Abstract
Purpose
To analyse the consequences of using different radiographic measurements and different threshold values for hip screening in children with cerebral palsy (CP).
Methods
In a total sample of children with CP a standardised radiological follow-up of the hips was carried out as a part of a hip prevention programme. Acetabular index (AI) and migration percentage (MP) were measured on all radiographs. In this study, 1,067 radiographs of 272 children born 1992–1998 were analysed.
Results
Lateral displacement of the femoral head was common without acetabular dysplasia, and acetabular dysplasia occurred at a later stage than femoral head lateralisation. Hip dysplasia without lateral displacement of the femoral head was rare. In 16 of the 56 hips (29%) with AI ≥ 27° and in 23 of the 71 (32%) hips with MP ≥ 33% the values decreased below the threshold value without operative treatment. In hips with AI ≥ 30° only 2 of 31 hips (6%) and in hips with MP ≥ 40% only 5 of 44 hips (11%) decreased below the threshold values without operative treatment.
Conclusions
Radiographic follow-up with only measurement of the MP seems sufficient in screening for dislocation in children with CP. MP ≥ 33% is recommended as threshold for reaction or intensified observation. In children with MP ≥ 40%, the lateral displacement increased over time in most hips, thus indicating the need for operative intervention. In children with MP 33–40%, treatment should be based on other clinical signs and the progression of MP over time.
Keywords: Cerebral palsy, Children, Hip dislocation, Prevention, Radiograph
Introduction
Hip dislocation in children with cerebral palsy (CP) is preventable by radiographic screening and early preventive treatment [1, 2]. The CP hip is normal at birth. The change that occurs in the structure of the joint, either on the femoral or acetabular side, is the result of eccentric forces from spastic adductors and flexors acting on the developing hip [3]. Migration percentage (MP) [4] and acetabular index (AI) [5] are the most commonly used measurement for hip lateralisation and acetabular dysplasia in these children. However, recommended threshold values for reaction or intervention vary in the literature: for MP between 30 and 40% [1, 4, 6], and for AI between 27° and 30° [1, 7]. The reason for this variation is probably that no study of a total population of children with CP has analysed the prognostic risk of progression to dislocation in relation to the degree of femoral head lateralisation or acetabular dysplasia.
In a screening programme comprising all children with CP born in southern Sweden from 1992 the children’s hips were radiographically examined from age at diagnosis, and both AI and MP were measured on all radiographs [2]. We analysed the consequences of using different threshold values in this sample.
Materials and methods
All children with CP in the study area in southern Sweden, with a population of 1.3 million, were identified and offered participation in the prevention programme [8]. In this study, radiographs of the children born 1992–1998 were analysed. There was a total of 283 children with CP in this age group. All but seven participated in the follow-up programme. Of the 276 children, three children died within two years of the first examination and were excluded. One boy who had a dislocated hip when he moved into the area was also excluded, making 272 children remaining for the study (Table 1).
Table 1.
Number of children above different threshold values in relation to CP subtype and gross motor function classification system (GMFCS) level. Number of children in total sample for comparison
| AI ≥ 27° | AI ≥ 30° | MP ≥ 30% | MP ≥ 33% | MP ≥ 40% | Total material | |
|---|---|---|---|---|---|---|
| Subdiagnosis | ||||||
| Spastic | ||||||
| Hemiplegia | 2 | 0 | 3 | 2 | 1 | 88 |
| Diplegia | 32 | 15 | 44 | 38 | 21 | 112 |
| Tetraplegia | 11 | 7 | 15 | 14 | 12 | 17 |
| Ataxic | 0 | 0 | 1 | 1 | 1 | 12 |
| Dyskinetic | ||||||
| Athetotic | 2 | 2 | 4 | 3 | 1 | 12 |
| Dysthonic | 9 | 7 | 13 | 12 | 7 | 26 |
| Not classified | 0 | 0 | 1 | 1 | 1 | 5 |
| Total | 56 | 31 | 81 | 71 | 44 | 272 |
| GMFCS level | ||||||
| I | 7 | 2 | 8 | 7 | 2 | 127 |
| II | 5 | 1 | 6 | 4 | 3 | 34 |
| III | 12 | 6 | 20 | 17 | 9 | 34 |
| IV | 17 | 11 | 24 | 21 | 12 | 39 |
| V | 15 | 11 | 23 | 22 | 18 | 35 |
| Not classified | 3 | |||||
| Total | 56 | 31 | 81 | 71 | 44 | 272 |
AI acetabular index, MP migration percentage
The programme included a continuous follow-up of the child’s diagnosis, gross motor function, clinical findings and treatment [9, 10]. The subtype of CP was determined according to Hagberg et al. [11]. Gross motor function was classified according to the gross motor function classification system (GMFCS) [12]. A standardised radiological follow-up of the hips was carried out [2]. The hips were examined on anteposterior radiographs at diagnosis, then at least once a year in children with diplegic, tetraplegic or dystonic type of CP until eight years of age, then on an individual basis. Children with spastic hemiplegia or pure ataxia were examined at four years of age. If the radiograph was normal, no further examinations were undertaken, unless the clinical follow-up showed decreasing range of motion of the hips.
Migration percentage and AI were measured on all radiographs (Fig. 1). All measurements were performed by one of the authors. In hips with a Gothic arch formation of the lateral margin, the midpoint of the arch was used as reference point according to Cooke et al. [7] (Fig. 2).
Fig. 1.
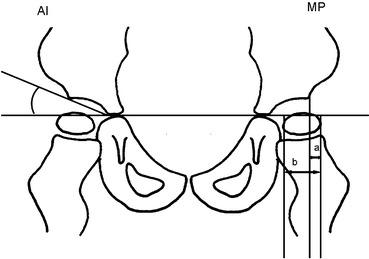
Measurement of the acetabular index (AI) and migration percentage (MP; MP = a/b × 100)
Fig. 2.
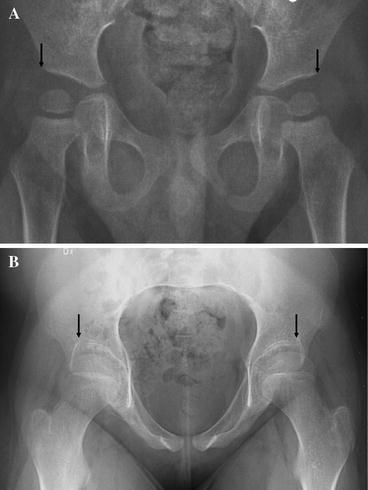
The point used for measuring the lateral margin of the acetabulum (a). In radiographs with oblique view of the pelvis, the top of the Gothic arch, which corresponds to the apex of acetabulum, was used (b), and if possible repeat examination with positioning using fluoroscopy
On 1 June 2005, 1,067 radiographs had been taken of the 272 children. The children were 6.5–13.5 years of age at latest examination. The hips with AI and/or MP exceeding different threshold values (AI 27° and 30°, MP 30, 33 and 40%) were further analysed. Only one hip in each child was included, that with the highest MP or AI. Once a hip had been operated, it was excluded from further analysis. In the analysis of the different threshold values we have used cumulative data, which means that hips with MP and AI above the higher levels are included in the number of children above the lower threshold levels.
Results
Of the 272 children, 56 (21%) developed acetabular dysplasia with AI ≥ 27° and 81 (30%) developed lateral displacement of the femoral head with MP ≥ 30%. Of the 56 children with AI > 27°, 30 (54%) reached AI ≥ 30°. Of the 81 children with MP ≥ 30%, 71 (88%) reached MP ≥ 33% and 44 (54%) reached MP ≥ 40%. Most of the children with increased MP or AI had spastic diplegia, spastic tetraplegia, or the dystonic type of CP, and most of them were classified at GMFCS levels III, IV or V (Table 1).
The majority of hips with increased AI also showed an increased MP, while many hips with increased MP had an AI below 27° or 30° (Table 2).
Table 2.
Number of children with migration percentage (MP) and acetabular index (AI) above different threshold values
| Threshold | AI ≥ 27° | AI ≥ 30° | |
| Total number | 56 | 31 | |
| MP ≥ 30% | 81 | 49 | 31 |
| MP ≥ 33% | 71 | 45 | 30 |
| MP ≥ 40% | 44 | 34 | 25 |
In those hips with both increased MP and AI, the MP was most often seen earlier than, or simultaneously with, the increased AI (Table 3).
Table 3.
Time relation between MP and AI for different threshold values
| AI first | AI = MP | MP first | Total | |
|---|---|---|---|---|
| AI ≥ 27° and MP ≥ 30% | 4 | 17 | 28 | 49 |
| AI ≥ 27° and MP ≥ 33% | 5 | 18 | 22 | 45 |
| AI ≥ 27° and MP ≥ 40% | 11 | 11 | 12 | 34 |
| AI ≥ 30° and MP ≥ 30% | 2 | 10 | 19 | 31 |
| AI ≥ 30° and MP ≥ 33% | 3 | 10 | 17 | 30 |
| AI ≥ 30° and MP ≥ 40% | 6 | 7 | 12 | 25 |
In 16 of the 56 hips (29%) with AI ≥ 27° and in 29 of the 81 hips (36%) with MP ≥ 30% the values decreased below the threshold value without operative treatment. In hips with AI ≥ 30° only 2 of 31 hips (6%) and in hips with MP ≥ 40% only 5 of 44 hips (11%) decreased below the threshold values without operative treatment (Fig. 3). Of the 16 hips with AI ≥ 27° that decreased without operation, the decrease from the highest measured AI to the last examination (before closure of the triradiate growth plate) was on average 7.8° (range 4–14°). Of the 29 hips with MP ≥ 30% that decreased without operation, the decrease from the highest measured MP to the last examination was in average 10.8% (range 3–15%).
Fig. 3.
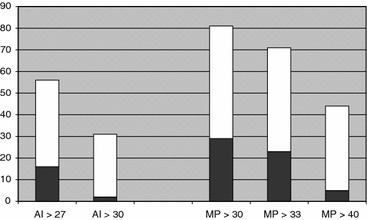
Number of children with decreased AI or MP below threshold value without operative treatment. White: total number above the threshold value. Black: decreased without surgery
Nonoperative treatment included intensified positioning in abduction and extension, and intensified standing in standing shells. Nonoperative treatment also included management of other problems resulting in increased spasticity as under nourishment, gastric reflux, or constipation. Sixteen children were treated with botulinum toxin of the adductor muscles. The indication for treatment was scissoring resulting in gait, sitting, or nursing problems. The numbers of treatments were one in 11 children, 1 in 2 children, four in 2 children and seven in one child. Of the 16 children, 10 had MP < 30%. The MP was ≥ 30% in six hips; four of these showed a decreasing lateral displacement and two increased and were operated with preventive surgery. The child with seven treatments had an MP < 30%.
Discussion
The findings are based on a total population of children with CP followed until 6.5–13.5 years of age. Most of the children had not completed growth. However, the risk of hip dislocation is highest before seven years of age [3].
Parrot et al. [13] demonstrated that an experienced rater would be expected to measure MP within 5.8% of the true value, and AI within 2.6°. Faraj et al. [14] analysed the error in the measurement of MP with less-experienced observers. The median intra-observer difference was 3.2–3.6%, and the median inter-observer difference was 3.3–5%. The upper 95% confidence interval for intra-examiner measurement was 13%, and for inter-examiner value was 22%. These two studies show that the measurement should be done by an experienced examiner and radiographs should be compared and measured by the same examiner.
It is important to have a horizontal view of the pelvis with the legs in a neutral position. A radiograph with the femur in adduction gives a false high MP; abduction gives a false low value [4]. In- or outward rotation doses not influence the measurement to the same extent [4]. In children with contractures or high muscle tone it may be necessary to take the radiograph under fluoroscopic control in order to get measurable pictures.
The AI was less than 27° in 40%, and less than 30° in 60% of the hips with MP ≥ 33%. In most of the hips with both increased MP and AI, the lateralisation of the femoral head preceded the acetabular dysplasia. This is contrary to the findings by Cooke et al. [7], who stated that increased AI is always seen before lateral displacement, and recommended AI for screening purpose.
Vidal et al. [15] and Wheeler et al. [16] both observed only 3–4° correction of the acetabular dysplasia after soft-tissue release. Cornell et al. [17] found poor results in 13 of 15 hips with preoperative AI ≥ 27°. In their sample seven hips were dislocated, and six hips had MP ≥ 60% before treatment. In our sample, 42 of the 56 hips (75%) with AI ≥ 27° improved to <27°; 16 hips improved without operative treatment and 26 improved after operative treatment of which only five included pelvic osteotomy. The highest measured AI in each of these 42 hips was 29.3° (27–36°). The remaining hips with increased AI are being followed in the prevention programme. Based on our findings, it seems that the dysplasia in most of the hips will decrease if the lateral displacement of the femoral head is corrected.
Of the hips with MP ≥ 33%, one out of three returned to <33% without operative treatment. Of the 44 hips with MP ≥ 40% five hips (11%) returned to <40% without operative treatment. The frequency of hips that decreased below the different threshold values without preventive surgery should be regarded as minimum values for nonoperative improvement, as it is not known whether any of those operated on would also have improved without surgery. However, in 10 of the 39 hips with MP ≥ 40% operated on, the MP progressed even after the first operation, and a second operation was needed to regain femoral head coverage. Most of the other hips also showed increasing lateral displacement before operative treatment.
The high number of hips with decreasing AI and MP without surgery has, to our knowledge, not been described earlier. One explanation could be a more-intense nonoperative treatment of the children in the prevention programme. In some children the degree of spasticity spontaneously decreases with increasing age, which may be another explanation. The number of children treated with botulinum toxin was too small to analyse further the significance. In some hips the decrease of MP and AI is within the range of measurement error.
There are a few earlier studies with recommendations of threshold values for preventive surgery. Onimus et al. [6] found the best results in children with MP < 33%, operated at 2–3 years of age. Based on our findings this can be explained by the fact that many of those children would have a good outcome even without preventive surgery.
Cornell et al. [17] showed better results in preventive surgery in hips with MP < 40%. The authors only analysed the results after soft-tissue surgery. Of the 30 hips with MP < 40% in their sample, 26 hips had a MP of 18–33% preoperatively, only four hips had MP between 33 and 40%.
Dobson et al. [1] recommended preventive surgery in those with MP ≥ 40%, AI ≥ 27° and in those with progression of MP of more than 10% in one year.
Some studies have shown better long-term results after preventive surgery at a young age [4, 15]; others did not find any correlation of the results in relation to age at surgery [16–18]. No correlation was seen in the present sample, indicating that the same threshold values can be used regardless of age in the studied period up to 13 years of age.
It seems that MP is the measurement of choice for hip screening in CP. Lateral dislocation is common without acetabular dysplasia, and lateral dislocation often precedes acetabular dysplasia. Acetabular dysplasia without lateral displacement of the femoral head is uncommon. MP ≥ 33% is recommended as a threshold for reaction or intensified observation. Hips with MP ≥ 40% have a high risk for further displacement, indicating the need for surgical intervention. In children with MP 33–40%, treatment should be based on other clinical signs and the progression of MP over time, which should be evaluated with repeat examinations analysed by the same examiner.
Acknowledgments
The study was supported by the Medical faculty, Lund University and Linnéa and Josef Carlssons stiftelse.
References
- 1.Dobson F, Boyd RN, Parrott J, Nattrass GR, Graham HK. Hip surveillance in children with cerebral palsy. J Bone Joint Surg Br. 2002;84-B:720–726. doi: 10.1302/0301-620X.84B5.12398. [DOI] [PubMed] [Google Scholar]
- 2.Hägglund G, Andersson S, Düppe H, Lauge Pedersen H, Nordmark E, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy. First ten years experience of a population based prevention program. J Bone Joint Surg Br. 2005;87-B:95–101. [PubMed] [Google Scholar]
- 3.Rang M, Silver R, de la Garza J. Cerebral palsy. In: Lovell WW, Winter RB, editors. Pediatric orthopaedics. 2nd. Philadelphia: JB Lippincott Co; 1986. pp. 345–396. [Google Scholar]
- 4.Reimers J. The stability of the hip in children: a radiological study of results of muscle surgery in cerebral palsy. Acta Orthop Scand. 1980;184:1–100. doi: 10.3109/ort.1980.51.suppl-184.01. [DOI] [PubMed] [Google Scholar]
- 5.Hilgenreiner H. Zur Frühdiagnose und Frühbehandlung der angeborenen Hüftgelenkverrenkung. Medizinische Klinik. 1925;21:1385–13425. [Google Scholar]
- 6.Onimus M, Allamel G, Manzone P, Laurain JM. Prevention of hip dislocation in cerebral palsy by early psoas and adductors tenotomies. J Pediatr Orthop. 1991;11:432–435. doi: 10.1097/01241398-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Cooke PH, Cole WG, Carey RPL. Dislocation of the hip in cerebral palsy. Natural history and predictability. J Bone Joint Surg Br. 1989;71-B:441–446. doi: 10.1302/0301-620X.71B3.2722938. [DOI] [PubMed] [Google Scholar]
- 8.Nordmark E, Hägglund G, Lagergren J. Cerebral palsy in southern Sweden. I. Prevalence and clinical features. Acta Paediatr. 2001;90:1271–1276. doi: 10.1111/j.1651-2227.2001.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 9.Hägglund G, Andersson S, Düppe H, Lauge Pedersen H, Nordmark E, Westbom L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: results of a population-based health care programme and new techniques to reduce spasticity. J Pediatr Orthop. 2005;14:268–272. doi: 10.1097/01202412-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Nordmark E, Hägglund G, Lagergren J. Cerebral palsy in southern Sweden. II. Gross motor function and disabilities. Acta Paediatr. 2001;90:1277–1282. doi: 10.1111/j.1651-2227.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg B, Hagberg G, Olow I. The changing panorama of cerebral palsy in Sweden 1954–1970. Acta Paediatr Scand. 1975;64:187–192. doi: 10.1111/j.1651-2227.1975.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 12.Palisano R, Rosenbaum P, Walter S, Russel D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 13.Parrott J, Boyd RN, Dobson F, Lancaster A, Love S, Oates J, Wolfe R, Nattrass GR, Graham HK. Hip displacement in spastic cerebral palsy: repeatability of radiologic measurement. J Pediatr Orthop. 2002;22:660–667. [PubMed] [Google Scholar]
- 14.Faraj S, Atherton WG, Stott NS. Inter- and intra-measurer error in the measurement of Reimer’s hip migration percentage. J Bone Joint Surg Br. 2004;86:434–437. doi: 10.1302/0301-620X.86B3.14094. [DOI] [PubMed] [Google Scholar]
- 15.Vidal J, Deguillaume P, Vidal M. The anatomy of the dysplastic hip in cerebral palsy related to prognosis and treatment. Int Orthop. 1985;9:105–110. doi: 10.1007/BF00266951. [DOI] [PubMed] [Google Scholar]
- 16.Weeler ME, Weinstein SL. Adductor tenotomy-obturator neurectomy. J Pediatr Orthop. 1984;4:48–51. doi: 10.1097/01241398-198401000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Cornell MS, Hatrick NC, Boyd R, Baird G, Spencer JD. The hip in children with cerebral palsy. Clin Orthop. 1997;340:165–171. doi: 10.1097/00003086-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Silver RI, Rang M, Chan J, de la Garza J. Adductor release in nonambulant children with cerebral palsy. J Pediatr Orthop. 1985;5:672–677. doi: 10.1097/01241398-198511000-00008. [DOI] [PubMed] [Google Scholar]


