Abstract
The evidence suggesting a role of epigenetics in the definition of complex trait diseases is rapidly increasing. The gender prevalence of multiple sclerosis, the low level concordance in homozygous twins and the linkage to several genetic loci, suggest an epigenetic component to the definition of this demyelinating disorder. While the immune etio-pathogenetic mechanism of disease progression has been well characterized, still relatively little is known about the initial events contributing to onset and progression of the demyelinating lesion. This article addresses the challenging question of whether loss of the mechanisms of epigenetic regulation of gene expression in the myelinating cells may contribute to the pathogenesis of multiple sclerosis, by affecting the repair process and by modulating the levels of enzymes involved in neo-epitope formation. The role of altered post-translational modifications of nucleosomal histones and DNA methylation in white matter oligodendroglial cells are presented in terms of pathogenetic concepts and the relevance to therapeutic intervention is then discussed.
Keywords: Epigenetics, Chromatin, Histone, DNA methylation, Multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) which affects about 2.5 million individuals worldwide, and whose cause remains unknown. MS affects mostly young adults with a higher prevalence in developed, industrialized countries. Disease-modifying therapies, such as interferon beta are available on the market and partially effective in slowing down and mitigating the disease course. However, these immunomodulatory or immunosuppressive strategies fall short of success in the long-run, and cannot ward-off indefinitely the accumulation of disability and chronic neurological damage.
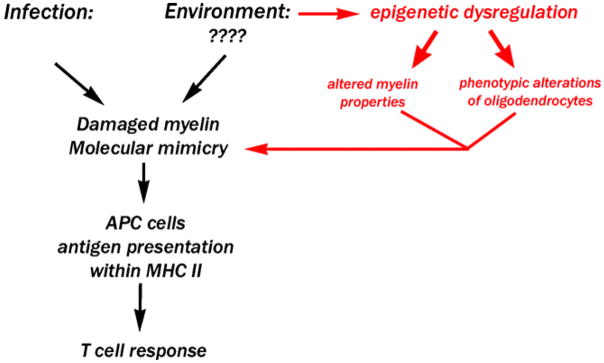
In this article we review the current literature suggesting a role for epigenetic regulation of gene expression in MS and contribute etio-pathogenetic concepts of disease progression with the intent to stimulate a dialogue for the development of new therapeutic solutions. Although the etiology of MS remains elusive (Barnett and Sutton, 2006; Sawcer, 2006; Sawcer and Compston, 2006), drug development has targeted inflammation and autoimmunity in order to manage clinical relapses (Calabresi et al., 2007; Elices, 2007; Koch et al., 2007; Korn et al., 2007; Linker et al., 2007; Vanderlocht et al., 2007). The current immunological hypothesis (as summarized in Fig. 1) advocates a critical role for cellular and humoral immunity in myelin damage and involves the role of cytotoxic cytokines (i.e. IFN-gamma, TNF, Fas and other death receptors; Duddy et al., 2007; Forte et al., 2006; Karni et al., 2006; Lopatinskaya et al., 2006; Xu et al., 2007). This model is supported by a large body of evidence, including genetic linkage data with specific HLA-DR2 loci that are critical for antigen presentation to T cells (Becher et al., 2006; Muller-Hilke and Mitchison, 2006; Olsson, 2006), the detection of high antibody titers and T-cell abnormalities in MS patients (Gestri et al., 2001;Carotenuto et al., 2006) and the neuropathological findings of perivascular plaques characterized by a strong lymphocytic component and myelin damage with sparing of oligodendrocytes (Lassmann et al., 1998).
Fig. 1.
Schematic drawing of the current hypotheses for MS etiologyaetiology. In black is a simplified drawing of the current pathogenetic hypothesis. In red is the hypothesis that is proposed in this review.
However the detection of plaques that are distant from blood vessels and are characterized by prevalence of microglial cells and myelin loss associated with significant oligodendrocytic apoptosis suggested the existence of alternative mechanisms of etio-pathogenesis (Barnett and Prineas, 2004; Kutzelnigg et al., 2007; Lucchinetti et al., 2000; Pittock and Lucchinetti, 2007; Roemer et al., 2007). The heterogeneity of the neuropathological findings and the relative lack of response to immunomodulatory therapy has led to the hypothesis that MS is a complex pathology characterized by demyelination as hallmark of the disease, possibly determined by multiple mechanisms of distinct etiology (Kornek and Lassmann, 2003).
The importance of myelin products in the pathogenesis of the disease has been supported by several studies (reviewed in Sospedra and Martin, 2005). Demyelination can be induced in rodents by immunization with myelin proteins or by transfer of T-cells stimulated with myelin peptides (i.e. MOG, PLP, MBP), (Kassiotis et al., 1999; Zargari et al., 2007). In addition, the detection of antibodies directed against myelin proteins in the brain of MS patients (Genain et al., 1999), and the presence of myelin-reactive T-cell and B-cells (McLaurin et al., 1995; Hafler, 2004; Klawiter and Cross, 2007; Nikbin et al., 2007) in the serum and CSF of MS patients (Tomassini et al., 2007; Berger et al., 2003) have suggested the importance of cell-mediated and humoral responses in the pathogenesis of the disease.
The nature of the triggering event leading to exposure of immunogenic myelin-derived peptides however remains unanswered. One hypothesis suggested that autoimmune demyelination is triggered by a mechanism of “molecular mimicry” to viral or bacterial proteins after a non-specific initial infection (Merkler et al., 2006). However, different forms of viral encephalomyelitis trigger an inflammatory response without widespread demyelination (Chen et al., 2005; Komoly, 2005; Mendez-Fernandez et al., 2003; Murray et al., 2001). Alternative hypotheses have implicated mechanisms that are intrinsic to the myelinating cells and depend on post-translational modifications of myelin proteins (Mastronardi and Moscarello, 2005). One of the best characterized molecular changes in a myelin component that has been associated with the exposure of an immunodominant epitope of the molecule is the deimination of myelin basic protein (Moscarello et al., 2007; Musse and Harauz, 2007) and it will be discussed later in this review. An alternative possibility has been suggested by the recent identification of autocatalytic anti-MBP antibodies in the sera of MS patients, characterized by proteolytic activity against the auto-antigen (Belogurov et al., 2008).
In this manuscript we will first review the epidemiological evidence supporting an epigenetic component to MS pathogenesis. We shall then discuss the potential role of epigenetic regulation of gene expression in modulating the ability of endogenous progenitors to repair the demyelinating lesion. We will conclude with a discussion on aberrant epigenetic modulation of gene expression in regulating the levels of enzymes responsible for the deimination of MBP and the generation of immunodominant neo-epitopes.
2. Evidence supporting the contribution of epigenetics to the pathogenesis of multiple sclerosis
Genetic linkage studies and genome-wide profiling arrays have contributed to the identification of multiple genes that may exert a combinatorial effect in affecting the susceptibility of individuals to develop the disease (Brynedal et al., 2007; Lundmark et al., 2007; Olsson, 2006; Zivadinov et al., 2007). However, it is becoming increasingly evident that gene expression based on DNA sequence alterations or mutations is not sufficient to explain the variety of manifestations observed in disease states (Dai and Rasmussen, 2007; Hake et al., 2007; Lu et al., 2006; Tabancay and Forsburg, 2006). If genetic information would be the sole determinant for susceptibility to MS, then it would be expected that homozygous twins, who are genetically identical, should display the same risk to develop the disease. In contrast, in MS the concordance rate for homozygous twins is only 20–30% (Hansen et al., 2005a,b; Poser, 2006; Willer et al., 2003). Although traditionally these differences have been attributed to exposure to environmental toxins, it is becoming evident that the effect of environmental factors is exerted by modulating the epigenome of the cells (Szyf et al., 2008). In this article we will use the term “epigenome” to define the higher-order, inheritable set of instructions determining gene expression (Weaver et al., 2006; Petronis, 2006), including modifications of chromatin components. We shall refer to “epigenetics” as the study of the “mechanisms modulating gene expression that do not involve mutations of DNA but are nevertheless inherited and persist in the absence of the signal that generated the event” (Ptashne, 2007). Additional support to the importance of epigenetics in MS is the parent-of-origin effect that predicts a higher rate of incidence of the disease in the offspring of parents of a specific sex. This concept was originally described in terms of parental transmission of disease susceptibility to the offspring (Ewen, 1995; Kantarci et al., 2006), although it has been recently reconsidered (Herrera et al., 2007). Finally, although the importance of sex hormones in MS has been well studied (Holmqvist et al., 2006; Tomassini and Pozzilli, 2006; Vukusic and Confavreux, 2006) it is important to stress the ability of sex steroids to epigenetically modulate gene expression by affecting chromatin components and transcriptional complexes (Kaminsky et al., 2006).
This article addresses the challenging question of whether altered control of gene expression, due to loss of mechanisms of epigenetic regulation within the myelinating cells, may contribute to the pathogenesis of multiple sclerosis.
3. Mechanisms of epigenetic regulation of gene expression
Chromatin is made of negatively charged DNA and of histone proteins with a net positive charge, due to the presence of arginine and lysine residues. Histones are disposed in an octamer (2 of each: H2A, H2B, H3 and H4), with globular bodies and tails irradiating from within.
Specific aminoacid residues in the histone tails are subject to post-translational modifications that affect gene expression, by modulating the degree of compactness of chromatin. The modifications include acetylation/deacetylation of lysine residues, methylation of lysine and arginine residues (Kouzarides, 2002), phosphorylation (Cheung et al., 2003), sumoylation (Shiio and Eisenman, 2003), ubiquitination (Jason et al., 2002) and citrullination (Mastronardi et al., 2006). The combinatorial arrangements of distinct histone modifications establish a specific “histone code” which, together with DNA methylation, is essential for the induction of specific patterns of epigenetic inheritance (Kouzarides, 2007; Turner, 2000). We shall first review some of the histone changes and the relative enzymatic activities catalyzing these changes and then discuss their relevance to the oligodendroglial lineage and myelin formation.
3.1. Histone acetylation/deacetylation of lysine residues
Acetylation of selected lysine residues in the tails of nucleosomal histones represents an efficient way to regulate gene expression (Strahl and Allis, 2000; Turner, 2000; Yang, 2004; Yoshida et al., 2003) and is regulated by two families of enzymes: histone acetyltransferases (HATS) and histone deacetylases (HDACs). High levels of acetylation have been correlated with the ultrastructural feature of dispersed euchromatin and the detection of “pale” nuclei. In contrast, histone deacetylation has been morphologically associated with the initiation of hetero-chromatin and it is often detected together with repressive histone methylation in “dense” nuclei, characterized by condensed chromatin (Casaccia-Bonnefil and Shen, 2008).
The HAT family of mammalian histone acetyl-transferases has the ability to promote gene expression, by transferring acetyl groups to lysines residues and thereby loosening the histone-DNA contacts, binding to specific transcription factors, as well as with basal components of the transcriptional machinery (Gregory et al., 2001; Kalkhoven, 2004; Roth et al., 2001). The HDAC family is responsible for the removal of acetyl groups from lysine residues in the histone tails and is highly conserved from yeast to human (de Ruijter et al., 2003; Michan and Sinclair, 2007; Thiagalingam et al., 2003). Based on sequence homology, mammalian HDACs can be broadly grouped into four major classes. Class I (HDAC-1, -2, -3 and -8), class II (HDAC-4, -5, - 6 -7, -9 and -10), class III (SIRT1–7), which is composed of the NAD-dependent family of sirtuins, and class IV (HDAC11), which has intermediate properties between class I and class II. In general terms, the removal of the negative acetyl groups from the histone tails has been associated with transcriptional repression and indeed class I HDAC family members form repressive complexes with transcription factors and co-repressors (Forsberg and Bresnick, 2001; Wade, 2001). In the oligodendrocyte global histone deacetylation has been defined as a prominent event during the differentiation of progenitors into myelinating oligodendrocytes (Marin-Husstege et al., 2002). This post-translational modification of histones was defined as necessary during a specific temporal window in oligodendrocyte maturation (Marin-Husstege et al., 2002). It was also shown that it is important for in vivo myelination because systemic administration of HDAC inhibitors to developing rodents (Shen et al., 2005) or zebrafish (Cunliffe and Casaccia-Bonnefil, 2006) arrested progenitors at an immature state. It remains to be determined whether similar events occur during the repair phase in demyelinating disorders.
3.2. Methylation of lysine residues
The functional role of histone methylation is dependent on the position of the modified lysine residue within the histone tail and on the number of methyl groups (one, two or three) added to the epsilon position of lysine residues (Ashraf and Ip, 1998; Bannister et al., 2002; Berger, 2002; Bird and Wolffe, 1999; Goll and Bestor, 2002; Kouzarides, 2002; Lachner and Jenuwein, 2002; Rice and Allis, 2001; Richards, 2002; Spencer and Davie, 1999; Zhang and Reinberg, 2001). The presence of three methyl groups on lysine 4 residue on histone H3 (Me-H3K4), for instance, has been associated with transcriptional activation. In contrast, the presence of three methyl groups on residue 9 or 27 has been associated with transcriptional repression. The enzymes responsible for activating and repressive methylation are also quite distinct. Methylation of K4, for instance, is catalyzed by MLL, a group of enzymes defined by the presence of specific structural motifs (Ruthenburg et al., 2007). Repressive methylation in contrast, is catalyzed by members of the Polycomb family, including EZH2 and other components of the PRC2 complex (Schuettengruber et al., 2007).
Histone methylation was originally considered a permanent modification, until the report of lysine-specific demethylase enzymatic activities, such as the flavin oxidase LSD1, whose substrates are mono- and di-methyl groups on lysine residues, and the Jumonji domain containing enzymes (JmjC), whose substrates are tri-methylated lysine residue (Anand and Marmorstein, 2007; Bannister and Kouzarides, 2005; Klose and Zhang, 2007; Kubicek and Jenuwein, 2004; Stavropoulos and Hoelz, 2007; Takeuchi et al., 2006; Wilson, 2007; Wysocka et al., 2005). Our group has reported the occurrence of global repressive histone methylation during the developmental window following histone deacetylation (Shen et al., 2005). We proposed that this event is critical for the stable down-regulation of stem cell markers and of transcriptional inhibitors of myelin gene expression (Shen and Casaccia-Bonnefil, 2008). We also suggested that a deficit of global histone methylation could interfere with the ability of progenitors to form new myelin or with the proper pattern of gene expression characterizing mature oligodendrocytes. Indeed, studies conducted in oligodendrocyte lineage cells in the brain of old mice have indicated the aberrant persistence of global histone acetylation and lack repressive histone methylation (Shen et al., 2008). We therefore hypothesized that an aberrant transcriptional response might occur in old mice during the repair phase following demyelination and lead to the persistence of high levels of transcriptional inhibitors of myelin gene expression and the inefficient differentiation of progenitors into myelin-forming cells (Shen, Sandoval and Casaccia-Bonnefil, unpublished).
3.3. Methylation and citrullination of arginine residues
Besides lysines, arginine residues can also be methylated by specific enzymatic activities and play a critical role in dynamic gene regulation (Bedford and Richard, 2005; Boisvert et al., 2005; Davie and Dent, 2002; Denman, 2005; Pahlich et al., 2006; Thompson and Fast, 2006; Wysocka et al., 2006). In mammals, the asymmetric transfer of methyl groups to arginine residues is catalyzed by the protein arginine methyltransferases PRMT1 and by the coactivator associated arginine methyltransferase 1 (CARM1). This asymmetric methylation of arginine residue 3 on histone H4 (H4R3) and arginine 17 on histone H3 (H3R17) results in gene activation (Bauer et al., 2002; Chen et al., 1999; Strahl et al., 2001; Wang et al., 2001). Repressive methylation of arginines is catalyzed by the enzyme PRMT5 that is responsible for the symmetric methylation of arginine 8 in histone H3 (H3R8) and of arginine 3 in histone H4 (H4R3; Pal et al., 2004; Wysocka et al., 2006). Arginine methylation is also reversible and can be removed by specific deiminases, such as human peptidylarginine deiminase 4 (PAD4), which converts arginine into citrulline (Wang et al., 2004). The reversibility of protein arginine methylation by PAD enzymes has more recently been disputed in an extensive in vitro biochemical analysis making the reversibility of this post-translational modification equivocal (Raijmakers et al., 2007). The functional significance of increased global histone citrullination in oligodendrocyte lineage cells remains to be clearly defined. However, increased levels of PAD4 and of citrullinated histone H3 have been detected in experimental models of demyelination and in the brain of MS patients compared to controls (Mastronardi et al., 2006).
3.4. DNA methylation
Individuals from any given species, and particularly humans, vary in methylation patterns. These patterns are transmissible and define inheritable patterns of gene expression through cell divisions. Methylation involves several reactions. The methyl group is transferred from tetrahydrofolate to vitamin B12 which in turn methylates homocysteine to form methionine. Methionine is then activated by ATP to form S-adenosylmethionine, which is the methyl donor in all biological methylation reactions (Stubbe, 1994). Methylation is an important regulatory mechanism for gene expression, which occurs on CpG sequences in the immediate 5′ promoter regions of genes. These regions have CpG contents greater than 60% compared to 40% for the bulk DNA. Methylation of cytosine in CpG sequences turns off promoter activity and thereby decreases gene expression, since methylation of DNA affects both DNA structure and the binding of transcription factors (Lamb et al., 1991; Nonkwelo and Long, 1993; Ohtani-Fujita et al., 1993). This event is regulated by the balance between DNA methyltransferase and DNA demethylases (Szyf, 1994). The possible contribution of altered DNA methylation in white matter cells in MS brains will be later discussed for its possible implications in modulating disease progression. Additional studies on methylation of HLA loci (Rakyan et al., 2004) and on the pattern of X-chromosome inactivation in patients with progressive forms of MS (Knudsen et al., 2007) further confirm that epigenetic modifications might be an important component of disease pathogenesis.
4. Epigenetic modulation of gene expression in demyelinating disorders
Disease state implies tissue damage and this local disruption of homeostasis by itself may elicit repair mechanisms recapitulating developmental pathways (Herrero-Herranz et al., 2007; John et al., 2002), a bit like phylogenesis is resumed in ontogenesis and embryology. Molecules and signalling pathways expressed during development are re-expressed in each case of altered tissue homeostasis. In urodeles (salamanders and newts) limb regeneration, for example, one of the few successful cases of actual regenerative ability in the animal kingdom, genes that are essential in embryological development are re-expressed with a modified pattern (Brown and Brockes, 1991; Savard et al., 1988; Savard and Tremblay, 1995). These events might attempt to restore balance through recapitulation (Guimaraes et al., 1975; Wizenmann and Bahr, 1997). Where adult cellular elements need to be replenished, new-born cells, derived from pools of precursors, need to migrate to the proper location and acquire mature phenotypes; once they have reached their destination they must interact with resident tissue cells and respond to local cues.
The existence of repair event in the brain of MS patients is supported by a large body of literature. One of the first reports of newly formed myelin in MS brains was presented by Prineas and Connell (1979) and several studies from other laboratories confirmed these findings (Prineas et al., 1984; Raine and Scheinberg, 1988; Compston, 1993, 1995; Chang et al., 2000).
It was suggested that the extent of remyelination events was affected by the frequency of occurrence of relapses (Prineas et al., 1993) and that overall remyelination was relatively rare within demyelinating lesions (Raine and Wu, 1993). The efficiency of new myelin formation is thought to be dependent on intrinsic signals within oligodendrocyte lineage cells and extrinsic signals from axons (Bruck et al., 2003) and astrocytes (John et al., 2002). Among these signals, it has been shown that developmentally regulated molecules (i.e. Notch) can be detected at the borders of the demyelinated plaques (John et al., 2002). This finding is consistent with the concept that re-activation of developmental programs occurs during remyelination and that these events are localized at the edges of the demyelinated lesions (Albert et al., 2007). Although the extent of spontaneous remyelination is variable, no correlation was found with the gender or age of disease onset (Patrikios et al., 2006), underscoring the functional importance of the intrinsic properties of cells involved in repair. A potential explanation for the inappropriate repair is the persistence of progenitors at an undifferentiated state due to inappropriate epigenetic regulation of a developmental program of gene expression. Further investigation will be necessary to prove the validity of this assumption and to determine whether the persistence of developmental pathways at the lesion site may inhibit further differentiation of oligodendrocyte progenitors (Wang et al., 1998, 2006; Mastronardi et al., 2004).
4.1. Aberrant recapitulation of developmental processes due to epigenetic disequilibrium: attempts to repair or contribution to MS disease progression?
In MS, myelin is lost in multifocal loci following episodes of neuroinflammation (Chaudhuri and Behan, 2004). These foci of demyelination, called “plaques”, are often characterized by the appearance of new myelin at the edge of the lesion (Prineas et al., 1993). However, disease pathology may not be exclusively limited to sites of active demyelination, since also the properties of myelin in the normal appearing white matter of MS brains are substantially different from those of normal myelin (Ludwin, 2006). Pathological studies on post-mortem tissue obtained from MS brains, for instance, revealed that the composition of myelin in the normal appearing white matter is different than the composition in non-MS brains and has the features of developmentally immature myelin (Moscarello et al., 1994). Therefore, the interpretation of biochemical analysis of tissue samples from MS brains needs to take into account the coexistence of multiple populations, including progenitors involved in repair and mature cells with altered properties, in the normal appearing white matter. For this reason it is critical to correlate biochemical studies with immunohistochemistry. Previous studies from our laboratory, for instance, had reported the up-regulation of stathmin, a microtubule destabilizing protein that keeps neural precursors in an immature morphology in the MS brain compared to controls (Liu et al., 2005). The expression of this protein in progenitors could indicate the presence of active repair, since high levels of stathmin promote progenitor migration. However, the persistence of this molecule in mature oligodendrocytes leads to the cytoskeleton destabilization and increased susceptibility to apoptosis (Liu et al., 2005). Together, these data led us to propose a speculative, and yet intriguing, hypothesis of disease progression resulting from the generation of neo-epitopes due to deregulated “epigenetic identity”. We have previously shown that histone deacetylase activity is necessary for the definition of the epigenetic signature of oligodendrocytes in developing rodents (He et al., 2007; Marin-Husstege et al., 2002; Shen et al., 2005). Our gene profiling studies defined the epigenetic down-regulation of genes encoding for cytoskeletal depolymerizing molecules (i.e. stathmin) and transcriptional inhibitors (i.e. Hes5) as pre-requisite for the attainment of a mature myelinating phenotype. We have discussed in Section 3 how transient global histone deacetylation during oligodendroglial development serves the important function of decreasing genes whose expression is characteristic of the progenitor stage (Liu et al., 2006, 2003, 2005). The re-expression of these genes in oligodendrocyte progenitors in the MS brains could be attributed to the occurrence of repair mechanisms. However, the persistence of progenitor markers in mature cells would indicate the defective establishment of the epigenetic identity, as observed in aging (Shen et al., 2008). For this reason, it will be important to experimentally characterize the cell types displaying aberrant epigenetic regulation in MS patients and correlate the findings to specific neuropathological features. It will also be important to correlate specific histone modifications to the developmental stage of oligodendrocyte lineage cells and their localization in reference to the demyelinating plaque. For instance, the persistence of histone acetylation and the inappropriate re-expression of stathmin (Liu et al., 2005) and Hes5 (John et al., 2002) in cells characterized by mature markers, would indicate the loss of the “epigenetic signature” of oligodendrocyte identity. In support of the concept that inappropriate establishment of epigenetic identity occurs during repair, previous studies on the appearance of nuclei of oligodendrocytic cells in chronic multiple sclerosis lesions suggested the predominance of large pale nuclei over the dense nuclei characteristic of mature oligodendrocytes (Wolswijk, 2000). Since the pale or dense appearance of nuclei is dependent on the state of chromatin condensation, the persistence of “pale” nuclei in oligodendrocytes would provide further support to the concept of inappropriate chromatin condensation during the repair phase of demyelinating lesions.
Potential causes for this aberrant regulation of chromatin condensation during disease states may include accelerated senescence due to exposure to proinflammatory cytokines, microglia activation, neuronal or axonal dysfunction. An additional possibility is that external stimuli, such as oxidative stress (Ceccatelli et al., 2007; Madhavan et al., 2006) or viral infections (Ogle et al., 2005), might induce changes in chromatin conformation that may halt (Odeberg et al., 2006) or prematurely induce differentiation (Covacu et al., 2006; Dietrich et al., 2004). Because the modifications of the epigenome are transmissible to progeny, it is intriguing to envision the propagation of these modifications to several daughter cells thereby resulting in progressively cumulative changes in the properties of the normal appearing white matter.
4.2. The controversy regarding the use of histone deacetylase inhibitors in demyelinating disorders
The immune hypothesis of MS proposes that it is a T-cell driven pathology defining inflammatory demyelinating lesions throughout the brain and spinal cord (Delgado and Sheremata, 2006; Hafler, 2004; Hafler et al., 2005; McDole et al., 2006; Sheremata et al., 2006). Based on this premise, the currently available therapies are mainly focused at controlling the inflammatory components by either preventing the access of activated lymphocytes in the brain or interfering with the production of cytotoxic cytokines by immune cells. Since HDAC inhibitors were shown to inhibit proliferation, activation and cytokine production by activated T cells (Dangond and Gullans, 1998; Moreira et al., 2003; Skov et al., 2003), they have been proposed as alternative immunomodulatory treatment for MS. Given the role of histone deacetylation in decreasing gene expression, it was suggested that favouring HDAC activity promotes an anti-inflammatory effect due to the down-regulation of pro-inflammatory genes. This concept was supported by the finding that the action of the most potent anti-inflammatory agents, the glucocorticoids, is dependent on HDAC activity. Glucocorticoids were shown to recruit HDAC to the promoter of pro-inflammatory genes (Adcock et al., 2005) and knockdown of HDAC2 function selectively impaired glucocorticoid function and rendered the cells insensitive to the action of these anti-inflammatory agents (Ito et al., 2006). Thus, these studies indicated that HDAC inhibitors had a pro-inflammatory effect, by antagonizing the effect of glucocorticoids and favouring the expression of pro-inflammatory molecules. Different conclusions were reached by other laboratories reporting elevated HDAC levels (especially HDAC3) after stimulation of peripheral blood lymphocytes (Dangond and Gullans, 1998). According to these authors, treatment of CD4+ T cells with HDAC inhibitors induced a decrease of the proliferative response and the suppression of the production of the pro-Th1 cytokine IFN-gamma (Dangond and Gullans, 1998; Skov et al., 2003; Su et al., 2008). It was also suggested that HDAC inhibitors favoured the shift from a Th1 to a Th2 phenotype; however, trichostatin A (TSA) attenuated T-cell suppressor effects in a mouse asthma model (Choi et al., 2005) and down-regulated various costimulatory adhesion molecules (Moreira et al., 2003). These studies suggested an anti-inflammatory role of HDAC inhibitors that was also supported by the recent results in the MOG induced model of monophasic, progressive EAE (Camelo et al., 2005).
It is important to mention, that the evidence supporting HDAC inhibitors as anti-inflammatory agents is quite controversial, especially in light of the fact that glucocorticoids, the most potent anti-inflammatory agents, require HDAC activity in order to shut down the synthesis of pro-inflammatory genes (Adcock et al., 2005; Ito et al., 2006). Besides the controversial findings related to the role of HDAC inhibitors as anti-inflammatory or pro-inflammatory agents, additional arguments against the use of these inhibitors in demyelinating disease were provided by studies conducted on microglial cells (Suuronen et al., 2003) and by studies conducted in oligodendrocyte progenitors, (Marin-Husstege et al., 2002) and neural stem cells (Hsieh et al., 2004; Kondo and Raff, 2004). Several studies reported that treatment with HDAC inhibitors has a negative impact not only on oligodendrocyte differentiation (Marin-Husstege et al., 2002) and developmental myelination (Shen et al., 2005), but also on the commitment of stem cells to the oligodendrocyte lineage (Cunliffe and Casaccia-Bonnefil, 2006; Hsieh et al., 2004; Kondo and Raff, 2004; Lyssiotis et al., 2007).
5. Alternative models of pathogenesis of demyelinating disorders: epigenetic deregulation of specific enzymes leads to the accumulation of neo-epitopes affecting the humoral response in MS
According to the autoimmune model of pathogenesis of MS, the cell-mediated and humoral response against myelin products would be initiated by the occurrence of damage following viral infections or myelin damage. We now propose an alternative model relating the formation of immunogenic peptides to the aberrant regulation of gene expression in myelinating oligodendrocytes. More specifically, we shall first review the evidence in support of aberrant methylation of the promoter of PAD2, a specific enzyme involved in deimination of myelin basic protein, and then discuss at least two potential mechanisms affecting the generation of neo-epitopes.
5.1. PAD2 as an example of aberrantly methylated promoter in MS brains
One of the important targets of hypomethylation in MS brain is the activation of the PAD2 locus. PAD2 is a peptidylarginine deiminase enzyme that was originally isolated from bovine brain white matter and that catalyzes the conversion of the guanidino group of arginine to citrulline in myelin basic protein(MBP; Lamensa and Moscarello, 1993). The PAD genes are clustered in a distinct genomic region in humans (1p36.1) and syntenic locations of rodent genomes (Vossenaar et al., 2003). PAD2 levels are elevated in MS (Mastronardi et al., 2007a) and in a transgenic animal model of MS (Moscarello et al., 2002). Its promoter has 74% GC content and studies involving bisulfite treatment followed by sequence analysis have revealed that this region is hypomethylated in MS brains compared to controls. Together with the findings of increased DNA demethylase, these data suggest that specific genes are reactivated in MS brains, due to epigenetic modulation.
5.2. The role of DNA methylation in MS
Studies on DNA isolated from white matter of MS brains contained only about 1/3 of the amount of methyl cytosine found in DNA from normal subjects (Mastronardi et al., 2007a). Decreased methylation of cytosines in CpG islands in white matter homogenates from MS brains was not the result of decreased methyltransferase activity, but rather the result of a two-fold higher DNA demethylase activity than that in homogenates from normal brain (Mastronardi et al., 2007a). It was specific for MS because DNA from thymus gland of the same MS patients and patients with Alzheimer’s, Parkinson’s and Huntington’s diseases were normally methylated and the DNA demethylase activity was unaffected (Mastronardi et al., 2007a). A summary of the hypothesis linking dysregulation of the PAD2 locus to the generation of immunogenic peptides is illustrated in Figs. 2 and 3.
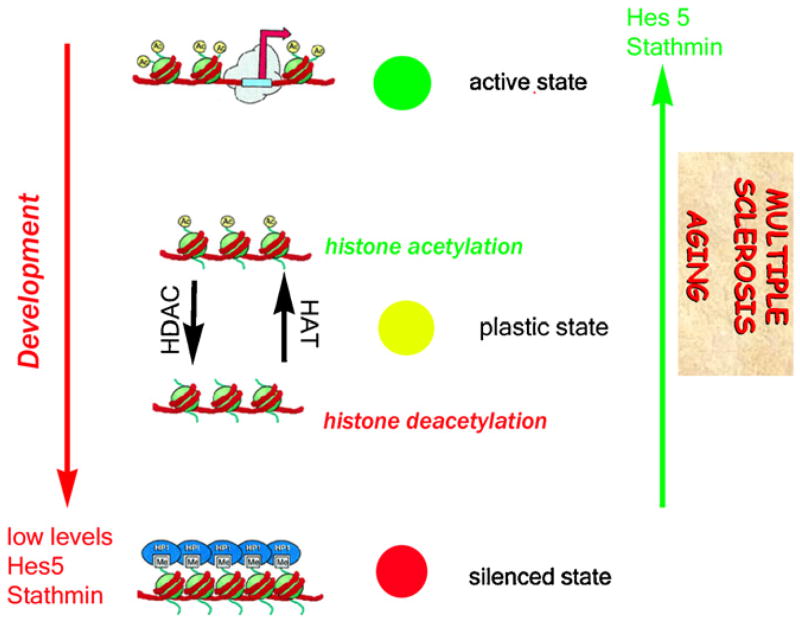
Fig. 2.
Aberrant recapitulation of developmental epigenetic landmarks in multiple sclerosis. This drawing illustrates the major epigenetic events occurring in differentiating oligodendroglial cells during development (green) and leading to repression of the differentiation inhibitors Hes5 and stathmin. In the brain of multiple sclerosis patients, in contrast, these repressive epigenetic events are replaced by activating events (red arrow) leading to re-expression of differentiation inhibitors (Hes5) and of molecules destabilizing the cytoskeletal network of oligodendroglial cells (stathmin).
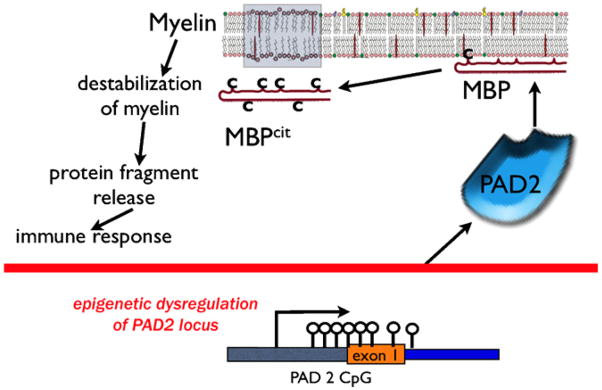
Fig. 3.
A Proposed epigenetic mechanism of PAD2 up-regulation in MS oligodendrocytes. The upstream regulatory region of the PAD2 gene contains a CpG island. In normal individuals the methylation and expression of PAD2 is under homeostatic regulation. However in MS white matter the cytosines in CG dinucleotides in the PAD2 promoter become demethylated via increased DNA demethylase activity. The result of this CpG demethylation might explain why there is hypercitrullination of arginine rich protein substrates like myelin basic protein. Myelin basic protein once citrullinated in the normal appearing white matter loses its interactions with myelin membranes, which fragment thereby leading to autoimmune progression and MS.
5.3. Consequences of increased PAD2 activity: citrullination of MBP
Protein substrates for PAD include polyarginine and MBP. While in normal brains only a very small proportion of MBP is citrullinated, in chronic MS, citrullinated MBP comprises approximately 40% of the total MBP (Moscarello et al., 1986, 1994). The relationship between the amount of citrullinated MBP and the severity of MS was suggested in a study which documented over 90% citrullination of MBP in a rare acute Marburg’s form of MS. In this acute case of MS 18 of 19 arginines were converted to citrulline residues. This dramatic conversion of the majority of arginines into citrullines, resulted in a visible apparent mass change of MBP (Wood et al., 1996) and in a net reduction of its positive charge, thereby affecting the interactions of this protein with other myelin components (Finch and Moscarello, 1972; Wood and Moscarello, 1989). Electron microscopy and immunogold localization of the citrulline containing MBP isomer with specific antibodies revealed aberrant localization of the citrullinated form of MBP in human brain white matter biopsies (McLaurin et al., 1993).
5.4. Citrullinated MBP and neo-epitope generation
Several studies support the importance of citrullination of MBP in modulating the immune response in MS (Tranquill et al., 2000; Zhou et al., 1995). There are at least two possible mechanisms explaining the contribution of citrullinated MBP to the enhanced immune response. Citrullination could increase the generation of immunodominant peptides, due to increased auto-cleavage of the protein (D’Souza et al., 2005). Alternatively, citrullination can disrupt the physical and chemical properties of MBP and thereby affect its localization within the myelin membrane (Musse et al., 2006).
Whereas very little auto-cleavage can be observed with the MBP from normal tissues, it is clear that citrullinated MBP from MS white matter undergoes auto-cleavage at slightly alkaline pH values (D’Souza et al., 2005; D’Souza and Moscarello, 2006). The conversion of arginine residues into citrullines is catalyzed by the enzyme PAD2, and it has been shown to render MBP more susceptible to proteolytic digestion, to compromise its ability to form compact lipid bilayers thereby resulting in a less compact myelin structure and in the generation of immunodominant peptides (Fig. 3). For this reason, we postulate that autocatalysis of citrullinated MBP during the early stages of MS may contribute to the sensitization of T-cells and thereby enhance the autoimmune response (D’Souza et al., 2005). Citrullinated MBP is also more readily digested by myelin associated proteases such as cathepsin D (Cao et al., 1999) and stromelysin compared to non-citrullinated MBP. Both proteases released immunodominant peptide epitopes. Whereas the immunodominant peptide released by cathepsin D is part of a large peptide (residues 40–89), the one released by stromelysin (MMP-3) is only 17 residues long and can be accommodated by MHC class II molecules (Pritzker et al., 2000).
An alternative possibility has been suggested by the work of Musse et al. (2006) who reported the importance of citrullination of MBP in neo-epitope formation. The current view regarding the identity of immunogenic peptides predicts that only molecules with aminoacid sequences extrinsic to the myelin membrane (such as MOG) would be able to elicit an immune response. This is true in physiological conditions, when the localization of MBP is within compact myelin. It is important to notice, however, that in pathological conditions MBP is predominantly citrullinated and this modification profoundly affects the physical and chemical properties of the molecule (Musse et al., 2006) likely resulting in membrane redistribution.
In conclusion, we suggest that citrullination of MBP caused by epigenetic deregulation of PAD2, leads to the release of modified and highly immunogenic forms of MBP from myelin which might significantly affect the progression of the demyelinating disorder (Mastronardi and Moscarello, 2005).
6. Future directions and concluding remarks
Disease induction and the onset of immune response in MS has been linked to mechanisms of myelin damage. In this review we discuss alternative models of disease onset suggesting that MS develops as the consequence of changes intrinsic to the whitematter oligodendroglial cells that favour their immunogenicity and promote the establishment of an autoimmune response. Within this review we discussed the evidence supporting the role of epigenetic regulation of gene expression in MS, and defined several relevant landmark changes occurring in the normal appearing white matter of MS brains that might contribute to disease progression.
We also discuss additional potential consequences of deregulated epigenetic changes in MS tissue and suggest that aberrant changes in the chromatin of oligodendroglial cells in the normal appearing white matter might lead to impaired remeyelination, besides the formation of neo-epitopes affecting the humoral response. Some of these critical changes have been documented in the normal appearing white matter of individuals with MS and in animal models (Liu et al., 2005; Shen et al., 2008; Mastronardi and Moscarello, 2005; Moscarello et al., 2007). Other changes may be more subtle, they are transmitted to daughter cells that remain functional but keep on accumulating the epigenetic changes within each cell division. This may in turn lead to increased susceptibility of oligodendroglial cells to damaging stimuli (Liu et al., 2005), by favouring the formation of myelin debris and stimulating the autoimmune process and disease progression. Based on the epigenetic hypothesis, focused investigations will need to address early epigenetic changes associated with MS within the white matter.
We also propose here that reprogramming the epigenome for the proper performance of oligodendroglial and immune cells is a potential therapeutic strategy for MS. It is important, however, to consider the potential repercussion that each treatment could have on both compartments. As hematopoietic stem cells have been shown to migrate from niche to niche (Kaplan et al., 2007) and have the potential to generate multiple cell types, and mesenchymal cells are able to instruct neurogenesis under many circumstances (Bai et al., 2007), it is likely that epigenetic interventions in favour of the neural tissue might also have repercussion on the immune system. It would therefore be prudent to target the 2 compartments, in the case of MS the myelin and immune compartment (Mastronardi and Moscarello, 2005), together, or more precisely, to intervene adequately in their cross-talk. A possible way to support this favourable outcome involves favouring methylation through vitamin B12 supplementation (Mastronardi et al., 2004, 2007b) along with administration of NAD, as nicotinamide, which is essential for all cellular metabolic processes and which has been recently shown to have protective and reparative functions in animal models of demyelination (Kaneko et al., 2006).
In conclusion, we suggest that MS is a phenotypically complex disease that may include multiple aetiologies, including epigenetic deregulation of the myelinating cells. We further suggest that therapeutic interventions that take into consideration the disease complexities, and promote epigenetic repression of multiple loci, in combination with other immunomodulatory therapies, may hold promise.
Acknowledgments
P.C.B. is supported by grants from NIH-NINDS (RO1NS042925), from the Multiple Sclerosis Research Foundation (MSRF) and the National Multiple Sclerosis Society (NMSS RG-3957). F.G.M. is supported by an MS Society of Canada grant as a co-applicant.
Abbreviations
- EAE
experimental allergic encephalomyelitis
- HAT
histone acetyl-transferase
- HDAC
histone deacetylase
- MBP
myelin basic protein
- MS
multiple sclerosis
- PAD
peptidylarginine deiminase
References
- Adcock IM, Ito K, Barnes PJ. Histone deacetylation: an important mechanism in inflammatory lung diseases. COPD. 2005;2:445–455. doi: 10.1080/15412550500346683. [DOI] [PubMed] [Google Scholar]
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Marmorstein R. Structure and mechanism of lysine specific demethylase enzymes. J Biol Chem. 2007 doi: 10.1074/jbc.R700027200. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, Ip YT. Transcriptional control: repression by local chromatin modification. Curr Biol. 1998;8:R683–686. doi: 10.1016/s0960-9822(98)70435-x. [DOI] [PubMed] [Google Scholar]
- Bai G, Sheng N, Xie Z, Bian W, Yokota Y, Benezra R, Kageyama R, Guillemot F, Jing N. Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell. 2007;13:283–297. doi: 10.1016/j.devcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109:801–806. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Sutton I. The pathology of multiple sclerosis: a paradigm shift. Curr Opin Neurol. 2006;19:242–247. doi: 10.1097/01.wco.0000227032.47458.cb. [DOI] [PubMed] [Google Scholar]
- Bauer UM, Daujat S, Nielsen SJ, Nightingale K, Kouzarides T. Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep. 2002;3:39–44. doi: 10.1093/embo-reports/kvf013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med (Berlin, Germany) 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18:263–272. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Belogurov AA, Kurkova IN, Friboulet A, Thomas D, Misikov VK, Zakharova MY, Suchov SV, Kotov SV, Alehin AI, Avalle B, Souslova EA, Norse H, Gabibov AG, Ponomarenko NA. Recognition and degradation of myelin basic proteins by serum autoantibodies: novel biomarkers for multiple sclerosis. J Immunol. 2008;180:1258–1267. doi: 10.4049/jimmunol.180.2.1258. [DOI] [PubMed] [Google Scholar]
- Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev. 2002;12:142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP. Methylation-induced repression—belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Boisvert FM, Chenard CA, Richard S. Protein interfaces in signaling regulated by arginine methylation. Sci STKE 2005. 2005:re2. doi: 10.1126/stke.2712005re2. [DOI] [PubMed] [Google Scholar]
- Brown R, Brockes JP. Identification and expression of a regeneration-specific homeobox gene in the newt limb blastema. Development (Cambridge, England) 1991;111:489–496. doi: 10.1242/dev.111.2.489. [DOI] [PubMed] [Google Scholar]
- Bruck W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Brynedal B, Duvefelt K, Jonasdottir G, Roos IM, Akesson E, Palmgren J, Hillert J. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS ONE. 2007;2:e664. doi: 10.1371/journal.pone.0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, Kappos L, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Lublin FD, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA. The incidence and significance of anti-natalizumab antibodies. Results from AFFIRM and SENTINEL. Neurology. 2007 doi: 10.1212/01.wnl.0000277457.17420.b5. [DOI] [PubMed] [Google Scholar]
- Camelo S, Iglesias AH, Hwang D, Due B, Ryu H, Smith K, Gray SG, Imitola J, Duran G, Assaf B, Langley B, Khoury SJ, Stephanopoulos G, De Girolami U, Ratan RR, Ferrante RJ, Dangond F. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Cao L, Goodin R, Wood D, Moscarello MA, Whitaker JN. Rapid release and unusual stability of immunodominant peptide 45–89 from citrullinated myelin basic protein. Biochemistry. 1999;38:6157–6163. doi: 10.1021/bi982960s. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil and Shen, 2008, Encyclopedia.
- Ceccatelli S, Tamm C, Zhang Q, Chen M. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav. 2007;92:87–92. doi: 10.1016/j.physbeh.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Proneas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Multiple sclerosis is not an autoimmune disease. Arch Neurol. 2004;61:1610–1612. doi: 10.1001/archneur.61.10.1610. [DOI] [PubMed] [Google Scholar]
- Chen AM, Khanna N, Stohlman SA, Bergmann CC. Virus-specific and bystander CD8 T cells recruited during virus-induced encephalomyelitis. J Virol. 2005;79:4700–4708. doi: 10.1128/JVI.79.8.4700-4708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- Compston A. Limiting and repairing the damage in multiple sclerosis. Schweizerische Medizinische Wochenschrift. 1993;123:1145–1152. [PubMed] [Google Scholar]
- Compston A. Brain repair. J Intern Med. 1995;237:127–134. doi: 10.1111/j.1365-2796.1995.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Covacu R, Danilov AI, Rasmussen BS, Hallen K, Moe MC, Lobell A, Johansson CB, Svensson MA, Olsson T, Brundin L. Nitric oxide exposure diverts neural stem cell fate from neurogenesis towards astrogliogenesis. Stem Cells. 2006;24:2792–2800. doi: 10.1634/stemcells.2005-0640. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT, Casaccia-Bonnefil P. Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech Dev. 2006;123:24–30. doi: 10.1016/j.mod.2005.10.005. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Moscarello MA. Differences in susceptibility of MBP charge isomers to digestion by stromelysin-1 (MMP-3) and release of an immunodominant epitope. Neurochem Res. 2006;31:1045–1054. doi: 10.1007/s11064-006-9116-9. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Wood DD, She YM, Moscarello MA. Autocatalytic cleavage of myelin basic protein: an alternative to molecular mimicry. Biochemistry. 2005;44:12905–12913. doi: 10.1021/bi051152f. [DOI] [PubMed] [Google Scholar]
- Dai B, Rasmussen TP. Global epiproteomic signatures distinguish ES cells from differentiated cells. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0131. [DOI] [PubMed] [Google Scholar]
- Dangond F, Gullans SR. Differential expression of human histone deacetylase mRNAs in response to immune cell apoptosis induction by trichostatin A and butyrate. Biochem Biophys Res Commun. 1998;247:833–837. doi: 10.1006/bbrc.1998.8891. [DOI] [PubMed] [Google Scholar]
- Davie JK, Dent SY. Transcriptional control: an activating role for arginine methylation. Curr Biol. 2002;12:R59–61. doi: 10.1016/s0960-9822(01)00674-1. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado S, Sheremata WA. The role of CD4+ T-cells in the development of MS. Neurol Res. 2006;28:245–249. doi: 10.1179/016164106X98107. [DOI] [PubMed] [Google Scholar]
- Denman RB. PAD: the smoking gun behind arginine methylation signaling? Bioessays. 2005;27:242–246. doi: 10.1002/bies.20205. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Blumberg BM, Roshal M, Baker JV, Hurley SD, Mayer-Proschel M, Mock DJ. Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties. J Neurosci. 2004;24:4875–4883. doi: 10.1523/JNEUROSCI.5584-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Elices MJ. A balancing act—making the right choices in anti-inflammatory drug development. Curr Opin Investig Drugs. 2007;8:362–363. [PubMed] [Google Scholar]
- Finch PR, Moscarello MA. A myelin protein fraction extracted with thioethanol. Brain Res. 1972;42:177–187. doi: 10.1016/0006-8993(72)90051-0. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Bresnick EH. Histone acetylation beyond promoters: long-range acetylation patterns in the chromatin world. Bioessays. 2001;23:820–830. doi: 10.1002/bies.1117. [DOI] [PubMed] [Google Scholar]
- Forte GI, Ragonese P, Salemi G, Scola L, Candore G, D’Amelio M, Crivello A, Di Benedetto N, Nuzzo D, Giacalone A, Lio D, Caruso C. Search for genetic factors associated with susceptibility to multiple sclerosis. Ann N Y Acad Sci. 2006;1067:264–269. doi: 10.1196/annals.1354.034. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Histone modification and replacement in chromatin activation. Genes Dev. 2002;16:1739–1742. doi: 10.1101/gad.1013902. [DOI] [PubMed] [Google Scholar]
- Gregory PD, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- Guimaraes JP, Klaczko LB, Hirano K, Vaz EM, Miguel MR. Reappearance of embryonal antigens in planarian regenerates. Rev Brasil Pesquisas Med Biol. 1975;8:255–259. [PubMed] [Google Scholar]
- Hafler DA. Multiple sclerosis. J Clin Investig. 2004;113:788–794. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, Slavik JM, Anderson DE, O’Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Hake SB, Xiao A, Allis CD. Linking the epigenetic ‘language’ of covalent histone modifications to cancer. Br J Cancer. 2007;96 (Suppl):R31–R39. [PubMed] [Google Scholar]
- Hansen T, Skytthe A, Stenager E, Petersen HC, Bronnum-Hansen H, Kyvik KO. Concordance for multiple sclerosis in Danish twins: an update of a nationwide study. Mult Scler (Houndmills, Basingstoke, England) 2005a;11:504–510. doi: 10.1191/1352458505ms1220oa. [DOI] [PubMed] [Google Scholar]
- Hansen T, Skytthe A, Stenager E, Petersen HC, Kyvik KO, Bronnum-Hansen H. Risk for multiple sclerosis in dizygotic and monozygotic twins. Mult Scler (Houndmills, Basingstoke, England) 2005b;11:500–503. doi: 10.1191/1352458505ms1202oa. [DOI] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera BM, Ramagopalan SV, Orton S, Chao MJ, Yee IM, Sadovnick AD, Ebers GC. Parental transmission of MS in a population-based Canadian cohort. Neurology. 2007;69:1208–1212. doi: 10.1212/01.wnl.0000268486.40851.d6. [DOI] [PubMed] [Google Scholar]
- Herrero-Herranz E, Pardo LA, Bunt G, Gold R, Stuhmer W, Linker RA. Reexpression of a developmentally restricted potassium channel in autoimmune demyelination: Kv1.4 is implicated in oligodendroglial proliferation. Am J Pathol. 2007;171:589–598. doi: 10.2353/ajpath.2007.061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas. 2006;54:149–153. doi: 10.1016/j.maturitas.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LJ, Moore SC, Lewis JD, Lindsey G, Ausio J. Histone ubiquitination: a tagging tail unfolds? Bioessays. 2002;24:166–174. doi: 10.1002/bies.10038. [DOI] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Kaminsky Z, Wang SC, Petronis A. Complex disease, gender and epigenetics. Ann Med. 2006;38:530–544. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Wang J, Kaneko M, Yiu G, Hurrell JM, Chitnis T, Khoury SJ, He Z. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J Neurosci. 2006;26:9794–9804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci OH, Barcellos LF, Atkinson EJ, Ramsay PP, Lincoln R, Achenbach SJ, De Andrade M, Hauser SL, Weinshenker BG. Men transmit MS more often to their children vs women: the Carter effect. Neurology. 2006;67:305–310. doi: 10.1212/01.wnl.0000225070.13682.11. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Karni A, Abraham M, Monsonego A, Cai G, Freeman GJ, Hafler D, Khoury SJ, Weiner HL. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J Immunol. 2006;177:4196–4202. doi: 10.4049/jimmunol.177.6.4196. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Pasparakis M, Kollias G, Probert L. TNF accelerates the onset but does not alter the incidence and severity of myelin basic protein-induced experimental autoimmune encephalomyelitis. Eur J Immunol. 1999;29:774–780. doi: 10.1002/(SICI)1521-4141(199903)29:03<774::AID-IMMU774>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Cross AH. B cells: no longer the nondominant arm of multiple sclerosis. Curr Neurol Neurosci Rep. 2007;7:231–238. doi: 10.1007/s11910-007-0035-1. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- Knudsen GP, Harbo HF, Smestad C, Celius EG, Akesson E, Oturai A, Ryder LP, Spurkland A, Orstavik KH. X chromosome inactivation in females with multiple sclerosis. Eur J Neurol. 2007;14:1392–1396. doi: 10.1111/j.1468-1331.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- Koch MW, Mostert JP, de Vries JJ, De Keyser J. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2007;68:1163. doi: 10.1212/01.wnl.0000261131.23708.1c. (author reply 1163–1164) [DOI] [PubMed] [Google Scholar]
- Komoly S. Experimental demyelination caused by primary oligodendrocyte dystrophy. Regional distribution of the lesions in the nervous system of mice [corrected] Ideggyogyaszati Szemle. 2005;58:40–43. [PubMed] [Google Scholar]
- Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18:2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelinspecific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kubicek S, Jenuwein T. A crack in histone lysine methylation. Cell. 2004;119:903–906. doi: 10.1016/j.cell.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Faber-Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol (Zurich, Switzerland) 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Lamb BT, Satyamoorthy K, Li L, Solter D, Howe CC. CpG methylation of an endogenous retroviral enhancer inhibits transcription factor binding and activity. Gene Exp. 1991;1:185–196. [PMC free article] [PubMed] [Google Scholar]
- Lamensa JW, Moscarello MA. Deimination of human myelin basic protein by a peptidylarginine deiminase from bovine brain. J Neurochem. 1993;61:987–996. doi: 10.1111/j.1471-4159.1993.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Linker R, Lee DH, Siglienti I, Gold R. Is there a role for neurotrophins in the pathology of multiple sclerosis? J Neurol. 2007;254 (Suppl 1):I33–I40. [Google Scholar]
- Liu A, Stadelmann C, Moscarello M, Bruck W, Sobel A, Mastronardi FG, Casaccia-Bonnefil P. Expression of stathmin, a developmentally controlled cytoskeleton-regulating molecule, in demyelinating disorders. J Neurosci. 2005;25:737–747. doi: 10.1523/JNEUROSCI.4174-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatinskaya L, Zwemmer J, Uitdehaag B, Lucas K, Polman C, Nagelkerken L. Mediators of apoptosis Fas and FasL predict disability progression in multiple sclerosis over a period of 10 years. Mult Scler (Houndmills, Basingstoke, England) 2006;12:704–709. doi: 10.1177/1352458506070826. [DOI] [PubMed] [Google Scholar]
- Lu Q, Qiu X, Hu N, Wen H, Su Y, Richardson BC. Epigenetics, disease, and therapeutic interventions. Ageing Res Rev. 2006;5:449–467. doi: 10.1016/j.arr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. The pathogenesis of multiple sclerosis: relating human pathology to experimental studies. J Neuropathol Exp Neurol. 2006;65:305–318. doi: 10.1097/01.jnen.0000225024.12074.80. [DOI] [PubMed] [Google Scholar]
- Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallstrom E, Khademi M, Oturai A, Ryder LP, Saarela J, Harbo HF, Celius EG, Salter H, Olsson T, Hillert J. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2007;104:14982–14987. doi: 10.1073/pnas.0707044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan L, Ourednik V, Ourednik J. Increased “vigilance”s of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells. 2006;24:2110–2119. doi: 10.1634/stemcells.2006-0018. [DOI] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi FG, Min W, Wang H, Winer S, Dosch M, Boggs JM, Moscarello MA. Attenuation of experimental autoimmune encephalomyelitis and nonimmune demyelination by IFN-beta plus vitamin B12: treatment to modify notch-1/sonic hedgehog balance. J Immunol. 2004;172:6418–6426. doi: 10.4049/jimmunol.172.10.6418. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, Moscarello MA. Molecules affecting myelin stability: a novel hypothesis regarding the pathogenesis of multiple sclerosis. J Neurosci Res. 2005;80:301–308. doi: 10.1002/jnr.20420. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, Noor A, Wood DD, Paton T, Moscarello MA. Peptidyl argininedeiminase 2 CpG island in multiple sclerosis white matter is hypomethylated. J Neurosci Res. 2007a;85:2006–2016. doi: 10.1002/jnr.21329. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, Tsui H, Winer S, Wood DD, Selvanantham T, Galligan C, Fish EN, Dosch HM, Moscarello MA. Synergy between paclitaxel plus an exogenous methyl donor in the suppression of murine demyelinating diseases. Mult Scler (Houndmills, Basingstoke, England) 2007b;13:596–609. doi: 10.1177/1352458506072167. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM, Probert L, Casaccia-Bonnefil P, Moscarello MA. Increased citrullination of histone [3H] in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26:11387–11396. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDole J, Johnson AJ, Pirko I. The role of CD8+ T-cells in lesion formation and axonal dysfunction in multiple sclerosis. Neurol Res. 2006;28:256–261. doi: 10.1179/016164106X98125. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Ackerley CA, Moscarello MA. Localization of basic proteins in human myelin. J Neurosci Res. 1993;35:618–628. doi: 10.1002/jnr.490350605. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Hafler D, Antel JP. Reactivity of normal T-cell lines to MBP isolated from normal and multiple sclerosis white matter. J Neurol Sci. 1995;128:205–211. doi: 10.1016/0022-510x(94)00224-c. [DOI] [PubMed] [Google Scholar]
- Mendez-Fernandez YV, Johnson AJ, Rodriguez M, Pease LR. Clearance of Theiler’s virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur J Immunol. 2003;33:2501–2510. doi: 10.1002/eji.200324007. [DOI] [PubMed] [Google Scholar]
- Merkler D, Horvath E, Bruck W, Zinkernagel RM, Del la Torre JC, Pinschewer DD. “Viral deja vu” elicits organ-specific immune disease independent of reactivity to self. J Clin Investig. 2006;116:1254–1263. doi: 10.1172/JCI27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello MA, Brady GW, Fein DB, Wood DD, Cruz TF. The role of charge microheterogeneity of basic protein in the formation and maintenance of the multilayered structure of myelin: a possible role in multiple sclerosis. J Neurosci Res. 1986;15:87–99. doi: 10.1002/jnr.490150109. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–256. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. Peptidylarginine deiminase: a candidate factor in demyelinating disease. J Neurochem. 2002;81:335–343. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Investig. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Hilke B, Mitchison NA. The role of HLA promoters in autoimmunity. Curr Pharm Des. 2006;12:3743–3752. doi: 10.2174/138161206778559759. [DOI] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Sathornsumetee S, Rodriguez M. Spontaneous remyelination following extensive demyelination is associated with improved neurological function in a viral model of multiple sclerosis. Brain. 2001;124:1403–1416. doi: 10.1093/brain/124.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musse AA, Boggs JM, Harauz G. Deimination of membrane-bound myelin basic protein in multiple sclerosis exposes an immunodominant epitope. Proc Natl Acad Sci U S A. 2006;103:4422–4427. doi: 10.1073/pnas.0509158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbin B, Bonab MM, Khosravi F, Talebian F. Role of B cells in pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:13–42. doi: 10.1016/S0074-7742(07)79002-5. [DOI] [PubMed] [Google Scholar]
- Nonkwelo CB, Long WK. Regulation of Epstein-Barr virus BamHI-H divergent promoter by DNA methylation. Virology. 1993;197:205–215. doi: 10.1006/viro.1993.1581. [DOI] [PubMed] [Google Scholar]
- Odeberg J, Wolmer N, Falci S, Westgren M, Seiger A, Soderberg-Naucler C. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol. 2006;80:8929–8939. doi: 10.1128/JVI.00676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- Ohtani-Fujita N, Fujita T, Aoike A, Osifchin NE, Robbins PD, Sakai T. CpG methylation inactivates the promoter activity of the human retinoblastoma tumorsuppressor gene. Oncogene. 1993;8:1063–1067. [PubMed] [Google Scholar]
- Olsson T. New era for MS treatment, Severe setback when it comes to enthusiasm—highly effective agent caused serious adverse effects. Lakartidningen. 2006;103:1282–1283. [PubMed] [Google Scholar]
- Pahlich S, Zakaryan RP, Gehring H. Protein arginine methylation: cellular functions and methods of analysis. Biochim Biophys Acta. 2006;1764:1890–1903. doi: 10.1016/j.bbapap.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Bruck W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Petronis A. Epigenetics and twins: three variations on the theme. Trends Genet. 2006;22:347–350. doi: 10.1016/j.tig.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF. The pathology of MS: new insights and potential clinical applications. Neurologist. 2007;13:45–56. doi: 10.1097/01.nrl.0000253065.31662.37. [DOI] [PubMed] [Google Scholar]
- Poser CM. The multiple sclerosis trait and the development of multiple sclerosis: genetic vulnerability and environmental effect. Clin Neurol Neurosurg. 2006;108:227–233. doi: 10.1016/j.clineuro.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR. Continual breakdown and regeneration of myelin in multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Revesz T, Kwon EE, Sharer L, Cho ES. Multiple sclerosis. Pathology of recurrent lesions Brain. 1993;116 (Pt 3):681–693. doi: 10.1093/brain/116.3.681. [DOI] [PubMed] [Google Scholar]
- Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- Ptashne M. On the use of the word ‘epigenetic’. Curr Biol. 2007;17:R233–236. doi: 10.1016/j.cub.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Richards EJ. Chromatin methylation: who’s on first? Curr Biol. 2002;12:R694–695. doi: 10.1016/s0960-9822(02)01208-3. [DOI] [PubMed] [Google Scholar]
- Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Savard P, Gates PB, Brockes JP. Position dependent expression of a homeobox gene transcript in relation to amphibian limb regeneration. EMBO J. 1988;7:4275–4282. doi: 10.1002/j.1460-2075.1988.tb03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard P, Tremblay M. Differential regulation of Hox C6 in the appendages of adult urodeles and anurans. J Mol Biol. 1995;249:879–889. doi: 10.1006/jmbi.1995.0345. [DOI] [PubMed] [Google Scholar]
- Sawcer S. A new era in the genetic analysis of multiple sclerosis. Curr Opin Neurol. 2006;19:237–241. doi: 10.1097/01.wco.0000227031.39834.31. [DOI] [PubMed] [Google Scholar]
- Sawcer S, Compston A. Multiple sclerosis: light at the end of the tunnel. Eur J Hum Genet. 2006;14:257–258. doi: 10.1038/sj.ejhg.5201561. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Casaccia-Bonnefil P. Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease. J Mol Neurosci. 2008;35:13–22. doi: 10.1007/s12031-007-9014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Liu A, Li J, Wolubah C, Casaccia-Bonnefil P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol Aging. 2008;29:452–463. doi: 10.1016/j.neurobiolaging.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremata WA, Jy W, Delgado S, Minagar A, McLarty J, Ahn Y. Interferon beta1a reduces plasma CD31+ endothelial microparticles (CD31+EMP) in multiple sclerosis. J Neuroinflamm. 2006;3:23. doi: 10.1186/1742-2094-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov S, Rieneck K, Bovin LF, Skak K, Tomra S, Michelsen BK, Odum N. Histone deacetylase inhibitors: a new class of immunosuppressors targeting a novel signal pathway essential for CD154 expression. Blood. 2003;101:1430–1438. doi: 10.1182/blood-2002-07-2073. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Ann Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Spencer VA, Davie JR. Role of covalent modifications of histones in regulating gene expression. Gene. 1999;240:1–12. doi: 10.1016/s0378-1119(99)00405-9. [DOI] [PubMed] [Google Scholar]
- Stavropoulos P, Hoelz A. Lysine-specific demethylase 1 as a potential therapeutic target. Exp Opin Ther Targets. 2007;11:809–820. doi: 10.1517/14728222.11.6.809. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Briggs SD, Brame CJ, Caldwell JA, Koh SS, Ma H, Cook RG, Shabanowitz J, Hunt DF, Stallcup MR, Allis CD. Methylation of histone [4H] at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol. 2001;11:996–1000. doi: 10.1016/s0960-9822(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Binding site revealed of nature’s most beautiful cofactor. Science (New York, NY) 1994;266:1663–1664. doi: 10.1126/science.7992049. [DOI] [PubMed] [Google Scholar]
- Su RC, Becker AB, Kozyrskyj AL, Hayglass KT. Epigenetic regulation of established human type 1 versus type 2 cytokine responses. J Allergy Clin Immunol. 2008;121:57–63. e53. doi: 10.1016/j.jaci.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Suuronen T, Huuskonen J, Pihlaja R, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by histone deacetylase inhibitors. J Neurochem. 2003;87:407–416. doi: 10.1046/j.1471-4159.2003.02004.x. [DOI] [PubMed] [Google Scholar]
- Szyf M. DNA methylation properties: consequences for pharmacology. Trends Pharmacol Sci. 1994;15:233–238. doi: 10.1016/0165-6147(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- Tabancay AP, Jr, Forsburg SL. Eukaryotic DNA replication in a chromatin context. Curr Top Dev Biol. 2006;76:129–184. doi: 10.1016/S0070-2153(06)76005-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Watanabe Y, Takano-Shimizu T, Kondo S. Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev Dynam. 2006;235:2449–2459. doi: 10.1002/dvdy.20851. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- Thompson PR, Fast W. Histone citrullination by protein arginine deiminase: is arginine methylation a green light or a roadblock? ACS Chem Biol. 2006;1:433–441. doi: 10.1021/cb6002306. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Pozzilli C. Sex hormones: a role in the control of multiple sclerosis? Exp Opin Pharmacother. 2006;7:857–868. doi: 10.1517/14656566.7.7.857. [DOI] [PubMed] [Google Scholar]
- Tomassini V, DeGiglio M, Reindl P, Russo I, Pestalozz P, Pantano T, Berger T, Pozzilli C. Anti-myelin antibodies predict the clinical outcome after a first episode suggestive of MS. Mult Scler. 2007;13:1086–1094. doi: 10.1177/1352458507077622. [DOI] [PubMed] [Google Scholar]
- Tranquill LR, Cao L, Ling NC, Kalbacher H, Martin RM, Whitaker JN. Enhanced T cell responsiveness to citrulline-containing myelin basic protein in Multiple Sclerosis. Mult Scler. 2000;6:220–225. doi: 10.1177/135245850000600402. [DOI] [PubMed] [Google Scholar]
- Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Vanderlocht J, Hellings N, Hendriks JJ, Stinissen P. The ambivalent nature of T cell infiltration in the central nervous system of patients with multiple sclerosis. Crit Rev Immunol. 2007;27:1–13. doi: 10.1615/critrevimmunol.v27.i1.10. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Confavreux C. Pregnancy and multiple sclerosis: the children of PRIMS. Clin Neurol Neurosurg. 2006;108:266–270. doi: 10.1016/j.clineuro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10:693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang ZQ, Xia L, Feng Q, Erdjument-Bromage H, Strahl BD, Briggs SD, Allis CD, Wong J, Tempst P, Zhang Y. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science. 2001;293:853–857. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Dulin J, Wu H, Hurlock E, Lee SE, Jansson K, Lu QR. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development (Cambridge, England) 2006;133:3389–3398. doi: 10.1242/dev.02522. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci U S A. 2003;100:12877–12882. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR. Targeting the JMJD2A histone lysine demethylase. Nat Struct Mol Biol. 2007;14:682–684. doi: 10.1038/nsmb0807-682. [DOI] [PubMed] [Google Scholar]
- Wizenmann A, Bahr M. Growth characteristics of ganglion cell axons in the developing and regenerating retinotectal projection of the rat. Cell Tissue Res. 1997;290:395–403. doi: 10.1007/s004410050946. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte survival. loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123:105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- Wood DD, Bilbao JM, O’Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40:18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- Wood DD, Moscarello MA. The isolation, characterization, and lipid-aggregating properties of a citrulline containing myelin basic protein. J Biol Chem. 1989;264:5121–5127. [PubMed] [Google Scholar]
- Wysocka J, Allis CD, Coonrod S. Histone arginine methylation and its dynamic regulation. Front Biosci. 2006;11:344–355. doi: 10.2741/1802. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Milne TA, Allis CD. Taking LSD 1 to a new high. Cell. 2005;122:654–658. doi: 10.1016/j.cell.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhou JY, Tainsky MA, Wu GS. Evidence that tumor necrosis factor related apoptosis-inducing ligand induction by 5-Aza-2′-deoxycytidine sensitizes human breast cancer cells to adriamycin. Cancer Res. 2007;67:1203–1211. doi: 10.1158/0008-5472.CAN-06-2310. [DOI] [PubMed] [Google Scholar]
- Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Matsuyama A, Komatsu Y, Nishino N. From discovery to the coming generation of histone deacetylase inhibitors. Curr Med Chem. 2003;10:2351–2358. doi: 10.2174/0929867033456602. [DOI] [PubMed] [Google Scholar]
- Zargari M, Allameh A, Sanati MH, Tiraihi T, Lavasani S, Emadyan O. Relationship between the clinical scoring and demyelination in central nervous system with total antioxidant capacity of plasma during experimental autoimmune encephalomyelitis development in mice. Neurosci Lett. 2007;412:24–28. doi: 10.1016/j.neulet.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhou SR, Moscarello MA, Whitaker JN. The effects of citrullination on variable aminoterminus acylation on the encephalitogenicity of human MBP in the Pl? J mouse J Neuroimmunol. 1995;62:147–152. doi: 10.1016/0165-5728(95)00112-3. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Uxa L, Bratina A, Bosco A, Srinivasaraghavan B, Minagar A, Ukmar M, Benedetto S, Zorzon M. HLA-DRB1*1501, -DQB1*0301, -DQB1*0302, -DQB1*0602, and -DQB1*0603 alleles are associated with more severe disease outcome on MRI in patients with multiple sclerosis. Int Rev Neurobiol. 2007;79:521–535. doi: 10.1016/S0074-7742(07)79023-2. [DOI] [PubMed] [Google Scholar]