Abstract
Purpose
Two common treatment options for congenital pseudarthrosis of the tibia (CPT) are intramedullary fixation following resection/shortening of the pseudarthrosis site and reconstruction with an Ilizarov external fixator following resection. We present in detail a narrative of two cases with similar degrees of tibial dysplasia associated with NF-1 treated using these different methods and followed to completion.
Methods
Technical issues and details of the treatment methods from case reports are discussed in depth. The eventual profoundly different outcomes are correlated to the technical variations used.
Results
Treatment with the Charnley–Williams rodding method and aggressive bone grafting supplemented by rh-BMP2 resulted in a normal functioning limb at maturity, while treatment with first, an ineffective version of IM rodding, followed by two sessions of bone transport using an Ilizarov fixator failed to gain useful union and eventually resulted in amputation.
Conclusions
Technical details, heretofore inadequately reviewed in the literature, are crucial to the success of either of these commonly utilized treatment methods for CPT.
Keywords: Congenital pseudarthrosis of the tibia, Charnley–Williams intramedullary rodding, Bone transport with Ilizarov fixator, rhBMP2 treatment for CPT
Prologue
Effective treatment for congenital tibial dysplasia with established pseudarthrosis—commonly referred to as congenital pseudarthrosis of the tibia (CPT)—remains elusive, because no one method has distinguished itself as superior to others in achieving the goals of obtaining and maintaining union over time so that the limb can be functional. Despite multiple methods of treatment having been reported over several decades, outcomes are too frequently unsatisfactory, and both patients and surgeons are disappointed and frustrated by repeated ineffective operations, re-fracture after apparent union, and poorly functioning limbs resulting from multiple operations and indefinite protective immobilization. This rare disorder, occurring in 1 in 140,000–190,000 patients, has received a seemingly inordinate amount of attention in the orthopedic literature, which merely emphasizes the absence of a reliable treatment to obtain a stable, functional extremity. How ironic it becomes when the ultimate capitulation by surgeon and patient is to proceed with amputation of the resistant pseudarthrosis in order to finally obtain a functional extremity.
Modern treatment of CPT commonly includes three well-described methods: (1) resection of the lesion with shortening, bone grafting, and internal stabilization by intramedullary fixation of both tibia and fibula; (2) external fixation of the tibia with compression across the pseudarthrotic lesion, with or without resection, acute shortening, or bone transport (Ilizarov method); and (3) microvascular transfer of a fibular graft, usually from the contralateral extremity, to the pseudarthrosis site. Depending on the success of each technique—or more accurately, the lack of success—the use of the other two methods may also be applied to the same extremity, in an all-out effort to obtain union.
In an attempt to delineate important and crucial treatment principles of two of these methods, we present in detail the following two cases of CPT, which initially appear to be essentially identical lesions but were treated using different methods.
It was the best of times....
A 2 year and 6-month-old male with a left CPT limped into clinic using a brace on his leg that had been prescribed by another orthopedist in another state at the age of 18 months. Shortly after he began walking, the CPT diagnosis had been made in response to a painless “fracture” which had failed to heal with casting.
The boy had multiple skin lesions of two varieties, diagnosed as systemic mastocytosis and as café au lait spots consistent with neurofibromatosis (Fig. 1). The presenting X-ray (Fig. 2a) showed frank pseudarthrosis of the tibia with a bony gap and atrophic bone ends, but the fibula was intact and even slightly hypertrophied. Despite the obvious limp, he had no apparent pain or reluctance to bear weight on his left leg when wearing the orthosis, although he was less content to bear weight without it. Because he had reasonably good painless function, the brace was continued, and no other treatment offered at that time. However, within 6 months, he became less active and refused to walk, even with the brace; so, at age 3 years and 2 months, he underwent a type-A intramedullary rod procedure [1], which included: (1) resection of the atrophic bone ends and surrounding periosteum including the hamartomatous “rind” strangulating the tibia at the pseudarthrosis site (Fig. 2b); (2) shortening and intramedullary (IM) rodding of both the tibia and fibula, removing a segment of the intact fibula to facilitate the shortening (Fig. 3a–c); and (3) autogenous bone grafting of the tibia with iliac crest graft, placing some of the graft along the interosseous membrane to attempt to create a cross-union to the fibula. In an effort to avoid transfixation of the ankle joint, the Williams technique [2] was modified, leaving the female portion of the Williams rod within the tibia only. In addition, a Steinmann pin was used to interlock the rod just proximal to the ankle (Fig. 3b–d). This custom rod was created by drilling an interlocking screw hole of appropriate diameter (1.5 mm) in the Williams rod in our machine shop. The patient was immobilized (Fig. 3d) in a non-weight-bearing, bent-knee cast, later changed to a weight-bearing, long-leg cast for a total of 4 months of immobilization after surgery.
Fig. 1.
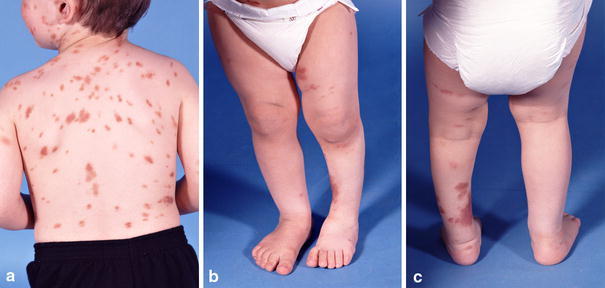
a–c Clinical appearance of patient at presentation. Cutaneous lesions include both mastocytosis lesions (darker) and cafe-au-lait spots (lighter)
Fig. 2.
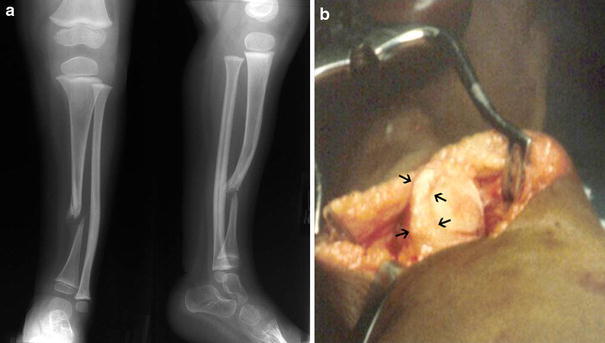
a Presenting X-ray at age 2 years and 6 months. b Intraoperative view of periosteal “rind” strangulating the tibia (arrows)
Fig. 3.
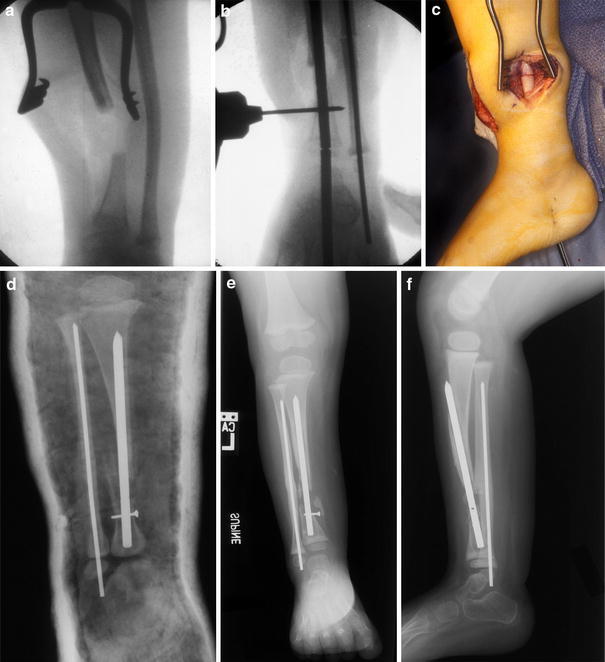
a Intraoperative X-ray showing amount of acute tibial resection at the pseudarthrosis site. b Williams rod technique. The male–female connection is at the distal tibia epiphysis, with the male portion to be unscrewed and removed through the bottom of the foot. The interlocking Steinmann pin is being placed. c Intraoperative view of fibula osteotomy and shortening. Note bone ends in contact. d Immediate postoperative X-ray of IM rod with Steinmann pin interlock, in cast. e, f Loss of distal fixation with persistent tibial discontinuity and healed fibula
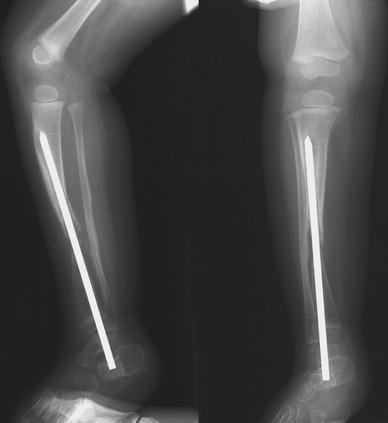
At 6 months postoperatively, the distal fixation had failed due to excessive movement (Fig. 3e, f), and the IM rod in the tibia was therefore revised, this time using a proximal entry site and passing the rod antegrade into the distal tibia, and interlocking the custom rod with two 1.5-mm screws, one in the metaphysis of the distal tibia and one in the epiphysis (Fig. 4a). An additional bone graft was added to the cavity created by the reaming effect of the loose distal fixation screw, as well as to the cross-union site. Four months later, with union proceeding, the epiphyseal screw was removed to “release” the distal tibial physis (Fig. 4b), which promptly grew off the end of the IM rod. The patient was released to full activity, which was resumed without further immobilization.
Fig. 4.
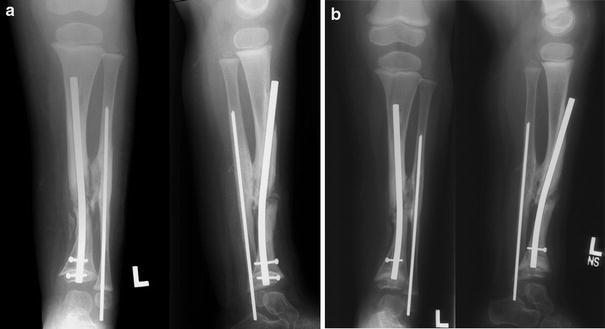
First revision at 4 years of age.a A new rod with two distal interlocking screw holes was inserted antegradely and locked in both metaphysis and epiphysis. An additional bone graft was also added to the pseudarthrosis and the synostosis sites.b Four months later, the epiphyseal locking screw was removed, and the distal tibial epiphysis grew off the end of the rod
He remained asymptomatic for 3 years, after which he began having some aching in his leg following normal activity (Fig. 5). The distal tibia had grown significantly off the end of the IM rod, and the remaining interlocking screw had broken, allowing rod migration. Because of the significant change in symptoms and function, a second revision was performed, requiring a new osteotomy in the proximal one-third of the tibia to regain alignment of the leg (Fig. 6). The original pseudarthrosis site was left undisturbed, and a new rod inserted antegrade from a proximal entry site near the proximal tibial physis. A new proximal fibular osteotomy was also performed and fixed with a new IM rod, to facilitate the tibial rod revision and insure that the new tibial osteotomy was not held distracted. The C-shaped IM rod (Fig. 7) provided excellent correction of the valgus and flexion of the tibia, with the proximal osteotomy allowing direct correction of the sagittal plane. By 2 months after this procedure, the proximal osteotomy had healed sufficiently to allow removal of the postoperative cast, and the patient was again allowed full activity as tolerated without external bracing (Fig. 7).
Fig. 5.
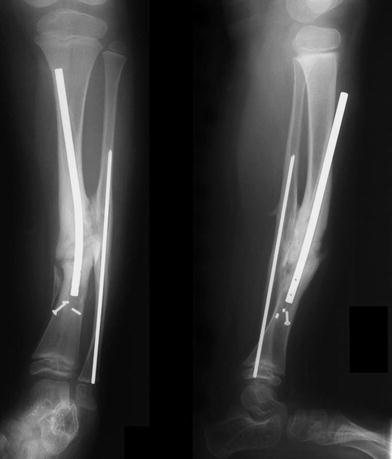
Recurrent symptoms 3 years later. Rod migration with loss of distal fixation are seen
Fig. 6.
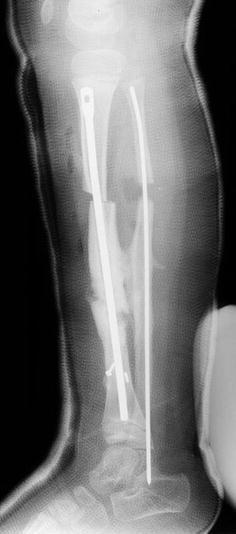
Second revision. A new C-shaped Ender nail has been placed from a new proximal entry site, and a proximal tibial osteotomy performed to assist sagittal plane correction, along with a new fibular osteotomy. The original pseudarthrosis site has been left undisturbed
Fig. 7.
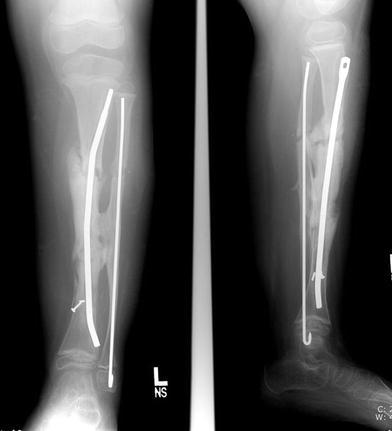
Healing of both osteotomies 2 months later
Subsequent follow-up over the next 7 years (to age 16 years) showed solid union with no evidence of any resorption, recurrence of deformity, or cortical atrophy (grade-1 outcome [1]). Both the proximal and distal physes grew off the IM rod appropriately, confirming normal distal tibial physeal function (Fig. 8). At the most recent follow-up, the patient was fully active and asymptomatic despite significant weight gain (Fig. 9). He also was noted to have lost most of the stigmata of the cutaneous mastocytosis.
Fig. 8.
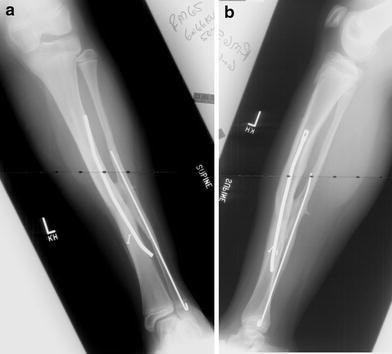
Most recent follow-up (age 16 years)
Fig. 9.
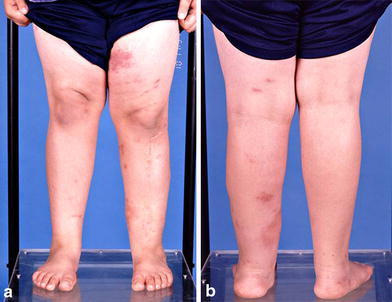
Clinical appearance at age 14 years. There is no atrophy of the left leg
It was the worst of times.....
A 9-month-old female infant presented for management of a fractured left tibia that had occurred with minimal trauma a week earlier. She had several café au lait spots, but there had been no previous evidence of a tibial deformity or lesion. The fibula remained intact despite the tibial lesion (Fig. 10). Initially placed in a long leg cast, there was no progress toward union after a 3-month period of immobilization; so, at the age of 1 year, she underwent a type-C procedure [1] consisting of resection of the tibial pseudarthrosis site and placement of a Williams rod with transfixation of the ankle. The intact fibula was not approached so that no shortening was performed or could occur. Iliac crest bone graft was harvested from the anterior hemipelvis and placed in the tibial defect. After 3 months of immobilization, no progress toward union was again noted (Fig. 11); thus, she underwent a second, posterolateral bone graft procedure, this time from the posterior iliac crest. She was placed in a long leg cast, which was converted to a KAFO fracture brace some 5 weeks later.
Fig. 10.
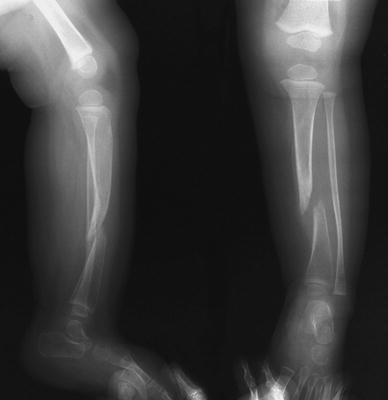
Initial X-ray appearance, left tibia, at age 9 months
Fig. 11.
Three months s/p initial surgery at age 1 year—type-C procedure. No evidence of callous formation. A second grafting procedure was performed
Over the ensuing 3 years, the patient was able to ambulate only while her leg was braced. At the age of 5 years, there was noticeable atrophy of the leg, motion at the pseudarthrosis site, and a varus and internal rotation deformity of the tibia (Figs. 12, 13). The Williams rod had “migrated” out of the ankle as the distal tibia grew off the rod, but the lack of union allowed the rotational deformity and proximal migration of the distal tibial segment (Fig. 13). Due to increasing symptoms and decreasing function, a bone transport procedure was performed using the Ilizarov technique. The leg was fixed in a tibial frame after resection/debridement (for the second time) of the pseudarthrotic bone ends; a proximal osteotomy of the tibia was performed; and the tibia transported distally using oblique olive wire longitudinal traction. The fibula, as in the earlier rodding procedure, was again left intact (Figs. 14, 15).
Fig. 12.
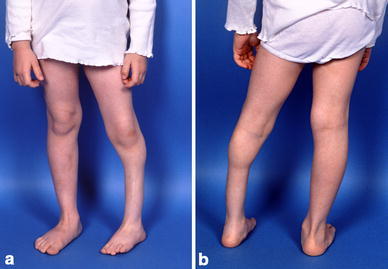
a, b Clinical photos at age 5 years with atrophy and varus deformity
Fig. 13.
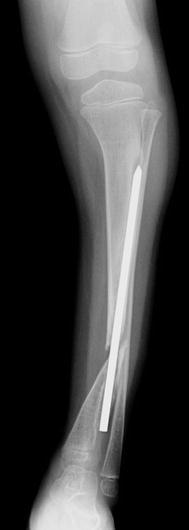
X-ray at age 5 years of persistent nonunion, with proximal migration of the distal tibia segment (preop. Ilizarov frame #1)
Fig. 14.
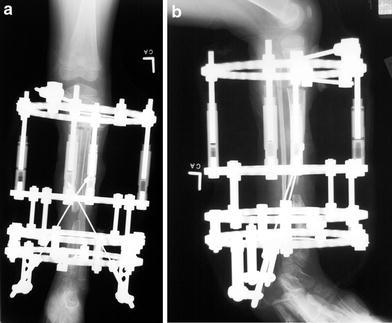
a, b Anteroposterior and lateral X-rays 3 weeks following transport frame placement. Slight distraction at the proximal corticotomy site has occurred, with distal movement of the transported segment of the tibia using oblique longitudinal olive wires
Fig. 15.
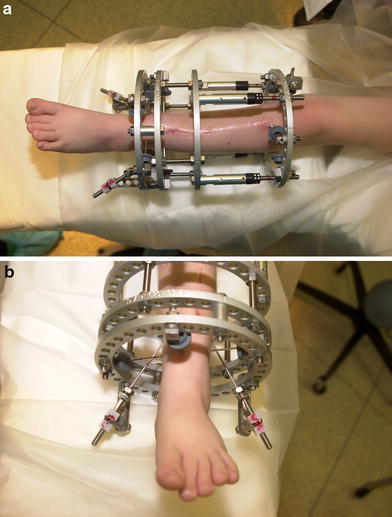
a, b Clinical appearance of transport frame with oblique longitudinal wires attached to the distal ring
After about 6 weeks of transport, the tibial bone ends were ready to be “docked” but were malaligned, having been deflected by interposed fibrous tissue that had grown in the original resection gap and presumed different rates of distraction on the olive wires. The pseudarthrosis was again exposed and debrided to bleeding bone ends; and, by applying new transversely oriented wires, the ends were coapted and placed under compression between rings proximal and distal to the resection site, with removal of the longitudinal wires (Fig. 16). An additional iliac crest graft (from a previously harvested posterior site) was added at the docking site (Fig. 16d, e). The frame was maintained for 4 months, during which time the patient also received bisphosphonate therapy in an attempt to thwart any ongoing bone resorption. After a total of 6 months in frame, it was removed and she was placed in a long leg cast and then braced (Fig. 17). Intravenous palmidronate and low-dose ultrasound at the pseudarthrosis site were also used to increase the likelihood of union. Unfortunately, the tenuous union did not hold up, and by 6 months following frame removal (1 year after frame application), the situation had essentially reverted to the preoperative state, with a gross pseudarthrosis in the original tibial site and an intact, indeed hypertrophied, fibula (Fig. 18). Due to the fact that she was relatively stable and asymptomatic while in brace, no further treatment was offered at that point.
Fig. 16.
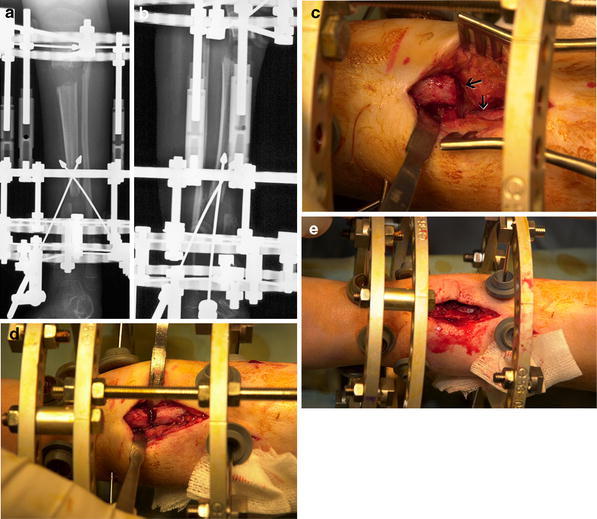
a, b X-ray nearing completion of transport. The bone ends are not aligned, requiring operative “open reduction” with frame revision to achieve “docking”. c Intraoperative view of offset bone ends (distal end left). The fibrous tissue deflecting the proximal bone end is seen (arrow). d, e Reduction and bone grafting of bone ends. The transported segment is now fixed to an intercalary “float” ring by transverse wires for segmental compression
Fig. 17.
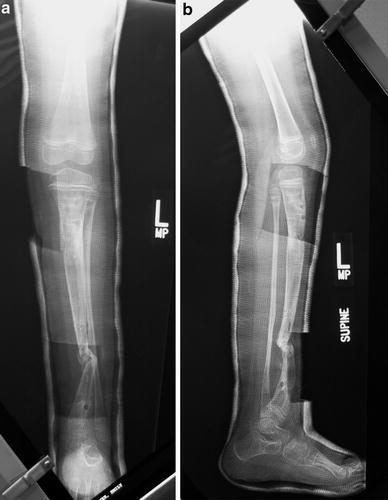
X-ray of tibia in cast at frame removal, age 5 years and 8 months. Windows in the cast were cut in order to place a low-pulse ultrasound generator at the pseudarthrosis site to stimulate healing
Fig. 18.
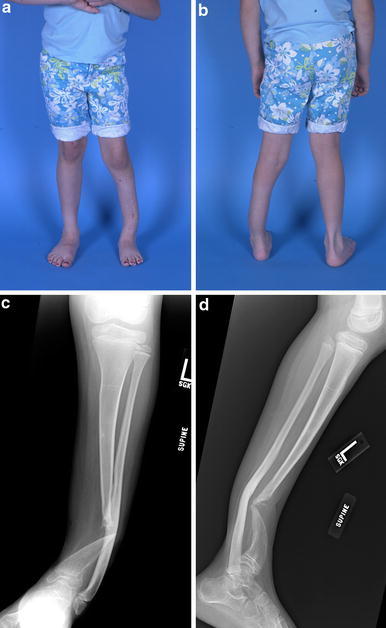
X-rays and clinical photos at age 9 years, with persistent non-union
She was followed until age 9 years, when she developed further pain and disability with progressive anterolateral bowing and ankle deformity. Gross motion was apparent at the pseudarthrosis site. Although in the intervening 2 years both intramedullary rodding and vascularized fibular transfer had been considered for the next treatment option, she underwent a second bone transport procedure, this time removing a fibular segment as well as resection of the tibial pseudarthrosis (for the fourth time). The gap created by the tibia resection—the bone was aggressively debrided to “normal” intramedullary canal—was acutely compressed as far as the soft tissues would permit without ischemia to tissues, and a similar amount was removed from the fibula, in an attempt to narrow the gap between bone ends as much as possible (Fig. 19a–c). The tibia was then osteotomized proximally and fixed by transverse half pins and wires to an intercalary transport ring. The fibula was not transported but simply fixed to the proximal and distal tibia–fibula rings (Fig. 19d, e). After 2 months of transport (Fig. 19f–h), with the bone ends again malaligned, open reduction/”docking” of the tibial bone ends was performed, with the application of additional iliac bone graft and bone morphogenic protein (rh-BMP) after another debridement of the site (Fig. 19i–k). The frame was placed under compression and maintained for an additional 5 months, at which time the pseudarthrosis appeared to have enough callous and the regeneration in the transport site proximally was satisfactory enough to allow frame removal with cast placement (Fig. 20). The tibia appeared to be solidly healed 6 months later (Fig. 21), for the first time ever, although there was a severe valgus ankle deformity with a suggestion of diastasis. This appeared to be related to the fibular non-union related to the omission of transporting the fibula during the last treatment, and a more proximal diaphyseal valgus (Fig. 22).
Fig. 19.
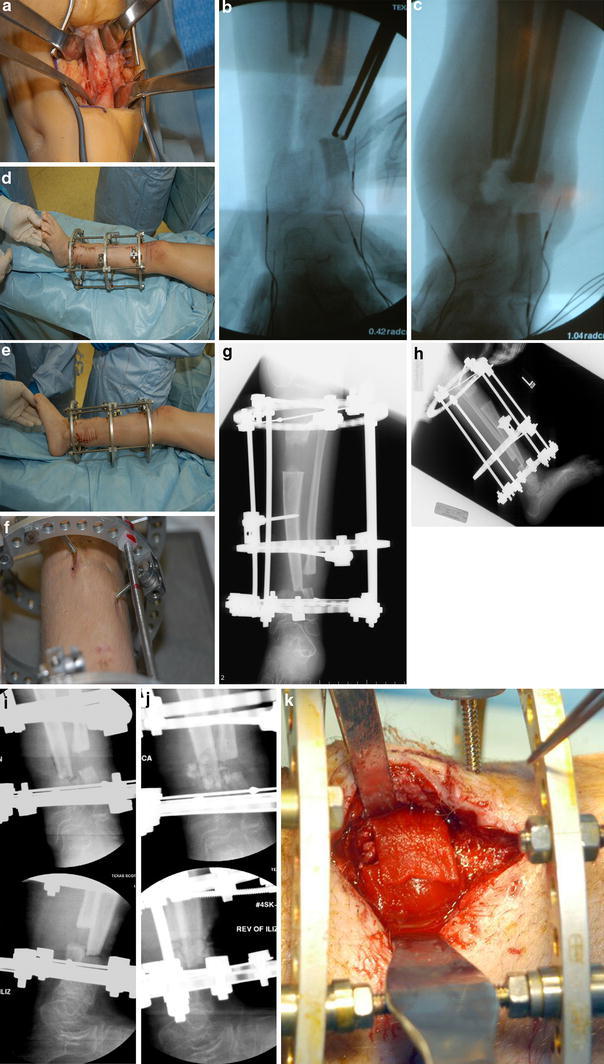
a Intraoperative view of pseudarthrosis site prior to resection: frame no.2 4/05. b Resection of tibial pseudarthrosis and fibulectomy prior to acute shortening. c Maximum acute shortening allowed by the surrounding soft tissues. d, e Tibia transport frame. The fibula is fixed to the proximal and distal rings only, with the tibia alone being transported by the middle ring. Bulging of the soft tissues around the resection site is from maximum allowed acute compression. f Pin tracts cutting the skin as distal transport of the tibia occurs (ankle, top). g, h Completion of tibial transport, with misalignment of the tibial ends in the coronal plane. The fibulectomy gap is unchanged from the beginning of tibia transport. i Intraoperative X-ray during open reduction of tibial ends to achieve “docking”. j Intraoperative X-ray following bone grafting, addition of BMP, and compression across the docking site. k Intraoperative view of sponges soaked with rh-BMP being applied to the docking site
Fig. 20.
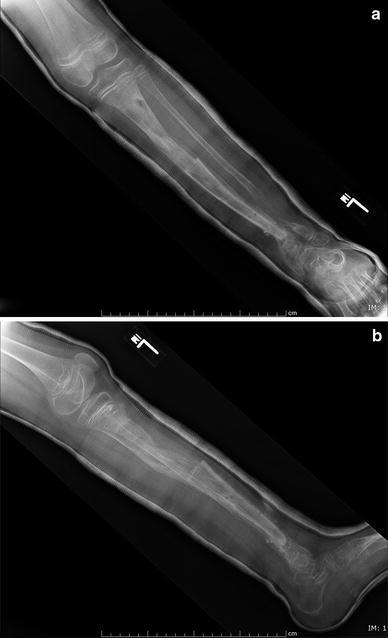
a, b X-rays in cast following frame no.2 removal
Fig. 21.
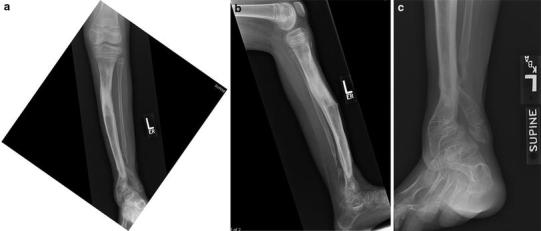
X-rays 6 months after frame removal, showing robust healing of the tibia but severe ankle valgus and fibular discontinuity
Fig. 22.
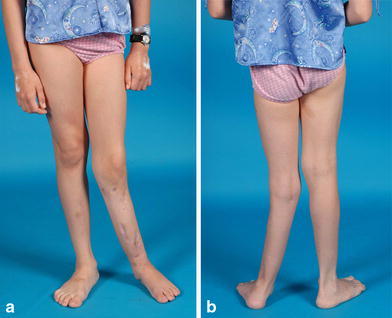
Clinical photos 6 months following frame no.2 removal. Diaphyseal and ankle valgus are readily apparent
Growth modulation via transient hemiepiphyseodesis of the proximal medial tibia and permanent hemiepiphyseodesis of the distal medial tibia, combined with creation of a distal tibia–fibula synostosis [3], were chosen to attempt correction of these residua and gain alignment. She was 10.5 years old and it was 9 months after frame no.2 removal (Fig. 23). Four months later, she fell while skating and wearing her protective fracture brace; she suffered a fracture through the screw holes of the distal tibia–fibula synostosis fixation, with one of the screws fracturing as well. Following another 4 months of cast immobilization, no callus was seen at the fracture site (Figs. 24, 25). The patient elected at this time to have a below-knee amputation.
Fig. 23.
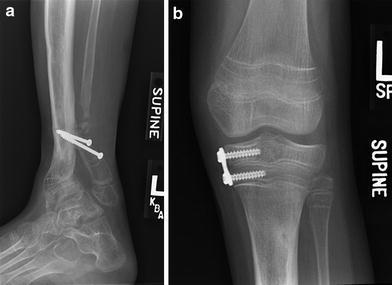
a X-ray following distal tibia-fibula synostosis and medial hemiepiphyseodesis. b Eight-plate hemiepihyseodesis of medial proximal tibia
Fig. 24.
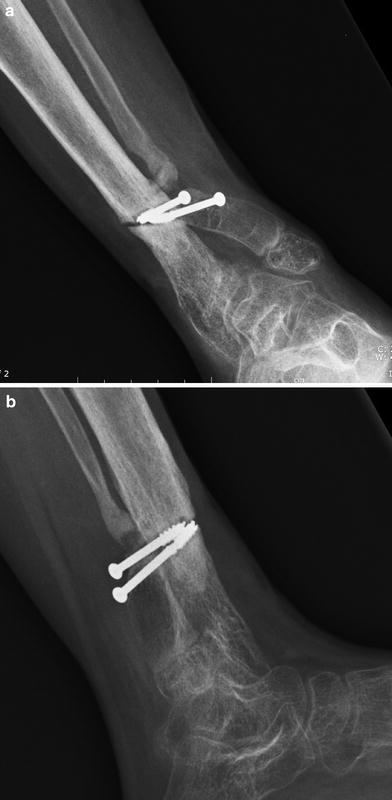
New fracture at distal tibia through screw holes
Fig. 25.
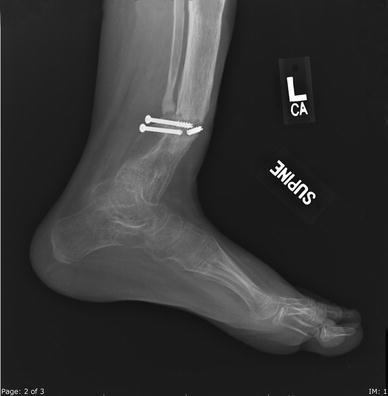
X-ray 4 months following fracture, with no evidence of healing
It was the age of wisdom......
How can one explain the radically different outcomes for these two apparently identical cases of CPT? Both children had NF-1, both fractured at or just before the age of ambulation, and both had established tibial pseudarthroses with intact fibulae at the time of the first surgical intervention (Figs. 26a, b, 27a). The only preoperative difference was the age at first surgery—in case 1, surgery was not performed until roughly age 3 years, due to the patient presenting and being initially treated elsewhere, and being functional in an orthosis; in case 2, operative treatment began at age 1 year, soon after cast immobilization was seen to be ineffective. The importance, or lack of it, of the age at first surgery on the eventual outcome had been debated both ways, with inconclusive results—Karol [4] showed a poorer prognosis for early-onset cases (first operation at ages less than 4 years), while Joseph [5] showed benefit for early surgery, and Johnston [1] showed no difference based on age at first surgery.
Fig. 26.
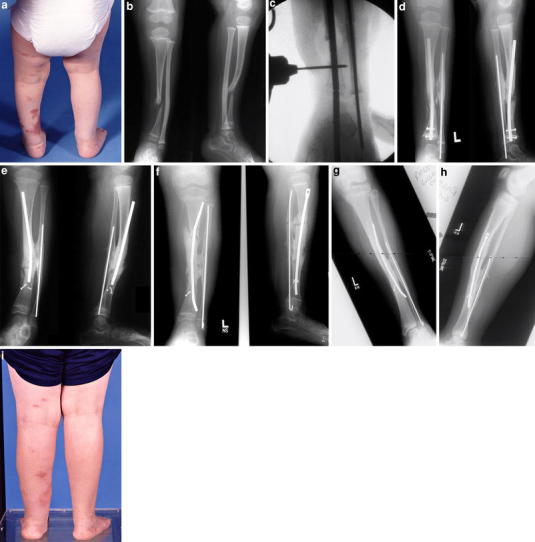
Summary of case 1:, a Clinical appearance at age 2 years and 6 months, b age 2 years and 6 months, c intraoperative age 3 years and 2 months, d age 4 years (s/p first revision), e age 7 years, f age 8 years (s/p second revision), g–i age 16 years (last f/u)
Fig. 27.
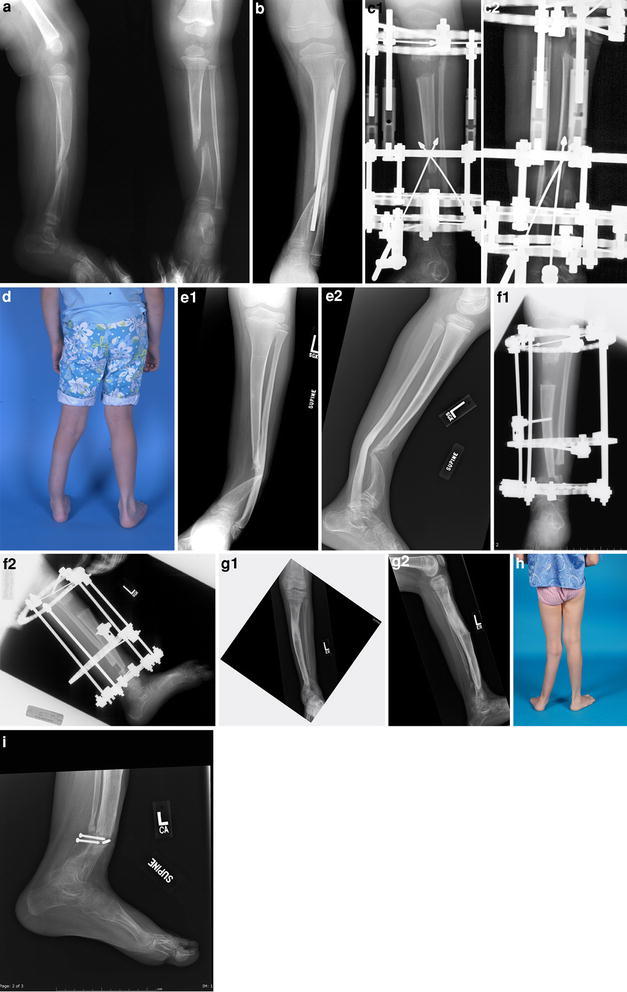
Summary of case 2: a age 1 year, b age 5 years and 3 months—s/p type-“C” procedure with non-union,c1, c2 age 5 years and 6 months—first transport, prior to “docking”, d age 6 years—clinical appearance,e1, e2 age 6 years,f1, f2 age 9 years—second transport, prior to “docking”,g1, g2 age 10.5 years—valgus + fibula non-union, h age 10.5 years—clinical appearance, i pathological fracture after 4-month casting
We would propose that the management of the pseudarthrosis site is a major element distinguishing the two cases. In case 1, the site was immediately shortened and compressed so that whatever advantage resection of hamartomatous tissue and early weight-bearing might provide toward healing could be exploited (Fig. 26c); in case 2, the bone ends were never in contact during the initial IM rod procedures (Fig. 27b), and only gradually came into contact after bone transport in the two subsequent Ilizarov sessions. The role of removing a source of distraction—the intact fibula—must be emphasized, as well as the debridement and devascularization of the bone ends in case 2 on multiple occasions. While other aspects of the treatments, to be discussed below, also must have played a role in the outcomes, the die was certainly cast in different directions by the two dramatically opposite approaches to obtaining union.
Case 1 represents as close to an ideal outcome for CPT as possible, based on the criteria of: achievement of union early in the course of treatment, so that limb function was preserved; maintenance of that union and function by the retention of IM fixation; revision of the IM rod with re-alignment of the tibia by osteotomy as soon as stabilization was lost (Figs. 5, 6, 26e); and achieving the alignment and permanent IM fixation without transfixation of the ankle joint (Fig. 26).
Details of the rodding technique deserve further comment. The resection of the strangulating periosteal tissue in the pseudarthrosis site has traditionally been thought to play a crucial role in removing a non-biological obstruction to the achievement of union, with some investigators regarding the periosteum as the primary pathological tissue [6–8]. The length of this resection of dysplastic bone obviously affects the ability to completely shorten the tibia due to constraints of the soft tissue envelope. What has perhaps not been emphasized adequately is the importance of osteotomy and shortening of the fibula, not only to allow acute tibial shortening, but to remove the intact fibula from holding the tibial bone ends distracted, similar to the situation in which a tibial fracture or osteotomy is delayed from healing by an intact or prematurely healed fibula. By osteotomy/shortening of both tibia and fibula, and IM fixation of both bones (type-A procedure [1]), the possibility of an intact fibula becoming an agent of persistent pseudarthrosis is generally eliminated. Furthermore, if the fibula is initially intact, there is little chance of a fibular pseudarthrosis developing if the fibular resection is appropriate to allow end to end contact of the bone ends (Fig. 3c).
The type-C procedure [1] performed initially for case 2 was unfortunately, but not unexpectedly, ineffective, even with additional bone grafting as a second separate procedure. Leaving the fibula intact for reasons of maintaining length and stability overlook the main pathological process, the presence of bone discontinuity. Even if the pseudarthrosis resection is minimized, the gap left by the failure to shorten due to lack of fibular ostectomy probably fills with interposed fibrous tissue during the time when the bone graft is attempting to re-establish tibial continuity. The type-C procedure is thus an invitation to delayed union (Fig. 27b) because of this gap to be overcome, a daunting task in the best of circumstances because of the underlying diagnosis (NF-1) and dysplastic bone. In addition, while waiting for the IM rod to migrate with growth out of the ankle joint, the extremity must be immobilized to protect the articular surfaces of the ankle from damage by the transfixation rod. Atrophy and stiffness of the calf and ankle are certain to occur in some form following this traditional Charnley–Williams method [2, 4, 9].
Aggressive tibial resection/shortening with fibular ostectomy theoretically increases instability of fixation in the distal tibial segment—hence the desire to maintain the “stability” provided by an intact fibula, as in case 2. The use of an interlocked rod, to avoid crossing the ankle joint, is a response to this need for additional stability, once the importance of the fibular ostectomy is accepted, as well as to the detrimental effect on ankle function by the Charnley–Williams rod crossing the ankle [4]. Such interlocked rods can be custom-made by simply drilling interlocking holes in existing implants. Due to diameter restrictions in young patients with dysplastic tibiae, the interlocking screws or threaded Steinmann pins are of even smaller diameter (typically 1.5–2.0 mm), and thus failure by screw fatigue is somewhat predictable (Figs. 3e, f, 5). Failed interlocking screws, with fixation loss and rod migration (Fig. 5), can always be revised using another rod. The importance of avoiding ankle transfixation in terms of long-term function is believed to outweigh the disadvantage of probable revision IM rodding. We are currently managing the predictable failure of the small-diameter interlocking screws by switching to curved flexible IM rods (Figs. 6, 7, 26f). The three-point fixation achieved by a curved nail provides adequate rotational and longitudinal fixation, especially with the fibula also stabilized. In a less-stable situation, e.g., first-time pseudarthrosis treatment, use of two flexible nails in divergent pattern in the distal fragment has shown short-term efficacy.
A comment about aggressive bone grafting is also appropriate. Once the decision for surgical treatment has been made, the surgeon must be prepared to graft and re-graft the pseudarthrosis site, including creation of synostosis proximal and distal to the site, to recruit the fibula into a greater stabilizing function. Lack of progress in achieving union after the second procedure in case 2 (Figs. 11, 13) was not addressed, converting the clinical evolution to one of chronic pseudarthrosis with no hope of functional improvement. Although unavailable at that time, biological enhancement of healing using rh-BMP (Fig. 19k) would now be attempted almost routinely in an all-out effort to obtain union, based on our institutional success with this modality [10]. Furthermore rh-BMP would be utilized in the primary procedure(s) rather than reserving it for recalcitrant cases, based on the assumption that the earlier functional union is achieved, the more normal will be the functional outcome of the leg. The anecdotal efficacy of rh-BMP is well illustrated in the final docking/frame revision in case 2 (Figs. 20, 21), where union was finally achieved after all of the previous failures, albeit union that was soon to succumb to severe malalignment (excessive valgus) and the presence of stress risers (Figs. 23, 24, 27h, i).
The devil is in the details....
The Ilizarov apparatus, used to effect a variety of reconstructive strategies for CPT, increases our armamentarium in the management of this condition. However, we should note that, in the majority of cases, the fundamental pathophysiology of this disorder continues unaltered even after “successful” treatment using Ilizarov reconstructive strategies. In fact, one of the most enthusiastic proponents of Ilizarov reconstruction for CPT includes the caveat of a 68% re-fracture rate [18].
Several details of the Ilizarov method in general, and its use in case 2, deserve discussion. The advantages that the Ilizarov method offers in the treatment of CPT include superior bone segment fixation of osteopenic bone with crossed, tensioned fine wires, the ability to gradually correct residual deformity by apparatus manipulation, the permission of joint motion and weight-bearing in the apparatus, and the production of increased blood flow to the limb by proximal tibial corticotomy. A tragic irony of CPT is the ability to perform an osteotomy and lengthening of the proximal tibia with relative impunity with the concomitant inability to effect union a few centimeters distally at the pseudarthrosis site (Fig. 27e).
The foot can be incorporated into the frame connected to the distal tibial segment rings if fixation of that segment alone is inadequate. Adequacy of distal segment fixation can be determined intraoperatively by plantarflexing and dorsiflexing the foot, and observing for motion of the distal tibia in the pseudarthrosis resection wound. In case 2, distal tibial fixation was deemed adequate on both occasions, and the foot was not incorporated. While some might regard this decision as a technical error, leaving the foot out of the apparatus has distinct advantages of less pain and increased weight bearing in the apparatus.
Critique of the bone transport method with the fibula intact (to preserve length) must begin with the observation that this method per se, described anecdotally as just one of several Ilizarov strategies, appears to be less successful in achieving union than alternatives involving acute shortening at the pseudarthrosis site [11]. As in other Ilizarov reports, failure of transport is often attributed to severity of pathology being salvaged, since many patients have been treated previously, and often repeatedly, with unsuccessful outcomes. A basic flaw in the method, however theoretical in nature but with high visibility in retrospect, might be illustrated as follows: resection/debridement of bone ends to create a gap and remove obstructive non-biological tissue then fills with fibrous tissue during roughly 6 weeks of transport. The bone ends are then “deflected” by this fibrous tissue filling the gap (Fig. 16), necessitating that the bone ends must be debrided again when “docking”—essentially an open reduction—is to occur (Fig. 27c, f).
Despite Ilizarov’s suggestions to the contrary, most experienced surgeons have recognized that after bone transport, the pseudarthrotic end of transported bone is avascular, thus encouraging delay in the very union that is to be produced. Debridement at the “docking site” is therefore planned, to excise this fibrous tissue and establish bleeding bone ends. We usually add autogenous bone graft and BMP at the time of this debridement (Figs. 16c, 19k). If oblique olive wires have been used for bone transport, we replace them with crossed wires (Figs. 16d, e). This wire exchange allows for better compression between the bone segments across the pseudarthrosis site.
Docking alignment problems might be resolved by intramedullary stabilization either during or at completion of transport. Recall that maintenance of union requires maintenance of alignment of the extremity, usually by some form of permanent stabilization. For reasons primarily involving the high likelihood of seeding intramedullary sepsis, IM rod placement is usually avoided with external fixation, even though its efficacy is assisting alignment for docking or stabilizing, and protecting tenuous union at a docking site would seem obvious. This inherent drawback—the relative incompatibility of IM rodding and trans-osseous fixation due to the problem of sepsis—is common to all Ilizarov methods applied to CPT.
Early descriptions of bone transport technique [12–14] reveal few details about the actual handling of the docking site, especially when the end of the transported segment does not coapt well to the target segment (Fig. 27c, f). In fact the latter situation—misalignment at docking—is mentioned only to the extent that it is caused by malalignment of the rings within the frame [13]. Oblique olive wires produce less soft tissue injury and scarring during bone transport than crossed wires on a transport ring. However, as much as possible, the paired wires must be at the same level in the bone, at the same angle, and pulled at the same rate to prevent angular distortion with lengthening (Fig. 14a). Deviations from these ideals will lead to malalignment between the bone segments (Fig. 16a). As already mentioned, uncontrollable soft tissue interposition within the pseudarthrosis resection gap also deflects the transport bone segment. Exchange of the oblique olive wires for crossed tension wires on a “float” ring incorporated in the original frame allows for coaptation of deviated bone ends at the same time as the debridement/bone grafting of the docking site (Figs. 16e, 19g–j).
However, the inevitable development of fibrous interposition tissue requiring debridement to obtain viable bleeding bone ends, which can then heal by contact compression, is an overlooked disadvantage of the transport method, because repeated debridements, both at the commencement of transport as well as at docking, are required. The specter of devascularization of the bone ends in this method is well represented in the clinical course for the first frame application of case 2. The EPOS multi-center study of 108 tibiae (194 procedures) treated using Ilizarov methods—the largest series ever compiled—reported the highest rate of tenuous or non-union following bone transport procedures (52%) when compared with either simple resection + acute shortening + compression or with resection + acute shortening + metaphyseal lengthening (25–35% tenuous or non-union) [11]. The EPOS group even commented, somewhat prophetically, that “...it was surprising to find that segmental bone transport resulted in a lower fusion rate than the other procedures” [11]. While the latter report did not include statistical analysis and is obviously retrospective and uncontrolled, the choice of segmental bone transport in case 2 can be criticized as the procedure least likely to obtain union, similar to the result (failure) of the original type-C procedure IM nailing.
Resection of the pseudarthrosis site with immediate shortening and compression, with lengthening via a proximal metaphyseal corticotomy, has been successfully advocated [15–17]. Immediate shortening and compression would theoretically have the advantage, similar to the A procedure for IM rodding, that no gap to fill with fibrous tissue is allowed to develop, and the “freshened” bone ends are immediately coapted, thus having the greatest chance to unite. The versatility of the Ilizarov frame would allow limb shortening to be addressed by the proximal corticotomy and lengthening, for which there is little concern of non-union developing at this site remote from the original pseudarthrosis.
The handling of the fibula during the bone transports must also be reviewed. Since the Ilizarov literature [12–14] provides few specifics concerning management of the fibula, caution must be exercised in drawing conclusions based on two treatment episodes. Suffice to say that maintaining an intact fibula during the first frame treatment contributed to the non-union by maintaining the docking ends in relative distraction following completion of transport (Fig. 17), similar to results from other studies employing both rodding and Ilizarov methods [1, 18]. More detrimental, perhaps, was the creation of a fibular non-union during frame no. 2, by virtue of failing to transport or re-establish fibular continuity, inviting the severe ankle valgus malalignment (Figs. 19, 21, 22, 27g), which necessitated additional treatment following union and arguably led to the final pathological fracture (Fig. 27i). While fibular osteotomy and appropriate shortening are crucial to healing of a tibial pseudarthrosis that is being resected and shortened, equal concern must be shown for re-establishing fibular continuity, so that pathological ankle valgus does not threaten a newly healed tibia.
Epilogue
These are obviously only single case examples of the extremes of outcome for CPT (Figs. 26, 27). The details of each method, however, are what determines in many instances the ultimate success or failure, and such details are often overlooked or omitted in the description of the techniques. We have devoted additional space to fully characterize the issues and offer comprehensive analysis of the pros and cons of the treatment options.
The goals of treatment of CPT are to obtain and maintain union in order to assure a functional limb. Toward these goals, immediate contact of bone ends with compression and ample bone graft and biological enhancers are likely to achieve them most frequently. Attention to internal fixation and fibular continuity are crucial for maintaining alignment and consequently union. We hope this review clarifies the efficacy controversies of each treatment method and will assist patients with an affected tibia and surgeons attempting to treat them to escape the winter of despair.
Acknowledgments
The authors wish to acknowledge the value of Charles Dickens’ perspective in the completion of this manuscript.
References
- 1.Johnston CE., II Congenital pseudarthrosis of the tibia. Results of technical variations in the Charnley–Williams procedure. J Bone Joint Surg Am. 2002;84:1799–1810. [PubMed] [Google Scholar]
- 2.Williams PF. Fragmentation and rodding in osteogenesis imperfecta. J Bone Joint Surg Br. 1965;47:23–31. [PubMed] [Google Scholar]
- 3.Langenskiöld A. Pseudarthrosis of the fibula and progressive valgus deformity of the ankle in children: treatment by fusion of the distal tibial and fibular metaphyses. Review of three cases. J Bone Joint Surg Am. 1967;49:463–470. [PubMed] [Google Scholar]
- 4.Karol LA, Haideri NF, Halliday SE, Smitherman B, Johnston CE., II Gait analysis and muscle strength in children with congenital pseudarthrosis of the tibia: the effect of treatment. J Pediatr Orthop. 1998;18:381–386. [PubMed] [Google Scholar]
- 5.Joseph B, Somaraju VV, Shetty SK. Management of congenital pseudarthrosis of the tibia under age 3 years of age: effect of early surgery on union of the pseudarthrosis and growth of the limb. J Pediatr Orthop. 2003;23:740–746. doi: 10.1097/01241398-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Aegerter EE. The possible relationship of neurofibromatosis, congenital pseudarthrosis and fibrous dysplasia. J Bone Joint Surg Am. 1950;32:618–626. [PubMed] [Google Scholar]
- 7.Briner J, Yunis E. Ultrastructure of congenital pseudarthrosis of the tibia. Arch Pathol. 1973;95:97–99. [PubMed] [Google Scholar]
- 8.Cui G, Lei W, Li J, et al. Histopathology of congenital pseudarthrosis of the tibia. Zhonghua Yi Xue Za Zhi. 2002;82:487–491. [PubMed] [Google Scholar]
- 9.Charnley J. Congenital pseudarthrosis of tibia treated by intramedullary nail. J Bone Joint Surg Am. 1956;38:283–290. [PubMed] [Google Scholar]
- 10.Richards BS, Welch RD, Shrader MW, Johnston CE (2005) Use of rhBMP-2 in congenital pseudarthrosis of the tibia. Presented at the Annual Meeting of the Pediatric Orthopedic Society of North America, Ottawa, May 12–15 2005
- 11.Grill F, Bollini G, Dungl P, Fixsen J, Hefti F, Ippolito E, Romanus B, Tudisco C, Weintroub S. Treatment approaches for congenital pseudarthrosis of the tibia: results of the EPOS multicenter study. J Pediatr Orthop B. 2000;9:75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop. 1992;280:81–93. [PubMed] [Google Scholar]
- 13.Green SA, Jackson JM, Wall DM, Marinow H, Ishkanian J. Management of segmental defects by the Ilizarov intercalary bone transport method. Clin Orthop. 1992;280:136–142. [PubMed] [Google Scholar]
- 14.Cattaneo R, Catagni M, Johnson EE. The treatment of non-unions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop. 1992;280:143–152. [PubMed] [Google Scholar]
- 15.Boero S, Catagni M, Donzelli O, Facchini R, Frediana PV. Congenital pseudarthrosis of the tibia associated with neurofibromatosis-1: treatment with Ilizarov’s device. J Pediatr Orthop. 1997;17:675–684. doi: 10.1097/01241398-199709000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem I, Damsin JP, Carlioz H. Ilizarov technique in the treatment of congenital pseudarthrosis of the tibia. J Pediatr Orthop. 1997;17:685–690. doi: 10.1097/01241398-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Keret D, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, Ippolito E, Romanus B, Tudisco C, Weintroub S. The fibula in congenital pseudarthrosis of the tibia: the EPOS multicenter study. J Pediatr Orthop B. 2000;9:69–74. doi: 10.1097/01202412-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 18.El-Rosasy MA, Paley D, Herzenburg JE. Congenital Pseudarthrosis of the Tibia. In: Rozbruch RS, Ilizarov S, editors. Limb lengthening and reconstruction surgery. New York: Informa Healthcare USA; 2007. pp. 485–493. [Google Scholar]


