Abstract
Postmortem analysis of five subjects with Parkinson’s disease9–14 years after transplantation of fetal midbrain cell suspensions revealed surviving grafts that included dopamine and serotonin neurons without pathology. These findings are important for the understanding of the etiopathogenesis of midbrain dopamine neuron degeneration and future use of cell replacement therapies.
Despite indirect evidence of long-term survival of fetal midbrain dopamine cell suspensions in people with Parkinson’s disease1, the question remains whether grafted neurons are affected by pathogenic factors intrinsic to the parkinsonian brain.
Prominent neuropathological features of Parkinson’s disease include dopaminergic neuron loss in the substantia nigra, the presence of dystrophic neurites (Lewy neurites)2 and the presence of Lewy bodies3,4. Ultimately, the durability of transplanted fetal ventral midbrain neurons in therapeutic approaches relies on their resistance to these neurodegenerative processes. Because many aspects of these processes remain unknown, it is important to understand the effects of neurodegeneration in the parkinsonian striatum upon transplanted fetal dopamine neurons. We report histopathological findings in the brains of three subjects (referred to as subjects 4, 5 and 6) with advanced idiopathic Parkinson’s disease who had received intracerebral transplantation of fetal ventral midbrain cell suspension grafts 9–14 years previously (Supplementary Tables 1 and 2 and Supplementary Results online). Additionally, we extend the pathological analysis to two subjects who died of unrelated causes 3–4 years after transplantation (subjects 1 and 2)5. This study provides a unique and in-depth long-term postmortem analysis of the effects of neurodegeneration on grafted fetal dopamine neurons in individuals with Parkinson’s disease.
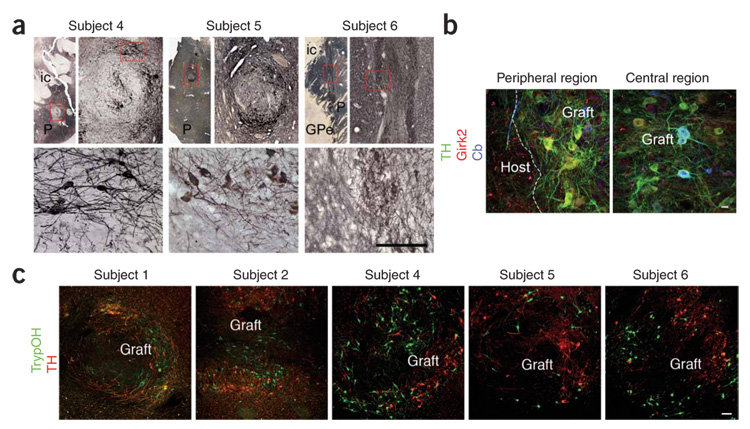
We grafted the subjects using our specific transplantation procedures (Supplementary Methods online). Postmortem analysis showed well-integrated grafts containing tyrosine hydroxylase–immunoreactive neurons with extensive neuritic outgrowth into the host putamen without displacement (Fig. 1a). The distribution of tyrosine hydroxylase–immunoreactive neurons in the grafts of subjects 4 and 5 was less homogeneous than in subjects 1, 2 and 6, in whomwe used a two-hole rotating cannula. In all grafts, the majority of tyrosine hydroxylase–immunoreactive neurons were located at the graft periphery and contained small amounts of neuromelanin; no extracellular neuromelanin was observed (Fig. 1 and Fig 2; for survival, cell preparation, injection technique and stereological analysis, see Supplementary Table 1 and Supplementary Methods).
Figure 1. Ventral midbrain cells transplanted as cell suspensions in the putamina of people with Parkinson’s disease survive for up to 14 years and show immunoreactivity for dopaminergic and serotoninergic markers.
(a) Fetal ventral midbrain cell suspension grafts in the post-commissural putamina of subjects 4, 5 and 6 contained tyrosine hydroxylase–immunoreactive (TH+) neurons that were well integrated with the host and did not cause any tissue displacement. Scale bar: 5 mm (top, left-hand images), 1,000 µm (top, right-hand images) and 150 µm (bottom) for each subject. The top right-hand image for each subject is an enlargement of the boxed area in the top left-hand image, and the bottom image is an enlargement of the boxed area in the top right-hand image. P, putamen; ic, internal capsule; GPe, globus pallidus, pars externus. (b) Representative confocal images of triple immunofluorescence staining of TH (green), Girk2 (red) and calbindin (Cb, blue) within a putaminal graft of subject 6, showing peripheral and central regions of the graft. TH+Girk2+ neurons were preferentially located in the peripheral areas of the graft, whereas TH+Cb+ neurons were preferentially located in central areas. Scale bar, 20 µm. (c) Representative confocal images of double immunofluorescence studies of TH+ (red) and tryptophan hydroxylase–immunoreactive (TrypOH+, a marker for serotoninergic neurons, green) neurons within graft deposits of subjects 1, 2, 4, 5 and 6. Colocalization between TrypOH and tyrosine hydroxylase immunoreactivity was rare, and tyrosine hydroxylase did not cross-react with TrypOH. To avoid ambiguity, all neurons labeled with TH were counted as dopaminergic neurons, and all TrypOH+ neurons that did not colocalize with tyrosine hydroxylase were counted as serotoninergic neurons. Scale bar, 100 µm. All studies were conducted under the strict guidelines of a protocol approved by the Queen Elizabeth II Health Sciences Centre Ethics Review Board, Nova Scotia, Canada. Fetal ventral midbrain tissue was collected with maternal consent. Informed consent for the transplantation procedures was obtained from each subject. Permission was granted by the subjects’ next of kin to retrieve the brains for histological analysis.
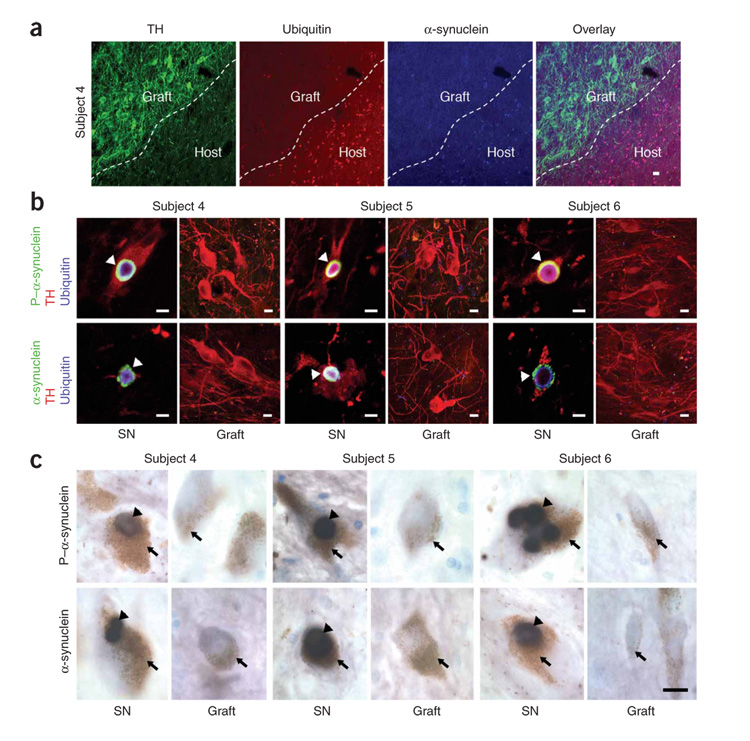
Figure 2. Transplanted dopamine neurons in people with Parkinson’s disease do not contain Lewy bodies.
(a) There was no evidence of α-synuclein– or ubiquitin-positive inclusions within the grafts, as typified by confocal images of triple immunofluorescence labeling of TH (green), α-synuclein (blue) and ubiquitin (red) in the grafted and adjacent host putamen of subject 4. Scale bar, 10 µm. (b) TH+ host nigral neurons (red) of all subjects showed α-synuclein (green), phosphorylated α-synuclein (P–α-synuclein) (green) and ubiquitin-positive (blue) Lewy body inclusions (arrowheads), consistent with the diagnosis of Parkinson’s disease (top and bottom left-hand images for each subject). In contrast, no Lewy body pathology was found in grafted TH+ neurons, as shown in these representative images from the putamina of subjects 4, 5 and 6 (top and bottom right-hand images for each subject). SN, substantia nigra. Scale bars, 20 µm. (c) Heavily neuromelanized dopamine neurons (brown, arrows) in the substantia nigras of the subjects with Parkinson’s disease contained Lewy bodies (black, arrowheads), the pathological hallmark of Parkinson’s disease, as seen in these representative images of phosphorylated α-synuclein (top left-hand images for each subject) and α-synuclein (bottom left-hand images for each subject) immunostaining in the substantia nigras of subjects 4, 5 and 6. In the subjects’ putamina, no grafted dopamine neurons, which were lightly neuromelanized, contained Lewy bodies (top and bottom right-hand images for each subject). Scale bar, 15 µm.
In normal aging, α-synuclein accumulation occurs as a nonpathological phenomenon in the substantia nigra, but not in other dopamine neuronal nuclei such as the ventral tegmental area6. This explains the absence of α-synuclein accumulation in young (9–14-year-old) grafted ventral midbrain neurons (Fig. 2). In the normal brain, α-synuclein is located in presynaptic terminals, creating a fine granularity in the neuropil, and is absent in the neuronal cytoplasm6. In Parkinson’s disease, the neurodegenerative process is characterized by the accumulation of proteinaceous intraneuronal inclusions4. Initially, α-synuclein appears as a pale and diffuse cytoplasmic inclusion. During neurodegeneration, perikaryal α-synuclein coalesces and incorporates polyubiquitin chains and other proteins, forming ‘pale bodies’ that fuse to form the Lewy body7. In the five subjects, triple immunostaining for tyrosine hydroxylase, α-synuclein and ubiquitin showed a clear boundary between fetal grafts and the host striatum (Fig. 2a). As expected, α-synuclein and ubiquitin aggregates were found in cell bodies and terminals within the substantia nigra (Fig. 2b,c). Similarly, α-synuclein and ubiquitin colocalized in the Parkinson’s disease brain regions including the upper raphe nucleus, neocortex and putamen, where they were broadly distributed throughout the neuropil (Fig. 2a and data not shown). In contrast, grafted dopamine and serotoninergic neurons did not contain α-synuclein, ubiquitin or lipofuscin inclusions, and there were no other morphological signs of neurode-generation in the graft neuropil (Fig. 2). The data from the fetal ventral midbrain grafts were congruent with our previous study of synuclein during development, in which no pathological aggregates were seen at up to 16 years of age8. In addition, α-synuclein can aggregate in nonpathological conditions and therefore requires ultrastructural (electron microscopy) evidence to identify the pathology6.
Coexpression of tyrosine hydroxylase and G protein–coupled inward rectifying current potassium channel type 2 (Girk2) defines the most vulnerable population of dopamine neurons in Parkinson’s disease5. This group of dopamine neurons is located in the ventral tier of adult substantia nigra pars compacta (A9), projecting axons and terminals to motor areas of the putamen5. In contrast, most tyrosine hydroxylase and calbindin coimmunoreactive neurons in the ventral tegmental area and substantia nigra (A10) are relatively spared in Parkinson’s disease and send their axons to mesolimbic brain regions1. Grafted tyrosine hydroxylase and Girk2 coimmunoreactive neurons were located close to the graft-host interface, whereas tyrosine hydroxylase and calbindin coimmunoreactive neurons populated central areas of the graft (Fig. 1b).
We have demonstrated the presence of serotonin neurons in grafted human fetal ventral midbrain tissue (Fig. 1c), which is consistent with our previous findings and those of other groups in rodents9. Although we did not observe graft-induced dyskinesias in any subjects, a recent report in a rodent model of Parkinson’s disease describes the role of serotonin neurons in the development of l-dopa–induced dyskinesias when dopamine neurons are depleted10. Given the potential role of serotonin neurons in the development of l-dopa–induced dyskinesia, our new finding highlights the need for controlling cell composition in clinical neural transplantation11.
Immunosuppression was withdrawn 6 months after transplantation in subjects 4, 5 and 6, and the transplanted fetal cell suspensions did not elicit a major immune reaction (Supplementary Fig. 1 online). This is consistent with the postmortem study of subjects 1 and 2 that showed a minimal microglial host reaction5 but contrasts with the pronounced microglial reactions to transplanted solid pieces of fetal ventral midbrain12,13. This reduced host response may be attributed to fewer major histocompatibility complex class I–containing donor blood vessels in suspension grafts and the predominance of host-derived angiogenic processes that arise from this cell preparation method1.
In summary, we show that grafted dopamine and serotonin neurons survive without signs of neurodegeneration for up to 14 years despite ongoing degeneration of midbrain dopamine neurons and other dopamine structures in the host parkinsonian brain. The lack of degeneration in the grafts does not imply that the disease will not affect these dopamine neurons eventually. Rather, this proves that under the appropriate conditions of integration and reduced inflammatory response obtained by our methods, grafted neurons can avoid significant degeneration long term. One cannot exclude the possibility for graft involvement in the neurodegenerative process in other transplantation methods or within other subject populations. These results have major implications for the etiopathogenesis of Parkinson’s disease, as the host brain does not necessarily create conditions that cause Parkinson’s disease–related neurodegeneration in the transplanted neurons. Moreover, these findings encourage the future use of fetal- and stem cell–derived dopamine neurons for people with Parkinson’s disease14,15.
Supplementary Material
Note: Supplementary information is available on the Nature Medicine website.
ACKNOWLEDGMENTS
This work was supported by the Canadian Institute of Health Research, Queen Elizabeth II Health Sciences Centre (I.M.), US National Institutes of Health NINDS Parkinson’s Disease Research Center of Excellence (P50 NS 39793 to O.I. and NS 053488 to J.Q.T.), The Michael Stern Foundation (O.I.), The Consolidated Anti-Aging Foundation (O.I.), The Orchard Foundation (O.I.) and the Harold and Ronna Cooper Family (O.I.). Fellowship support to K.M. was provided by the Dalhousie Medical Research Foundation and Parkinson Society Canada. We also thank O. Cooper for evaluation of tissue sections from study subjects.
Footnotes
Published online at http://www.nature.com/naturemedicine
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Isacson O, Bjorklund LM, Schumacher JM. Ann. Neurol. 2003;53 Suppl 3:S135–S148. doi: 10.1002/ana.10482. [DOI] [PubMed] [Google Scholar]
- 2.Lewy F. Dtsch. Z. Nervenheilkd. 1913;50:50–55. [Google Scholar]
- 3.Eriksen JL, Wszolek Z, Petrucelli L. Arch. Neurol. 2005;62:353–357. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Wakabayashi K. Neuropathology. 2001;21:315–322. doi: 10.1046/j.1440-1789.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- 5.Mendez I, et al. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba M, et al. Am. J. Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 7.Kuusisto E, Parkkinen L, Alafuzoff I. J. Neuropathol. Exp. Neurol. 2003;62:1241–1253. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- 8.Galvin JE, Schuck TM, Lee VM, Trojanowski JQ. Exp. Neurol. 2001;168:347–355. doi: 10.1006/exnr.2000.7615. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson T, Carta M, Winkler C, Bjorklund A, Kirik D. J. Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carta M, Carlsson T, Kirik D, Bjorklund A. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 11.Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Stem Cells. 2007;25:2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed CR, et al. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW, et al. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 14.Perrier AL, et al. Proc. Natl. Acad. Sci. USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy NS, et al. Nat. Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Medicine website.