–1191. [DOI] [PubMed] [Google Scholar]
- 35.Pinedo S., M. N. Vissers, K. von Bergmann, K. Elharchaoui, D. Lutjohann, R. Luben, N. J. Wareham, J. J. Kastelein, K. T. Khaw, and S. M. Boekholdt. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. J. Lipid Res. 48 139–144. [DOI] [PubMed] [Google Scholar]
- 36.Miettinen T. A., H. Gylling, T. Strandberg, and S. Sarna. 1998. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ. 316 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uusitupa M. I., T. A. Miettinen, P. Happonen, T. Ebeling, H. Turtola, E. Voutilainen, and K. Pyorala. 1992. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arterioscler. Thromb. 12 807–813. [DOI] [PubMed] [Google Scholar]
- 38.Miettinen T. A., and H. Gylling. 2002. Ineffective decrease of serum cholesterol by simvastatin in a subgroup of hypercholesterolemic coronary patients. Atherosclerosis. 164 147–152. [DOI] [PubMed] [Google Scholar]
- 39.Assmann G., F. Kannenberg, D. R. Ramey, T. A. Musliner, S. W. Gutkin, and E. P. Veltri. 2008. Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe-statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia. Curr. Med. Res. Opin. 24 249–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.