Abstract
Severe pain syndromes reduce quality of life in patients with inflammatory and neoplastic diseases, partly because reduced analgesic effectiveness with chronic opiate therapy (i.e., tolerance) leads to escalating doses and distressing side effects. Peroxynitrite mediated nitroxidative stress in the dorsal horn of the spinal cord plays a critical role in the induction and development of antinociceptive tolerance to morphine. This provides a valid pharmacological basis for developing peroxynitrite scavengers as potent adjuncts to opiates in the management of pain. The cationic Mn(III) ortho N-alkylpyridylporphyrins, MnTE-2-PyP5+ and MnTnHex-2-PyP5+, are among the most potent peroxynitrite scavengers with nearly identical scavenging rate constants (≫107 M−1 s−1). Yet, MnTnHex-2-PyP5+ is significantly more lipophilic, more bioavailable, and in turn, was 30-fold more effective in blocking the development of morphine antinociceptive tolerance than MnTE-2-PyP5+ using the hot plate test in a well characterized murine model. The hydrophilic MnTE-2-PyP5+ and the lipophilic MnTnHex-2-PyP5+ were 10-fold and 300-fold, respectively, more effective in inhibiting morphine tolerance than hydrophilic Fe(III) porphyrin, FeTM-4-PyP5+. Both Mn porphyrins decreased levels of TNF-α, IL-1β, and IL-6 to normal values. Neither of them affected acute morphine antinociceptive effect nor caused motor function impairment. Also neither was able to reverse already established morphine tolerance. We have recently shown that anionic porphyrin, Mn(III) tetrakis(4-carboxylatophenyl)porphyrin, MnTBAP is selective in removing ONOO− over O2•−, yet at ~ 2 orders of magnitude lower efficacy than MnTE-2-PyP5+ and MnTnHex-2-PyP5+, which in turn parallels up to 100-fold lower ability to reverse morphine tolerance. These data (1) support the role of peroxynitrite rather than superoxide as a major mechanism in blocking the development of morphine tolerance, and (2) show that lipophilicity is a critical parameter in enhancing the potency of such novel peroxynitrite scavengers.
Keywords: pain management, morphine tolerance, peroxynitrite, nitrotyrosine, Mn porphyrins, MnTBAP, MnTE-2-PyP, MnTnHex-2-PyP, peroxynitrite scavenging, cytokines
Introduction
Chronic, severe pain is a significant health problem [1]. One third of Americans suffer from some form of chronic pain, and in over 30% it is resistant to analgesic therapy [1]. The economic impact of pain is equally large at approximately $100 billion annually [1]. Opiate/narcotic analgesics, typified by morphine sulfate, are the most effective treatments for acute and chronic severe pain but their clinical utility is often hampered by the development of analgesic tolerance as well as by de novo painful hypersensitivity to innocuous and noxious stimuli, phenomena observed in both animal and human studies [2–4]. With respect to morphine in particular, tolerance necessitates escalating doses to achieve equivalent pain relief [5], even as morphine-induced hypersensitivity subverts the therapeutic impact of such dose increases [2–4]. This complex pathophysiological cycle contributes to decreased quality of life in the growing population of subjects with chronic pain because of oversedation, reduced physical activity, respiratory depression, constipation, potential for addiction, and other side-effects [5]. Accordingly, there is major interest in new approaches to maintain opiate efficacy during repetitive dosing for chronic pain, without engendering tolerance or unacceptable side-effects.
The mechanisms by which prolonged opiate exposure induces tolerance and hypersensitivity remain unclear, although a role for peroxynitrite (ONOO−), the product of the interaction between superoxide (O2•−) and nitric oxide (•NO) has been demonstrated [6]. To this end, repeated administration of morphine in mice promotes the nitration and enzymatic inactivation of spinal manganese superoxide dismutase (MnSOD) which provides a critical source of spinal ONOO− in turn contributing to the development of morphine antinociceptive tolerance through three well defined biochemical pathways within the dorsal horn of the spinal cord: (1) post-translational nitration of proteins involved in glutamate homeostasis, (2) neuroimmune activation (release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6)), and (3) apoptosis [6].
Due to the increasing importance of oxidative stress in a number of diseases, redox able compounds have been actively thought such as Mn cyclic polyamines [7–9], Mn salen derivatives [10], nitroxides [11], mitochondrially targeted drugs such as MitoQ [12] and others. Among them metalloporphyrins have the highest rate constants for scavenging O2•− and ONOO− and were shown to ameliorate all conditions that have oxidative stress in origin such as diabetes, cancer, radiation injury, central nervous system injuries, including morphine antinociceptive tolerance [6,13–22]. Incidentally, the most potent ONOO− scavengers and SOD mimics reported so far are cationic Mn(III) N-alkylpyridylporphyrins (Figure 1) [23–26]. Originally metalloporphyrins, both Fe and Mn ones have been developed as catalysts for O2•− dismutation based on structure-activity relationship between the kcat for O2•− dismutation and redox property of the metal site [23–25]. It was later shown that the ONOO− reduction by metalloporphyrin is governed by the similar relationships as O2•− dismutation, as the ability of the metalloporphyrin to reduce ONOO− parallels their ability to catalyze the O2•− dismutation [27,28]. Given that •NO reacts with O2•− to yield ONOO− very rapidly with rate constant higher (2 × 1010 M−1s−1 [29]) than that of the self (log k ~5.7 [30]) or enzymatic (log kcat ~ 8.84–9.3 [31–36]) dismutation of O2•−, it is, thus, likely that in the presence of •NO the predominant mode of action of metalloporphyrins in vivo will be the decomposition of ONOO− rather than dismutation of O2•−. Importantly, scavenging ONOO− in vivo is coupled with cellular reductants and is thus catalytic in nature [37]. Of note, Mn porphyrins were also found to be able to efficiently remove CO3•−, which is the degradation product of the ONOO− adduct with CO2 [27]. Finally, by removing reactive species cationic ortho Mn(III) N-alkylpyridylporphyrins have been shown to suppress not only the primary oxidative event but also the cellular transcriptional activity (inhibiting HIF-1α, NF-κB, AP-1) [13, 22–26, 38–43] and thereby the secondary inflammatory and immune responses and levels of related cytokines as well.
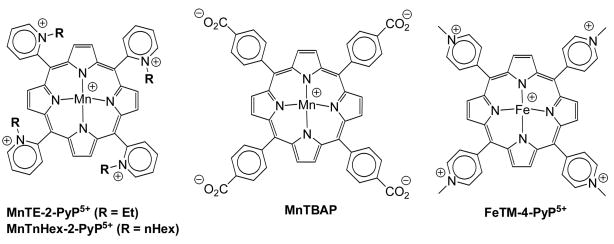
Figure 1.
Structures of the complexes relevant to this study: MnTE-2-PyP5+, MnTnHex-2-PyP5+, MnTBAP and FeTM-4-PyP5+.
Salvemini et al [6] have already shown that Fe(III) cationic para N-methylpyridylporphyrin, FeTM-4-PyP5+ (Figure 1) is able to block the development of morphine antinociceptive tolerance. Yet, the use of Fe porphyrins has limitations due to the high likelihood of Fenton-related toxicity, as well as due to their lower ability to reduce ONOO− (Table 1). Further, their richer axial coordination chemistry in comparison with Mn porphyrins enables them to exert more efficiently other in vivo functions, such as cyt P450-like actions [44].
Table 1.
The catalytic rate constant for the dismutation of O2•− and ONOO− decomposition at pH 7.4 and 37°C and the therapeutic dose needed to reverse morphine tolerance at ≥ 80%. Morphine was given ip chronically for 4 days along with compounds.
Another critical parameter for the in vivo efficacy of Mn porphyrins is their bioavailability [21,45]. We have developed more lipophilic porphyrins while maintaining the same antioxidant potential, which is defined by the catalytic rate constants [44,46]. A convenient strategy was to extend the alkyl side chain length to increase lipophilicity, while keeping the positively charged nitrogens in ortho positions of the meso pyridyl groups to maintain the thermodynamic and electrostatic facilitations [24–26,46]. MnTnHex-2-PyP5+ (Figure 1), a hexyl analogue of the lead compound MnTE-2-PyP5+ was found to be considerably more lipophilic than MnTE-2-PyP5+ [46] and ~30-fold more effective in protecting aerobic growth of SOD-deficient E. coli [45]. The effect parallels the significantly increased uptake of MnTnHex-2-PyP5+ by E. coli [45]. MnTnHex-2-PyP5+ was also up to 120-fold more efficacious in other models of oxidative stress injuries [21,47; Spasojević et al., 2008, unpublished; Batinić-Haberle et al., 2007, unpublished; Crow and Batinić-Haberle, 2006, unpublished]. In a stroke model our most recent data indicate an enhanced distribution of MnTnHex-2-PyP5+ in central nervous system; the blood to brain partition ratio is 1:8 with MnTnHex-2-PyP5+ and 1:100 with MnTE-2-PyP5+ [Spasojević et al., 2008, unpublished]. We thus hypothesized that MnTnHex-2-PyP5+ would be superior to the less lipophilic porphyrins in blocking the development of morphine antinociceptive tolerance. This study aimed at assessing the impact of lipophilicity on the efficacy of Mn porphyrins in blocking the development of morphine antinociceptive tolerance.
Materials and methods
Mn porphyrins
MnTE-2-PyP5+ and MnTnHex-2-PyP5+ were synthesized and characterized as previously described [23,46,48].
Induction of morphine-induced antinociceptive tolerance in mice
Male CD-1 mice (24–30g, Charles River) were housed and cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the St. Louis University Health Science Center and in accordance with the National Institute of Health Guidelines on Laboratory Animal and the University of Messina, in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with the EEC regulations (O.J. of E.C. L 358/1 12/18/1986). Mice were housed 5–7 per cage and maintained under identical conditions of temperature (21±1°C), humidity (60±5%) with a 12-hr light-dark cycle and allowed food ad libitum. Nociceptive/pain thresholds were determined by measuring latencies (in seconds, s) of mice placed in a transparent glass cylinder on a hot plate (Ugo Basile, Italy) maintained at 52°C. Responses indicative of nociception included intermittent lifting and/or licking of the hindpaws or escape behavior. Determination of antinociception/pain relief effects was assessed between 7:00 and 10:00 AM. All injections were given by intraperitoneal (ip) or subcutaneous (sc) means in a 0.1 mL volume at approximately 7 AM and 4 PM. Drugs or their vehicle (saline) were given before each dose of morphine. Hot plate latencies were taken in mice from all groups on day five before (baseline latency) and 40 min after an acute dose of morphine (0.3–3 mg/kg) or its vehicle (saline) (response latency). Baseline values from all groups as measured on day five before injection of the acute dose of morphine or its vehicle, were statistically insignificant and ranged between 6–8 s. Results are expressed as percent maximal possible antinociceptive effect (% MPE) calculated as follows: (response latency − baseline latency)/(cut off latency − baseline latency) × 100. A cut-off latency of 20 s was employed to prevent tissue damage. Six to twelve mice per group were used and all experiments were conducted with the experimenters blinded to treatment conditions. Unless specified, all drugs were purchased from Sigma. The following experimental groups were used.
Naïve group
In this group, mice were injected twice a day with an ip injection of saline (the vehicle used to deliver the drugs used in this study over four days) and an sc injection of saline (vehicle used to deliver morphine over four days). On day five, mice received an ip injection of saline followed fifteen minutes later by an sc injection of saline.
Naïve + drug group
In this group, mice were injected twice a day with an ip injection of the highest dose of MnTE-2-PyP5+ (3 mg/kg/day) or MnTnHex-2-PyP5+ (0.1 mg/kg/day) and sc injection of saline. On day five, mice received an ip injection of MnTE-2-PyP5+ (1.5 mg/kg/day) or MnTnHex-2-PyP5+ (0.05 mg/kg/day) followed fifteen minutes later by an sc injection of saline.
Vehicle group
In this group, mice were injected twice a day with an ip injection of saline and an sc injection of saline. On day five, mice received an ip injection of saline followed fifteen minutes later by an sc injection of acute morphine eliciting near-to-maximal antinociception (3 mg/kg).
Vehicle + drugs group
In this group, mice were injected twice a day with an ip injection of the highest dose of MnTE-2-PyP5+ (3 mg/kg/day) or MnTnHex-2-PyP5+ (0.1 mg/kg/day) and an sc injection of saline. On day five, mice received an ip injection of MnTE-2-PyP5+ (1.5 mg/kg/day) or MnTnHex-2-PyP5+ (0.05 mg/kg/day) followed fifteen minutes later by sc doses of acute morphine giving between 10 and 95% antinociceptive responses within 40 minutes of administration (0.1–3 mg/kg).
Morphine (Mor) group
In this group, mice were injected twice a day with an ip injection of saline and sc injection of morphine (20 mg/kg/day). On day five, mice received an ip injection of saline followed fifteen minutes later by an sc dose of acute morphine (3 mg/kg).
Morphine + drugs group
In this group, mice were injected twice a day with an ip injection of varying doses of MnTE-2-PyP5+ (0.3–3 mg/kg/day) or MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day) followed by the sc injection of morphine (20 mg/kg/day). On day five, mice received an ip dose of MnTE-2-PyP5+ (1.5 mg/kg/day) or MnTnHex-2-PyP5+ (0.05 mg/kg/day) followed fifteen minutes later by the sc dose of acute morphine (3 mg/kg).
In another set of experiments, and in order to address whether MnTE-2-PyP5+ or MnTnHex-2-PyP5+ reverse the expression of tolerance, mice were treated twice a day with morphine as described above and on day five they received a single intraperitoneal dose of MnTE-2-PyP5+ (3 mg/kg/day) or MnTnHex-2-PyP5+ (0.1 mg/kg/day) followed fifteen minute later by the acute dose of morphine (3 mg/kg).
On day five and after the behavioral tests, spinal cord tissues from the lumbar enlargement segment of the spinal cord (L4-L6) and dorsal horn tissues were removed and tissues processed for immunohistochemical, Western blot and biochemical analysis.
Rotarod test
Mice (n=4 per group) were trained before experimentation for their ability to remain for 120 sec on a revolving Rotarod apparatus (accelerating units increase from 3.5 to 35 rpm in 5 min). Mice were injected with an intraperitoneal injection of the highest dose of MnTE-2-PyP5+ or MnTnHex-2-PyP5+ (3 mg/kg and 0.1 mg/kg respectively) found to fully block antinociceptive tolerance or its vehicle (saline) and subsequently tested and examined for motor impairments on the Rotarod at 15, 30 and 60 minutes after drug administration. The latency time to fall off the Rotarod was determined (cut-off time used was 120 seconds).
Cytokines
The TNF-α, IL-1β and IL-6 were measured by ELISA using commercially available kits as described previously [49].
Statistics
For paired group analysis Student’s t-tests were performed. For paired multiple groups, analyses of variance followed by Student-Newman-Keuls test were employed to analyze the data. Results are expressed as mean±sem for n animals. A statistically significant difference was defined as a P value <0.05.
Results
The development of morphine-induced tolerance is blocked by Mn porphyrin-based peroxynitrite decomposition catalysts, MnTE-2-PyP5+ and MnTnHex-2-PyP5+
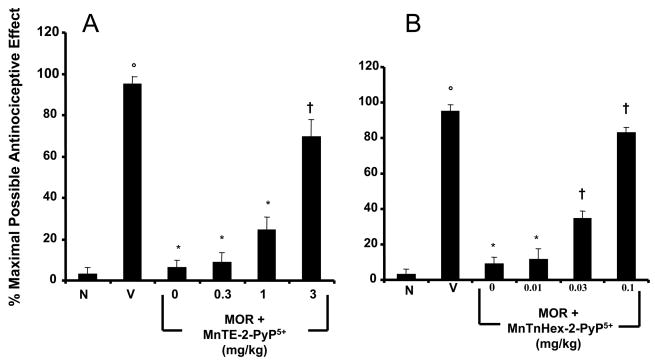
When compared to animals receiving an equivalent injection of its vehicle (naïve group), acute injection of morphine (3 mg/kg) in animals that received saline over four days (vehicle group) produced a significant and near-to-maximal antinociceptive response [percent maximal possible antinociceptive effect (%MPE) ranging between 90–95%] (Fig. 2A, B). On the other hand, when compared to the antinociceptive response to acute morphine in animals that received saline over four days, repeated administration of morphine over the same time course (Morphine group) led to the development of antinociceptive tolerance as evidenced by a significant loss of its antinociceptive response (Fig. 2A, B). Baseline latencies in groups treated with saline or morphine over four days before acute administration of morphine on day five were statistically insignificant from each other and ranged between 6–8 seconds (n=6).
Figure 2.
Inhibition of morphine antinociceptive tolerance with MnTE-2-PyP5+ or MnTnHex-2-PyP5+. On day five, acute injection of morphine (3 mg/kg) in animals that received saline over four days produced a significant antinociceptive response when compared to responses observed in animals that received an equivalent volume of saline (A,B, Vehicle, V vs Naïve, N). On the other hand, a significant loss to the antinociceptive effect of the acute injection of morphine was observed in animals that received repeated administration of morphine over four days (Morphine group) (A,B). Co-administration of morphine over four days with MnTE-2-PyP5+ (0.3–3 mg/kg/day, n=6; A) or MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day, n=6; B) inhibited the development of tolerance in a dose-dependent manner. Results are expressed as mean ± s.e.m for six animals. °P<0.001 for Vehicle, V vs Naïve, N; *P<0.001 for Morphine alone vs Vehicle; †P<0.001 for Morphine + drug treated vs Morphine alone.
Co-administration of morphine with MnTE-2-PyP5+ (Fig. 2A) or MnTnHex-2-PyP5+ (Fig. 2B) inhibited in a dose-dependent manner, the development of antinociceptive tolerance to approximately the same degree. Given that MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day, n=6) was administered at ~30-fold lower doses than MnTE-2-PyP5+ (0.3–3 mg/kg/day, n=6), this shows that the hexyl analogue is ~30-fold more potent than the ethyl analogue. Although both drugs have nearly identical intrinsic catalytic activity in decomposing ONOO− (rate constant of log k = 7.53 and 7.11 for MnTE-2-PyP5+ and MnTnHex-2-PyP5+) (Table 1), the effective increase in potency of MnTnHex-2-PyP5+ in vivo is most likely due to its higher lipophilicity (i.e., relative accumulation) when compared to MnTE-2-PyP5+. This behavior is reminiscent of the relative bioavailability of these compounds to E. coli [45]. The remarkable difference in the lipophilicity of ethyl and hexyl analogues was determined by TLC [46].
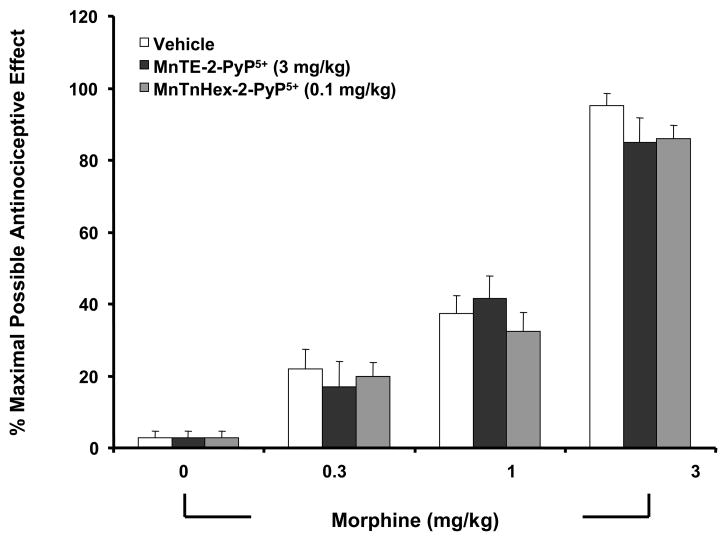
When tested alone at the highest dose used, MnTE-2-PyP5+ (3 mg/kg/day, n=6) or MnTnHex-2-PyP5+ (0.1 mg/kg/day, n=6) had no antinociceptive effects. Thus, on day five, hot plate latencies following a subcutaneous (sc) injection of saline in animals that received saline over four days or in animals that received MnTE-2-PyP5+ or MnTnHex-2-PyP5+ were statistically insignificant and ranged between 6–7 s (n=6, not shown). The inhibitory effects of MnTE-2-PyP5+ or MnTnHex-2-PyP5+ were not attributable to acute antinociceptive interactions between MnTE-2-PyP5+ or MnTnHex-2-PyP5+ and acute morphine since the response to acute morphine given at three different doses (0.3–3 mg/kg, n=6) in animals treated with the highest dose of MnTE-2-PyP5+ or MnTnHex-2-PyP5+ (3 and 0.1 mg/kg/day respectively, n=6) or their vehicle over four days was statistically insignificant (Fig. 3).
Figure 3.
On day five, acute injection of different doses of morphine (0.3–3 mg/kg) in animals that received saline over four days produced a dose-dependent significant antinociceptive response when compared to responses obtained in animals receiving an equivalent volume of its vehicle. The antinociceptive response to morphine was not altered in animals that were treated over four days with MnTE-2-PyP5+ or MnTnHex-2-PyP5+ (3 and 0.1 mg/kg/day respectively, n=6) indicating lack of acute interaction between morphine and these compounds. Results are expressed as mean ± s.e.m for six animals. *P<0.001 for the vehicle group when compared to values obtained in the absence of morphine.
MnTE-2-PyP5+ or MnTnHex-2-PyP5+ do not reverse established morphine tolerance
Loss of the antinociceptive effect of morphine observed on day 5 in animals that received repeated administration of morphine over 4 days was not restored by a single administration of MnTE-2-PyP5+ (3 mg/kg, n=4) or MnTnHex-2-PyP5+ (1 mg/kg, n=4) given by ip injection 15 min before the acute dose of morphine (3 mg/kg). Thus, the %MPE was 95±2%, 8±2%, 10±3%, 7±2% mean±sem for the vehicle, morphine, morphine+ MnTE-2-PyP5+ and morphine+ MnTnHex-2-PyP5+ groups respectively (P<0.5 for all). Such results suggest that these pharmacological agents inhibit the development and not the expression of tolerance.
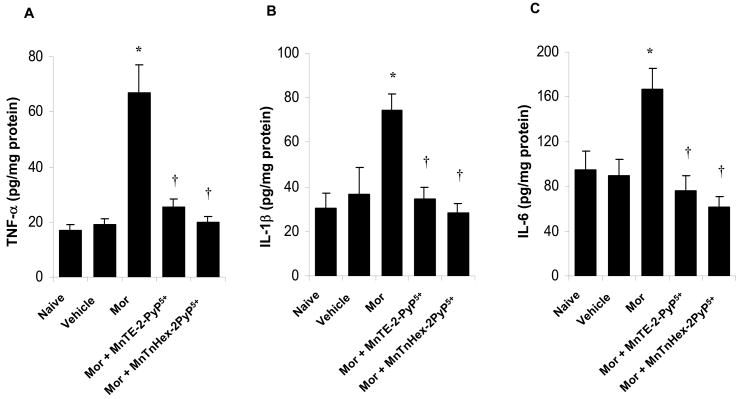
The development of morphine-induced tolerance is associated with increased cytokine formation which is inhibited by MnTE-2-PyP5+ or MnTnHex-2-PyP5+
On day five, and when compared to animals receiving an injection of saline (naïve group), acute injection of morphine (3 mg/kg, n=6) in animals that received saline over four days (vehicle group) did not increase dorsal horn tissue levels of TNF-α, IL-1β and IL-6 (Fig. 4A–C). On the other hand, acute injection of morphine in animals that received repeated administration of morphine (Morphine group) led to a significant increase in TNF-α, IL-1β and IL-6 (n=6) (Fig. 4A–C) in dorsal horn tissues. This increase was attenuated by co-administration of morphine over four days with MnTE-2-PyP5+ (3 mg/kg/day, n=6) and MnTnHex-2-PyP5+ (0.1 mg/kg/day, n=6) (Fig. 4A–C).
Figure 4.
Acute injection of morphine (3 mg/kg) on day five in animals that received saline over four days (Vehicle group) did not increase dorsal horn tissue levels of TNF-α (A), IL-1β (B) and IL-6 (C) when compared to animals that received an equivalent volume of its vehicle (naïve group). On the other hand, acute administration of morphine in animals that received repeated administration of morphine (Morphine group, Mor) led to a significant increase in TNF-α, IL-1β and IL-6 in dorsal horn tissues (A–C). This increase was attenuated by co-administration of morphine over four days with MnTE-2-PyP5+ or MnTnHex-2-PyP5+ (3 and 0.1 mg/kg/day respectively, n=6) (A–C). Results are expressed as mean ± s.e.m for 6 animals. *P<0.001 for Morphine alone vs Vehicle; †P<0.001 for Morphine + drug-treated vs Morphine alone.
Lack of effect of MnTE-2-PyP5+ or MnTnHex-2-PyP5+ on the Rotarod test
In order to establish whether these inhibitors used to block antinociceptive tolerance cause motor function impairment, mice were treated with the highest dose of MnTE-2-PyP5+ (3 mg/kg) or MnTnHex-2-PyP5+ (0.1 mg/kg, n=4) used to block antinociceptive tolerance and then tested on the Rotarod for potential motor function deficits at 15, 30 and 60 min after drug administration. When compared to the vehicle-treated group, these drugs did not show signs of Rotarod deficits over the observed time frame (120±0, 120±0 and 120±0 latency on rotarod in sec±sem for vehicle, MnTE-2-PyP5+ and MnTnHex-2-PyP5+ treated mice respectively, n=4).
Discussion
Chronic administration of morphine promotes neuroimmune activation as evidenced by activation of spinal cord glial cells, production of proinflammatory cytokines such as TNF-α, IL-1β and IL-6 and spinal sensitization [50–52]. Thus, inhibitors of glial cell metabolism and/or anti-cytokine approaches block morphine-induced antinociceptive tolerance and hyperalgesia [50–52]. The possible mechanisms for chronic morphine-induced glial cell activation are not known with certainty. The μ-opiate receptors are present on microglia and astrocytes [53] but acute administration of morphine does not activate these cells [50]. On the other hand, morphine primes glial cells for enhanced production of proinflammatory cytokines [54]. We have recently reported that formation of spinal ONOO− is critical to the development of morphine antinociceptive tolerance and that ONOO− as a signaling molecule is involved in the increased formation of TNF-α, IL-1β and IL-6 that is typically observed during tolerance [6]. A mechanism by which ONOO− leads to the generation of such proinflammatory cytokines is through activation of redox-sensitive transcription factors such as NF-κB and AP-1 as well as activation of MAPK kinases such as p38 kinase [55–57]. An iron porphyrin-based superoxide and peroxynitrite scavenger, FeTM-4-PyP5+ [58,59], inhibits the development of antinociceptive tolerance with IC50 of approximately 5 mg/kg [6].
The roles of ONOO− in nociception are emerging. We now know that direct intraplantar injection of O2•−, or ONOO− itself in rats evokes potent thermal hyperalgesia [60,61]. Removal of ONOO− with peroxynitrite scavengers blocks hyperalgesia associated with: (1) inflammation [60,62]; (2) spinal activation of the N-methyl-D-aspartate (NMDA) receptor [63]; and (3) repeated administration of morphine administration (morphine induced antinociceptive tolerance) [6–8]. In these settings, central sensitization is sustained by the nitration and enzymatic inactivation of spinal MnSOD, which provides a source for ONOO− [6,60]. In addition, ONOO− is an important component of hyperalgesia associated with arthritis [64] and O2•− is also increased in dorsal horn neurons during neuropathic pain induced by spinal nerve ligation [65] and neurogenic induced hyperalgesia induced by capsaicin [66]. Furthermore, the use of non-selective pharmacological probes such as phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL), for ameliorating nitroxidative stress in inflammatory pain [62] and neuropathic pain also have been documented [67–71]. These agents as well as other antioxidants have also been reported to be effective against trigeminal pain, fibromyalgia and temporomandibular joint dysfunction [72,73], chronic pancreatitis [74], post-irradiation of breast cancer fibrosis [75] and neurogenic hyperalgesia [66]. Collectively these data clearly point to the prominent role of nitroxidative stress in pain of many etiologies. Recognizing that the role of ONOO− and nitroxidative stress in pain has been recent, limited data are available to help us decipher the molecular and biochemical pathways engaged by ONOO− and related nitroxidative species. ONOO− contributes to peripheral and central sensitization by (1) increasing production of pro-inflammatory cytokines, (2) activating poly-ADP-ribose polymerase (3) activating and/or inducing cycloooxygenases (COX-1 and COX-2) and (4) by post-translational nitration and modification of key proteins that are themselves involved in central and peripheral sensitization such as glutamate transporters [6,60,62,63]. Additionally, species arising from nitroxidative stress may be involved more subtly in central sensitization at least in part by sensitizing wide dynamic range neurons in the dorsal horn [76].
Here we have shown that co-administration of morphine MnTE-2-PyP5+ and MnTnHex-2-PyP5+ blocked antinociceptive tolerance with the latter being approximately 30-fold more potent than the former (Fig. 2); the reminiscence of the difference in protecting E. coli. The increase in efficacy of MnTnHex-2-PyP5+ in comparison with MnTE-2-PyP5+, is most likely due to its higher lipophilicity (and thus its increased ability to cross blood brain barrier [Spasojević et al., 2008, unpublished]), as both have nearly identical catalytic activity in decomposing ONOO− (rate constant of log k = 7.53 and 7.11 for MnTE-2-PyP5+ and MnTnHex-2-PyP5+, Table 1). Neither porphyrin interferes with the ability of morphine, if given acutely, to exert antinociceptive effect (Fig. 3). Also, neither of them alone causes impairment of mouse motor function. Also neither porphyrin was able to reverse already established morphine tolerance. The inhibition of antinociceptive tolerance by both porphyrins was associated with the full normalization of the levels of inflammatory cytokines, TNF-α, IL-1β, and IL-6 (Fig. 4) at the dose where maximal antinociceptive effects was observed. Our findings confirm our previous observations suggesting that attenuation of morphine tolerance by ONOO− decomposition catalysts is secondary to the suppression of repeated morphine-induced spinal neuroimmune activation promoted by ONOO− [6].
We have already shown that MnTE-2-PyP5+ distributes into heart mitochondria at levels high enough to protect it against peroxynitrite-based damage [37,77]. MnTnHex-2-PyP5+ given at single intravenous dose of 0.05 mg/kg prevented inactivation of MnSOD in a rat renal ischemia/reperfusion model [21], which is indicative of its localization in mitochondria. We have further shown that MnTE-2-PyP5+ distributes in all organs; brain included. The accumulation in brain proceeded after its levels in all other organs started to decline, presumably being driven by the negatively charged phospholipids [78]. MnTnHex-2-PyP5+ distributes in brain at more than 10-fold higher levels than does MnTE-2-PyP5+ [Spasojević et al., 2008, unpublished] (see Introduction). Based on all above said, along with the data obtain in this study, we can safely assume that MnTnHex-2-PyP5+ distributes at a higher extent in spinal cord mitochondria than does MnTE-2-PyP5+ protecting its constituents and antioxidant systems from oxidative damage resulting from chronic morphine.
Although direct comparisons between the in vitro rate constants and the therapeutic doses (Table 1) of the cationic Mn porphyrins with the anionic MnTBAP (Figure 1) and the iron complex FeTM-4-PyP5+ are tempting, these are not as straightforward cases as the comparison within the cationic Mn porphyrin group. These three groups of porphyrins vary greatly with respect to their overall chemical reactivity, steric demands, and electrostatics, which are expect to influence significantly their in vivo recognition patterns, cellular uptake and biodistribution. Despite these obvious limitations, some very qualitative guidance for understanding the biological chemistry of metalloporphyrins in vivo and the mechanistic aspects of morphine tolerance may emerge from this study.
Salvemini et al have shown that the anionic porphyrin, MnTBAP is able to block the development of morphine antinociceptive tolerance [6]. We have recently improved the general understanding of the frequently reported beneficial effects of MnTBAP (Fig. 1) in decreasing oxidative stress-related injuries [79], which have often been wrongly assigned to its SOD-like activity. Due to the lack of both electrostatic and thermodynamic facilitation for O2•− dismutation, MnTBAP possesses no SOD activity [79]. Yet it has modest ability to decompose ONOO− (kcat = 1.04 × 105 M−1 s−1) and can thus serve for mechanistic purposes to distinguish between these two pathways [28]. The present data on the cationic Mn porphyrins and those previously reported on MnTBAP [6] show that all these compounds share a common mechanism, which is, therefore, conceivably through peroxynitrite scavenging pathways [28]. Such findings further shed some light on the nature of MnTBAP effect in vivo, support the key role of ONOO− rather than O2•− in morphine tolerance and justify the advance of potent ONOO− scavengers as adjuvant in pain treatment.
The ability of FeTM-4-PyP5+ to dismute O2•− and scavenge ONOO− is comparable to that of the cationic Mn(III) porphyrins at pH 7.8 (Table 1) [23]. Yet, it is 10 or 300-fold less efficient in the reversal of morphine tolerance than MnTE-2-PyP5+ and MnTnHex-2-PyP5+ (Table 1). The reasons for this has not been investigated in details, as Fe porphyrins may be a source of iron, which may, in turn, result in the Fenton-related production of deleterious hydroxyl radicals [80,81]. The axial coordination requirements of Fe(III) porphyrins may also impose some restrictions regarding the cell recognition and uptake, which may differ greatly from those of Mn porphyrins. Additionally, intercalation/conjugation of FeTM-4-PyP5+ to RNA/DNA in close resemblance to that observed with MnTM-4-PyP5+ [82] may result in cellular toxicity. Conversely, it is worth noting that ortho substitution in MnTE-2-PyP5+ and MnTnHex-2-PyP5+, and the peripheral negative charges in MnTBAP minimize strong interactions of these porphyrins with nucleic acids.
Concluding remarks
In summary, we have: (1) provided evidence that ONOO− rather than O2•− is a predominant player in the development of morphine antinociceptive tolerance; (2) pointed to the lipophilicity as a critical parameter that determines the efficacy of the drug in reversing morphine tolerance and decreasing level of inflammatory cytokines, TNF-α, IL-1β and IL-6. With high ability to eliminate ONOO− along with enhanced lipophilicity, MnTnHex-2-PyP5+ (0.1 mg/kg/day) is 300-fold more effective in blocking the development of morphine antinociceptive tolerance than Fe porphyrin, FeTM-4-PyP5+, 100-fold better than MnTBAP and 30-fold better than its hydrophilic ethyl analogue, MnTE-2-PyP5+. Consequently it appears to be the most promising candidate as adjuvant in the opioid-based clinical management of pain.
The potential use of novel non-narcotic peroxynitrite-targeted approaches in pain alone or in combination with opiates or non-selective COX-1/COX-2 inhibitors or selective COX-2 inhibitors [6,59,61] needs also to be pursued aggressively, as these studies will provide a more comprehensive scientific foundation for an issue of major clinical and socio-economic importance, while laying the basis for interventions with strong therapeutic potential. Of note, Mn porphyrins will be valuable in assuring pain relief with cancer patients also as they do not protect tumors; in fact they were shown to suppress tumor growth [14,15,83].
Acknowledgments
Ines Batinić-Haberle and Júlio S. Rebouças are grateful to the financial support from NIH U19 AI67798-01/pilot project and the financial support from The Wallace H. Coulter Translational Partners Grant Program. Ivan Spasojević acknowledges NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29). Daniela Salvemini is grateful to the financial support from Saint Louis University Seed Funds. Daniela Salvemini and Ines Batinić-Haberle thank the support from NIH R01 DA024074.
Abbreviations
- SOD
superoxide dismutase
- MnP
MnIIITE-2-PyP5+, Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (AEOL-10113)
- MnTnHex-2-PyP5+
Mn(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- FeTM-4-PyP5+
Fe(III) meso-tetrakis(N-methylpyridinium-4-yl)porphyrin
- MnTBAP
[MnIIITCPP]3−, Mn(III) meso-tetrakis(4-carboxyphenyl)porphyrin (also [MnIIITBAP]3−)
- HIF-1α
hypoxia inducible factor-1, NF-κB, nuclear factor κB
- AP-1
activator protein-1
- TNF-α
tumor necrosis factor-α
- IL-1β
interleukin 1β
- IL-6
interleukin 6
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Renfrey S, Downton C, Featherstone J. The painful reality. Nat Rev Drug Discov. 2003;2:175–176. doi: 10.1038/nrd1038. [DOI] [PubMed] [Google Scholar]
- 2.Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids – a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 3.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 4.Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- 5.Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- 6.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvemini D, Wang Z-Q, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpetidyl mimic pf superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 8.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 9.Samlowski WE, Petersen R, Cuzzocrea S, Macarthur H, Burton D, McGregor JR, Salvemini D. A nonpeptidyl mimic of superoxide dismutase, M40403, inhibits dose-limiting hypotension associated with interleukin-2 and increases its antitumor effects. Nat Med. 2003;9:750–755. doi: 10.1038/nm874. [DOI] [PubMed] [Google Scholar]
- 10.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytopritrective agents: Structure-activity relationship studies. J Med Chem. 2002;45:4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein S, Samuni A, Hideg K, Merenyi G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J Phys Chem. 2006;110:3679–3685. doi: 10.1021/jp056869r. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Chaiswing L, Oberley TD, Batinić-Haberle I, StClair W, Epstein CJ, StClair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 14.Moeller BJ, Batinić-Haberle I, Spasojević I, Rabbani ZN, Anscher MS, Vujasković Z, Dewhirst MW. Effects of a catalytic metalloporphyrin antioxidant on tumor radioresponsiveness. Int J Rad Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of oxygenation, free radicals and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 16.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jian C, Batinić-Haberle I, Vujasković Ž. Comparison of two Mn porphyrin-based mimics of superoxide-dismutase (SOD) in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinić-Haberle I, Abdul HM, Butterfield A, StClair DK. A neuronal model of Alzheimer’s disease: An insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008;153:120–130. doi: 10.1016/j.neuroscience.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackensen GB, Patel M, Sheng H, Calvi CL, Batinic-Haberle I, Day BJ, Liang LP, Fridovich I, Crapo JD, Pearlstein RD, Warner DS. Neuroprotection from Delayed Post-Ischemic Administration of a Metalloporphyrin Catalytic Antioxidant in the Rat. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Tarpey MM, Patel RP, Batinić-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric-oxide dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csont T, Viappiani S, Sawicka J, Slee S, Altarejos JY, Batinić-Haberle I, Schulz R. The involvement of superoxide and iNOS-derived NO in cardiac dysfunction induced by pro-inflammatory cytokines. J Mol Cell Card. 2005;39:833–840. doi: 10.1016/j.yjmcc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Saba H, Batinić-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batinić-Haberle I, Haskins K. A metalloporphyrin superoxide dismutase mimetic (SOD mimetic) inhibits autoimune diabetes. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 23.Batinić-Haberle I, Benov L, Spasojević I, Hambright P, Crumbliss AL, Fridovich I. The Relationship Between Redox Potentials, Proton Dissociation Constants of Pyrrolic Nitrogens, and in Vitro and in Vivo Superoxide Dismutase Activities of Manganese(III) and Iron(III) Cationic and Anionic Porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 24.Rebouças JS, DeFreitas-Silva G, Idemori YM, Spasojević I, Benov L, Batinić-Haberle I. Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: Protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier. Free Radic Biol Med. 2008;45:201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebouças JS, Spasojević I, Tjahjono DH, Richaud A, Mendez F, Benov L, Batinić-Haberle I. Redox modulation of oxidative stress by Mn porphyrin-based therapeutics: The effect of charge distribution. Dalton Trans. 2008:1233–1242. doi: 10.1039/b716517j. [DOI] [PubMed] [Google Scholar]
- 26.Spasojević I, Batinić-Haberle I, Rebouças JS, Idemori YM, Fridovich I. Electrostatic Contribution in the Catalysis of O2•− Dismutation by Superoxide Dismutase Mimics. J Biol Chem. 2003;278:6831–6837. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer-Sueta G, Vitturi D, Batinić-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 28.Batinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.09.042. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nauser T, Koppenol WH. The rate constant of the reaction of superoxide and nitrogen monoxide: approaching the diffusion limit. J Phys Chem A. 2002;106:4084–4086. [Google Scholar]
- 30.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford; 1999. [Google Scholar]
- 31.Lawrence GD, Sawyer DT. Potentiometric titrations and oxidation-reduction potentials of manganese and copper-zinc superoxide dismutases. Biochemistry. 1979;18:3045–3050. doi: 10.1021/bi00581a021. [DOI] [PubMed] [Google Scholar]
- 32.Barrette WC, Jr, Sawyer DT, Free JA, Asada K. Potentiometric titrations and oxidation-reduction potentials of several iron superoxide dismutases. Biochemistry. 1983;22:624–627. doi: 10.1021/bi00272a015. [DOI] [PubMed] [Google Scholar]
- 33.Ellerby RM, Cabelli DE, Graden JA, Valentine JS. Copper-Zinc Superoxide Dismutase: Why Not pH-Dependent? J Am Chem Soc. 1996;118:6556–6561. [Google Scholar]
- 34.Vance CK, Miller AF. A Simple Proposal That Can Explain the Inactivity of Metal-Substituted Superoxide Dismutases. J Am Chem Soc. 1998;120:461–467. [Google Scholar]
- 35.Michel E, Nauser T, Sutter B, Bounds PL, Koppenol WH. Kinetic properties of Cu, Zn-superoxide dismutases as a function of metal. Arch Biochem Biophys. 2005;439:234–240. doi: 10.1016/j.abb.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu, Zn-superoxide dismutase as a function of metal content–Order restored. Free Radic Biol Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer-Sueta G, Hannibal L, Batinić-Haberle I, Radi R. Reduction of manganese Porphyrins by flavoenzymes and submitochondrial particles and the catalytic redox cycle of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Jackson IL, Batinić-Haberle I, Vujasković Z. Superoxide dismutase mimic reduces hypoxia induced-O2•−, TGF-β and VEGF production by macrophages. Free Radic Res. 2007;41:8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- 39.Jackson IL, Batinić-Haberle I, Sonveaux P, Dewhirst MW, Vujasković Z. Superoxide production and angiogenic regulation by macrophages in response to heat. Int J Hypothermia. 2006;22:263–273. doi: 10.1080/02656730600594027. [DOI] [PubMed] [Google Scholar]
- 40.Tse H, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation/reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 42.Batinić-Haberle I, Spasojević I, Fridovich I. Tetrahydrobiopterin Rapidly Reduces SOD Mimic, Mn(III) tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radic Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Rabbani ZN, Vasquez-Vivar J, Spasojević I, Whitsett J, Selvakumar B, Haberle S, Vujasković Z, Dewhirst MW, Batinić-Haberle I. Anti-tumor Effects of Mn(III) Ortho Tetrakis N-ethylpyridylporphyrin, MnTE-2-PyP5+ in a Mice Model of Breast Tumor. Antiangiogenic Impact of Nitric Oxide Synthase Cofactor, Tetrahydrobiopterin; Presented at the American Association of Cancer Research Annual Meeting; San Diego. April 12–16; 2008. [Google Scholar]
- 44.Spasojević I, Colvin OM, Warshany KR, Batinić-Haberle I. New approach to the activation of anti-cancer pro-drug by metallporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. J Inorg Biochem. 2006;100:1897–1902. doi: 10.1016/j.jinorgbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Okado-Matsumoto A, Batinić-Haberle I, Fridovich I. Complementation of SOD deficient Escherichia coli by manganese porphyrin mimics of superoxide dismutase. Free Radic Biol Med. 2004;37:401–410. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 46.Batinić-Haberle I, Spasojević I, Stevens RD, Hambright P, Fridovich I. Manganese(III) Meso Tetrakis Ortho N-alkylpyridylporphyrins. Synthesis, Characterization and Catalysis of O2•− Dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 47.Pollard JM, Rebouças JS, Durazo A, Kos I, Gralla EB, Valentine JS, Batinić-Haberle I, Gatti RA. Radio-protective effects of manganese-containing superoxide dismutase mimics on ataxia telangiectasia cells. Free Radic Biol Med. 2004 doi: 10.1016/j.freeradbiomed.2009.04.018. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rebouças JS, Spasojević I, Batinić-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre clinical mechanistic/therapeutic purposes. J Pharm Biomed Anal. 2008 doi: 10.1016/j.jpba.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 50.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 51.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 54.Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- 55.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 56.Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem. 2002;277:2330–2335. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- 57.Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–193. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- 58.Stern MK, Jensen MP, Kramer K. Peroxynitrite Decomposition Catalysts. J Am Chem Soc. 1996;118:8735–8736. [Google Scholar]
- 59.Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: Therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci U S A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 61.Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 Contribute to Peroxynitrite-Mediated Inflammatory Pain Hypersensitivity. FASEB J. 2008 doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- 62.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Bezerra MM, Brain SD, Girão VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- 65.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008 doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 68.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2004;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, Erdogan C. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 73.Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–78. doi: 10.1016/j.brainres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 74.Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499–503. doi: 10.1016/j.gassur.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 75.Campana F, Zervoudis S, Perdereau B, Gez E, Fourquet A, Badiu C, Tsakiris G, Koulaloglou S. Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J Cell Mol Med. 2004;8:109–116. doi: 10.1111/j.1582-4934.2004.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spasojević I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, St Clair DK, Batinić-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spasojević I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouças JS, St Clair DK, Batinić-Haberle I. Pharmacokinetics of the potent redox modulating manganese porphyrin, MnTE-2-PyP5+ in plasma and major organs of B6C3F1 mice. Free Radic Biol Med. 2008a doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rebouças JS, Spasojević I, Batinić-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: A case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Inorg Biol Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 80.Imlay JA. Cellular Defenses Against Superoxide and Hydrogen Peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohse T, Nagaoka S, Arakawa Y, Kawakami H, Nakamura K. Cell death by reacytive oxygen species generated from water-soluble cationic metalloporphyrins as superoxide dismutase mimics. J Inorg Biochem. 2001;85:201–208. doi: 10.1016/s0162-0134(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 82.Batinić-Haberle I, Benov L, Spasojević I, Fridovich I. The Ortho Effect makes manganese (III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin (MnTM-2-PyP) a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 83.Gridley DS, Makinde AY, Luo X, Rizvi A, Crapo JD, Dewhirst MW, Moeller BJ, Pearlstein RD, Slater JM. Radiation and a metalloporphyrin radioprotectant in a mouse prostate tumor model. Anticancer Res. 2007;27:3101–3110. [PubMed] [Google Scholar]
- 84.Jensen MP, Riley DP. Peroxynitrite decomposition activity of iron porphyrin complexes. Inorg Chem. 2002;41:4788–4797. doi: 10.1021/ic011089s. [DOI] [PubMed] [Google Scholar]
- 85.Lee J, Hunt JA, Groves JT. Mechanism of iron porphyrin reaction with peroxynitrite. J Am Chem Soc. 1998;120:7493–7501. [Google Scholar]