Abstract
Background
Alteration of the circadian rhythm and increased vascular senescence are linked to cardiovascular disease. Per2, a circadian gene, is known to regulate endothelium-dependent vasomotion. However, the mechanism by which Per2 affects endothelial function is unknown. We hypothesize that endothelial dysfunction in Per2 mutant (Per2m/m) mice is mediated in part by increased vascular senescence and impaired endothelial progenitor cell (EPC) function.
Methods and Results
Endothelial cells from Per2m/m mice exhibit increased protein kinase Akt signaling, greater senescence, and impaired vascular network formation and proliferation. Indeed, Per2m/m mice have impaired blood flow recovery and developed autoamputation of the distal limb when subjected to hind-limb ischemia. Furthermore, matrigel implantation into Per2m/m mice resulted in less neovascularization. Because EPCs contribute to angiogenesis, we studied the role of Per2 in these cells using bone marrow transplantation. Basal EPC levels were similar between wild-type and Per2m/m mice. However, compared with wild-type bone marrow transplantation mice, EPC mobilization was impaired in Per2m/m bone marrow transplantation mice in response to ischemia or VEGF stimulation. Bone marrow transplantation or infusion of wild-type EPC restored blood flow recovery and prevented autoamputation in Per2m/m mice.
Conclusion
These findings indicate that mutation of Per2 causes Akt-dependent senescence and impairs ischemia-induced revascularization through the alteration of EPC function.
Keywords: angiogenesis, circadian rhythm, endothelium, ischemia, senescence
Epidemiological studies have shown that shift or nighttime workers have a higher incidence of cardiovascular disease. 1–3 Because blood pressure,4 heart rate,5 endothelial function,6 and the onset of myocardial infarction and stroke7,8 have distinct circadian patterns, it has been suggested that disruption of circadian rhythm may contribute to cardiovascular disease. The primary control of the circadian clock is located in the hypothalamic suprachiasmatic nuclei, which are used to synchronize peripheral clocks in organs and tissues. This synchronization is accomplished through a series of tightly regulated circadian genes such as CLOCK, BMAL1, NPAS2, Cry1/2, and Per1/2.9,10 Mutations in or knockout of these circadian genes leads to diverse pathophysiological disorders, including metabolic syndrome and obesity,11 premature aging,12 and abnormal sleep cycle.13 In particular, mice with a Per2 mutation (Per2m/m) suffer multiple abnormalities, including shortened lifespan, defect in thymocyte apoptosis,14 increased carcinogenesis,15 greater bone formation, 16 increased alcohol consumption,17 and impaired endothelial function.6 The diverse phenotype observed in Per2m/m mice likely reflects the broad downstream targets of Per2, which include c-myc, Cyclin D1, Cyclin A, Mdm-2, and Gadd45α.14 In the cardiovascular system, the production of nitric oxide (NO) and vasoactive prostaglandins is altered in Per2m/m mice.6 However, the vascular consequences of Per2 mutation on NO-mediated angiogenesis and the underlying mechanism responsible for the phenotype have not been investigated or characterized in Per2m/m mice.
Cardiovascular risks and disease occur at much higher rates in individuals with advanced age and vascular senescence. Vascular senescence as defined by a state of irreversible growth arrest, expression of negative regulators of cell cycle (such as p53 and p16), and increased senescence-related β-galactosidase staining is an important indicator of vascular dysfunction.18,19 Vascular senescence is associated with atherosclerosis, 20 endothelial dysfunction,21 increased reactive oxygen species,22 and alteration of telomerase activity.23 However, the precise mechanisms contributing to vascular senescence are not known. We hypothesize that alteration of circadian rhythm leads to vascular senescence, decreased NO bioavailability, and impaired angiogenesis. Using Per2m/m mice, we investigated the effects of Per2 mutation on vascular senescence and the angiogenic response to distal limb ischemia.
Methods
Animals
Per2m/m mice with C57BL/6-Tyrc-Brd background were obtained from The Jackson Laboratory (Bar Harbor, Me) and backcrossed to C57BL/6 background for 6 generations before experimental use. The wild-type (WT) and Per2m/m mice littermates were generated from heterozygote cross-breedings and kept in a regular light/dark cycle (ie, 12-hour cycle).
Cumulative Population Doublings and Senescence Stain
The lung and aortic endothelial cells were passaged every 3 to 4 days. Cumulative population doubling at each passage was calculated from the cell count with the following equation:24
where N1 is the inoculum number, NH is the cell harvest number, and X is population doublings. The population doublings were added to yield cumulative population doubling. Replicative senescence is defined as X < 1 for 3 weeks. Senescence-associated β-galactosidase staining was performed as previous described at pH 5.5.25
Statistical Analysis
Results are expressed as mean ± SEM. Differences between 2 groups were examined by the Student t test. Laser Doppler perfusion images were analyzed with repeated-measures ANOVA with posthoc analysis.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Impaired Proliferation and Vascular Network Formation in Endothelial Cells From Per2 Mice
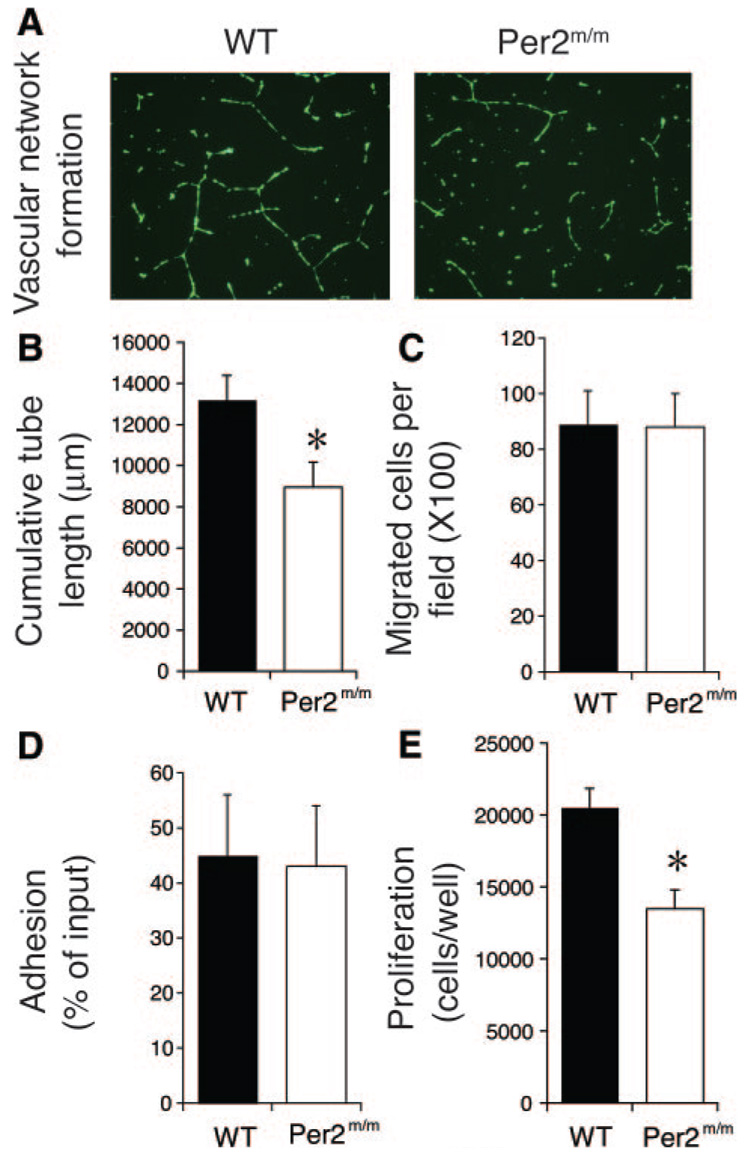
To determine whether mutation of Per2 affects endothelial function and angiogenesis, we isolated endothelial cells from WT and Per2m/m mice. Cells from WT and Per2m/m mice have similar appearances and sizes (cell diameters: WT, 16.1±0.4 µm; Per2m/m, 15.9±0.3 µm; n=120; P=NS). However, the ability of endothelial cells from Per2m/m mice to form vascular networks (ie, total length of cords) was substantially reduced compared with endothelial cells from WT mice (WT, 13 246±1420 µm; Per2m/m, 8181±1192 µm; n=9; P<0.05) (Figure 1A and 1B). Interestingly, endothelial cell migration and adhesion were not altered by Per2 mutation (Figure 1C and 1D). In agreement with decreased vascular network formation, cell proliferation was impaired in endothelial cells from Per2m/m mice (WT, 20 520±1319 cells per well; Per2m/m, 13 473±1291 cells per well; n=11; P<0.05) (Figure 1E). These results indicate that mutation of Per2 impairs the ability of endothelial cells to proliferate and form vascular networks.
Figure 1.
Impaired proliferation and vascular network formation in endothelial cells from Per2m/m mice. A, Vascular network formation is decreased in Per2m/m lung endothelial cells. Representative images of 9 separate experiments. B, Quantitative assessment of vascular network formation (tube length) (n=9). C, Migration of lung endothelial cells in wound healing assay is maintained (n=4). D, Adhesion of lung endothelial cells is maintained in Per2m/m cells (n=4). E, Cell proliferation is decreased in Per2m/m lung endothelial cells in crystal violet assay (n=11). *P<0.05 vs WT.
Per2 Mutation Leads to Vascular Senescence
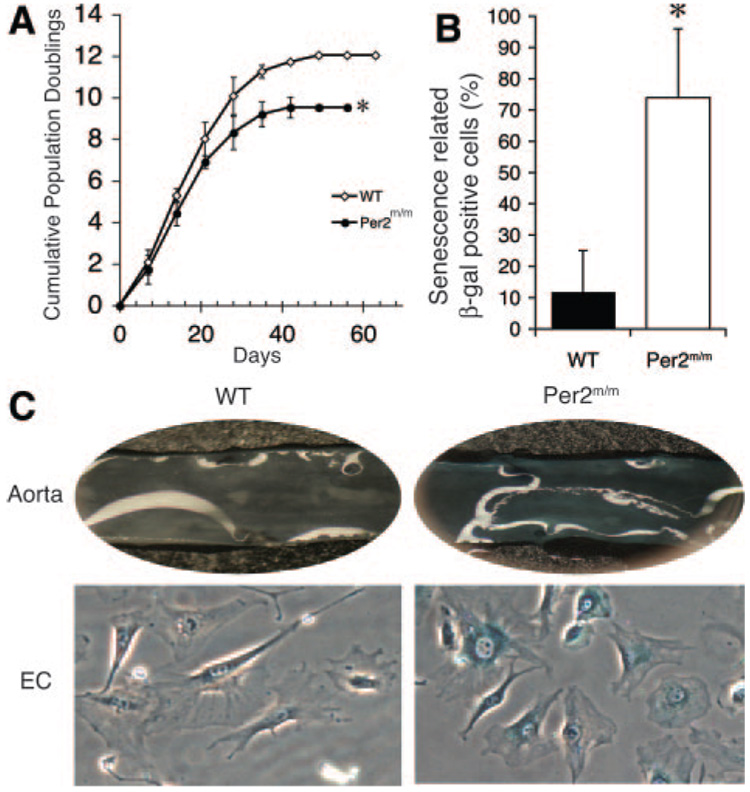
To determine whether mutation of Per2 leads to senescence, endothelial cells from WT and Per2m/m mice were subjected to serial passages to test for replicative capacity. WT endothelial cell cultures undergo growth arrest after 10 to 12 passages (Figure 2A). However, Per2m/m endothelial cells exhibited earlier growth arrest at passages 8 to 10. Similar results were obtained from 4 independent Per2m/m and 2 independent WT endothelial cell cultures. To determine whether endothelial cells from Per2m/m mice show increased senescence compared with WT mice, the aortas from 12-month-old mice were stained for senescence-related β-galactosidase activity. The aortas from Per2m/m mice showed increased β-galactosidase staining compared with WT aortas (Figure 2B). When endothelial cells from 12-month-old Per2m/m mice were isolated, they also showed an increased percentage of β-galactosidase–stained cells compared with WT mice (Per2m/m, 74±22%; WT, 12±13%; n=6; P<0.05) (Figure 2C). Furthermore, the expression of endothelin-1, plasminogen activator inhibitor-1, thromboxane A2, hypoxia inducible factor-1α, and vascular endothelial growth factor (VEGF) in endothelial cells from WT and Per2m/m mice was not different (data not shown). These findings indicate that Per2 mutation is associated with increased endothelial cell senescence.
Figure 2.
Mutation of Per2 leads to vascular senescence. A, Cumulative population doubling is decreased in murine lung endothelial cells (EC) isolated from 8-week-old Per2m/m mice (n=4). B, One-year-old Per2m/m mice have increased percentage of senescence-related β-galactosidase staining positive compared with mice (n=4). C, Representative images of en face aorta or endothelial cells with senescence-related β-galactosidase staining (n=6). *P<0.05 vs WT.
Increased Akt Signaling in Per2m/m Mice
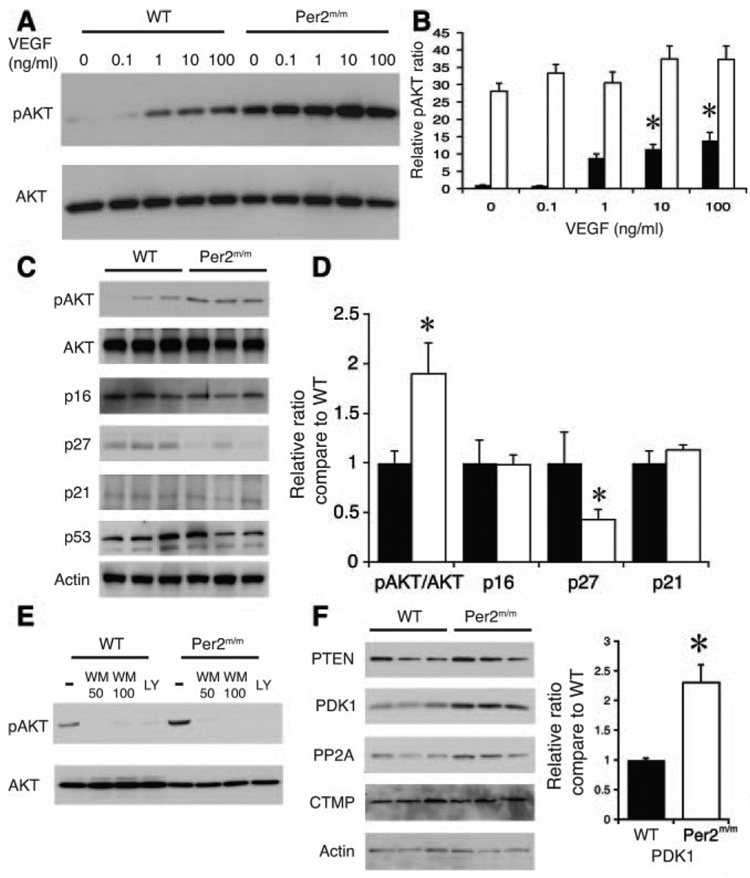
The Per2m/m mice have a shortened lifespan and are prone to developing teratomas and lymphomas.14 Furthermore, their thymocytes are relatively resistant to apoptosis in response to γ-radiation. Because oncogenesis and senescence have been shown to be associated,26 they are sometimes regulated by similar signaling pathways. We investigated whether Per2 could regulate an oncogene that could be responsible for cellular senescence. We postulate that protein kinase B/Akt, which was initially found to be an oncogene in murine thymic lymphomas, mediates vascular senescence. Compared with endothelial cells from WT mice, endothelial cells from Per2m/m mice exhibited high constitutive levels of Akt Ser473 phosphorylation (pAkt; Figure 3A and 3B). Treatment with VEGF increased pAkt levels in WT endothelial cells but not in Per2m/m endothelial cells (Figure 3A and 3B). Similarly, aortas from Per2m/m mice have 2-times-higher pAkt levels and >50% lower p27 levels (Figure 3C and 3D).
Figure 3.
Increased Akt activation in Per2m/m mice. A, Western blot showing concentration-dependent pAkt by VEGF in WT and Per2m/m aortic endothelial cells. Representative blots from 3 separate experiments. B, Quantitative analysis of Akt phosphorylation from Western blots (n=3). *P<0.05 vs no VEGF. C, Western blots showing expression of pAkt, p16, p21, p27, and p53 in WT and Per2m/m aortas. Representative blots from 3 separate experiments from 9 total aortas. D, Quantitative analysis of Akt phosphorylation from Western blots (n=3). *P<0.05 vs WT. E, Western blots showing pAkt level after treatment with wortmannin (WM; 50 or 100 nmol/L) and LY294002 (LY; 10 µmol/L) in WT and Per2m/m endothelial cells. Representative blots from 3 separate experiments. F, Aortic phosphatase and tensin homolog (PTEN), PDK1, protein phosphatase 2A (PP2A), and carboxyl-terminal modulator protein (CTMP) levels from WT and Per2m/m mice. Representative blots from 6 separate experiments. Quantification of PDK1 levels in WT and Per2m/m aortas. *P<0.01 vs WT; n=6.
Because Akt phosphorylation is regulated by phosphatidylinositol 3-kinase (PI3K) and the mammalian target of rapamycin–rictor pathway,27 we examined whether these 2 pathways are altered in Per2m/m mice. Compared with WT mice, the protein levels of mammalian target of rapamycin components, rictor, raptor, and p70 S6 kinase, were not different in Per2m/m mouse aorta (data not shown). Treatment with the PI3K inhibitors LY294002 (LY) and wortmannin decreased pAkt levels in both WT and Per2m/m endothelial cells (Figure 3E), suggesting that constitutive pAkt in Per2m/m mice is due in part to chronic PI3K activation. However, both phosphatase and tensin homolog (P=0.04) and protein phosphatase 2A (P=0.014), which negatively regulate Akt phosphorylation, showed compensatory increases in Per2m/m mice. In contrast, the levels of phosphatidylinositol-dependent kinase-1 (PDK1) in Per2m/m mice are ≈2.3 times higher than those of WT mice (Figure 3F). These findings suggest that increased PDK1/Akt signaling in Per2m/m mice may contribute to the observed increase in vascular senescence.
Decreased Angiogenesis in Per2m/m Mice
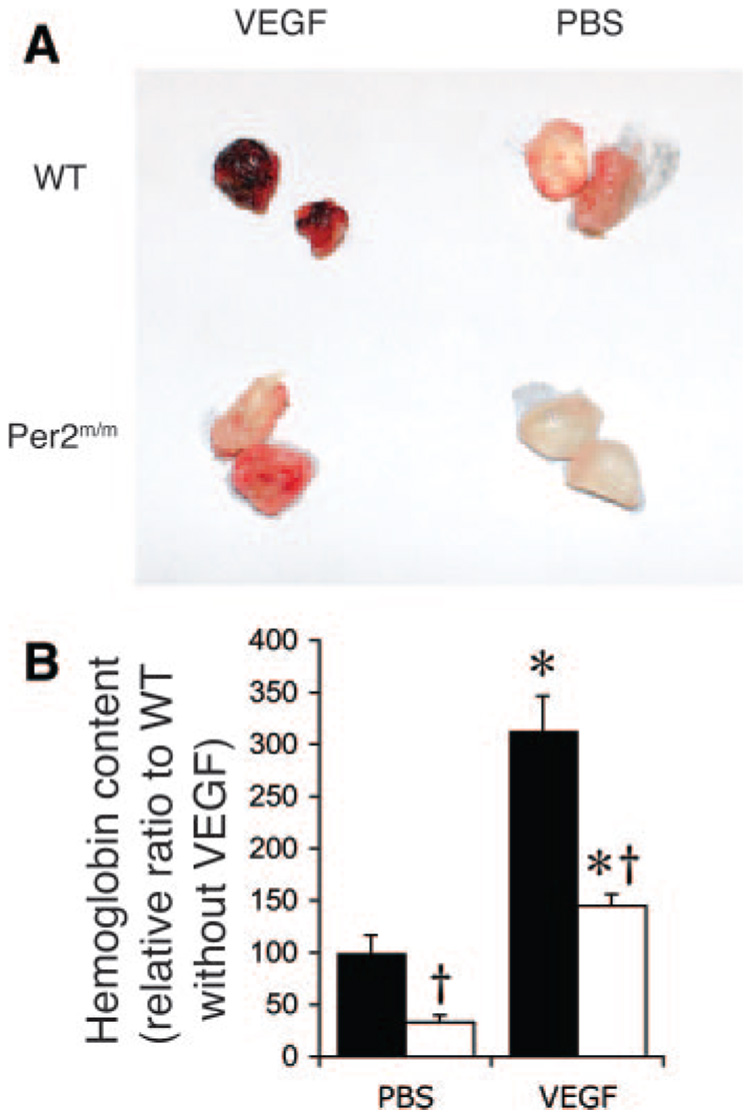
Because Per2m/m mice exhibit endothelial dysfunction6 and senescence, we investigated whether angiogenic function in Per2m/m mice is altered. To study the angiogenic response in vivo, we performed subcutaneous implantation of matrigel with or without VEGF. The matrigel was removed 5 days after implantation, and the incorporated hemoglobin, which correlates with vessel formation, was then quantified. Compared with WT mice, the hemoglobin content of the matrigel was reduced in the Per2m/m mice in the absence of VEGF (Per2m/m, 33±7%; WT, 100±17%; n=6; P<0.05) (Figure 4A and 4B). Although treatment with VEGF produced a similar induction of hemoglobin content in matrigel implanted into WT and Per2m/m mice, the absolute amount of hemoglobin content was substantially less in Per2m/m mice compared with that of WT mice (Per2m/m, 145±11%; WT, 313.6±33%; n=6; P<0.05). These findings indicate that Per2 mutation leads to impaired angiogenesis in vivo.
Figure 4.
Decreased angiogenesis in Per2m/m mice. A, Decreased in vivo angiogenesis in matrigel in Per2m/m mice. Representative matrigels after implantation for 5 days in WT and Per2m/m mice with or without VEGF (60 ng/mL). Experiments were performed 6 times with similar results. B, Hemoglobin content in matrigel as determined by Drabkin’s method. Solid bar indicates WT; open bar, Per2m/m. *P<0.01 vs PBS; †P<0.05 compared with WT; n=6.
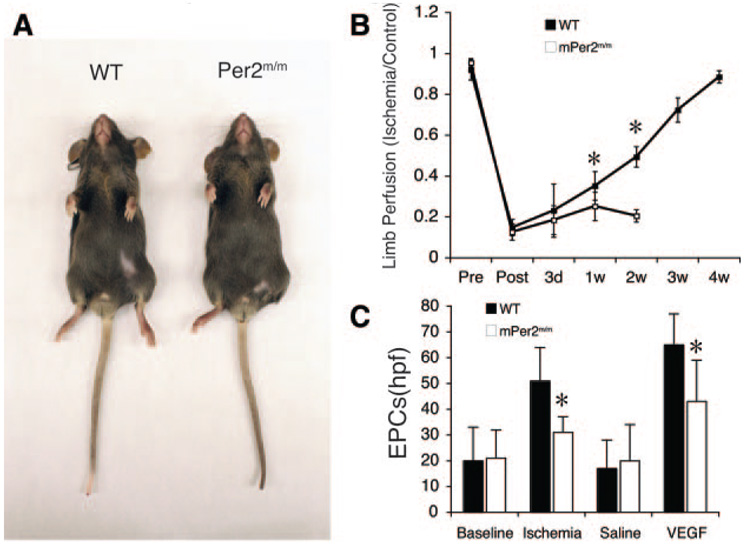
To determine the in vivo relevance of Per2 mutation in postnatal ischemia-induced neovascularization, we performed hind-limb ischemia in WT and Per2m/m mice. Surprisingly, all Per2m/m mice developed autoamputation following hind-limb ischemia after 2 weeks, whereas none of the WT mice developed autoamputation (Figure 5A). This correlated with impaired blood flow recovery after ischemia in Per2m/m mice compared with WT mice after 1 week (Figure 5B). These findings indicate that the circadian gene, Per2, is essential for postischemic blood flow recovery in adult mice.
Figure 5.
Ischemia-induced neovascularization is impaired in Per2m/m mice. A, Autoamputation of Per2m/m mice after hind-limb ischemia (arrow). Representative figures for 6 WT and Per2m/m mice. B, Quantitative analysis of ischemic-to-nonischemic laser Doppler blood flow ratio in WT and Per2m/m mice (WT, n=6; Per2m/m, n=6 for before, after, and 3 days after; n=5 for 1 week; n=3 for 2 weeks). Because of autoamputation in all Per2m/m mice, laser Doppler imaging was not possible in this group of mice after 2 weeks. C, Quantitative analysis of EPC levels at baseline and after ischemia or VEGF stimulation. *P<0.05 vs WT; n=9.
Because bone marrow (BM) cells may contribute to ischemia-induced revascularization, we investigated the role of Per2 mutation in BM-derived endothelial progenitor cells (EPCs). We first investigated EPC levels in WT and Per2m/m mice. EPCs were characterized by positive staining for acetylated low-density lipoprotein, lectin, and endothelial NO synthase.28 Interestingly, there were no significant differences in the baseline EPC levels between WT and Per2m/m mice (Per2m/m, 21±11 per high-power field [hpf]; WT, 20±13 per hpf; n=9; P>0.05). Furthermore, the ability of EPCs from Per2m/m mice to proliferate in vitro was not different compared with that of EPCs from WT mice (data not shown). However, 5 days after hind-limb ischemia, the increase in EPC was substantially less in Per2m/m mice compared with WT mice (Per2m/m, 31±6 per hpf; WT, 51±13 per hpf; n=9; P<0.05) (Figure 5C). To further characterize the EPC response, we administered VEGF165 (500 µg · kg−1 · d−1 SC for 3 days) to WT and Per2m/m mice. The increase in EPC numbers in Per2m/m mice was much less compared with EPC numbers in WT mice (Per2m/m, 43±16 per hpf; WT, 65±12 per hpf; n=9; P<0.05) (Figure 5C).
Mutation of Per2 in BM-Derived Cells Impairs Angiogenesis
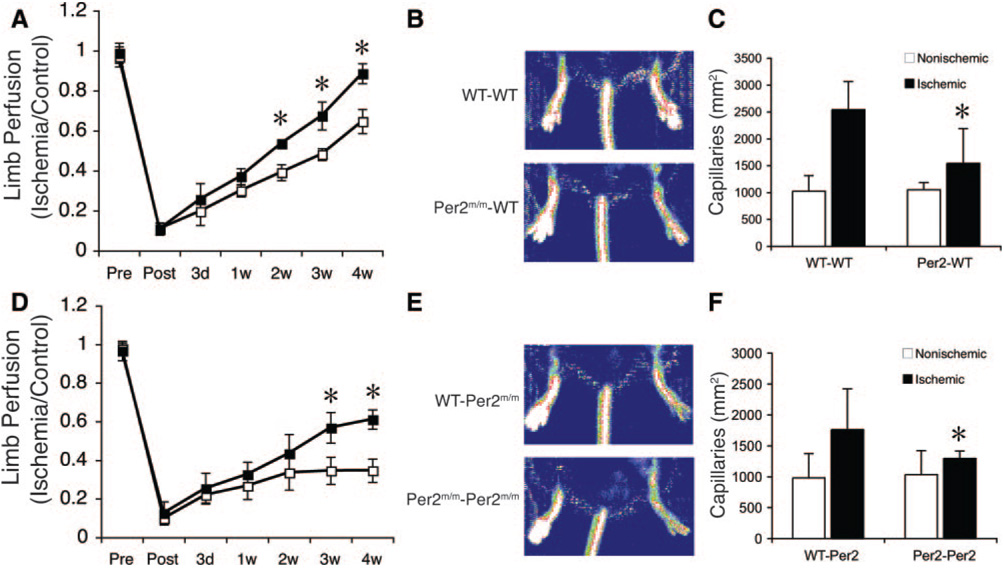
To determine the role of Per2 in ischemia-induced neovascularization, we performed BM transplantation (BMT) from WT or Per2m/m mice into irradiated recipient WT mice. Four weeks after BMT, the number of BM-derived EPCs was similar in the 2 groups of recipient mice (Per2m/m, 17±9 per hpf; WT, 16±12 per hpf; n=6; P=0.25), and hind-limb ischemia was then induced. Compared with mice receiving WT BM (WT→WT BMT mice), Per2m/m→WT BMT mice exhibited 27±0.9% less blood flow recovery at 28 days after surgery (Figure 6A and 6B). The microcapillary density in the ischemic adductor skeletal muscle tissue also was substantially less in Per2m/m→WT BMT mice compared with WT→WT BMT mice (Per2m/m→WT, 1543±652/mm2; WT→WT, 2542±523/mm2; n=6; P<0.05) (Figure 6C). These findings suggest that mutation of Per2 in BM-derived cells impairs ischemia-induced neovascularization.
Figure 6.
Per2 in BM-derived cells mediates ischemia-induced neovascularization and restoration of blood flow recovery in Per2m/m mice. A, Decreased neovascularization after ischemia in WT mice transplanted with Per2m/m BM. Quantitative analysis of the ischemic-to-nonischemic laser Doppler blood flow ratio in WT→WT BMT or Per2m/m→WT BMT mice (n=6 in each group). *P<0.05 vs WT→WT BMT mice. B, Representative laser Doppler blood flow images of WT→WT and Per2m/m→WT BMT mice at 28 days after induction of hind-limb ischemia (n=6 in each group). C, Microcapillary density analysis in ischemic adductor muscle tissue of WT→WT and Per2m/m→WT BMT mice at 28 days (n=6 in each group). Capillary density was expressed as the number of capillaries stained with Isolectin IB4 per 1 mm2. *P<0.05 vs WT→WT BMT mice. D, Decreased neovascularization after ischemia in Per2m/m mice infused with Per2m/m EPCs. Quantitative analysis of the ischemic-to-nonischemic laser Doppler blood flow ratio in WT→Per2m/m or Per2m/m mice (Per2m/m→Per2m/m) (n=6 in each group). *P<0.05 vs WT→Per2m/m EPC mice. E, Representative LDBF images of WT→Per2m/m and Per2m/m→Per2m/m EPC mice at 28 days after induction of hind-limb ischemia. F, Microcapillary density analysis in ischemic adductor muscle tissue of WT→Per2m/m and Per2m/m→Per2m/m EPC mice at 28 days. *P<0.05 vs WT→Per2m/m BMT mice; n=6.
To determine whether infusion of WT EPCs rather than BMT can restore the angiogenic response and prevent the autoamputation observed in Per2m/m mice after hind-limb ischemia, we infused EPCs (2×105 cells) from either WT or Per2m/m mice into recipient Per2m/m mice and then monitored these mice (WT→Per2m/m and Per2m/m→Per2m/m mice) for blood flow recovery and autoamputation. Surprisingly, the autoamputation phenotype was completely rescued in both groups of mice (Figure 6D and 6E). However, consistent with our findings, the Per2m/m mice receiving EPCs from Per2m/m mice showed less blood flow recovery compared with mice receiving EPCs from WT mice (Per2m/m→Per2m/m, 34±6%; WT→Per2m/m, 61±15%; n=6; P<0.05). Quantitative histological analysis of neovascularization revealed that capillary density was lower in the ischemic adductor muscle of Per2m/m mice receiving EPCs from Per2m/m mice compared with similar mice receiving EPCs from WT mice (Per2m/m→Per2m/m, 1298±118/mm2; WT→Per2m/m, 1762±661/mm2; n=6; P<0.05) (Figure 6F). Taken together, our findings indicate that the autoamputation observed in Per2m/m mice could be rescued by intravenous infusion of EPCs from either WT or Per2m/m mice but that, in contrast to EPCs from Per2m/m mice, only EPCs from WT mice have the ability to restore ischemia-induced neovascularization and blood flow recovery in Per2m/m mice. These findings strongly suggest that Per2 in BM-derived cells, particularly in EPCs, mediates ischemia-induced revascularization.
Inhibition of Akt Signaling Rescues the Impairment of Cellular Function in Per2m/m Mice
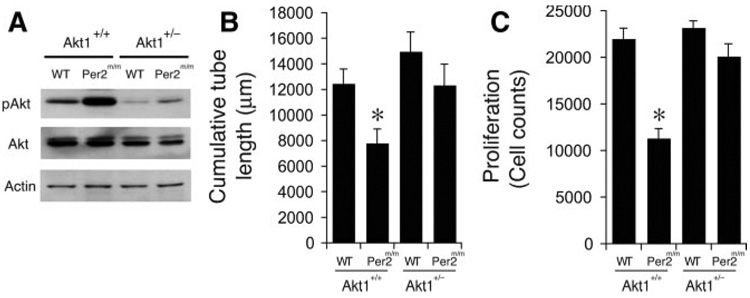
Next, we examined whether inhibition or knockdown of excessive Akt signaling could restore and improve endothelial function such as proliferation or vascular network formation in Per2m/m mice. Akt1 is the predominant isoform in endothelium, and Akt1 haploinsufficiency leads to less Akt activation.29 Because Akt1 also mediates tumor and ischemic angiogenesis,30 we investigated the role of Akt1 in Per2m/m mice by crossing Per2m/m mice with Akt1−/− mice to generate Per2m/m/Akt1+/− mice. Consistent with previous reports, we found that Akt1+/− mice have less pAkt (Figure 7A). Despite less Akt activity, there were no significant differences in endothelial cell proliferation or vascular network formation between Akt1+/+ and Akt1+/− mice (Figure 7B and 7C). Haploinsufficiency of Akt1, however, restored the impaired vascular network formation and endothelial cell proliferation in Per2m/m mice (Figure 7B and 7C). These findings suggest that excessive Akt activation in Per2m/m mice is probably responsible for the impaired vascular function in these mice.
Figure 7.
Inhibition of Akt signaling rescues the impairment of cellular function in Per2m/m mice. A, Decreased pAkt level in Akt1+/− mice (n=4). B, Quantitative assessment of vascular network formation (tube length) (n=9). C, The decrease in cell proliferation in Per2m/m mice is rescued when Per2m/m mice are crossed onto Akt1+/− background. (n=10). *P<0.05 compared to WT.
Discussion
An interesting and important finding of this study is that Per2 is a critical mediator of vascular senescence, angiogenesis, and EPC function. This finding is in agreement with previous studies showing that Per2 is an important mediator of endothelial function because mutation of Per2 impairs NO release and vascular relaxation.6 However, the mechanism and pathophysiological consequences of endothelial dysfunction in Per2m/m mice were not known. In this study, we provide a potential underlying mechanism by showing that Per2 mutation leads to premature vascular senescence, which correlates with impaired EPC function and angiogenesis. The association of disruption or mutation of the circadian rhythm with senescence or aging remains controversial, despite evidence suggesting that the robustness and integrity of the circadian rhythm decrease with age.31 For example, BMAL1 knockout mice exhibit decreased lifespan, sarcopenia, cataracts, and other aging symptoms.12 It is not known if the circadian rhythm itself is important in the aging process or if a specific circadian gene is responsible for the aging phenotype. In support for gene specificity rather than general circadian rhythm disturbance is the observation that Cry1/Cry2 double-knockout mice, which have a disturbance in the circadian rhythm, do not exhibit the vascular senescence phenotype that Per2m/m mice do (C.-Y.W. and J.K.L., unpublished observation).
Decreased replicative capacity refers to a condition when cells lose their ability to replicate after a finite number of cell divisions. Reduced cellular replicative capacity is a marker of senescence and correlates with aging. Although a change in cellular replicative capacity often is associated with aging or senescence, it also is observed sometimes in tumor suppression.26,32,33 In this study, we have shown that endothelial cell senescence as defined by decreased replicative capacity is associated with impaired vascular function and angiogenesis.
Our findings indicate that vascular senescence and dysfunction are associated with chronic upregulation of Akt signaling. Overactivity of Akt has been linked to increased vascular senescence through increased reactive oxygen species generation and decreased NO bioavailability.34 Indeed, excessive Akt activation and impaired angiogenesis are observed in VEGF receptor-1 (VEGFR-1 or flt-1) haploinsufficient mice as a result of unmitigated VEGFR-2 (flk-1/KDR) signaling.35 We find that mutation of Per2 leads to greater Akt phosphorylation via the PI3K-dependent pathway and upregulation of PDK1. PDK1 is an important regulator of insulin and growth factor–stimulated protein kinases such as Akt,36 p70 ribosomal S6 kinase,37 and p90 ribosomal S6 kinase.38 PDK1 also is important in regulating murine development and multiple cellular functions.39 A potential downstream target of PDK1/Akt that may mediate senescence is FOXO. For example, the insulin/PI3K/Akt/FOXO pathway has been shown to alter the lifespans of organisms from worms to mice,40,41 and insulin signaling has been shown to regulate circadian rhythm through FOXO.42 Thus, insulin signaling, aging, and circadian rhythm may be linked through chronic, excessive PDK1/Akt activation. However, further studies are required to determine how Per2 regulates Akt activation through PI3K/PDK1.
We found that mutation of Per2 leads to decreased EPC levels in response to ischemia or VEGF. However, the ability of EPCs from Per2m/m mice to proliferate in vitro was not different compared with that of EPCs from WT mice. These findings suggest that the defect in EPC function in Per2m/m mice is due to EPC mobilization rather than proliferation. This is in agreement with previous studies showing that NO is required for EPC mobilization43 and that NO bioavailability is impaired in Per2m/m mice.6 Furthermore, several studies suggest that the circadian rhythm affects stem cell function.44,45 For example, hematopoiesis and phagocytosis by BM-derived cells have circadian variations.46,47 Thus, disturbances or disruption in circadian genes is likely to affect the function of BM-derived cells, including EPCs. Because Per2 mutation leads to both endothelial senescence and decreased EPC mobilization, both phenotypes may contribute to the autoamputation observed in Per2m/m mice after hind-limb ischemia. Indeed, blood flow recovery after hind-limb ischemia in WT→Per2m/m BMT mice was still substantially lower than that of WT→WT BMT mice (ischemic-to-nonischemic limb ratio, 0.35 versus 0.62), suggesting that impaired intrinsic vascular function, in addition to impaired EPC function, contributes importantly to the altered angiogenic response and autoamputation in Per2m/m mice.
We find that alteration of the circadian rhythm leads to vascular senescence. Although Per2 mutation leads to increased Akt signaling and excessive Akt activation is linked to increased vascular senescence by oxidative stress,34 Akt may not be the only factor that mediates the senescence phenotype. Because Per2m/m mice exhibit a wide array of phenotypic abnormalities, other factors that are downstream of Per2 may cooperate with Akt in causing replicative senescence. These factors may include genes that regulate the cell cycle such as c-myc, p27Kip1, Cyclin D1, and Cyclin A.16 In particular, p27Kip1 is important in cell cycle control, and disruption of the cyclin-dependent kinase-inhibitory domain of p27 enhances growth of mice.48 Disruption of p27 increases cell proliferation, cell cycle progression,49 and senescence, which is compatible to our findings. As mentioned, the decrease in NO bioavailability in Per2m/m mice6 also may contribute to senescence, although senescence itself may ultimately lead to decreased NO bioavailability and endothelial dysfunction. Regardless of the precise mechanism by which mutation of Per2 causes vascular senescence, Per2m/m mice have circadian rhythm disturbances and have impaired EPC function and angiogenic response to limb ischemia. These findings suggest that polymorphisms or alteration of circadian genes may be linked to cardiovascular disease. It remains to be determined, however, whether restoration of circadian gene function could ameliorate age-related vascular diseases.
CLINICAL PERSPECTIVE
Alteration of the circadian rhythm is associated with increased incidence of pathological diseases such as sleep disorders, cardiovascular diseases, metabolic syndromes, and cancers. However, the mechanism by which alteration of the circadian rhythm leads to impaired vascular function is not known. In this study, we showed that mutation of the circadian gene, Per2, in mice leads to increased vascular senescence or aging through excessive activation of protein kinase Akt. This was associated with decreased endothelial cell proliferation and impaired vascular network formation. Indeed, compared with normal or wild-type mice, Per2 mutant (Per2m/m) mice exhibit defective angiogenic response to distal limb ischemia as a result of endothelial dysfunction and impaired mobilization of endothelial progenitor cells. When Per2m/m mice were injected with bone marrow–derived endothelial progenitor cells or mated with haploinsufficient Akt1+/− mutant mice to reduce excessive Akt activation, the impaired angiogenic response, endothelial cell proliferation, and vascular network formation were completely rescued or restored. These findings suggest that polymorphisms or alteration of circadian genes may be linked to cardiovascular disease through increased vascular senescence, endothelial dysfunction, and impaired bone marrow stem cell function. Thus, restoration of circadian gene function and prevention of excessive Akt activation may lead to mitigation of age-related vascular diseases.
Acknowledgments
Sources of Funding
This work was supported in part by grants from the National Institutes of Health (HL052233 and HL080187 to Dr Liao) and Chang Gung Memorial Hospital (CMRPG34016 and CMRPG370811 to Dr Wang).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
The online-only Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.108.790469/DC1.
Disclosures
None.
References
- 1.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–455. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerstedt T, Knutsson A, Alfredsson L, Theorell T. Shift work and cardiovascular disease. Scand J Work Environ Health. 1984;10:409–414. doi: 10.5271/sjweh.2302. [DOI] [PubMed] [Google Scholar]
- 3.Knutsson A, Boggild H. Shiftwork and cardiovascular disease: review of disease mechanisms. Rev Environ Health. 2000;15:359–372. doi: 10.1515/reveh.2000.15.4.359. [DOI] [PubMed] [Google Scholar]
- 4.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik M, Farrell T, Camm AJ. Circadian rhythm of heart rate variability after acute myocardial infarction and its influence on the prognostic value of heart rate variability. Am J Cardiol. 1990;66:1049–1054. doi: 10.1016/0002-9149(90)90503-s. [DOI] [PubMed] [Google Scholar]
- 6.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 7.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 8.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 9.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–1705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 10.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 11.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee CC. Tumor suppression by the mammalian Period genes. Cancer Causes Control. 2006;17:525–530. doi: 10.1007/s10552-005-9003-8. [DOI] [PubMed] [Google Scholar]
- 16.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner C, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 18.Erusalimsky JD, Fenton M. Further in vivo evidence that cellular senescence is implicated in vascular pathophysiology. Circulation. 2002;106:e144. doi: 10.1161/01.cir.0000037130.06003.11. [DOI] [PubMed] [Google Scholar]
- 19.Minamino T, Yoshida T, Tateno K, Miyauchi H, Zou Y, Toko H, Komuro I. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation. 2003;108:2264–2269. doi: 10.1161/01.CIR.0000093274.82929.22. [DOI] [PubMed] [Google Scholar]
- 20.Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 21.Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi H, Orimo M, Okada S, Komuro I. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res. 2006;98:532–539. doi: 10.1161/01.RES.0000204504.25798.a8. [DOI] [PubMed] [Google Scholar]
- 22.Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation. 2004;110:856–861. doi: 10.1161/01.CIR.0000138743.09012.93. [DOI] [PubMed] [Google Scholar]
- 23.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 27.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 29.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 31.Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 34.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishi JI, Minamino T, Miyauchi H, Nojima A, Tateno K, Okada S, Orimo M, Moriya J, Fong GH, Sunagawa K, Shibuya M, Komuro I. Vascular endothelial growth factor receptor-1 regulates postnatal angiogenesis through inhibition of the excessive activation of Akt. Circ Res. 2008;103:261–268. doi: 10.1161/CIRCRESAHA.108.174128. [DOI] [PubMed] [Google Scholar]
- 36.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 37.Avruch J, Belham C, Weng Q, Hara K, Yonezawa K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog Mol Subcell Biol. 2001;26:115–154. doi: 10.1007/978-3-642-56688-2_5. [DOI] [PubMed] [Google Scholar]
- 38.Frodin M, Jensen CJ, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 41.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X, Yang Z, Yue Z, Alvarez JD, Sehgal A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc Natl Acad Sci U S A. 2007;104:15899–15904. doi: 10.1073/pnas.0701599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 44.Tsinkalovsky O, Smaaland R, Rosenlund B, Sothern RB, Hirt A, Steine S, Badiee A, Abrahamsen JF, Eiken HG, Laerum OD. Circadian variations in clock gene expression of human bone marrow CD34+ cells. J Biol Rhythms. 2007;22:140–150. doi: 10.1177/0748730406299078. [DOI] [PubMed] [Google Scholar]
- 45.Tsinkalovsky O, Filipski E, Rosenlund B, Sothern RB, Eiken HG, Wu MW, Claustrat B, Bayer J, Levi F, Laerum OD. Circadian expression of clock genes in purified hematopoietic stem cells is developmentally regulated in mouse bone marrow. Exp Hematol. 2006;34:1249–1261. doi: 10.1016/j.exphem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Smaaland R, Sothern RB, Laerum OD, Abrahamsen JF. Rhythms in human bone marrow and blood cells. Chronobiol Int. 2002;19:101–127. doi: 10.1081/cbi-120002594. [DOI] [PubMed] [Google Scholar]
- 47.D’Hondt L, McAuliffe C, Damon J, Reilly J, Carlson J, Dooner M, Colvin G, Lambert JF, Hsieh CC, Habibian H, Stencel K, Quesenberry PJ. Circadian variations of bone marrow engraftability. J Cell Physiol. 2004;200:63–70. doi: 10.1002/jcp.20032. [DOI] [PubMed] [Google Scholar]
- 48.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 49.Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]