Abstract
The cells of innate and adaptive immunity, although activated by different ligands, engage in cross talk to ensure a successful immune outcome. Toll-like receptors (TLRs) are key components of the innate immune system and have the ability to detect microbial infection and trigger host defence responses. Otherwise, human T lymphocytes are able to produce most TLRs. Thus, we analyze the capability of some TLR ligands to modulate the function of highly-purified CD4+ T cells. We found that agents acting via TLRs (poly I:C, a TLR3 ligand; flagellin, a TLR5 ligand; and R848, a TLR7/8 ligand) are able to regulate the expression of costimulatory molecules both on purified antigen presenting cells and on purified T lymphocytes. Moreover, the activation mediated by TLRs determines a kinetic expression of B7-family members such as through an inhibition of T lymphocytes delayed proliferation. These findings suggest a functional role of some invading microorganisms in regulating acquired immunity.
Key Words: B7-family receptors, T lymphocytes, Toll-like receptors.
INTRODUCTION
Host defense against invading microbial pathogens is elicited by the immune system: innate and acquired immunity. Both components of immunity recognize invading microorganisms as non-self, which triggers immune responses to eliminate them. In acquired immunity B and T lymphocytes utilize antigen receptors such as immunoglobulins and T cell receptors to recognize the non-self.
At the end of the 20th century, Toll was shown to be an essential receptor for host defense against fungal infection in Drosophila, which only has innate immunity [1]. Soon after, a mammalian homolog of the Toll receptor (now termed Toll-like receptor-4, TLR4) was shown to induce expression of genes involved in inflammatory responses [1]. These studies lead innate immunity to become a very attractive subject of research. To date, at least eleven members of the Toll family have been identified [2]. This type I transmembrane proteins family is characterized by an extracellular domain with leucine-rich repeats and a cytoplasmic domain with homology to the type I IL-receptor. Functionally, a critical role of TLR4 when recognizing the microbial component (i.e. LPS) was initially characterized. TLR4 physically associates with another molecule called MD-2, along with CD14. This complex is responsible for LPS recognition and signalling [3].
In this article, we focus our attention on some of the other TLR family members such as the TLR3 which recognizes double stranded (ds) RNA and induces both the activation of NF-kB through MyD88-dependent and independent pathways and the production of type I interferons (IFN-α /β) [4, 5]. TLR4, as well as TLR2, are pattern recognition receptors and signal molecules in response to bacterial lipoproteins and have been implicated in innate immunity and inflammation [6]. TLR5 recognizes bacterial flagellin [7]. TLR7 and TLR8 are structurally highly conserved proteins, and recognize a nucleic acid-like structure of the virus [8].
Costimulatory molecules are considered as critical in eliciting cellular proliferation and cytokine production upon stimulation. Our knowledge of the signals mediated through B7 family molecules (CD80, CD86, B7-H1 and B7-DC) and their corresponding receptors (CD28, CD152, and PD-1) have become more complex and it is now clear that they play critical roles in immune regulation. CD28 is present on resting T cells and is therefore likely to be related to initial costimulatory activity. CD152 (CTLA-4) is an inducible inhibitory receptor homologous to CD28 and its engagement triggers phosphatases which dephosphorylate molecules of the CD3/TCR activation cascade. Conversely, blockade of CD152 by specific mAb increases proliferation of CD4+ T cell blasts and clones [9-13]. CD152 mRNA is detectable within one hour after T cell activation and cell surface expression of the protein, although weak, reaches a peak one to three days later [14]. In addition, the engagement of CD152 inhibits specific target cell lysis mediated by CD8+ T lymphocytes [15], down-regulates proliferation of T cells as well as IL-2, IFN-γ, IL-4, and IL-13 production, and up-regulates the production of IL-10 and TGF-β [16]. PD-1 is expressed by B cells, T lymphocytes (activated but not resting), and myeloid cells, in contrast to the more restricted expression of CD28 and CD152. PD-1 is also expressed in the thymus primarily on CD4–CD8– T cells, during this transition from double negative to double positive [17]. B7-H1 binds its receptor PD-1 along with another ligand, B7-DC. The overall expression of B7-H1 and B7-DC transcripts is similarly found in various lymphoid and non-lymphoid tissues, whereas the expression profiles of their proteins are quite distinct [18-22].
The immunomodulating activity of bacterial components may be related to their ability to induce expression of reactive molecules on responding cells. For this reason, we verified the in vitro capacity of flagellin, poly (I:C) and R848 to induce the expression of the costimulatory molecules, such as CD80, CD86, B7-H1, B7-DC on DC and CD28, CD152, PD-1 on T cells. The further understanding of these pathways will create new and exciting possibilities for therapeutic fine-tuning of the immune system.
MATERIALS AND METHODOLOGY
Cell Cultures
Human peripheral blood samples were obtained from healthy donors according to institutional guidelines. Peripheral Blood Mononuclear Cells (PBMC) were purified by Ficoll-Hypaque density gradient centrifugation. Monocytes and CD4+ T lymphocytes were isolated by positive selection with magnetic beads coated with mAb to CD14 and CD4 (MACS, Miltenyi Biotec, Auburn, CA, USA). To induce DC differentiation, purified monocytes were cultured for 4 days in the presence of 200 ng/ml rhGM-CSF (Shering-Plough Research Institute, Kenilworth, NJ) and 10ng/ml rhIL-4 (Immunotools, Friesoythe, Germany). These cells are termed immature DCs. Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS, 5 mM L-glutamine and 50 IU/ml penicillin-streptomycin (from here on referred to as complete medium).
Antibodies
The following mAbs were used for immunophenotypic and functional analyses: anti-human B7-DC, anti-human PD-1 (clone MIH 18, and clone JI16 eBiosciences, San Diego, CA, USA); PE-conjugated anti-HLA-DR, anti-CD80, anti-CD86 and anti-CD152 (BD Pharmingen, San Diego, CA, USA). Anti-CD28 mAb was a gift from D. Olive (Marseille, France).
In Vitro Stimulation
The activating factors used in the final concentrations were the following: 10 ng/ml Flagellin from S. thyphimurium (InvivoGen, San Diego, CA, USA); 1 μg/ml LPS from Escherichia coli, serotype RE515 (referred to as LPS-RE515, Alexis-Italy, Florence, Italy) or 1 μg/ml Escherichia coli LPS (referred to as LPS, Sigma-Aldrich, Milan, Italy); 25 μg/ml poly (I:C) (Polyinosinic:polycytidylic acid; Sigma-Aldrich); and 10 μM R-848 (imidazoquinoline resiquimod; GLSynthesis, Worcester, MA, USA). All of these TLR ligands were previously tested in order to obtain doses that repeatedly generate the same maximum response (data not shown). While studying the activation of DCs via TLR, several samples were treated with 7.5 μg/ml rabbit anti-human IFN-γ antibody (Prodotti Gianni, Milan, Italy) to neutralize the IFN-γ. DCs and T lymphocytes were collected after 24, 48 and 72 hrs, and cell surface markers were tested by flow cytometry.
Flow Cytometry
The surface phenotype of monocyte-derived DCs was assessed by flow cytometric analysis (FACSCalibur, Becton Dickinson). The secondary reagent was PE-labelled goat anti-mouse (GAM) antiserum (Southern Biotechnology Associates, Birmingham, AL, USA).
Quantification of IFN-γ
The level of IFN-γ in the supernatant was measured using an ELISA kit according to the manufacture’s protocol (Alexis-Italia, Milano, Italy). The deviation between triplicates was <10% for any reported value.
Mixed Leukocyte Reaction
Two-way MLR was used to test functional activity following the activation via TLR. The cells used in these experiments were extracted from a panel of HLA typed laboratory volunteers and were selected to provide two HLA-DR mismatches. DCs and T lymphocytes were pre-treated for three days, as previously described. After in vitro stimulation DCs and T lymphocytes were harvested, washed, and resuspended in complete medium. Then 105 purified allogeneic T cells were harvested, washed, and incubated with 104 DCs in a 0.2 ml volume in 96-well round-bottom plates with 5% CO2 at 37°C. Triplicate cultures were set up and incubated for 5 days. The cultures were then pulsed with 0.5 mCi 3H-thymidine and harvested 18 h later.
Neutralizing anti-human-CD80, -CD86, -CD152, -B7-H1, B7-DC, PD-1 mAb, and irrelevant isotype-matched control mAb were added into the MLR as indicated.
Statistical Analyses
Statistical analyses were performed in Microsoft Excel 5.0 (Microsoft) using two-tailed Student’s t tests. The p value <0.05 was considered statistically significant.
RESULTS
Flagellin, LPS, poly (I:C) and R848 are able to regulate the expression of co-stimulatory receptors on the membrane of T lymphocytes.
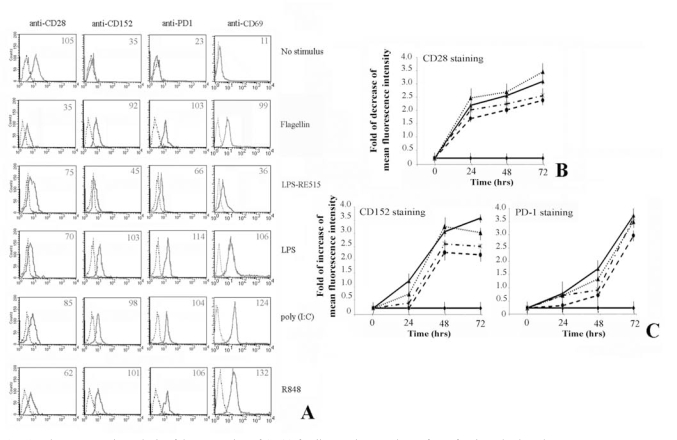
First, we analysed the effect of TLR stimulation on freshly isolated CD4+ T lymphocytes (Fig. 1). Results show that the stimulation leads to a decrease of CD28 expression, whereas the CD152 and PD-1 expression increases after 72 hrs of treatment (Fig. 1A). In addition, expression of CD69 was evaluated as a positive control of activation.
Fig. (1).
Flow cytometric analysis of the expression of CD28-family members on the surface of activated T lymphocytes.
Human CD4+ T lymphocytes, purified from peripheral blood cells, were cultured as described in Materials and Methods.
Panel A. Shows that T cells activation via TLRs is able to up-regulate the expression of CD152 and PD-1 at the membrane cell level. Expression of CD69 was a positive control for T lymphocytes activation. Numbers indicate the Mean Fluorescence Intensity of each curve.
Panel B. The expression of CD28 decreases following treatment of T cells with flagellin, poly (I:C) and R848. Data are shown as means ± standard deviations (error bars) of five experiments.
Panel C. Shows the increasing of respectively CD152 and PD-1 expression. It is interesting to note that CD152 reaches the maximum of expression after 48 hrs, whereas PD-1 after 72 hrs. Data are shown as means ± standard deviations (error bars) of five experiments.
Experimental conditions are: ♦ no stimulus; ▼ flagellin; ▲ + LPS; * poly (I:C); ● R848.
The kinetic of CD28 molecules expression shows that the greatest decrease is reached after 72 hrs of stimulation (Fig. 1B). Interestingly, the CD152 reaches its maximum expression on the membrane of T cells after 48 hrs of stimulation, whereas the maximum for PD-1 is achieved after 72 hrs (Fig. 1C). In addition, the expression of these inhibitory molecules rapidly decreases after 96 hrs (data not shown).
Although gene knockout mouse demonstrates that TLR-4 is the key receptor of LPS [23], in vitro transfection assay shows that many commercial preparations of LPS [24-25] including the E. coli LPS from Sigma [26] possess both TLR-4 and TLR-2 activities. For this reason we utilized LPS-RE515, a strong activator of TLR-4, that does not activate TLR-2 or other TLRs [27]. As can be depicted from Fig. 1A, LPS modulates the expression of co-stimulatory molecules on T lymphocytes, whereas LPS-RE515 was slightly less effective. Thus, we could presume these effects were a result of a specific activation via TLR-2 ligand, excluding a role mediated by TLR-4 [28-29].
Flagellin, LPS, poly (I:C) and R848 can regulate the expression of co-stimulatory molecules on the DC membrane.
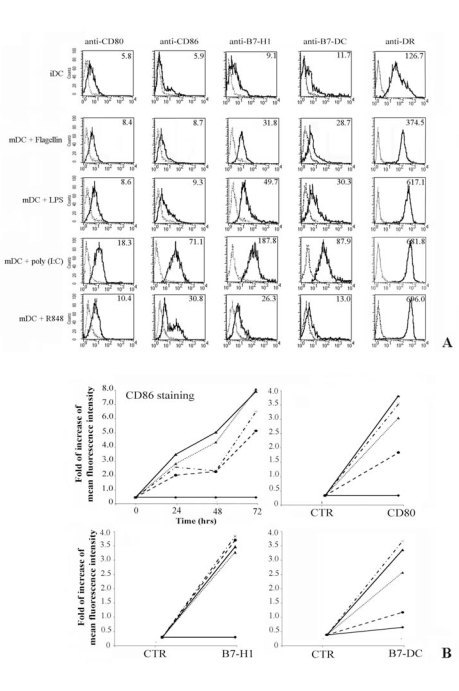
Fresh monocytes, isolated by positive selection using magnetic beads coated with anti-CD14 mAb, were cultured for 4 days with GM-CSF and IL-4 (immature DCs, iDC), and further stimulated for up to 3days with flagellin, LPS, poly (I:C) and R848 (mature DCs, mDC). We analyzed the expression of CD80, CD86, B7-H1 and B7-DC at the cell surface level of iDC and mDC (Fig. 2A). The experiment shown represents the study of five donors. The iDC expressed very low amount of B7 family receptors and CD80 was the most represented (Fig. 2A). In addition, our results demonstrate that flagellin, LPS, poly (I:C) and R848 selectively up-regulate the expression of all tested costimulatory molecules on mDC, although poly (I:C) seems to have a greater effect on the induction of B7 family member expressions (compare different rows in Fig. 2A). Expression of HLA-DR was evaluated through positive control of activation,
Fig. (2).
Flow cytometric analysis of costimulatory molecules expression on DC.
Dendritic cells (DC) were obtained by in vitro differentiation of monocytes. For cellular stimulations, DC were cultured for 24, 48 and 72hrs with medium alone or in the presence of different stimuli.
Panel A. Shows the immunophenotype of DC cultured in complete medium (iDC), first row. The other rows represent the membrane expression of B7-family members on mature DC (mDC) after 72 hrs of treatment with flagellin, poly (I:C) and R848 respectively. Expression of MHC-Class II was a positive control for iDC activation.
Panel B. Only the kinetic of CD86 expression is shown, whereas the expression of the other costimulatory molecules is displayed at the best time point (72 hrs). Data are expressed as increasing of Mean fluorescence intensity. Data are shown as means ± standard deviations (error bars) of five experiments.
Experimental conditions are: ♦ no stimulus; ▼ flagellin; ▲ + LPS; * poly (I:C); ● R848.
Figure 2B shows both the kinetic of the CD86 expression over a period of 72 hrs and the increasing of the mean fluorescence intensity of differently treated DC stained with anti-CD80, -B7-H1 and -B7-DC mAb also over a 72 hrs period.
Effect of IFN-γ Neutralization on the Expression of Co-Stimulatory Molecules
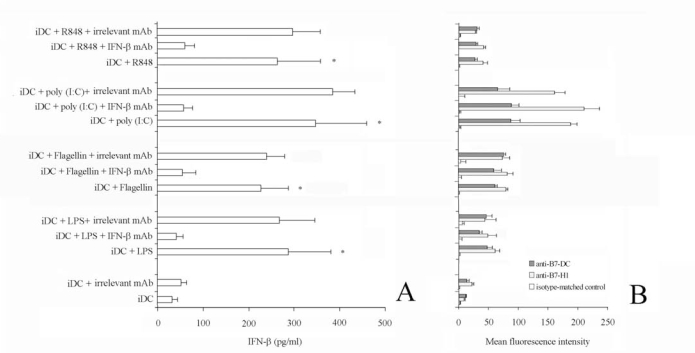
Viral, as well as bacteria, infections induce innate immune responses including the production of type I interferons, IFN-α/β. Thus, these agents could induce costimulatory molecules up-regulation in response to type I interferon signalling. Otherwise, up-regulation of costimulatory molecules on airway epithelium does not seem to depend on these inteferons [29]. For this reason, we measured IFN-γ concentration in the supernatant of activated DC (Fig. 3A). The concentration of IFN-γ was markedly increased in the supernatant of TLR-stimulated cells up to 448 pg/ml (stimulation via poly (I:C), compared with that of unstimulated cells). Interestingly, although the pretreatment of cell culture with saturing amount of anti-IFN-γ antibody completely neutralized IFN-γ in the supernatant, it failed to suppress the TLR-induced upregulation of B7-H1 and B7-DC on DC (Fig. 3B).
Fig. (3).
Effect of IFN-b neutralization on B7-H1 and B7-DC expression.
iDC were stimulated with flagellin, LPS, poly (I:C) and R848 for 48 h in the presence or absence of anti-IFN-b antibody (7.5 mg/ml).
Panel A. IFN-b in the supernatant was quantified by ELISA. Data are shown as means ± standard deviations (error bars) of five experiments.
*Samples that are significantly different (p < 0.05) from the control culture (DC alone). The pre-treatment of cell culture with saturing amount of anti-IFN-b antibody completely neutralized IFN-b in the supernatant.
Panel B. Representative histograms showing the Mean fluorescence intensity of iDC stimulated flagellin, LPS, poly (I:C) or R848 alone, in the presence of anti-IFN-b mAb, or in the presence of the isotype-matched control mAb, stained with the indicted mAb. Of note, pre-treatment with saturing amount of anti-IFN-b antibody failed to suppress the TLR-induced upregulation of B7-H1 and B7-DC on iDC. Data are shown as means ± standard deviations (error bars) of five experiments.
The Interaction of CD152 and PD-1 with Their Natural Ligands Inhibits T Cell Proliferation
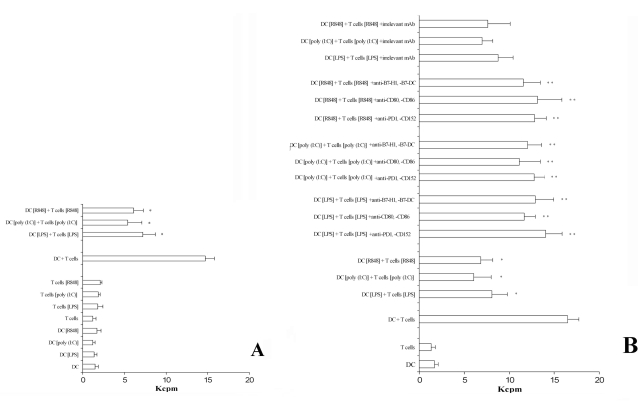
Because TLRs ligation induced the expression of co-stimulatory molecules on DC as well as the expression of inhibitory receptors on T cells, we next examined the immunoregulatory properties of flagellin, LPS, poly (I:C), and R848 by determining their effects on two-way MLR. Results are shown in Fig. 4A. Only the pre-treatment of DCs and T lymphocytes with the TLR-acting agents evidence a decrease of thymidine incorporation. Otherwise, T lymphocytes and DCs, not pre-activated via TLRs, added to the double-MLR assay were able to normally proliferate (Fig. 4A). Finally, in order to evidence the role played by CD152 and PD-1 receptors in inhibiting T cell proliferation, we set up a similar experiment adding a cocktail of mAb specific to CD80, CD86, CD152 and B7-H1, B7-DC, PD-1. Results (Fig. 4B) proved that the interfering mAb reconstitute the normal ability of T cells to proliferate. These results were obtained independently when the cocktail mAb used were directed to the inhibitory receptors (i.e. CD152 or PD-1) or to their ligands (i.e. CD80, CD86, B7-H1 or B7-DC). Thus it seems that the interaction between inhibitory receptors and ligands is blocked by these mAb. Furthermore, the adding to the proliferation test of isolated mAb showed a partial reconstitution effect (data not shown). Thus, the inhibitory effect seems to be related principally to the induction of the expression of these B7-family members on the membrane level in this experimental model.
Fig. (4).
The pre-activation of T lymphocytes and DC via TLRs down-regulates the proliferative ability in a two-MLR system.
Panel A. Pre-treatment of DC and T cells with agents acting via TLRs is able to significatively inhibit a two-way MLR. A positive control was the measure of thymidine incorporation of cells not treated (DC + T cells). The data represent the mean of quadruplicate samples. The averages ± standard deviations (error bars) of three different experiments are shown. Similar results were obtained in three separate experiments from a variety of donor/recipient combinations. *, Samples that are significantly different (p < 0.05) from the control culture (DC + T cells).
Panel B. A cocktail of mAb specific for the B7/CD28-family members added to the assay is able to revert the capability of T lymphocytes to proliferate. In addition, the presence of a cocktail of irrelevant isotype-matched mAb does not perturbate the inhibitory effect observed. The averages ± standard deviations (error bars) of three different experiments are shown. Similar results were obtained in three separate experiments from a variety of donor/recipient combinations.
*Samples that are significantly different (p < 0.05) from the control culture (DC + T cells).
**Samples that are significantly different (p < 0.05) from the control culture (DC + T cells + TLR activation).
DISCUSSION
The immunomodulating activity of bacterial components may be related to their ability to induce expression of reactive molecules on responding cells. The mechanisms by which the antigen receptors recognize foreign antigens have been intensively analyzed, and the major mechanisms, such as diversity, clonality and memory, have been well characterized. However, these receptors are present only in vertebrates, and therefore we do not fully understand the mechanism for non-self recognition in invertebrate organisms. The important role of individual TLRs during innate immunity has been established, above all in recognizing specific microbial components derived from pathogens including bacteria, fungi, protozoa and viruses and in inducing antimicrobial immune response and furthermore, activation of innate immunity is a critical step to the development of antigen-specific acquired immunity [30-31].
DCs are central to T lymphocyte activation and differentiation into T helper and cytotoxic T lymphocyte effectors. The generation of acquired immunity begins with DCs capturing microbial antigens in the peripheral tissues. Subsequently, DCs migrate to the draining lymph nodes to present the processed peptides to naïve T lymphocytes in the context of MHC molecules. Once inside the lymph nodes, DCs migrate to the T cell areas, seek out antigen-specific T cells and induce their activation and differentiation into effector cells. This migration is mediated by TLR-induced downregulation of inflammatory chemokine receptors and upregulation of the receptors for lymphoid chemokines [32-34].
Signals through TLRs generally result in the activation and maturation of all DCs, as measured by enhanced expression of the costimulatory molecules CD80, CD86 and CD40. Induction of the costimulatory molecules expression on the surface of DCs is a particularly important step in the initiation of acquired immunity. The originally described members of the B7 family, B7-1 and B7-2 (CD80 and CD86), both costimulate through CD28. However, T cell activity is down-modulated by a second receptor for B7-1 and B7-2, CD152 [35]. While B7-1 and B7-2 were once thought to represent the major source of signal 2 by DCs, the recent discovery of additional B7 family members highlights the molecular complexity of costimulation. The overall expression of B7-H1 and B7-DC transcripts is similarly found in various lymphoid and non-lymphoid tissues, whereas the expression profiles of their proteins are quite distinct. The expression of B7-H1 protein, although virtually absent in normal tissues except for macrophage-like cells, could be induced in a variety of tissues and cell types, such as APCs, B cells, T cells, epithelial cells, muscle cells, trophoblast, endothelial cells and tumour cells. In contrast, expression of B7-DC was only detected on DCs and monocytes. B7-H1 was first identified as a T cell costimulatory molecule that augments human T cell proliferation and IL-10 secretion in the presence of either anti-CD3 or alloantigens [36-37]. B7-DC was also found to have similar functions in vitro [38]. However, the receptor PD-1 is believed to be an inhibitory receptor because of its phenotypes of lymphoproliferative/autoimmune diseases in PD-1-deficient mice [39]. This seemingly contradictory data, however, could be best interpreted by expression of additional costimulatory receptor on T cells other than PD-1. In addition, it is known that the induction of type I interferon (i.e. IFN-γ constitutes a primary signal for upregulation of costimulatory molecules induced by LPS and (ds) RNA [40, 41]. Nevertheless, we show that the neutralization of IFN-γ did not block the upregulation of B7-H1 and B7-DC, suggesting that their expression on DCs may not depend, in these experimental conditions, on the induction of IFN-γ.
TLR messages have been reported to be present in T cells. TLRs have been associated principally with the T helper cell response [42]. For all of these reasons, we first addressed our attention on the capability of TLR-mediated signals to modulate the expression of some B7-famly members at the membrane level of DCs and T lymphocytes. Subsequently, we have analyzed the functional roleof TLR-mediated signals on DC maturation and T cells activation. The induction of the expression of inhibitory receptors, such as CD152 and PD-1, may represent a mechanism able to regulate the intensity of T cell response. What is of interest is the kinetic of expression of these two molecules and of their counter-receptors, showing the possibility to down-regulate the on-going of the cellular-mediated immune-response. In fact, we observed an inhibition of T cells proliferation in a two-way MLR when both CDs and T lymphocytes were preactivated via TLRs. In addition, this effect was partially abrogated when mAb specific to B7-family members were added to the assay. Thus, we evidenced a possible functional roleof TLRs-stimulation in regulating acquired immunity acting both on DCs and T cells level. Finally, the using of purified LPS indicates a prevalent role of TLR2 in activating T cells (obtained by non-purified LPS), partially excluding the effects mediated by TLR4 [28].
An interesting observation is due to the effects of flagellin in the modulation of costimulatory molecules, both on T lymphocytes and DC. These results could suggest that the bacterial protein flagellin, the primary structural component of flagella, plays a role in mediating gut inflammation associated with infection by enteric pathogens and in inflammatory bowel disease. Recent findings revealed that flagellin, via ligation of TLR-5, is a major means of activating the innate immune responses defining active intestinal inflammation [43-44]. In addition, human studies demonstrated that flagellin is also a major antigenic target of immune responses associated with Crohn’s disease, suggesting that immune responses to flagellin are not only associated with Crohn’s disease, but can promote the pathogenic response. Thus, we can hypothesize that the innate immune response to flagellin provides a degree of direct protection against flagellated microbes promoting inflammation and causing perturbations of immune cell homeostasis to lead to development of both protective adaptive immunity and, under select circumstances, a pathogenic disease. The ability to appropriately respond to pathogenic assault is critical to survival, and the TLRs are the first point of contact that we have with invading organisms.
The mechanisms regulating the TLR response must be controlled tightly, first to respond properly to the pathogenic challenge and second, to avoid excessive activation of the TLR signaling pathway. Therefore control damaging injury to the host system following TLR activation. Thus, a better understanding of the mechanisms behind TLR activation will hopefully lead to the development of novel therapeutic strategies in the treatment of inflammatory diseases and autoimmunity.
ETHICS APPROVAL
Blood samples were taken after informed verbal consent.
ACKNOWLEDGEMENTS
This work was supported by a grant from Fondazione CARIGE, Genova, and from Progetti di Ateneo 2007, University of Genova, Italy to D.S.
We thank R. Piccardo and J. Lee Patricia Delgado for secretarial assistance.
REFERENCES
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immun. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 3.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–9. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-B by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 6.Calvano JE, Agnese DM, Um JY, et al. Modulation of the lipopolysaccharide receptor complex (CD14, TLR4, MD-2) and toll-like receptor 2 in systemic inflammatory response syndromepositive patients with and without infection: relationship to tolerance. Shock. 2003;20(5):415–9. doi: 10.1097/01.shk.0000092269.01859.44. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 8.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 9.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. Ann Rev Immunol. 1991;7:191–212. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 12.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune response. Ann Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 13.Alegre ML, Shiels H, Thompson CB, Gajewski TF. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol. 1998;161:3347–56. [PubMed] [Google Scholar]
- 14.Alegre M-L, Noel PJ, Eisfelder BJ, et al. Regulation of surface and intracellular expression of CTLA-4 on mouse T cells. J Immunol. 1996;157:4762–70. [PubMed] [Google Scholar]
- 15.Saverino D, Tenca C, Zarcone D, et al. CTLA-4 (CD152) inhibits the specific lysis mediated by human cytolytic T lymphocytes in a clonally distributed fashion. J Immunol. 1999;162:651–8. [PubMed] [Google Scholar]
- 16.Saverino D, Merlo A, Bruno S, Pistoia V, Grossi CE, Ciccone E. Dual effect of CD85/leukocyte Ig-like receptor-1/Ig-like transcript 2 and CD152 (CTLA-4) on cytokine production by antigen-stimulated human T cells. J Immunol. 2002;168:207–15. doi: 10.4049/jimmunol.168.1.207. [DOI] [PubMed] [Google Scholar]
- 17.Foell J, Hewes B, Mittler RS. T cell costimulatory and inhibitory receptors as therapeutic targets for inducing anti-tumor immunity. Curr Cancer Drug Targets. 2007;7:55–70. doi: 10.2174/156800907780006841. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 19.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Freeman GL, Long AL, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latchman Y, Wood CR, Chernove T, et al. PD-L2, a novel B7 homologue, is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–68. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 22.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dentritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poltorak A, Smirnova I, He X, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–55. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 24.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–8. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 25.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 26.Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M . Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–7. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber M, Kalis C, Keck S J, et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36:701–11. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 29.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of acquired immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 30.Coutinho A, Poltorack A . Innate immunity: from lymphocyte mitogens to Toll-like receptors and back. Curr Opin Immunol. 2003;15:599–602. doi: 10.1016/j.coi.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Caamaño J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–29. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–69. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins MK, Khoruts A, Ingulli E, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Linsley PS, Peach R, Gladstone P, Bajorath J . Extending the B7 (CD80) gene family. Protein Sci. 1994;3:1341–43. doi: 10.1002/pro.5560030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong H, Zhu G, Tamada K, Chen L . B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 37.Tamura H, Dong H, Zhu G, et al. B7-H1 costimulation preferentially enhances CD28-independent T-help cell function. Blood. 2001;97:1809–6. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen LT, Radhakrishnan S, Ciric B, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–8. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Okazaki T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38:353–7. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda M, Matsumoto K, Inoue H, et al. Expression of B7-H1 and B7-DC on the airway epithelium is enhanced by double-stranded RNA. Biochem Biophys Res Commun. 2005;330:263–70. doi: 10.1016/j.bbrc.2005.02.161. [DOI] [PubMed] [Google Scholar]
- 41.Allam JP, Peng WM, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121:368–74. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 42.Liew FY, Komai-Koma M, Xu D. A toll for T cell costimulation. Ann Rheum Dis. 2004;63(Suppl 2):ii76–ii78. doi: 10.1136/ard.2004.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gewirtz AT. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol. 2006;22:8–12. doi: 10.1097/01.mog.0000194791.59337.28. [DOI] [PubMed] [Google Scholar]
- 44.Gewirtz AT. TLRs in the Gut III Immune responses to flagellin in Crohn's disease: good, bad, or irrelevant? Am J Physiol Gastrointest Liver Physiol. 2007;292:G706–10. doi: 10.1152/ajpgi.00347.2006. [DOI] [PubMed] [Google Scholar]