Abstract
Purpose
Traditionally, angular deformities are treated by means of osteotomy. In patients who are skeletally immature, this major intervention can be avoided by influencing or guiding the growth of the affected physis. Recently, a new device was presented as an alternative to the widely used Blount staple. Stevens developed a technique using a two-hole, non-locking plate with two screws to perform temporary hemiepiphysiodesis in children. We studied the effectiveness of this new device in correcting angular deformities in children even younger than 5 years of age.
Methods
We evaluated our first series of 11 patients (17 eight-Plates) who underwent treatment for angular deformities of various origins and were followed to completion of correction. The average age at hemiepiphysiodesis was 10 years and 2 months (age range 4 years and 11 months–13 years and 8 months). The device was inserted in the lateral distal femur (two cases), the medial distal femur (eight cases), the lateral proximal tibia (two cases), the medial proximal tibia (four cases), and the medial distal tibia (one case).
Results
The eight-Plate was inserted for an average of 9.5 months (range 5–13 months). The joint orientation angles and the mechanical axis improved in all patients, with the exception of one 13-year and 8-month-old boy with a resected osteosarcoma and a compromised growth plate. In valgus cases (12 limbs, 13 eight-Plates), the mechanical axis deviation improved by an average of 30.7 mm (range 13–55 mm). In varus cases (four limbs, four eight-Plates), the mechanical axis deviation improved by an average of 38.8 mm (range 0–74 mm). No hardware failures, extrusion, growth arrest, or other complications were observed. None of our patients required an osteotomy or repeat eight-Plate insertion.
Conclusions
We consider the eight-Plate to be an ideal tool for treatment of angular deformities in growing children. It allows for precise insertion and is reliable. It is also less likely to extrude like the Blount staple.
Keywords: Hemiepiphysiodesis, Angular deformity, Children, Eight-Plate
Introduction
Adults with angular deformities of the long bones are treated by means of osteotomy [1]. Growing children can undergo a less traumatic option: hemiepiphyseal arrest with staples. Stapling was first reported by Blount and Clarke [2] in 1949 to treat angular deformities and length discrepancies of the lower limbs. The next advance in reversible hemiepiphyseal arrest was proposed by Métaizeau et al. [3], who used transphyseal screws. However, it is unclear whether epiphysiodesis with transphyseal screws truly is reversible. Nouth and Kuo [4] used the Métaizeau method to treat limb-length discrepancy with growth arrest and angular deformities with hemiepiphyseal arrest. Eidelman and D’Agostino [5] recently described a new Blount staple design that has grooves to allow for more accurate, Kirschner-wire guided insertion.
It has been presumed that staples could be left in place for as long as 2 years without causing permanent growth arrest. However, numerous complications have been observed with staples, including staple failure, breakage, and extrusion [2, 3, 6–10]. To avoid these complications, Dr. Peter M. Stevens of the University of Utah devised a novel device comprised of two screws and a two-hole plate (Fig. 1) and coined the term guided growth to describe its action on the growth plate [11]. The design of the eight-Plate Guided Growth System (Orthofix, McKinney, TX, USA) is thought to be an improvement over the Blount staple because it theoretically does not compress the growth plate like a staple and it is more resistant to spontaneous extrusion. Stevens [11] recently reported his results, but his work has yet to be corroborated by other researchers. We present our early experience with this new device.
Fig. 1.
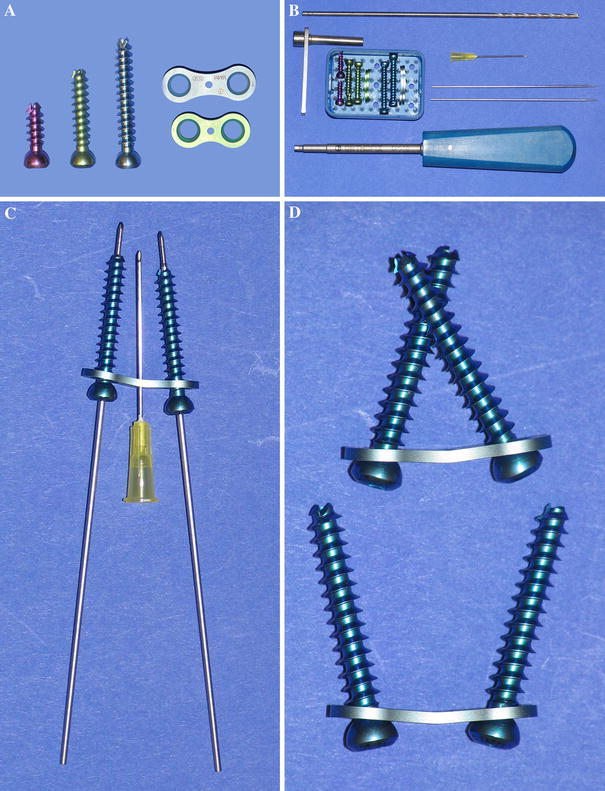
a Photograph shows two sizes of the eight-Plate Guided Growth System: 12 and 16 mm. The eight-Plate kit includes three sizes of cannulated screws (16, 24, and 32 mm). b Photograph shows the complete eight-Plate set (clockwise from top): a 3.2-mm cannulated drill bit, a 21-gauge needle, two 1.5-mm wires with 1.2-mm threaded tips, a screwdriver, four eight-Plates, ten cannulated screws, and a drill-guide. New instrumentation kits include a 3.2-mm cannulated step drill and a guide to limit penetration to 5 mm. c Guide wires, 21-gauge needle, cannulated screws, and an eight-Plate. The cannulated screws are inserted over the guide wires. The 21-gauge needle provides temporary fixation. d Photograph shows that the screws are able to pivot within the eight-Plate (greater than 45° of rotation is possible)
Materials and methods
Between August 2004 and December 2005, we inserted 54 eight-Plates to correct angular deformities of the lower extremities in 42 children. As of January 2006, 17 eight-Plates in 11 patients had been electively removed. We are reporting the results from this group of patients who have completed treatment. The remaining 31 patients are still undergoing treatment and will eventually be the subject of a subsequent long-term follow-up report. All patients had genu valgum or varum deformity, and one with genu valgum also had an ankle valgus deformity. Standing radiographs and anteroposterior and lateral view radiographs were obtained before deciding to perform hemiepiphysiodesis. Preoperative planning was performed to measure the magnitude of the deformity before surgery and at follow-up [12]. No attempt was made to time the intervention so that the end of correction coincided with skeletal maturity. The specific timing of intervention was not felt to be critical because the eight-Plate method is reversible. All children were predicted to have at least 12 months of remaining growth. The goal of this study was to determine whether the eight-Plate can effectively correct angular deformities of the long bones in growing children. For this initial study, follow-up ended at the time of eight-Plate removal.
Surgical technique
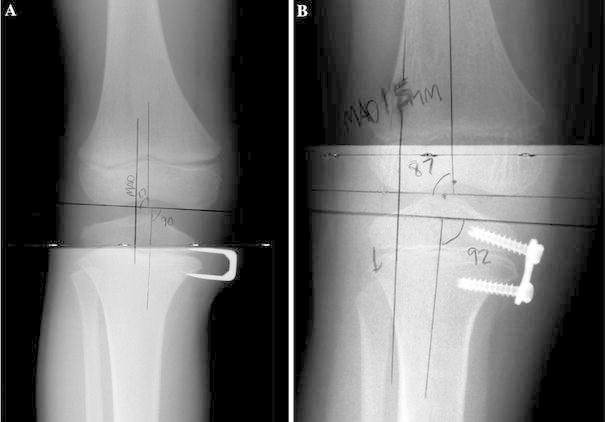
This surgery is performed under general anesthesia and tourniquet control, with the patient positioned supine on a radiolucent table. The physis is localized with the image intensifier in both the frontal and sagittal planes, and a 2.5-cm vertical skin incision is made. During dissection, the surgeon avoids injuring cutaneous nerves such as the saphenous nerve in the distal medial femur, the infrapatellar branch of the saphenous nerve at the medial distal knee, and the saphenous nerve at the ankle. Successive layers of tissue are longitudinally split until reaching the periosteum, which is left intact. A useful rule of thumb is that if the structure can be lifted with forceps, it should be split. The periosteum is tightly adhered to the bone and cannot be lifted with forceps. The epiphyseal vessels are left undisturbed. We use a 12-mm eight-Plate for the proximal or distal tibia and a 16-mm eight-Plate for the distal femur. The eight-Plate is suspended off a 20-gauge needle or small-diameter wire through the central guide hole, which is placed so that it is centered on the growth plate (Fig. 2a). The position of the eight-Plate is verified in the anteroposterior and lateral views using the image intensifier, and the eight-Plate is adjusted until the desired position is reached (Fig. 2b). The eight-Plate should straddle the physis in the anteroposterior view and be centered midway in the sagittal plane. The 1.5-mm temporary guide wires are inserted in both holes of the eight-Plate under image intensifier control to direct them away from the growth plate. Ideally, they should be as parallel as possible. A 3.2-mm cannulated drill bit is then used to ream the outer cortex to a depth of 5 mm over each of the two guide wires. A special step drill and calibrated drill guide are available for this step. Cannulated screws with a length of either 24 or 32 mm are inserted and gently tightened to hold the eight-Plate in position (Fig. 2c). We recommend the 32-mm screws, particularly in the metaphyseal side, because the remodeling of the metaphysis during growth can cause the shorter screw to appear to pull out. Before final tightening, the soft tissue is carefully examined to ensure that the mobile structures such as the pes anserinus and the fascia are not trapped under the eight-Plate. The guide wires are removed. Final radiographs in the anteroposterior and lateral views are obtained with an image intensifier (Fig. 2d). The tourniquet is deflated, hemostasis is obtained with electrocautery, and a solution of 0.5% bupivacaine with epinephrine is injected into the wound edges. The incision is closed in layers with absorbable suture and subcuticular skin closure. A Tegaderm transparent dressing (Nexcare, St. Paul, MN, USA) and a light compressive dressing are applied. A bag of ice or other cold delivery system can be intermittently applied to prevent swelling, especially for distal femoral application. The patient can be discharged to home or spend a night in the hospital, in accordance with pain management requirements and the social situation. Crutches are optional and can be used for 3–7 days, after which progressive activities are permitted as tolerated. Physical therapy is prescribed only if the child is not bending the knee well during the first follow-up visit (7–14 days after surgery) when the wound is examined. In our experience, physical therapy is more likely to be needed after femoral rather than tibial applications, as there might be a knee hemarthrosis. Return to full activity, including contact sports, is allowed 4 weeks after surgery.
Fig. 2.

Application of the eight-Plate. a Anteroposterior view radiograph shows a 1.5-mm Kirschner wire being used to position the eight-Plate so that it straddles the physis. b Lateral view radiograph shows that the position of the eight-Plate is monitored so that it is not positioned too anterior or posterior in the sagittal plane. c Anteroposterior view radiograph shows two guide wires with threaded tips inserted in the two holes of the eight-Plate. Cannulated screws are then inserted over the guide wires. d Anteroposterior view radiograph shows the final position of the eight-Plate and screws. The longer 32-mm screws depicted here are preferred over the shorter screws, especially when inserted in the metaphysis
Patients with femoral eight-Plates should be seen at intervals of 3–4 months, and patients with tibial eight-Plates should be seen at intervals of 4–6 months to monitor growth and deformity correction. This is because the femur achieves correction faster than the tibia because of its more rapid growth rate. As the correction occurs, the screws diverge or pivot. Erect-limb radiographs are obtained and measured to determine the change in the joint orientation angles and the mechanical axis of the limb (i.e., Mikulicz line) [12]. A slight over correction of the Mikulicz line might be recommended (Figs. 3, 4) because some rebound growth can occur after eight-Plate removal. In our study, correction or slight over correction was achieved 5–13 months after insertion and the eight-Plate was then removed.
Fig. 3.
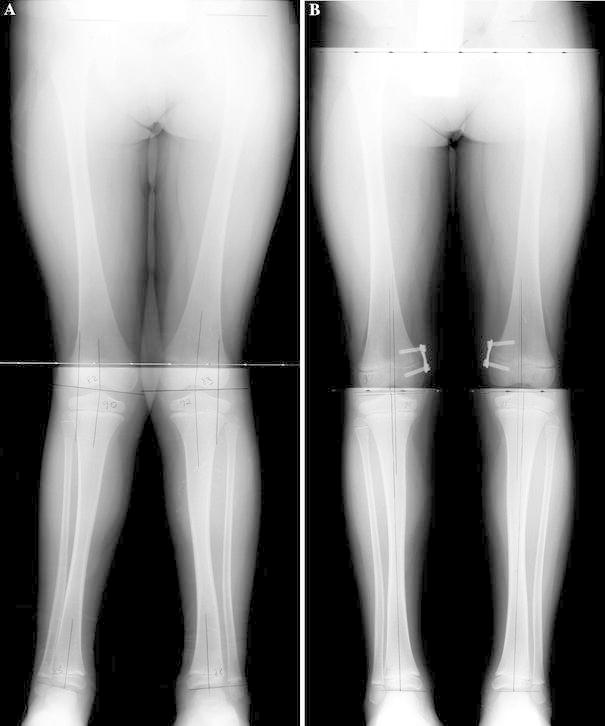
A 5-year and 7-month-old boy with bilateral insertion of an eight-Plate in the medial distal femur. a Preoperative anteroposterior view radiograph shows significant bilateral mechanical axis deviation (MAD) of 20 mm. b Anteroposterior view radiograph shows the patient after undergoing 10 months of treatment. The patient has slight bilateral over correction to 4 mm medial MAD
Fig. 4.
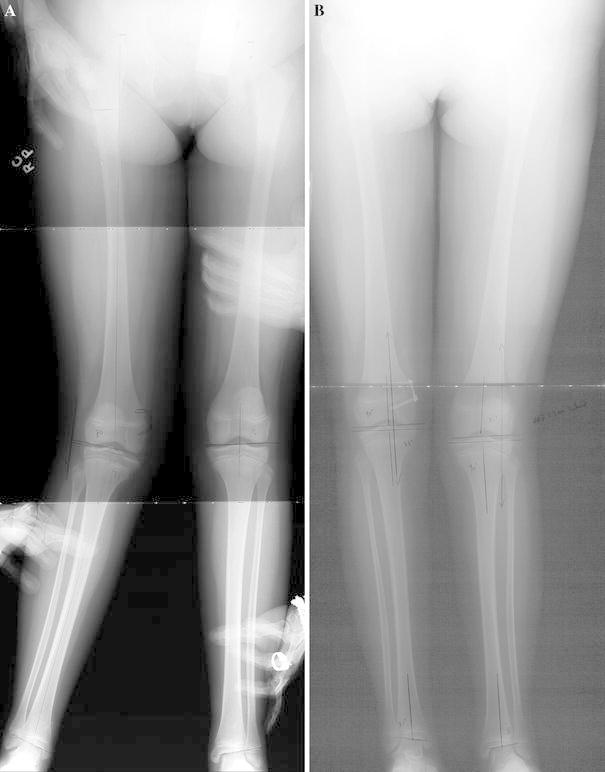
A 13-year-old girl with valgus deformity treated with an eight-Plate in the right distal medial femur. a Anteroposterior view radiograph shows the right limb with significant mechanical axis deviation (MAD) of 49 mm lateral. b Anteroposterior view radiograph shows the patient after 10 months of treatment. The patient has a slight over correction to 6 mm medial MAD
The removal is performed under general anesthesia and under tourniquet control. The scar is sharply divided and can be excised if desired. In most cases, the eight-Plate is easily found after dissecting the subcutaneous and deep tissues. However, we recommend that an image intensifier is available in case the surgeon experiences any difficulty locating the eight-Plate. It is important to always preserve the integrity of the periosteum and the perichondrial ring to minimize the risk of physeal bar formation. Return to full activity, including contact sports, is allowed within 4 weeks after eight-Plate removal. After removal, the patient should attend regular follow-up visits until skeletal maturity. During follow-up visits, erect-limb radiographs are obtained so that the surgeon can monitor for recurrence of the original deformity. If recurrence is experienced, the eight-Plate can be reinserted.
Results
A total of 11 patients (17 eight-Plates) were followed through completion of treatment and underwent eight-Plate removal; 9 underwent treatment for genu valgum deformity and 2 for genu varum deformity. The primary diagnoses included clubfoot (n = 3), idiopathic genu valgum (n = 1), hip dysplasia (n = 1), fibular hemimelia (n = 1), congenital femoral deficiency (n = 1), Perthes disease (n = 1), osteosarcoma (n = 1), lower-limb deficiency combined with thrombocytopenia-absent radius syndrome (TAR syndrome) (n = 1), and Marfan syndrome (n = 1). The average age at time of eight-Plate insertion was 10 years and 2 months (age range 4 years and 11 months–13 years and 8 months). The average time between insertion and removal of the eight-Plate was 9.5 months (range 5–13 months). Of the 17 eight-Plates, 8 were inserted in medial distal femora (2 bilateral cases) (Fig. 3) and 3 in medial proximal tibiae (1 bilateral case). One patient with valgus deformity had 2 eight-Plates inserted in the right limb: 1 in the medial proximal and 1 in the medial distal tibia. Of the remaining 4 eight-Plates, 2 were inserted in both lateral distal femora of the patient with TAR syndrome. The other 2 eight-Plates were inserted bilaterally in the patient who had an osteosarcoma in the right limb. The patient underwent lateral proximal tibial treatment for bilateral genu valgum.
All patients who underwent eight-Plate removal achieved complete deformity correction (Table 1; Figs. 5, 6) with the exception of one limb of a 13-year and 8-month-old boy whose growth plate had been damaged from a prior juxtaphyseal osteosarcoma resection and did not experience sufficient growth. Complete correction was defined as reaching neutral mechanical axis deviation (MAD); however, we often attempted to slightly over correct the deformity in cases in which rebound was anticipated.
Table 1.
Patients' data. LLeft, lat lateral, ldf lateral distal femur, LDTA lateral distal tibial angle, lpt lateral proximal tibia, MAD mechanical axis deviation, mdf medial distal femur, mdt medial distal tibia, med medial, mLDFA mechanical lateral distal femoral angle, mpt medial proximal tibia, MPTA medial proximal tibial angle, R right, TAR thrombocytopenia-absent radius
| Patient (Gender) | 1 ♀ | 2 ♀ | 3 ♀ | 4 ♂ | 5 ♀ | 6 ♀ | 7 ♀ | 8 ♂ | 9 ♂ | 10 ♂ | 11♂ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Delayed development | Marfan syndrome | Idiopathic clubfoot | Idiopathic genu valgum | Idiopathic genu valgum | Perthes disease | Fibular hemimelia | Osteosarcoma | TAR syndrome | Femoral deficiency | Idiopathic clubfoot |
| Age at initial surgery (years + months) | 13 + 0 | 7 + 5 | 4 + 11 | 13 + 8 | 5 + 7 | 11 + 7 | 8 + 9 | 13 + 8 | 8 + 9 | 12 + 7 | 12 + 5 |
| Position of eight-Plate | R mdf | R mdf | R mpt + R mdt | Bilateral mpt | Bilateral mdf | Bilateral mdf | L mpt | Bilateral lpt | Bilateral ldf | L mdf | L mdf |
| Total time from insertion to removal (months) | 9 | 11 | 12 | 6 | 10 | 5 | 7 | 10 | 12 | 12 | 13 |
| Preoperative mLDFA (normal, 85°–90°) | 79° | 81° | – | – | R 82° L 83° |
R 84° L 84° |
– | – | R 100° L 105° |
79° | 81° |
| Postoperative mLDFA (normal, 85°–90°) | 90° | 90° | – | – | R 90° L 94° |
R 90° L 89° |
– | – | R 90° L 95° |
90° | 91° |
| Change in mLDFA | 11° | 9° | – | – | R 8 L 11° |
R 6° L 5° |
– | – | R 10° L 10° |
11° | 10° |
| Preoperative MPTA (normal, 85°–90°) | – | – | 95° | R 94° L 94° |
– | – | 95° | R 79° L 83° |
– | – | – |
| Postoperative MPTA (normal, 85°–90°) | – | – | 91° | R 84° L 87° |
– | – | 87° | R 79° L 90° |
– | – | – |
| Change in MPTA | – | – | 4° | R 10° L 7° |
– | – | 8° | R 0° L 7° |
– | – | – |
| Preoperative LDTA (normal, 86°–92°) | – | – | 82° | – | – | – | – | – | – | – | – |
| Postoperative LDTA (normal, 86°–92°) | – | – | 92° | – | – | – | – | – | – | – | – |
| Change in LDTA | – | – | 10° | – | – | – | – | – | – | – | – |
| Preoperative MAD (mm) | lat 49 | lat 32 | lat 17 | R lat 15 L lat 22 |
R lat 19 L lat 20 |
R lat 21 L lat 22 |
lat 30 | R med 29 L med 26 |
R med 52 L med 44 |
lat 38 | lat 30 |
| Postoperative MAD (mm) | med 6 | lat 2 | lat 4 | R med 14 L med 0 |
R med 4 L med 4 |
R med 11 L med 7 |
med 8 | R med 29 L lat 3 |
R lat 22 L lat 8 |
lat 3 | med 8 |
| Change in MAD (mm) | 55 | 30 | 13 | R 29 L 22 |
R 23 L 24 |
R 32 L 29 |
38 | R 0 L 29 |
R 74 L 52 |
35 | 38 |
| Change in MAD per month (mm) | 6.1 | 2.7 | 1.1 | R 4.8 L 3.7 |
R 2.3 L 2.4 |
R 6.4 L 5.8 |
5.4 | R 0 L 2.9 |
R 6.2 L 4.3 |
3.5 | 3 |
Fig. 5.
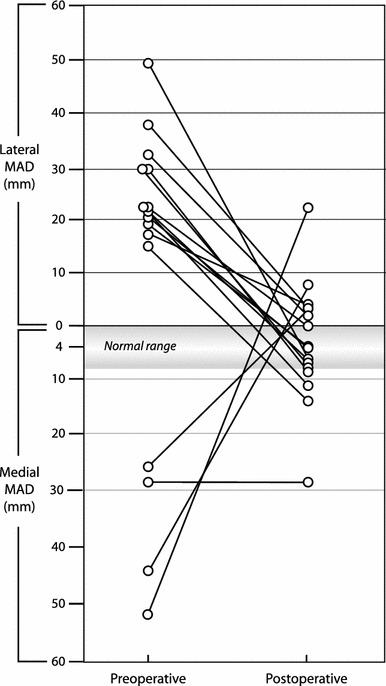
Change in the mechanical axis deviation (MAD) for 17 eight-Plates (16 limbs) measured preoperatively and postoperatively. The average normal MAD is 4.1 ± 4 mm medial [12]
Fig. 6.
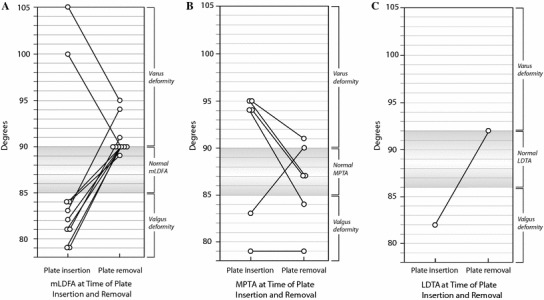
Joint orientation angles at the time of eight-Plate insertion and removal. a Mechanical lateral distal femoral angle (mLDFA) of cases in which the eight-Plate was inserted in the distal femur. b Medial proximal tibial angle (MPTA) of cases in which the eight-Plate was inserted in the proximal tibia. c Lateral distal tibial angle (LDTA) of cases in which the eight-Plate was inserted in the distal tibia
No perioperative or postoperative complications, such as infections, wound dehiscence, reactive synovitis, or hardware failures, were observed. We did not observe any instance of inadvertent physeal closure.
Alignment improved in all but one case. The average MAD, mechanical lateral distal femoral angle (mLDFA), medial proximal tibial angle (MPTA), and lateral distal tibial angle (LDTA) improved 32.7 mm, 9°, 7°, and 10°, respectively. When the eight-Plate was inserted in the medial distal femur (eight cases), the mLDFA changed on average from 82° to 91°, which constitutes an average change of 9° (range 5°–11°). When the eight-Plate was inserted in the lateral distal femur (one bilateral case) to correct a varus deformity, the mLDFA changed on average from 103° to 93°, which constitutes an average change of 10° (both limbs changed 10°). Correcting valgus deformities by inserting the eight-Plate in the medial proximal tibia (four cases) caused the MPTA to change from an average of 94° to 87°, which constitutes an average change of 7° (range 4°–10°). The eight-Plate was used to correct bilateral tibial varus deformity in a patient who underwent a resection of an osteosarcoma in the tibia. In this case, the proximal tibia experienced no further growth; therefore, deformity correction was not achieved. Presumably, the treatment for the osteosarcoma (i.e., resection, transport with Ilizarov device) led to permanent damage of the growth plate. However, the MPTA of the normal contralateral limb of this patient improved from 83° to 90°. In the single case in which the eight-Plate was inserted in the medial distal tibia, the LDTA improved from 82° to 92°.
Nine patients (12 limbs, 13 eight-Plates) with valgus deformity had an average MAD of 26.3 mm lateral before surgery. On average, the axis deviation improved to 4.4 mm medial (average improvement 30.7 mm; range 13–55 mm). The two patients (four limbs, four eight-Plates) with varus deformity had an average deviation of 37.8 mm medial and improved to an average 1.0 mm lateral (average improvement 38.8 mm; range 0–74 mm). None of the patients required an osteotomy or repeat eight-Plate insertion.
Discussion
Phemister [13] developed the concept of epiphysiodesis to stop physeal growth in order to equalize limb length. Permanent physeal arrest is still a well-accepted and safe method [14]. Bowen et al. [15] described hemiepiphysiodesis to correct angular deformity. Epiphysiodesis for angular deformity correction requires exact preoperative planning; but, even with accurate planning, an exact prediction of growth might not be possible [16]. When using the Bowen method, calculation of timing of hemiepiphysiodesis is based on a certain magnitude of deformity and age. If the formulas lead to postponement of the procedure, the magnitude may, in certain instances, progress, leading to an incorrect calculation in the future.
Haas [17] was the first to propose reversible epiphysiodesis. Blount and Clarke [2], in 1949, presented the results of controlling growth retardation by epiphyseal stapling. The original Blount staple was made of stainless steel, but it often broke. To prevent this, Blount made the staple of Vitallium instead and reinforced the shoulders with fillets. These staples are marketed by Zimmer (Warsaw, IN, USA) and are still widely used [2, 6–10, 18–21].
Inserting a Blount staple is a simple procedure that is associated with minimal risks, especially when compared with osteotomy. Complications of stapling primarily occur because of improper staple selection and/or violations of technique [9]. One controversial question remains: how long can staples remain in the patient before the physis closes permanently? Many investigators have studied the mechanical and biological responses of the physis to compression and have concluded that all changes caused by stapling of the physis should be reversible [17, 22–27]. Aykut et al. [28] have studied clinical as well as experimental aspects of the risk of inducing physeal bar formation. In clinical practice, it is prudent to assume that the risk of physeal bar formation will increase after 24 months of staple presence [9]. According to Mielke and Stevens [9], the physis is probably more resilient in patients who are younger than 10 years of age. However, stapling in young children can be problematic [9].
Bowen et al. [15] proposed a method to calculate optimal timing of epiphysiodesis in which correction and skeletal maturity would be achieved at the same time. We do not subscribe to this strategy for several reasons. First, the magnitude of the deformity might change. Thus, a calculation or prediction done at one age may not be accurate when the child reaches the age when the Bowen formula instructs the hemiepiphysiodesis to be done. Second, we do not feel that it is reasonable to make young children wait until they are close to adolescence before correcting their deformities.
When a staple is inserted in the limb of a young child, there is a tendency for the staple to back out (Fig. 7) [9]. Extrusion occurs because the epiphysis is largely unossified in young children, so only the tip of each tine is lodged in bone. Most of the length of each tine is embedded in unossified cartilage. Adding barbs to the tines would theoretically reduce the risk of extrusion, but the barbs would also make it much more difficult to remove the staples after the angular deformity has been corrected. Such staple designs exist in the form of tissue staples designed for osteotomy or ligament fixation. The eight-Plate eases the problem of extrusion by using screws to anchor the eight-Plate into the bone. Screws offer much better purchase than smooth staples.
Fig. 7.
a Photograph shows spontaneous extrusion of a Blount staple in a young child. b Photograph shows the eight-Plate in a 5-year and 6-month-old child. The screws provide better fixation than staples in the partially ossified epiphysis
Breakage was not observed in our series. We think this is because the screws of the eight-Plate are free to pivot in the plate holes. Theoretically, the ability of the screws to pivot might apply less pressure to the physis. The eight-Plate might preserve the physis and therefore reduce the risk of physeal bar formation.
Recently, Stephens [11] published his preliminary results with the eight-Plate. He reported 34 patients, of which 32 were successfully treated. Two patients with Blount disease had “sick physes” and might have needed a tibial osteotomy because they experienced insufficient correction. Stevens discussed the issue of potential rebound after removal but does not recommend over correction. However, two of his patients who were successfully treated developed so much rebound that they required another surgery for bilateral reinsertion (four eight-Plates).
Of the 11 patients reported who underwent eight-Plate insertion and removal at our center, full deformity correction was achieved in all but one limb. In retrospect, the one exception can be explained by poor patient selection, as the growth plate in one limb had been irreversibly damaged by the original osteosarcoma resection and an Ilizarov bone transport of 18 cm. Despite this, the remaining deformity was judged not significant enough to require osteotomy.
The eight-Plate has the potential to extend the indication for hemiepiphyseal stapling to a younger age group because it has a reduced risk of breakage and extrusion. Indeed, in our series, we used the eight-Plate in patients as young as 4 years and 11 months. Stevens [11] used it in patients as young as 1 year and 7 months. From a cost standpoint, the eight-Plate is more expensive than a Blount staple (approximately US $475 for the eight-Plate, screws, and guide pins compared with US $75 for a Blount staple).
This paper presents our preliminary results with a small sample size. Additional studies need to be conducted with a larger sample size and longer follow-up to determine whether the results from this study can be validated. Longer follow-up would be needed to study the problem of rebound deformity after removal of the implant and to evaluate subsequent growth. The studies could also examine whether the correction is reversible and whether growth resumes after removal. Further follow-up studies are needed to confirm that growth will reliably resume after eight-Plate removal. Parents should be cautioned that subsequent growth could be asymmetrical, leading to relapse of the deformity. Our results have shown that the eight-Plate with its pivoting screw and plate design is a valid concept and that it effectively corrects angular deformity. The only failure in our study resulted from poor patient selection, not device malfunction. Additional studies should be conducted to compare the speed of angular correction obtained using staples with that of the eight-Plate and to determine the different mode of action on the physis.
At this time, we can say that the greatest advantage of the eight-Plate is its tenacious grasp to the bone, which reduces the risk of extrusion and thereby extends the indications to patients who are younger and have largely unossified epiphyses. We observed clinically that the precise insertion technique for the eight-Plate is superior to the Blount staple insertion technique. With both the eight-Plate and the Blount staple, it is possible to straddle the growth plate easily on the anteroposterior view with intraoperative image intensifier control. However, it is more challenging to place the Blount staple in the middle of the growth plate on the lateral view. The eight-Plate is suspended from a needle, and the position checked prior to inserting the screws. The Blount staple is inserted first and then the insertion tool is disassembled to allow the surgeon to check the Blount staple position on the lateral view. If the position is too anterior or posterior on the lateral view, which could lead to inadvertent recurvatum or procurvatum, we generally add a second staple to balance the effect. With the eight-Plate, we avoid this problem.
The eight-Plate has largely supplanted the Blount staple at our institution. One of the few remaining indications for the Blount staple is when the patient has limited remaining growth. We feel that the eight-Plate tethers growth after a few months, while the Blount staple tethers growth immediately.
Acknowledgments
The authors wish to thank Alvien Lee for photographic expertise, Joy Marlowe, MA, for graphic assistance, and Amanda Chase, MA, for editorial assistance.
Footnotes
None of the authors received financial support for this study.
The study was approved by our institutional human research committee.
Level of evidence: Level II, retrospective study.
References
- 1.Volkmann R. Surgical experiences over bone bucklings and bone growth. Archiv f Pathol Anat. 1862;24:512–540. doi: 10.1007/BF01879454. [DOI] [Google Scholar]
- 2.Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling: a preliminary report. J Bone Joint Surg Am. 1949;31:464–478. [PubMed] [Google Scholar]
- 3.Métaizeau JP, Wong-Chung J, Bertrand H, Pasquier P. Percutaneous epiphysiodesis using transphyseal screws (PETS) J Pediatr Orthop. 1998;18:363–369. [PubMed] [Google Scholar]
- 4.Nouth F, Kuo LA. Percutaneous epiphysiodesis using transphyseal screws (PETS): prospective case study and review. J Pediatr Orthop. 2004;24:721–725. doi: 10.1097/01241398-200411000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Eidelman M, D’Agostino P. Hemiepiphysiodesis around the knee by percutaneously guided and grooved staple. J Pediatr Orthop B. 2005;14:434–435. doi: 10.1097/01202412-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Brockway A, Craig WA, Cockreli BR., Jr End-result study of sixty-two stapling operations. J Bone Joint Surg Am. 1954;36:1063–1070. [PubMed] [Google Scholar]
- 7.Frantz CH. Epiphyseal stapling: a comprehensive review. Clin Orthop Relat Res. 1971;77:149–157. [PubMed] [Google Scholar]
- 8.Fraser RK, Dickens DR, Cole WG. Medial physeal stapling for primary and secondary genu valgum in late childhood and adolescence. J Bone Joint Surg Br. 1995;77:733–735. [PubMed] [Google Scholar]
- 9.Mielke CH, Stevens PM. Hemiepiphyseal stapling for knee deformities in children younger than 10 years: a preliminary report. J Pediatr Orthop. 1996;16:423–429. doi: 10.1097/01241398-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Stevens PM, Maguire M, Dales MD, Robins AJ. Physeal stapling for idiopathic genu valgum. J Pediatr Orthop. 1999;19:645–649. [PubMed] [Google Scholar]
- 11.Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop. 2007;27:253–259. doi: 10.1097/BPO.0b013e31803433a1. [DOI] [PubMed] [Google Scholar]
- 12.Paley D (2005) Frontal plane mechanical and anatomic axis planning. In: Paley D (ed) Principles of deformity correction, 1st edn Corr. 3rd printing. Revised edn. Springer, Berlin, pp 61–97
- 13.Phemister DB. Operative arrestment of longitudinal growth of bones in the treatment of deformities. J Bone Joint Surg Am. 1933;15:1–15. [Google Scholar]
- 14.Horton GA, Olney BW. Epiphysiodesis of the lower extremity: results of the percutaneous technique. J Pediatr Orthop. 1996;16:180–182. doi: 10.1097/01241398-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Bowen JR, Leahey JL, Zhang ZH, MacEwen GD. Partial epiphysiodesis at the knee to correct angular deformity. Clin Orthop Relat Res. 1985;198:184–190. [PubMed] [Google Scholar]
- 16.Westh RN, Menelaus MB. A simple calculation for the timing of epiphysial arrest: a further report. J Bone Joint Surg Br. 1981;63:117–119. doi: 10.1302/0301-620X.63B1.7204464. [DOI] [PubMed] [Google Scholar]
- 17.Haas SL. Retardation of bone growth by a wire loop. J Bone Joint Surg Am. 1945;27:25–36. [Google Scholar]
- 18.Blount WP. A mature look at epiphyseal stapling. Clin Orthop Relat Res. 1971;77:158–163. [PubMed] [Google Scholar]
- 19.Christensen NO (1973) Growth arrest by stapling: an experimental study of longitudinal bone growth and morphology of the growth region. Acta Orthop Scand Suppl:3–78 [PubMed]
- 20.Farnum CE, Nixon A, Lee AO, Kwan DT, Belanger L, Wilsman NJ. Quantitative three-dimensional analysis of chondrocytic kinetic responses to short-term stapling of the rat proximal tibial growth plate. Cells Tissues Organs. 2000;167:247–258. doi: 10.1159/000016787. [DOI] [PubMed] [Google Scholar]
- 21.Kramer A, Stevens PM. Anterior femoral stapling. J Pediatr Orthop. 2001;21:804–807. [PubMed] [Google Scholar]
- 22.Alberty A, Peltonen J, Ritsilä V. Effects of distraction and compression on proliferation of growth plate chondrocytes: a study in rabbits. Acta Orthop Scand. 1993;64:449–455. doi: 10.3109/17453679308993665. [DOI] [PubMed] [Google Scholar]
- 23.Arkin AM, Katz JF. The effects of pressure on epiphyseal growth; the mechanism of plasticity of growing bone. J Bone Joint Surg Am. 1956;38:1056–1076. [PubMed] [Google Scholar]
- 24.Bylski-Austrow DI, Wall EJ, Rupert MP, Roy DR, Crawford AH. Growth plate forces in the adolescent human knee: a radiographic and mechanical study of epiphyseal staples. J Pediatr Orthop. 2001;21:817–823. [PubMed] [Google Scholar]
- 25.Ehrlich MG, Mankin HJ, Treadwell BV. Biochemical and physiological events during closure of the stapled distal femoral epiphyseal plate in rats. J Bone Joint Surg Am. 1972;54:309–322. [PubMed] [Google Scholar]
- 26.Hall-Craggs EC, Lawrence CA. The effect of epiphyseal stapling on growth in length of the rabbit’s tibia and femur. J Bone Joint Surg Br. 1969;51:359–365. [PubMed] [Google Scholar]
- 27.Herwig J, Schmidt A, Matthiab HH, Kleemann H, Buddecke E. Biomechanical events during stapling of the proximal tibial epiphyseal plate in pigs. Clin Orthop Relat Res. 1987;218:283–289. [PubMed] [Google Scholar]
- 28.Aykut US, Yazici M, Kandemir U, Gedikoglu G, Aksoy MC, Cil A, Surat A. The effect of temporary hemiepiphyseal stapling on the growth plate: a radiologic and immunohistochemical study in rabbits. J Pediatr Orthop. 2005;25:336–341. doi: 10.1097/01.bpo.0000152906.23669.d8. [DOI] [PubMed] [Google Scholar]


