Abstract
Class B GPCR’s are activated by peptide ligands, typically 30-40 amino acid residues, that are involved in major physiological functions such as glucose homeostasis (glucagon and glucagon-like peptide 1), calcium homeostasis and bone turnover (parathyroid hormone and calcitonin), and control of the stress axis (corticotropin-releasing factor). Peptide therapeutics have been developed targeting these receptors but development of nonpeptide ligands, enabling oral administration, has proved challenging. Allosteric modulation of these receptors provides a potential route to developing nonpeptide ligands that inhibit, activate, or potentiate activation of these receptors. Here the known mechanisms of allosteric modulators targeting Class B GPCR’s are reviewed, particularly nonpeptide antagonists of the corticotropin-releasing factor 1 receptor and allosteric enhancers of the glucagon-like peptide-1 receptor. Also discussed is the potential for antagonist ligands to operate by competitive inhibition of one of the peptide binding sites, analogous to the Charniere mechanism. These mechanisms are then used to discuss potential strategies and management of pharmacological complexity in the future development of allosteric modulators for Class B GPCR’s.
Key Words: Allosteric, class B, G-protein-coupled receptor, secretin, corticotropin-releasing factor, nonpeptide, glucagon-like peptide, parathyroid hormone.
INTRODUCTION
The class B G-protein-coupled receptor (GPCR) family is a small group of receptors, 15 in the human genome, that are activated by intermediate sized peptides of typically 30-40 amino acid residues (Table 1) [17, 21]. These peptides mediate a diverse array of important homeostatic processes and other physiological functions, acting as hormones, autocrine factors and neuromodulators (Table 1). For example, parathyroid hormone and calcitonin reciprocally regulate calcium homeostasis and bone turnover, activating PTH1 and calcitonin receptors, respectively [54, 66]. Glucagon regulates hepatic glucose output [45, 52, 77], and post-prandial glucose homeostasis is modulated by the incretin peptides glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [18, 52, 77]. Corticotropin-releasing factor is the principal regulator of the stress axis, acting peripherally via activation of the hypothalamic-pituitary-adrenal (HPA) axis and centrally by means of modulating behavioral responses to stress [2, 20, 32, 81].
Table 1.
Human Class B GPCR’s and Their Peptide Ligands
| Receptor | Peptide Ligand | Principal Biological Action | Major Disease Indication | Peptide Therapeutic | Refs. |
|---|---|---|---|---|---|
| CRF1 | CRF UCN 1 |
Stress responses Stress responses |
Depression (antagonist) | [2,43, 92] | |
| CRF2 | UCN 1 UCN 2 UCN 3 |
Stress responses Cardiac contractility Hearing |
Heart failure (agonist) | [2] | |
| GHRH | GHRH | Growth hormone release | [52,77] | ||
| GIP | GIP | Insulin secretion | Type 2 diabetes (agonist) | [52,77] | |
| Glucagon | Glucagon | Glucose homeostasis | Type 2 diabetes (antagonist) | [52,77] | |
| GLP-1 | GLP-1 | Insulin secretion | Type 2 diabetes (agonist) | Byetta (Exanatide) | [19,52,77] |
| GLP-2 | GLP-2 | Gut mucosal growth | [52,77] | ||
| PTH1 | PTH PTHrP |
Ca2+ homeostasis Developmental regulator |
Osteoporosis (agonist) | Forteo (PTH(1-34)) | [5,58,73, 90] |
| PTH2 | TIP39 | Hypothalamic secretion,Nociception | [88] | ||
| Secretin | Secretin | Pancreatic secretion | [52,77] | ||
| VPAC1 | VIP PACAP |
Neuroendocrine functions Neuroendocrine functions |
[30,77] | ||
| VPAC2 | VIP PACAP |
Neuroendocrine functions Neuroendocrine functions |
[30,77] | ||
| PAC1 | PACAP | Neuroendocrine functions | [30,77] | ||
| Calcitonin | Calcitonin | Ca2+ homeostasis | Osteoporosis (agonist) | Miacalcin (calcitonin) | [54] |
| Calcitonin/ RAMP1 | CGRP Amylin |
Vasodilation Feeding |
Migraine (antagonist) | [57,67] | |
| Calcitonin / RAMP3 | Amylin | Feeding | [57,67] | ||
| CL / RAMP1 | CGRP | Vasodilation | [57,67] | ||
| CL / RAMP2 | Adrenomedullin | Vasodilation | [57,67] | ||
| CL / RAMP3 | Adrenomedullin CGRP |
Vasodilation Vasodilation |
[57,67] |
Abbreviations:CRF- corticotropin-releasing factor; UCN - urocortin; GHRH - growth hormone-releasing hormone; GIP - glucose-dependent insulinotropic peptide; GLP - glucago nlike peptide;PTH- parathyroidhormone;PTHrP - parathyroid hormone-related protein; TIP39 - tuberoinfundibular peptide of 39 residues; VIP - vasoactive intestinal peptide; PACAP- pituitary adenylate cyclase-activating polypeptide; CGRP - calcitonin gene-related peptide; RAMP - receptor activity modifying protein; CL - calcitonin receptor-like receptor.
At least eight of the fifteen human Class B GPCR’s have received attention as potential targets for the treatment of disease (Table 1). In some instances therapeutic agents have been developed from the peptides themselves. Calcitonin and parathyroid hormone are in clinical use for treatment of osteoporosis [54, 58]. A reptilian analogue of GLP-1, Exanatide (Byetta), has recently been developed as a mechanistically novel therapeutic for the management of Type 2 diabetes [15, 19]. The major physiological and therapeutic function of these peptides has stimulated considerable understanding of their receptor binding and receptor activation mechanisms. Intrinsic to these mechanisms for Class B GPCR’s is the potential for allosteric regulation by nonpeptide ligands, which could aid the development of orally bioavailable modulators of Class B GPCR’s to circumvent practical and potential compliance issues with injections of the peptide therepeutics. Also intrinsic to the peptide binding mechanism is the potential for a modulator to act by competitive blockade of one of the peptide binding sites, a mechanism analogous to the Charniere model [69]. This mechanism challenges the utility of commonly-used analytical pharmacology methods to precisely define the mechanism of action of the modulator. Here the peptide binding mechanisms are briefly reviewed, the modulator mechanisms evaluated with reference to specific examples, and then this information is applied to discuss methods and analytical issues in the future development of nonpeptide allosteric ligands targeting Class B GPCR’s.
TWO-DOMAIN MODEL OF PEPTIDE LIGAND INTERACTION WITH CLASS B GPCR’S
Class B GPCR’s likely share a similar secondary and tertiary structure [17]. The extracellular N-terminal region, herein termed the N-domain, comprises approximately 100-160 amino acid residues. The NMR structure of the N-domain of the CRF2(b) receptor indicates a folded structure stabilized by three disulfide bonds, a hydrophobic core and an internal salt bridge [25]. The folded structure is comprised of β-sheets in an orientation that is described as a short consensus repeat, or Sushi domain [25, 62]. The key residues involved in maintaining this structure are highly conserved throughout the Class B GPCR family, suggesting this structure is common to all Class B GPCR’s [25]. The remaining juxtamembrane domain of the receptor (J-domain) comprises seven predicted membrane-spanning α-helices with intervening intracellular and extracellular loops [17]. The structure of the J-domain has not been determined directly. It shares little primary structural homology with rhodopsin (for which the X-ray structure has been determined [60]) but mutagenesis and zinc-bridging studies suggest certain tertiary structure-stabilizing elements might be similar between Class A and Class B GPCR’s [22, 76].
The orientation and mechanism of peptide interaction with Class B GPCR’s has been studied extensively using peptide structure-activity relationships (SAR) [7, 46, 79], receptor and ligand fragments [1, 38, 49, 61, 63, 79], chimeric receptors [3, 41, 74], site-directed mutagensis [33, 46], photochemical cross-linking [11, 16, 51, 65], NMR structure determinations [4, 25, 55, 62] and molecular modeling [4, 11, 25, 65, 72]. Almost all of the data are consistent with a low-resolution molecular orientation of binding in which the carboxyl-terminal portion of the peptide binds to the N-domain of the receptor and the amino-terminal portion of the peptide binds and activates the J-domain (Fig. 1A). For example, a chimeric peptide formed of the carboxyl-terminal portion of calcitonin and the amino-terminal portion of parathyroid hormone activates a chimeric receptor comprising the N-domain of the calcitonin receptor and J-domain of the PTH1 receptor [3]. The same orientation was inferred using the reciprocal chimeras [3], and with glucagon/GLP-1 ligand and receptor chimeras [74]. The carboxyl-terminal portion of the peptides typically form an α-helix in binding to the N-domain of the receptor [55]. This interaction is of moderate-to-high affinity (1-100nM) [1, 36, 38, 39, 61] and does not appear to be directly involved in receptor activation [14]. As a result, carboxyl-terminal fragments act as high-affinity antagonists [49, 68, 87]. Interaction between the amino-terminal region of the peptide with the J-domain is of much lower affinity (in the high μM range). This interaction activates the receptor, stimulating intracellular signaling [50, 59, 78, 79]. Class B GPCR’s signal predominantly through GS-coupled pathways and, to a more limited extent, through Gq/11 and Gi family G-proteins.
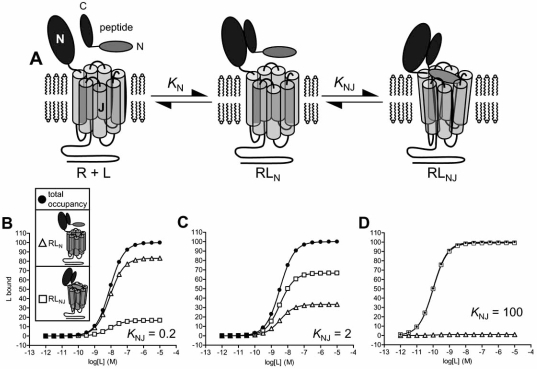
Fig. (1).
Two domain model of peptide interaction with Class B GPCR’s. A. Schematic representation of the two-domain model. The carboxyl-terminal portion of the peptide binds the extracellular N-terminal domain (N-domain) of the receptor, forming RLN, defined by the equilibrium constant KN. This interaction acts as an affinity trap, promoting interaction of the amino-terminal portion of the ligand with the juxtamembrane domain (J-domain) of the receptor, forming RLNJ. The J-domain interaction is defined by the isomerization constant KNJ, defining the RLNJ : RLN concentration ratio. B-D: Simulation of receptor occupancy, N-domain occupancy and J-domain occupancy by peptide ligand with varying strength of J-domain interaction. Occupancy of the receptor was defined by eq. 1 (Appendix), occupancy of the N-domain alone by eq. 2, and occupancy of the J-domain (with concomitant occupancy by the N-domain) by eq. 3. The parameter values used were: KN = 1×108 M-1, [R]TOTAL = 100, and values of KNJ of 0.2 (B, weak J-domain interaction), 2 (C, moderate interaction)and 100 (D, strong interaction).
The two-domain model provides a simple and tractable mechanistic framework for interpreting peptide and allosteric modulator binding mechanisms [1, 33, 79]. One formulation of the model is presented in Fig. (1A). Peptide ligand binds the receptor N-domain, defined by the equilibrium association constant KN. This interaction provides an affinity trap, increasing the local concentration of the amino-terminal portion of the peptide in the vicinity of the J-domain, overcoming the low-affinity of this interaction to enable significant binding to occur. This binding event is represented here as an isomerization between RLN, ligand bound to the N-domain alone, and RLNJ, ligand bound to both N- and J-domains (Fig. 1A). The strength of this interaction is described here by an isomerization constant, KNJ, representing the RLNJ : RLN concentration ratio at equilibrium.
In order to understand mechanisms of allosteric modulation, it is instructive to consider the extent of peptide occupancy of N- and J-domains, under different conditions, for different receptors, and for different peptides binding the same receptor. In Fig. (1A), ligand occupancy of the N-domain alone is represented by RLN, and ligand occupancy of the J-domain is represented by RLNJ. The extent of receptor occupancy by the N-domain is largely dependent on the peptide concentration and KN. The fraction of peptide-bound receptors in which peptide is bound to the J-domain is dependent on KNJ. This binding energy varies between Class B GPCR’s and is dependent on the conformational state of the receptor. (Similar to most Class A GPCR’s the conformational state of Class B GPCR’s regulated by receptor-G-protein interaction [71], reflected in the higher agonist affinity at the G-protein coupled state (RG) compared with the uncoupled state (R).) At the R state, the inferred value of KNJ is quite low for some Class B GPCR’s (e.g. <1 at PTH1 and CRF1 receptors [35, 38]). Under these conditions, the majority of peptide-occupied receptors have ligand bound only to the N-domain (Fig. 1B), with only a small fraction having ligand bound to J-domain (Fig. 1B). Importantly, under these conditions peptide ligand cannot saturate the J-domain of the receptor, even at ligand concentrations that saturate the receptor through occupancy the N-domain (Fig. 1B). Consequently, even at saturating peptide concentrations unoccupied J-domain will be available for other ligands to bind to, a significant property in understanding modulator mechanisms (see below). Slightly stronger interaction with the J-domain (KNJ = 2) increases the fraction of occupied receptors in which peptide is bound the J-domain (Fig. 1C). Finally for some receptors a high value of KNJ has been inferred at the R state (e.g. 30 for urocortin 2 interaction with the CRF2 receptor [36]). Under these conditions peptide is bound to the J-domain in almost all ligand-occupied receptors (Fig. 1D). G-protein interaction with the receptor likely increases the strength of ligand binding to the J-domain, which can be represented by an increase of KNJ [38, 39]. As evident from comparing Fig. (1B-D), this effect of G-protein increases the fraction of occupied receptors in which ligand is bound to the J-domain. This inference is important in comparing modulator actions at R and RG states (see below).
ALLOSTERIC MODULATORS OF THE CRF1 RECEPTOR
Allosteric modulators have been identified and developed for numerous Class A and Class C GPCR’s (see reviews in this issue). Mechanistic studies including analytical pharmacology, ligand SAR and receptor modification have elaborated general concepts and specific mechanisms of how allosteric ligands produce their modulatory effects. These methods have been applied to identify allosteric modulators of Class B GPCR’s. Low-molecular weight, nonpeptide allosteric modulators have been identified for CRF1, glucagon and GLP-1 receptors (Fig. 2) [6, 24, 43-45, 75, 80, 86]. Mechanistic studies, particularly with the CRF1 receptor, have demonstrated that knowledge of the mechanism of peptide binding is highly useful in elaborating the mechanisms of allosteric modulation of Class B GPCR’s.
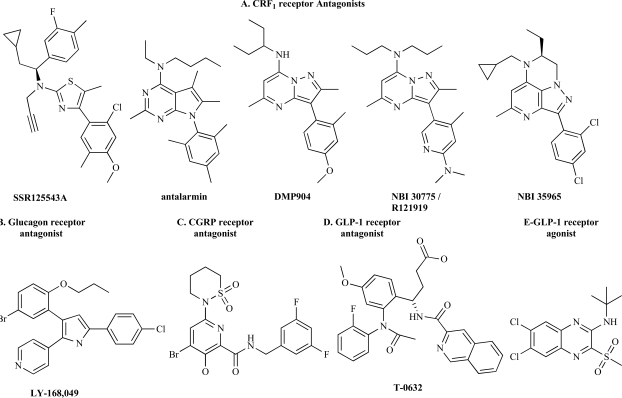
Fig. (2).
Chemical structure of allosteric modulators of Class B GPCR’s. A. CRF1 receptor antagonists (SSR125543A [27]; antalarmin [89]; DMP904 [23]; NBI 30775 [8]; NBI 35965 [29]). For a review of CRF1 receptor antagonist chemical structure see ref [43]. B. Glucagon receptor antagonist [6, 13]. C. CGRP antagonist [75]. D. GLP-1 receptor antagonist [86]. E. GLP-1 receptor agonist [44].
Allosteric modulators of the CRF1receptor have been studied in the most detail, in terms of ligand SAR [24, 43, 53], in vivo efficacy [28] and allosteric mechanism of action. Antagonism of central CRF1 receptors has been proposed as a potential novel mechanism for the treatment of anxiety, depression and other stress-related disorders, such as irritable bowel syndrome [28, 40, 56, 85]. This proposal has stimulated the discovery and development of a broad array of orally-available, CNS-penetrating nonpeptide antagonists that bind with high affinity (low nonomolar) to the CRF1receptor. Prototypical examples include CP-154,526 [9], antalarmin [89], DMP696 [31], DMP904 [23], SR125543A [27] and NBI 30775 [8] (also known as R121919) (Fig. 2). Nonpeptide antagonists are active in animal models of CRF- and environmentally-induced responses to stress [24, 28, 43, 53]. NBI 30775 has been tested in human subjects. This compound significantly reduced Hamilton depression and anxiety scores in severely depressed individuals in a small open-label Phase IIa clinical trial [92].
The first evidence that nonpeptide antagonists of the CRF1 receptor act allosterically was provided by receptor mutation studies to identify the ligand binding site [48]. Mutation of two residues within the predicted membrane-spanning region of the receptor (H199V and M276I) reduced binding of the nonpeptide antagonist NBI 27914 without affecting binding of peptide agonists (e.g. CRF). This finding suggests the binding sites for nonpeptide antagonist and peptide ligand are at least partially distinct. This hypothesis is supported by subsequent findings that strongly imply M276 is proximal to the bound nonpeptide ligand [34]. In addition, the peptide binding determinants that have been identified to date are located within extracellular regions of the receptor – the N-domain and the extracellular loops of the J-domain (reviewed in refs [12, 25, 34, 62]. Taken together these findings suggest CRF1 receptor nonpeptide antagonists bind within the membrane-spanning region of the J-domain and peptide ligands bind to sites further towards the extracellular face of the receptor, implying allostetric interaction between peptide and nonpeptide ligand.
Radioligand binding studies are consistent with an allosteric interaction between nonpeptide antagonist and peptide ligands at the CRF1 receptor [37, 91]. In radioligand dissociation assays, nonpeptide ligands modulate the dissociation of radiolabeled peptides from the receptor and, reciprocally, peptide ligands modulate dissociation of radiolabeled nonpeptides [37]. In equilibrium binding assays, peptide ligands do not fully inhibit specific binding of radiolabeled nonpeptides [37, 91]. Nonpeptide ligands decrease the apparent affinity of peptide ligands but this decrease of affinity approaches a limit as the concentration of nonpeptide ligand increases [37]. All of these features are consistent with the allosteric ternary model described for Class A GPCR’s such as muscarinic acetylcholine receptors [47, 84]. In this model, modulator can bind to the receptor occupied by endogenous ligand, and vice versa, forming a ternary complex between receptor, modulator and endogenous ligand.
The peptide-receptor interactions that are modulated by nonpeptide antagonists have been studied using receptor and peptide fragments [37, 38, 59, 64]. Binding of peptide agonists to the CRF1 receptor is well-described by the two domain model described above and illustrated schematically in Fig. (1A) [25, 38, 64]. Nonpeptide binding determinants are borne largely if not exclusively by the J-domain;. nonpeptide antagonist affinity for a J-domain fragment is not significantly different from that for the full-length receptor and the ligands do not displace radiolabed peptide binding to a N-domain fragment [38]. The peptide interaction that is blocked by nonpeptide antagonists appears to be that between the amino-terminal region of the peptide and the J-domain of the receptor. Binding of a radiolabeled amino-terminal-truncated peptide ([125I] astressin, a CRF(12-41) analogue) to the wild-type CRF1 receptor is not appreciably inhibited by nonpeptide ligands [37, 38, 64], and nonpeptide antagonists completely block CRF-stimulated activation of a J-domain fragment [38, 59]. Taken together these findings are consistent with the model in Fig (3A). In this model, CRF binding is described by the two-domain mechanism. Nonpeptide ligand binds within the membrane-spanning region of the J-domain, a site distinct from the CRF binding regions located further towards the extracellular face of the J-domain (Fig. 3A). Nonpeptide binding does not affect CRF binding to the N-domain, but allosterically inhibits CRF binding to the J-domain. Since CRF interaction with the J-domain is required for receptor activation, blocking this interaction with nonpeptide ligand effectively antagonizes CRF-stimulated signaling. The behavior of this model, an extended variant of the allosteric ternary model, was simulated and rationalized as described in the Appendix and presented in Fig. (3B-E). In these simulations, nonpeptide ligand does not fully inhibit equilibrium binding of peptide ligand (Fig. 3B) and reciprocally peptide does not fully inhibit binding of nonpeptide (Fig. 3C). In radioligand dissociation experiments nonpeptide modulates dissociation of peptide ligand (Fig. 3D), and vice versa (Fig. 3E).
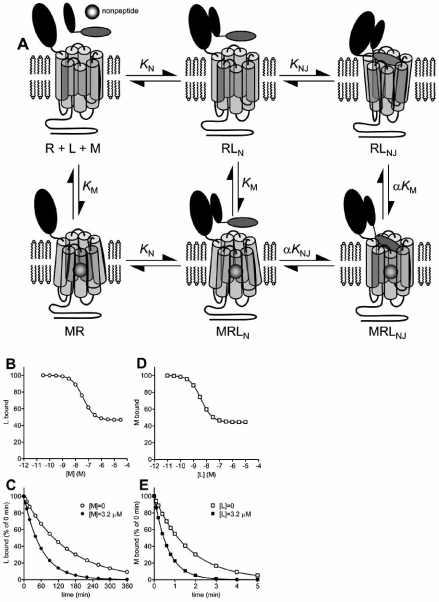
Fig. (3).
Allosteric modulation of peptide binding to Class B GPCR’s via the J-domain. A. Schematic representation of the model. Peptide binding is described by the two domain model (Fig. 1A). Allosteric modulator binds the J-domain of the receptor at a site spatially distinct from the peptide binding regions, defined by the equilibrium constant KM. Binding of modulator allosterically regulates binding of peptide to the J-domain, defined by the cooperativity factor α, without affecting peptide binding to the N-domain. B-E. Manifestation of the model in binding assays for an allosteric inhibitor, simulated using equations in the Appendix. Binding parameters were: KN = 1×108 M-1, [R]TOTAL = 100, KNJ = 2, KM = 1×108 M-1 and α = 0.1 (allosteric inhibition, negative cooperativity). B. Modulation of equilibrium binding of peptide (L) by modulator (M), simulated using eq. 5, [L] = 1×10-9 M. C. Modulation of peptide dissociation by modulator, simulated using eq. 7. The dissociation rate of peptide from the N-domain (k-N) was set at 0.02 min-1. D. Modulation of equilibrium binding of modulator by peptide ligand, simulated using eq. 8, [M] = 2×10-9 M. E. Modulation of modulator dissociation by peptide, simulated using eq. 12. The dissociation rate of modulator (k-M) was set at 0.6 min-1 and the dissociation rate of modulator from MRLNJ (k-M(L)) was set at 6.0 min-1.
This model can explain an interesting feature of nonpeptide modulator action at the CRF1 receptor. In inhibiting peptide binding, nonpeptide ligands display a much greater allosteric effect (negative cooperativity) at the G-protein-coupled state of the receptor (RG) compared with the uncoupled state (R) [37]. Nonpeptides near-fully inhibit peptide binding to the RG state but only partially inhibit peptide binding to R. This effect can be explained by a stronger peptide-J-domain interaction at the RG state compared with the R state. CRF binds with moderate affinity to the N-domain (approximately 50nM [38, 61]). At the R state, interaction with the J-domain is weak (KNJ <1), whereas at the RG state the J-domain interaction is much stronger (KNJ >100) [38]. Consequently the extent of peptide occupancy of the J-domain is predicted to differ dramatically between R and RG states, with low J-domain occupancy at the R state (e.g. Fig. 1B) and high occupancy at the RG state (e.g. Fig. 1D). Consequently, inhibition of J-domain occupancy by nonpeptide ligand does not dramatically affect total receptor occupancy by peptide at the R state, because only a minor fraction the occupied receptors has peptide bound to the J-domain (Fig. 1B). In contrast, at the RG state, inhibition of J-domain occupancy by nonpeptide ligand strongly reduces total receptor occupancy by peptide because almost all of the occupied receptors have peptide bound to the J-domain (Fig 1D).
NONPEPTIDE ALLOSTERIC MODULATORS OF OTHER CLASS B GPCR’S
Nonpeptide antagonists have been identified for glucagon [6, 45], CGRP [75] and GLP-1 receptors [44, 86] that have been shown or inferred to act allosterically (Fig. 2B-E). The mechanisms by which these ligands modulate their receptors have not been established in detail. Antagonism of the glucagon receptor has received interest as a potential mechanism for managing hyperglycemia in the treatment of Type 2 diabetes [45]. Numerous structural classes of high-affinity nonpeptide antagonists have been identified, with examples including L-168,049 (Fig. 2B), Bay 27-9955 and NNC 25-2504 [45]. L-168,049 has been shown to allosterically modulate glucagon interaction with the glucagon receptor [6]. The ligand slows dissociation of [125I]glucagon, and two mutations within the membrane-spanning region of the receptor reduce affinity of the antagonist without affecting binding of glucagon. These findings are potentially consistent with the ligand acting by a similar allosteric mechanism as that identified for nonpeptide antagonists of the CRF1receptor (Fig. 3A). By contrast, a nonpeptide antagonist of the GLP-1 receptor, T-0632 (Fig. 2D), modulates the receptor by a different mechanism [86]. This low-affinity (1μM) ligand binds the N-domain of the GLP-1 receptor, at a site that appears at least partially distinct from the peptide binding sites; W33S mutation in the N-domain decreases T-0632 affinity 120-fold without appreciably affecting binding of peptide ligand. Finally, a low-affinity (3μM) nonpeptide antagonist of the CGRP receptor (Fig. 2C) has been inferred to act allosterically from studies of chimeric receptors [75].
Recently, the first allosteric agonist of a Class B GPCR was reported [44]. A representative of the compound class is shown in Fig. (2E). This nonpeptide ligand enhances the binding of [125I]GLP-1 to the GLP-1 receptor and directly activates the receptor, with potency of approximately 100nM. This direct agonist activity is apparent for the receptor expressed endogenously and is GLP-1 receptor mediated: The modulator stimulates insulin release from pancreatic islets and perfused pancreas, and does not affect insulin release from islets isolated from GLP-1 receptor knockout mice [44]. Although the modulator enhances the binding of GLP-1 it does not detectably enhance the signaling activity of the peptide.
POTENTIAL FOR ‘CHARNIERE’ TYPE MODULATION OF CLASS B GPCR’S
Inherent to the two-domain mechanism of peptide binding to Class B GPCR’s is a modulatory mechanism that can appear allosteric but which actually arises from competitive inhibition at one of the peptide binding sites. An explicit description of this type of phenomenon is the ‘Charniere’ effect [69]. In this mechanism an antagonist bears two functional groups, connected by a hinge-region, one that binds the agonist binding site and the other that binds a distinct site not bound by the agonist. This model was developed to explain two unusual actions of some antagonists – persistent blockade after washout and subsequent treatment with agonist, and a time-course of the subsequent agonist response that is independent of the concentration of agonist [69, 70]. This model was applied to explain blockade of histamine responses [70], and blockade of acetylcholine by lachesine at muscarinc receptors of guinea pig ileum [69], and has been subsequently applied to other receptor systems, such as blockade of angiotensin-stimulated tachyphylaxis of rat uterine smooth muscle by a chlorambucil-substituted peptide antagonist [83]. The Charniere concept can be expanded to include persistently-binding large agonists, which are blocked at their readily-reversible site of interaction by smaller antagonists. For example, salmeterol is a β2 adrenergic agonist that binds the endogenous-ligand binding site, blocked by classical antagonists, and a second ‘exosite’ to which it persistently binds and which is not blocked by antagonists [26, 42]. A similar mechanism has also been used to explain the characteristics of xanomeline receptor interaction with the M1 muscarinc acetylcholine receptor [10]
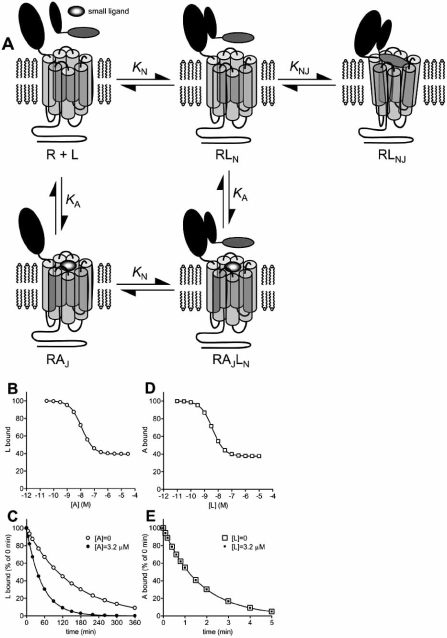
Intrinsic to the two domain model is the potential for a Charniere-type modulation because a small ligand could competitively inhibit peptide binding to one of the two domains without affecting peptide interaction with the other. The behavior of one potential mechanism within this general model was evaluated. In Fig. (4A), a small ligand binds the J-domain at the same site that binds the amino-terminal region of peptide ligand, competitively inhibiting peptide-J-domain interaction. The small ligand does not affect binding of the carboxyl-terminal portion of the peptide to the N-domain of the receptor. Importantly, in this model there are no allosteric interactions between the small ligand and the peptide. Fig. (4B-E) and the Appendix describe the manifestation of this model in ligand binding experiments typically used to identify allosteric modulators. In this simulation it was assumed the strength of peptide binding to the J-domain was moderate (KNJ= 2), such that occupancy of the J-domain represented a significant fraction of total receptor occupancy by peptide ligand (Fig. 1C). Under these conditions, the small ligand inhibits binding of the peptide but saturating concentrations of the small ligand only partially inhibit peptide binding (Fig. 4B). The residual receptor occupancy by peptide is comprised of peptide bound only to the N-domain of the receptor. In peptide ligand dissociation experiments, the small ligand can accelerate dissociation of the peptide ligand (Fig. 4C). This effect is a result of peptide only dissociating from the RLN state, rather than the RLNJ state, in the model. Dissociation of peptide from RLN is slowed by formation of RLNJ. The small ligand inhibits peptide binding to the J-domain of the receptor, preventing the formation of RLNJ that slows peptide dissociation, and consequently accelerating dissociation of peptide. In Fig. (4D), peptide inhibits small ligand binding but saturating concentrations of peptide do not fully inhibit small ligand binding. This effect is a result of the only partial occupancy of the J-domain by peptide ligand at saturating concentrations of peptide ligand (Fig. 1C); the remaining receptors are available to be bound by the small ligand. Finally, in dissociation experiments peptide ligand cannot affect the observed dissociation rate of the small ligand (Fig. 4E). The presence of pre-bound small ligand prevents binding of the peptide ligand to the J-domain, and peptide binding to the N-domain does not affect dissociation of the small ligand.
Fig. (4).
Charniere-type model of modulator interaction with Class B GPCR’s. A. Schematic representation of the model. In this model, a small ligand binds to one of the two sites of peptide interaction, in this specific example the J-domain. Binding of small ligand to this site, defined by the equilibrium constant KA, competitively inhibits peptide interaction with the J-domain but does not affect peptide binding to the N-domain. Note that in this model there is no allosteric interaction between the small ligand and peptide. B-E. Manifestation of the model in binding assays, simulated using equations in the Appendix. Binding parameters were: KN = 1×108 M-1, [R]TOTAL = 100, KNJ= 2, and KA =1×108. B. Modulation of equilibrium binding of peptide (L) by small ligand (A), simulated using eq. 13, [L] = 1×10-9 M. C. Modulation of peptide dissociation by small ligand, simulated using eq. 15. The dissociation rate of peptide from the N-domain (k-N) was set at 0.02 min-1. D. Modulation of equilibrium binding of small ligand by peptide ligand, simulated using eq. 17, [A] = 2×10-9 M. E. Modulation of small ligand dissociation by peptide, simulated using eq. 19. The dissociation rate of small ligand (k-A) was set at 0.6 min-1. Note that this model does not allow peptide to modulate dissociation of small ligand, in contrast to the allosteric model (Fig. 3E).
It is instructive to compare the potential consequences of this model, in which no allosteric interaction is involved, with the allosteric model presented in Fig. 3. The models are similar in that the modulating ligand binds the J-domain of the receptor but they differ in the mechanism by which the modulating ligand inhibits J-domain binding of the peptide (competitive in the direct interaction model, Fig. 4A, allosteric in the allosteric model, Fig. 3A). Both mechanisms can result in partial inhibition of equilibrium binding of peptide (Figs. 3B and 4B), acceleration of peptide dissociation from the receptor (Figs. 3C and 4C) and partial inhibition of modulator binding by the peptide (Figs. 3D and 4D). Consequently, identifying these patterns of behavior in binding assays is potentially insufficient, in the absence of other data, to define a ligand as an allosteric modulator. The principle difference between the models is that peptide ligand can modulate dissociation of an allosteric modulator, but not a competitive inhibitor of a peptide binding site. (Fig. 3E and 4E). In addition, two other results are only possible with the allosteric mechanism – enhanced binding of peptide by modulator (and vice versa), resulting from positive cooperativity, and slowing of peptide dissociation by modulator (see Appendix). These considerations suggest that, for Class B GPCR’s, care should be employed in the interpretation of results from binding experiments typically used to define a compound as an allosteric modulator. As described below, mischaracterizing the mechanism of action of a ligand could impact further ligand optimization.
The potential ambiguity between these mechanisms has arisen at least twice in the literature. In the first case, certain characteristics of nonpeptide antagonists of the CRF1 receptor could potentially be explained by both mechanisms; nonpeptide antagonists only partially inhibit peptide ligand binding, and peptides only partially inhibit radiolabeled nonpeptide ligand binding [37]. Defining these compounds as acting allosterically was possible because the peptide ligands modulate radiolabeled nonpeptide dissociation from the receptor, nonpeptides slow dissociation of radiolabeled peptides [37], and perhaps most importantly mapping the binding sites by site-directed mutagenesis identified them as being spatially distinct on the receptor [34, 48]. In the second case, an amino-terminal analogue of parathyroid hormone (a modified PTH(1-14) fragment) only partially displaced binding of [125I]PTH(3-34) [35]. Reciprocally, PTH(1-34) only partially inhibited interaction of the PTH(1-14) analogue. This interaction between the two ligands was formerly described as allosteric [35] but in the absence of ligand dissociation data this conclusion cannot be reliably drawn. The model described in Fig. (4A) is a more likely explanation for the data, given the location of the ligand binding sites [39, 50, 79]; PTH(3-34) binds the N domain and weakly binds the J-domain, and PTH(1-14) analogues bind only to the J-domain.
IMPLICATIONS OF CLASS B GPCR LIGAND BINDING MECHANISMS IN THE FUTURE DEVELOPMENT OF ALLOSTERIC MODULATORS
Peptide therapeutics have been developed that target Class B GPCR’s (Table 1), but their use can be complicated by the route of administration, typically injection. For example, Byetta is administered by injection twice daily [19]. In addition, peptides of the size that bind Class B GPCR’s do not readily cross the blood-brain barrier, limiting their use to peripheral disease indications. Allosteric modulation of Class B GPCR’s offers the opportunity of developing low molecular weight, nonpeptide agents that could be administered orally, and that could penetrate the blood-brain barrier to treat CNS disorders. Knowledge of the peptide binding mechanism could prove to be highly valuable in developing such ligands.
Broadly, two types of allosteric modulation could be employed for Class B GPCR’s – allosteric inhibition of peptide binding, and allosteric enhancement. Allosteric inhibitors could be developed as antagonists, targeting the CRF1receptor as a potential treatment of stress disorders, the glucacon receptor for managing hyperglycemia, and the CGRP receptor as an alternative mechanism for treating migraine (Table 1). The two-domain model of peptide binding implies that two different regions of the receptor could be targeted – the J-domain, blocking peptide-stimulated receptor activation (e.g. CRF1receptor antagonists), and the N-domain, blocking the principle binding interaction between peptide and receptor. Allosteric enhancers could be developed to potentiate receptor signaling stimulated by the endogenous agonist. Allosteric enhancers of this type have been successfully developed for Class C GPCR’s, e.g. Cinacalcet, an enhancer of the calcium sensing receptor used to treat secondary hyperparathyroidism [82]. The two-domain model could accommodate at least two different enhancer mechanisms – enhancement of peptide affinity for the N-domain, increasing receptor occupancy by the endogenous agonist and so enhancing the signaling output, and enhancement of peptide interaction with the J-domain, directly enhancing the peptide interaction required for receptor activation.
Complexities of ligand binding to Class B GPCR’s could also impact specific stages of drug development. In high-throughput screening using a labeled peptide, the nature of the peptide binding mechanism could affect the outcome. Use of a peptide that binds predominantly to the N-domain could preclude identification of nonpeptide ligands that bind the J-domain. For example, [125I] astressin binds to the N-domain of the CRF1receptor and is not appreciably displaced by nonpeptide ligands that bind the J-domain [37, 64]. The use of [125I]CRF enabled detection of the initial lead compounds because this radioligand binds strongly to the J-domain of the CRF1 receptor [38]. In optimizing leads, the nature of the nonpeptide mechanism might need to be considered to define the parameters used to define compound SAR. For example, if a nonpeptide partially blocks peptide binding by a Charniere mechanism, it would be fruitless to attempt to improve maximal inhibition of peptide binding. This parameter is defined by solely by the peptide binding energy for the J-domain, not by an allosteric effect. In selecting compounds to test in vivo, the criteria need to be carefully considered. For example, a nonpeptide might only slightly inhibit binding by a Charniere mechanism but could fully antagonize the receptor if the interaction it blocks is required for receptor activation. The use of cellular signaling assays can provide an alternative means to address these issues, from high-throughput screening, through lead optimization and selection for in vivo testing.
CONCLUDING REMARKS
Although the Class B GPCR family is relatively small compared with the Class A family, at least half of the members of the Class B family are attractive therapeutic targets. Allosteric modulation of these receptors represents a potential strategy for the development of low molecular-weight agents as first-generation therapeutics (e.g. for the CRF1 receptor) or second-generation alternatives to peptides already in clinical use (e.g. for the GLP-1 receptor). The two-domain interaction of the endogenous peptides with Class B GPCR’s is highly amenable to allosteric modulation by nonpeptide ligands, through modulating the principle binding interaction with the N-domain or the principle activation interaction with the J-domain. Further understanding of these modulatory mechanisms should facilitate the future discovery and optimization of nonpeptide ligands targeting Class B GPCR’s.
APPENDIX
Formulation of the Two-Domain Model of Peptide Binding
Peptide binding to Class B GPCR’s can be represented by the two-domain model (Fig. 1A), in which the carboxyl-terminal portion of the ligand binds the N-domain of the receptor, and the aminoterminal region binds the J-domain [33]. This model assumes peptide ligand (L) binds the receptor N-domain forming RLN, defined by the equilibrium association constant KN. This interaction concentratesL in the vicinity of the J domain, promoting formation of RLNJ.This interaction can be described as a reversible isomerization between RLN and RLNJ, defined by the isomerization constant KNJ([RLNJ/[RLN]).The model assumes interaction between L and the J domain is weak, such that L binding to the J-domain alone (RLJ) does not contribute significantly to the overall occupancy of receptor by L.
The equilibrium concentration of ligand-bound receptor ([L]BOUND)is given by:
| (1) |
where KL = KN (1 + KNJ) and [R]TOTAL is total receptor concentration ([R]+[RL]N+[RL]NJ]). The equilibrium concentration of RLN, ligand bound only to the N-domain, is given by:
| (2) |
The equilibrium concentration of RLNJ, in which L is bound to both the N-domain and J-domain, is given by:
| (3) |
Dissociation of L from R is described by eq. 4, derived by assuming L only dissociates fromand isomerization between RL RLN and isomerization between RLN and RLNJ is the dissociation rate constant for L dissociation from is sufficiently rapid to be at steady-state during the dissociation phase. k-N is the rate constant for L dissociation from RLN and [L]BOUND = [RLN]+[RLNJ]
| (4) |
Eq. 4 indicates the observed dissociation rate is slowed by isomerization of RLN to RLNJ, anticipated since the observed dissociation rate of L from the receptor is first-order with respect to RLN.
Allosteric Modulators that Bind the J-domain of Class B GPCR’s
In this model, formulated in Fig. (3A), the modulator M binds a site on the J domain that is spatially distinct from the peptide binding site on the J-domain. Modulator binding does not affect binding of peptide to the N domain but can allosterically modulate peptide binding to the J-domain, defined by the cooperativity constant α.
In Fig. (3B and C), the effect of M on the binding of L was simulated. Equilibrium binding of a fixed concentration of L was simulated in the presence of a range of concentrations of M (Fig. 3B) using eq. 5, derived using the same logic used for eq. 1:
| (5) |
where [L]BOUND = [RLN]+[RLNJ]=[MRLN]+[MRLNJ], and KL = KN(1 + KNJ) (see eq. 1). KM is the equilibrium association constant of M binding to R. In the presence of saturating concentrations of M, binding of L is defined by:
| (6) |
Visual inspection of eq. 6 indicates that a saturating concentration of M does not reduce binding of L to zero when M is an allosteric inhibitor ( α< 1). In addition, modulator can enhance binding of L ( α>1).
In Fig. (3C) the effect of M on dissociation of L from the receptor was simulated using eq. 7. This derivation assumes, during the time course of L dissociation, a steady-state of isomerization between RLN and RLNJ, and between MRLN and MRLNJ, and no disruption of equilibrium between M and the receptor [47]. The derivation also assumes M does not affect the dissociation rate of L from RLN.
where k-N is the dissociation rate constant for L dissociation from RLN. The concentration of each L-bound component is given by:
| (7) |
In Fig. (3D and E), the effect of L on the binding of M was simulated. Equilibrium binding of a fixed concentration of M was simulated in the presence of a range of concentrations of L (Fig.3D) using eq. 8:
| (8) |
where [M]BOUND = [MR]+[MRLN]+[MRLNJ] and . In the presence of saturating concentrations of L, binding of M is defined by:
| (9) |
Visual inspection of eq. 9 indicates that negative cooperativity (α<1) decreases binding of M, since the numerator is less than the denominator, and reciprocally positive cooperativity (α>1) increases binding of M.
In Fig. (3E), the effect of L on dissociation of M was simulated.This derivation makes a number of simplifying assumptions to enable an explicit derivation. As described in ref [47], allosteric modulation of ligand dissociation can result in complex effects on the ligand dissociation curve depending on the relative kinetics of the two ligands. The following derivation assumes that dissociation of L from RLN is much slower (30-fold) than M dissociation from MR, that the kinetics of isomerization between RLN and RLNJ are rapid compared with L dissociation from RLN, and that L binding to the J domain accelerates dissociation of M [47]. Under these conditions,MR behaves as a distinct population from the peptide-bound species MRLN and MRLNJ. Dissociation of M from MR is described by:
| (10) |
where k-M is the rate of dissociation of M from MR. Dissociation of M from MRLN and MRLNJ is described by:
| (11) |
where k-M(L) is the dissociation rate of M from MRLNJ and where:
Dissociation of M in the presence of L is the sum of the two exponential equations 10 and 11, as follows:
| (12) |
Charniere-Type Model Applied to Class B GPCR’s
In the Charniere model, an antagonist ligand can bind two sites,the first a ‘specific’ site that can be bound by agonist and the second an ‘unspecific’ site that can only be bound by the antagonist [69]. Two separate, hinged regions of the antagonist molecule bind the two distinct sites. This model can be applied to Class B GPCR’s. The formulation (Fig. 3A) only considers ligand binding,disregarding agonistic or antagonistic properties of the ligands.Full-length peptide ligand (L) binds according to the two-domain model (Fig. 1A, eqs. 1-4). A second small ligand (A) binds the peptide-binding site on the J domain, defined by the equilibrium association constant KA. This interaction competitively inhibits peptide binding to the J-domain of the receptor but does not affect peptide binding to the N-domain. Importantly, there is no allosteric interaction between the two ligands in this model.
In Fig. (4B and C), the effect of A on the binding of L was simulated. Equilibrium binding of a fixed concentration of L was simulated in the presence of a range of concentrations of A (Fig.4B) using eq. 13:
| (13) |
where [L]BOUND=[RLN]+[RLNJ]+[RLNAJ], and KL = KN(1+KNJ (see eq. 1). In the presence of saturating concentrations of A, binding of L is defined by:
| (14) |
Visual inspection of eq. 14 indicates that saturating concentrations of A do not reduce L binding to zero. Eq. 14 also indicates that A can inhibit binding of L but cannot enhance binding of L, since the denominator is the sum of the numerator and KNJ.
In Fig. (4C) the effect of A on dissociation of L from the receptor was simulated using eq. 15. This derivation assumes, during the time course of L dissociation, a steady-state of isomerization between RLN and RLNJ and no disruption of equilibrium between A and the receptor [47].
where k-N is the dissociation rate constant for L dissociation from RLN. By substitution and re-arrangement:
| (15) |
In the presence of saturating concentrations of A, the observed dissociation rate of L is given by:
| (16) |
Eq. 16 indicates that A accelerates the observed dissociation of L, anticipated since A increases the level of RLN by inhibiting its isomerization to RLNJ. Saturating concentrations of ligand A increase the observed rate to that for dissociation from RLN because A competitively inhibits formation of RLNJ.
In Fig. (4D and E), the effect of L on the binding of A was simulated. Equilibrium binding of a fixed concentration of A was simulated in the presence of a range of concentrations of L (Fig.3D) using eq. 17:
| (17) |
where[A]BOUND =[RAJ]+[RLNAJ]and . In the presence of saturating concentrations of L, binding of A is defined by:
| (18) |
Visual inspection of eq. 18 indicates that saturating concentrations of L do not reduce A binding to zero. Eq. 18 also indicates that L can inhibit binding of A but cannot enhance binding of A,since the denominator is the sum of the numerator and KNJ.
In Fig. (3E) dissociation of A from the receptor was simulated using Eq. 19:
| (19) |
where k-A is the dissociation rate constant for A dissociation from RAJ. From the derivation and Fig. 4A) it is evident that L cannot affect dissociation of A because binding of L to form RLNAJ does not affect the dissociation rate of A from RAJ, and RAJ cannot isomerize to a different bound state.
CONFLICT OF INTEREST
The author is an employee and stock holder of Neurocrine Biosciences, Inc.
REFERENCES
- 1.Al-Sabah S, Donnelly D. A model for receptor-peptide binding at the glucagon-like peptide-1 (GLP-1) receptor through the analysis of truncated ligands and receptors. Br. J. Pharmacol. 2003;140:339–346. doi: 10.1038/sj.bjp.0705453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TL, Vale WW. CRF CRF receptors role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 3.Bergwitz C, Gardella TJ, Flannery MR, Potts JT Jr, Kronenberg HM, Goldring SR, Juppner H. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin Evidence for a common pattern of ligand-receptor interaction. J. Biol. Chem. 1996;271:26469–26472. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- 4.Bisello A, Adams AE, Mierke DF, Pellegrini M, Rosenblatt M, Suva LJ, Chorev M. Parathyroid hormone-receptor interactions identified directly by photocross-linking and molecular modeling studies. J. Biol. Chem. 1998;273:22498–22505. doi: 10.1074/jbc.273.35.22498. [DOI] [PubMed] [Google Scholar]
- 5.Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. In: Larsen P.R, editor. Textbook of Endocrinology. Philadelphia: W.B. Saunders Company; 1998. pp. 1155–1210. [Google Scholar]
- 6.Cascieri MA, Koch GE, Ber E, Sadowski SJ, Louizides D, de Laszlo SE, Hacker C, Hagmann WK, MacCoss M, Chicchi GG, Vicario PP. Characterization of a novel, non-peptidyl antagonist of the human glucagon receptor. J. Biol. Chem. 1999;274:8694–8697. doi: 10.1074/jbc.274.13.8694. [DOI] [PubMed] [Google Scholar]
- 7.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:16084–16089. doi: 10.1073/pnas.0503942102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Wilcoxen KM, Huang CQ, Xie YF, McCarthy JR, Webb TR, Zhu YF, Saunders J, Liu XJ, Chen TK, Bozigian H, Grigoriadis DE. Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylaminopyrazolo[1 ,5-a]pyrimidine (NBI 30775/R121919) and structure--activity relationships of a series of potent and orally active corticotropin-releasing factor receptor antagonists. J. Med. Chem. 2004;47:4787–4798. doi: 10.1021/jm040058e. [DOI] [PubMed] [Google Scholar]
- 9.Chen YL, Mansbach RS, Winter SM, Brooks E, Collins J, Corman ML, Dunaiskis AR, Faraci WS, Gallaschun RJ, Schmidt A, Schulz DW. Synthesis and oral efficacy of a 4-(butylethylamino) pyrrolo[2,3- d]pyrimidine: a centrally active corticotropin-releasing factor1 receptor antagonist. J. Med. Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- 10.Christopoulos A, Parsons AM, El-Fakahany EE. Pharmacological analysis of the novel mode of interaction between xanomeline and the M1 muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 1999;289:1220–1228. [PubMed] [Google Scholar]
- 11.Couvineau A, Tan YV, Ceraudo E, Lacapere JJ, Murail S, Neumann JM, Laburthe M. The human VPAC1 receptor: identification of the N-terminal ectodomain as a major VIP-binding site by photoaffinity labeling and 3D modeling. Ann. N. Y. Acad. Sci. 2006;1070:205–209. doi: 10.1196/annals.1317.015. [DOI] [PubMed] [Google Scholar]
- 12.Dautzenberg FM, Kilpatrick GJ, Hauger RL, Moreau J. Molecular biology of the CRH receptors-- in the mood. Peptides. 2001;22:753–760. doi: 10.1016/s0196-9781(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 13.de Laszlo SE, Hacker C, Li B, Kim D, MacCoss M, Mantlo N, Pivnichny JV, Colwell L, Koch GE, Cascieri MA, Hagmann WK. Potent, orally absorbed glucagon receptor antagonists. Bioorg. Med. Chem. Lett. 1999;9:641–646. doi: 10.1016/s0960-894x(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 14.Dean T, Linglart A, Mahon MJ, Bastepe M, Juppner H, Potts JT Jr, Gardella TJ. Mechanisms of ligand binding to the parathyroid hormone (PTH)/PTH-related protein receptor selectivity of a modified PTH(1-15) radioligand for GalphaS-coupled receptor conformations. Mol. Endocrinol. 2006;20:931–943. doi: 10.1210/me.2005-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 16.Dong M, Miller LJ. Use of photoaffinity labeling to understand the molecular basis of ligand binding to the secretin receptor. Ann. N. Y. Acad. Sci. 2006;1070:248–264. doi: 10.1196/annals.1317.023. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly D. The arrangement of the transmembrane helices in the secretin receptor family of G-protein-coupled receptors. FEBS Lett. 1997;409:431–436. doi: 10.1016/s0014-5793(97)00546-2. [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–2940. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 20.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann. N. Y. Acad. Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 21.Foord SM, Jupe S, Holbrook J. Bioinformatics and type II G-protein-coupled receptors. Biochem. Soc. Trans. 2002;30:473–479. doi: 10.1042/bst0300473. [DOI] [PubMed] [Google Scholar]
- 22.Gardella TJ, Luck MD, Fan MH, Lee C. Transmembrane residues of the parathyroid hormone (PTH)/PTH-related peptide receptor that specifically affect binding and signaling by agonist ligands. J. Biol. Chem. 1996;271:12820–12825. doi: 10.1074/jbc.271.22.12820. [DOI] [PubMed] [Google Scholar]
- 23.Gilligan PJ, Baldauf C, Cocuzza A, Chidester D, Zaczek R, Fitzgerald LW, McElroy J, Smith MA, Shen HS, Saye JA, Christ D, Trainor G, Robertson DW, Hartig P. The discovery of 4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyrimidine a corticotropin-releasing factor (hCRF1) antagonist. Bioorg. Med. Chem. 2000;8:181–189. doi: 10.1016/s0968-0896(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 24.Gilligan PJ, Robertson DW, Zaczek R. Corticotropin releasing factor (CRF) receptor modulators: progress and opportunities for new therapeutic agents. J. Med. Chem. 2000;43:1641–1660. doi: 10.1021/jm990590f. [DOI] [PubMed] [Google Scholar]
- 25.Grace CR, Perrin MH, DiGruccio MR, Miller CL, Rivier JE, Vale WW, Riek R. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc. Natl. Acad. Sci. USA. 2004;101:12836–12841. doi: 10.1073/pnas.0404702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green SA, Spasoff AP, Coleman RA, Johnson M, Liggett SB. Sustained activation of a G protein-coupled receptor via "anchored" agonist binding.Molecular localization of the salmeterol exosite within the 2-adrenergic receptor. J. Biol. Chem. 1996;271:24029–24035. doi: 10.1074/jbc.271.39.24029. [DOI] [PubMed] [Google Scholar]
- 27.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl] 5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist II. Characterization in rodent models of stress-related disorders. J. Pharmacol. Exp. Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 28.Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opin. Ther. Targets. 2005;9:651–684. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- 29.Gross RS, Guo Z, Dyck B, Coon T, Huang CQ, Lowe RF, Marinkovic D, Moorjani M, Nelson J, Zamani-Kord S, Grigoriadis DE, Hoare SR, Crowe PD, Bu JH, Haddach M, McCarthy J, Saunders J, Sullivan R, Chen T, Williams JP. Design and synthesis of tricyclic corticotropin-releasing factor-1 antagonists. J. Med. Chem. 2005;48:5780–5793. doi: 10.1021/jm049085v. [DOI] [PubMed] [Google Scholar]
- 30.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology.XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- 31.He L, Gilligan PJ, Zaczek R, Fitzgerald LW, McElroy J, Shen HS, Saye JA, Kalin NH, Shelton S, Christ D, Trainor G, Hartig P. 4-(1,3-Dimethoxyprop-2-ylamino)-2,7-dimethyl-8-(2,4-dichlorophenyl)pyra-zolo[1,5-a]-1,3,5-triazine a potent, orally bioavailable CRF(1) receptor antagonist. J. Med. Chem. 2000;43:449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- 32.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 33.Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov. Toda. 2004;10:417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- 34.Hoare SR, Brown BT, Santos MA, Malany S, Betz SF, Grigoriadis DE. Single amino acid residue determinants of non-peptide antagonist binding to the corticotropin-releasing factor1 (CRF1) receptor. Biochem. Pharmacol. 2006;72:244–255. doi: 10.1016/j.bcp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Hoare SR, Gardella TJ, Usdin TB. Evaluating the signal transduction mechanism of the parathyroid hormone 1 receptor Effect of receptor-G-protein interaction on the ligand binding mechanism and receptor conformation. J. Biol. Chem. 2001;276:7741–7753. doi: 10.1074/jbc.M009395200. [DOI] [PubMed] [Google Scholar]
- 36.Hoare SR, Sullivan SK, Fan J, Khongsaly K, Grigoriadis DE. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides. 2005;26:457–470. doi: 10.1016/j.peptides.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Hoare SR, Sullivan SK, Ling N, Crowe PD, Grigoriadis DE. Mechanism of corticotropin-releasing factor type I receptor regulation by nonpeptide antagonists. Mol. Pharmacol. 2003;63:751–765. doi: 10.1124/mol.63.3.751. [DOI] [PubMed] [Google Scholar]
- 38.Hoare SR, Sullivan SK, Schwarz DA, Ling N, Vale WW, Crowe PD, Grigoriadis DE. Ligand affinity for amino-terminal and juxtamembrane domains of the corticotropin releasing factor type I receptor: regulation by G-protein and nonpeptide antagonists. Biochemistry. 2004;43:3996–4011. doi: 10.1021/bi036110a. [DOI] [PubMed] [Google Scholar]
- 39.Hoare SR, Usdin TB. Molecular mechanisms of ligand recognition by parathyroid hormone 1 (PTH1) and PTH2 receptors. Curr. Pharm. Des. 2001;7:689–713. doi: 10.2174/1381612013397825. [DOI] [PubMed] [Google Scholar]
- 40.Holsboer F. Stress hypercortisolism and corticosteroid receptors in depression: implicatons for therapy. J. Affect. Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 41.Holtmann MH, Hadac EM, Miller LJ. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors.Studies of chimeric receptors. J. Biol. Chem. 1995;270:14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- 42.Jack D. The 1990 Lilly Prize Lecture A way of looking at agonism and antagonism: lessons from salbutamol, salmeterol and other beta-adrenoceptor agonists. Br. J. Clin. Pharmacol. 1991;31:501–514. doi: 10.1111/j.1365-2125.1991.tb05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehne J, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr. Drug Target CNS Neurol. Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- 44.Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, Holst JJ, Jeppesen CB, Johnson MD, de Jong JC, Jorgensen AS, Kercher T, Kostrowicki J, Madsen P, Olesen PH, Petersen JS, Poulsen F, Sidelmann UG, Sturis J, Truesdale L, May J, Lau J. Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc. Natl. Acad. Sci. USA. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurukulasuriya R, Link JT, Madar DJ, Pei Z, Richards SJ, Rohde JJ, Souers AJ, Szczepankiewicz BG. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr. Med. Chem. 2003;10:123–153. doi: 10.2174/0929867033368556. [DOI] [PubMed] [Google Scholar]
- 46.Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Recept. Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- 47.Lazareno S, Birdsall NJ. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors interactions of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- 48.Liaw CW, Grigoriadis DE, Lorang MT, De Souza EB, Maki RA. Localization of agonist- and antagonist-binding domains of human corticotropin-releasing factor receptors. Mol. Endocrinol. 1997;11:2048–2053. doi: 10.1210/mend.11.13.0034. [DOI] [PubMed] [Google Scholar]
- 49.Lopez de Maturana R, Willshaw A, Kuntzsch A, Rudolph R, Donnelly D. The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J. Biol. Chem. 2003;278:10195–10200. doi: 10.1074/jbc.M212147200. [DOI] [PubMed] [Google Scholar]
- 50.Luck MD, Carter PH, Gardella TJ. The (1-14) fragment of parathyroid hormone (PTH) activates intact and amino-terminally truncated PTH-1 receptors. Mol. Endocrinol. 1999;13:670–680. doi: 10.1210/mend.13.5.0277. [DOI] [PubMed] [Google Scholar]
- 51.Mannstadt M, Juppner H, Gardella TJ. Receptors for PTH and PTHrP their biological importance and functional properties. Am. J. Physiol. 1999;277:F665–675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 52.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology XXXV.The glucagon receptor family. Pharmacol. Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy JR, Heinrichs SC, Grigoriadis DE. Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications. Curr. Pharm. Des. 1999;5:289–315. [PubMed] [Google Scholar]
- 54.McDermott MT, Kidd GS. The role of calcitonin in the development and treatment of osteoporosis. Endocr. Rev. 1987;8:377–390. doi: 10.1210/edrv-8-4-377. [DOI] [PubMed] [Google Scholar]
- 55.Mesleh MF, Shirley WA, Heise CE, Ling N, Maki RA, Laura RP. NMR structural characterization of a minimal peptide antagonist bound to the extracellular domain of the corticotropin-releasing factor1 (CRF1) receptor. J. Biol. Chem. 2007;282:6338–6346. doi: 10.1074/jbc.M609816200. [DOI] [PubMed] [Google Scholar]
- 56.Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system a life span perspective. Dev. Psychopathol. 2001;13:565–580. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- 57.Morfis M, Christopoulos A, Sexton PM. RAMPs 5 years on where to now? Trends Pharmacol. Sci. 2003;24:596–601. doi: 10.1016/j.tips.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen SM, Nielsen LZ, Hjorth SA, Perrin MH, Vale WW. Constitutive activation of tethered-peptide/corticotropin-releasing factor receptor chimeras. Proc. Natl. Acad. Sci. USA. 2000;97:10277–10281. doi: 10.1073/pnas.97.18.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 61.Perrin MH, DiGruccio MR, Koerber SC, Rivier JE, Kunitake KS, Bain DL, Fischer WH, Vale WW. A Soluble Form of the First Extracellular Domain of Mouse Type 2beta Corticotropin-releasing Factor Receptor Reveals Differential Ligand Specificity. J. Biol. Chem. 2003;278:15595–15600. doi: 10.1074/jbc.M210476200. [DOI] [PubMed] [Google Scholar]
- 62.Perrin MH, Grace CR, Riek R, Vale WW. The three-dimensional structure of the N-terminal domain of corticotropin-releasing factor receptors sushi domains and the B1 family of G protein-coupled receptors. Ann. N. Y. Acad. Sci. 2006;1070:105–119. doi: 10.1196/annals.1317.065. [DOI] [PubMed] [Google Scholar]
- 63.Perrin MH, Sutton S, Bain DL, Berggren WT, Vale WW. The first extracellular domain of corticotropin releasing factor-R1 contains major binding determinants for urocortin and astressin. Endocrinology. 1998;139:566–570. doi: 10.1210/endo.139.2.5757. [DOI] [PubMed] [Google Scholar]
- 64.Perrin MH, Vale WW. Corticotropin-releasing factor receptors and their ligand family. Annals New York Academy of Sciences. 1999. pp. 312–328. [DOI] [PubMed]
- 65.Pham V, Dong M, Wade JD, Miller LJ, Morton CJ, Ng HL, Parker MW, Sexton PM. Insights into interactions between the alpha-helical region of the salmon calcitonin antagonists and the human calcitonin receptor using photoaffinity labeling. J. Biol. Chem. 2005;280:28610–28622. doi: 10.1074/jbc.M503272200. [DOI] [PubMed] [Google Scholar]
- 66.Potts JT. Parathyroid hormone past and present. J. Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- 67.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology XXXII.The mammalian calcitonin gene-related peptides adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 68.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 69.Rocha e Silva M. A thermodynamic approach to problems of drug antagonism I.“The Charniere theory”. Eur. J. Pharmacol. 1969;6:294–302. doi: 10.1016/0014-2999(69)90188-5. [DOI] [PubMed] [Google Scholar]
- 70.Rocha e Silva M, Beraldo WT. Dynamics of recovery and measure of drug antagonism.Inhibition of smooth muscle by lysocithin and antihistaminics. J. Pharmacol. Exp. Ther. 1948;93:457–469. [PubMed] [Google Scholar]
- 71.Rodbell M, Krans HM, Pohl SL, Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver 3.Binding of glucagon method of assay and specificity. J. Biol. Chem. 1971;246:1861–1871. [PubMed] [Google Scholar]
- 72.Rolz C, Pellegrini M, Mierke DF. Molecular characterization of the receptor-ligand complex for parathyroid hormone. Biochemistry. 1999;38:6397–6405. doi: 10.1021/bi9829276. [DOI] [PubMed] [Google Scholar]
- 73.Rubin MR, Bilezikian JP. New anabolic therapies in osteoporosis. Endocrinol. Metab. Clin. North Am. 2003;32:285–307. doi: 10.1016/s0889-8529(02)00056-7. [DOI] [PubMed] [Google Scholar]
- 74.Runge S, Wulff BS, Madsen K, Brauner-Osborne H, Knudsen LB. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br. J. Pharmacol. 2003;138:787–794. doi: 10.1038/sj.bjp.0705120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salvatore CA, Mallee JJ, Bell IM, Zartman CB, Williams TM, Koblan KS, Kane SA. Identification and pharmacological characterization of domains involved in binding of CGRP receptor antagonists to the calcitonin-like receptor. Biochemistry. 2006;45:1881–1887. doi: 10.1021/bi052044w. [DOI] [PubMed] [Google Scholar]
- 76.Sheikh SP, Vilardarga JP, Baranski TJ, Lichtarge O, Iiri T, Meng EC, Nissenson RA, Bourne HR. Similar structures and shared switch mechanisms of the beta2-adrenoceptor and the parathyroid hormone receptor Zn(II) bridges between helices III and VI block activation. J. Biol. Chem. 1999;274:17033–17041. doi: 10.1074/jbc.274.24.17033. [DOI] [PubMed] [Google Scholar]
- 77.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu M, Carter PH, Gardella TJ. Autoactivation of type-1 parathyroid hormone receptors containing a tethered ligand. J. Biol. Chem. 2000;275:19456–19460. doi: 10.1074/jbc.M001596200. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu N, Guo J, Gardella TJ. Parathyroid hormone (PTH)-(1-14) and -(1-11) analogs conformationally constrained by alpha-aminoisobutyric acid mediate full agonist responses via the juxtamembrane region of the PTH-1 receptor. J. Biol. Chem. 2001;276:49003–49012. doi: 10.1074/jbc.M106827200. [DOI] [PubMed] [Google Scholar]
- 80.Sloop KW, Michael MD, Moyers JS. Glucagon as a target for the treatment of Type 2 diabetes. Expert Opin. Ther. Targets. 2005;9:593–600. doi: 10.1517/14728222.9.3.593. [DOI] [PubMed] [Google Scholar]
- 81.Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 82.Steddon SJ, Cunningham J. Calcimimetics and calcilytics--fooling the calcium receptor. Lancet. 2005;365:2237–2239. doi: 10.1016/S0140-6736(05)66782-7. [DOI] [PubMed] [Google Scholar]
- 83.Stewart JM, Freer RJ, Rezende L, Pena C, Matsueda GR. A pharmacological study of the angiotensin receptor and tachyphylaxis in smooth muscle. Gen. Pharmacol. 1976;7:177–183. doi: 10.1016/0306-3623(76)90058-6. [DOI] [PubMed] [Google Scholar]
- 84.Stockton JM, Birdsall NJ, Burgen AS, Hulme EC. Modification of the binding properties of muscarinic receptors by gallamine. Mol. Pharmacol. 1983;23:551–557. [PubMed] [Google Scholar]
- 85.Tache Y. Corticotropin releasing factor receptor antagonists potential future therapy in gastroenterology? Gut. 2004;53:919–921. doi: 10.1136/gut.2003.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tibaduiza EC, Chen C, Beinborn M. A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. J. Biol. Chem. 2001;276:37787–37793. doi: 10.1074/jbc.M106692200. [DOI] [PubMed] [Google Scholar]
- 87.Tregear GW, Van Rietschoten J, Greene E, Keutmann HT, Niall HD, Reit B, Parsons JA, Potts JT Jr. Bovine parathyroid hormone: minimum chain length of synthetic peptide required for biological activity. Endocrinology. 1973;93:1349–1353. doi: 10.1210/endo-93-6-1349. [DOI] [PubMed] [Google Scholar]
- 88.Usdin TB, Dobolyi A, Ueda H, Palkovits M. Emerging functions for tuberoinfundibular peptide of 39 residues. Trends Endocrinol. Metab. 2003;14:14–19. doi: 10.1016/s1043-2760(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 89.Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 90.Wysolmerski JJ, Stewart AF. The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu. Rev. Physiol. 1998;60:431–460. doi: 10.1146/annurev.physiol.60.1.431. [DOI] [PubMed] [Google Scholar]
- 91.Zhang G, Huang N, Li YW, Qi X, Marshall AP, Yan XX, Hill G, Rominger C, Prakash SR, Bakthavatchalam R, Rominger DH, Gilligan PJ, Zaczek R. Pharmacological characterization of a novel nonpeptide antagonist radioligand, (+/-)-N-[2-methyl-4-methoxyphenyl]-1-(1-(metho-xymethyl) propyl)-6-methyl-1H-1,2,3-triazolo[4,5-c]pyridin-4-amine ([3H] SN003) for corticotropin-releasing factor1 receptors. J. Pharmacol. Exp. Ther. 2003;305:57–69. doi: 10.1124/jpet.102.046128. [DOI] [PubMed] [Google Scholar]
- 92.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression the first 20 patients treated. J. Psychiatr. Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]