Abstract
Purpose
Our current understanding of the rate and pattern of physeal closure is based on roentgenographic, magnetic resonance imaging, and qualitative histological studies. The purpose of this report is to provide a detailed histomorphometric/stereological analysis of a distal tibial human growth plate in the process of physiological epiphysiodesis.
Methods
A human distal tibial growth plate was sampled in three regions (anterior, central, and posterior), with each region further separated medially, in the middle, and laterally. The regions were assessed for the location and extent of bony bar formation as well as for physeal height. Companion sections from optimally fixed tissue in the distal 100 µm of the hypertrophic zone were analyzed for hypertrophic chondrocytic volumes.
Results
Physis closure started in the middle of the central region of the growth plate, with 46% of the volume in this area occupied by trans-physeal bridging bone. The growth plate was also narrowed with the lowest physeal heights evident in the middle of the central and anterior regions of the physis. Disruption of the regular columns of the physis was evident with the cells arranged in clusters with intervening areas of acellularity. The average hypertrophic cell volume was 5,900 µm3 and did not significantly differ between different areas of the physis.
Conclusions
This is the first characterization of closure in a human distal tibial growth plate via optimum fixation and stereological techniques. The studied physis was during the earliest phases of closure and provides stereological support that the distal tibial physis closes in a central to medial direction.
Keywords: Epiphysiodesis, Physis, Histomorphometry
Introduction
Traumatic injuries to the distal tibial physis are common and growth arrest can result in leg length discrepancy or angular malformation. The pattern of physeal closure is clinically important because it places the adolescent patient at risk of transitional injuries, such as the tri-plane or Tillaux fractures. Our current understanding of the rate and pattern of physeal closure is based on roentgenographic [1–3], magnetic resonance imaging [4], and qualitative histological [3, 5] studies. The purpose of this report is to provide a stereological analysis of a distal tibial human growth plate in the process of physiological epiphysiodesis.
Methods
Patient history
This study was approved by the Institutional Review Board, and informed consent was obtained from the patient and the patient’s family. The patient had been diagnosed with osteosarcoma in the left distal femur at 11 years of age. The local tumor was treated with wide excision and limb-sparing surgery using a large osteoarticular allograft and plating. The patient received preoperative and postoperative chemotherapy with cisplatin, doxorubicin, and methotrexate. For over 1-year, the patient was fully ambulatory, clinically assessed approximately by normal loading on the distal tibia. By 18 months postoperatively, the patient was diagnosed with a local recurrence and subsequently underwent an above-knee amputation at the age of 12 years and 11 months. The distal tibial specimen was harvested and immediately processed for histological analysis.
Fixation, embedment, sectioning, and stereology
The distal end of the tibia was sectioned into a series of medial to lateral slabs that were 2 mm thick (Bone saw, Gryphon Model C-40, Gryphon Corporation,12417 Foothill Blvd., Sylmar, CA 91342, USA). Slabs included the entire growth plate, and epiphyseal and metaphyseal bone. Three slabs—one from each of anterior, central, and posterior regions—were chosen for further processing and analysis. These three slabs (in their entirety) were fixed in 10% neutral-buffered formalin, decalcified (citrate-buffered formic acid), vacuum infiltrated, and embedded in paraffin. Sections 5 μm thick were made of the entire (medial-to-lateral) growth plate and stained with either Hematoxylin–Eosin–Phloxine or Safranin-O.
For stereological analysis of growth plate chondrocytes; companion anterior, central, and posterior region slabs were prepared for optimal chemical fixation. These slabs were further trimmed into 1 × 1 × 2-mm blocks and fixed in 2% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.35) with 0.7% ruthenium hexamine trichloride (RHT) [6, 7]. Tissue blocks were oriented, such that growth plates would be sectioned vertically [8], embedded in Epon-araldite, sectioned at 1.5 µm, and stained with a polychrome stain eliminating methylene blue-azure II to enhance the digital discrimination of chondrocytes versus matrix [9]. One central slab was prepared with a combination of techniques including RHT fixation followed by 70% ethanol fixation, embedded in polymethyl methacrylate, and undecalcified sections were cut using a precision saw (Isomet Plus, Buehler Lake Bluff, IL, USA) and ground to 100 μm thickness (EXAKT Technologies Inc, Oklahoma City, OK 73116, USA). In addition, prior to processing, fine detail microradiography was performed (model 43855A; Hewlett-Packard Faxitron, McMinnville, OR, USA).
Stereology: growth plate height and distribution of bony bridges
Using sections of the entire width of the growth plate, camera lucida maps were made of the anterior, central, and posterior regions. Because of the confounding influence of the ‘ossification groove’ (of Ranvier) and the ‘perichondrial ring’ (of Lacroix), the medial and lateral 5% of each map were omitted from the analysis. The map of each region was then separated into medial, middle, and lateral thirds of the growth plate (Fig. 1). In each third of each region, the height of the growth plate (epiphyseal bone to chondro-osseous junction) was measured at four unbiased locations. The columnar arrangement of growth plate chondrocytes was used to align the measurement. Quantitative measures of the extent and distribution of bony bridges were made using a standard point counting volume fraction stereological analysis [10–12].
Fig. 1.

Photographic montage of the central region of the physis demonstrating the division into medial, middle and lateral regions after exclusion of the peripheral 5% of the physis. This specimen was optimally fixed in RHT, undecalcified, embedded in polymethyl methacrylate and stained with toluidine blue
Stereology: volume of hypertrophic chondrocytes
Companion sections from optimally fixed tissue in the distal 100 μm of the hypertrophic zone were analyzed for hypertrophic chondrocytic volumes (v(c)hypertrophic chondrocytes); Fig. 2). This data was collected using the point-sampled, mean linear intercept method from three different angles of intercept [10, 11]. In a prior preliminary study, we demonstrated that there is no significant difference in our estimates of hypertrophic chondrocytic volumes using the angles of 46, 57, and 66° in comparison with our previous time-consuming method of using 8 or 16 angles [6, 10]. The measurements were made using MatLab custom image-digitizing software (courtesy of Dr. Ian Stokes, University of Vermont, Burlington Vermont) written in MatLab (MathWorks, Natick, MA, USA).
Fig. 2.
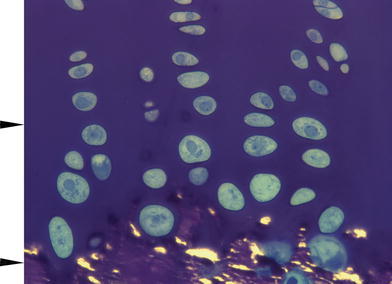
Micrograph of optimally fixed tissue at level of chondro-osseus junction (lateral sample from central region). Arrowheads are spaced 100 µm apart indicating the cellular field used for hypertrophic volumetric measurements
Results
Transphyseal bone formation
Examination of the microradiographic images demonstrate physeal bar formation in the middle of the physis (Fig. 3) and, by stereological, volume fraction measurement, most of the volume of the bar formation of the physis that is occupied by bridging bone is found in the very center of the physis (middle of the central region), representing 46% of the volume in this area. There was a minor amount of bridging bone found in the middle and medial aspects of the anterior region (Fig. 4). At this stage of the patient’s life, there was no bridging bone found in any of the other sections.
Fig. 3.
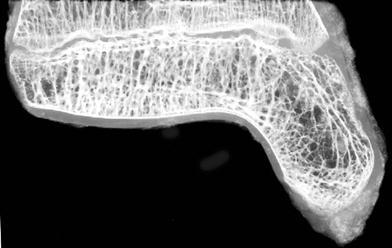
Microradiograph of the central region of the distal tibial physis demonstrates bridging bone in the middle of the specimen
Fig. 4.

Percentage volume of each of the nine regions of the distal tibia physis occupied by bridging bone. Bridging bone is found almost exclusively in the middle of the central region, occupying 46% of the volume in this area
Physeal height
The average height of the distal tibial physis was 980 μm. The average height for each of the nine sampling regions is illustrated in Fig. 5. The most significant narrowing was found in the middle of the anterior and central regions.
Fig. 5.

Average height of the distal tibial physis by region. The greatest narrowing is seen in the middle of the anterior and central regions
Hypertrophic cell volumes
The overall average hypertrophic cell volume was 5,900 μm3. The volumes ranged from 3,600 μm 3 in the medial aspect of the anterior region to 8,400 μm3 in the lateral aspect of the posterior region. There were no significant differences among the hypertrophic cell volumes in the nine regions sampled.
Qualitative observations
Histological observations of this physis demonstrated several interesting findings, especially when contrasted to what is known about an actively growing physis. The chondrocytes were organized into small clusters of cells with large areas of intervening hypocellularity (Fig. 6a). The cellular columns are relatively disorganized and it is difficult to define a clear hypertrophic zone. At the metaphyseal border there is a horizontally oriented layer of bone covering much of this surface of the physis. In the middle of the central region almost half of the physeal area is occupied by extensions of bone and marrow fat from the adjacent metaphysis and epiphysis (Fig. 6b).
Fig. 6.

a Photomicrograph of the distal tibia demonstrating the clusters of cells with large intervening areas of acellularity. b Photomicrograph showing the bridging bone in the middle of the central region
Discussion
Our current understanding of the pattern of closure of the distal tibial physis is based on radiographic, magnetic resonance imaging, and qualitative histological studies. Kleiger and Mankin in 1964 reviewed the radiographs of 22 ankles in the process of physeal closure and found that it proceeded in an asymmetrical pattern. The physis appears to close in the middle first, then on the medial side, and then the lateral portion. This assessment has been reaffirmed by the work of Kump [2] and Ogden [1]. Chung and Jarmillo [4] analyzed the magnetic resonance imaging sequences of 14 patients who were felt to be undergoing normal physiological epiphysiodesis. These authors observed, by means of MRI, that closure appeared to start at the undulation in the anteromedial physis and proceed laterally. Love et al. [5] performed a routine (non-optimized) histological examination of 44 distal tibiofibular composites from cadaver or amputation specimens from age birth to 15 years. In this study, physiological epiphysiodesis occurred between the ages of 12 and 14 years in girls and between the ages of 15 and 18 years in boys. Closure was seen, characteristically, to occur in a medial to lateral pattern over a period of 1.5 years.
The subject of the current study was almost 13 years of age at the time of amputation. Her chronological age would suggest that she was in the period of physiological epiphysiodesis, and our findings support this. Our patient’s growth plate was closing in the central portion, a finding which corresponds to previous results in the literature. We anticipated that the medial portion would close before the lateral side, in concordance with previous reports.
This study provided a unique opportunity to examine a human distal tibial physis with optimal chemical fixation in the presence of RHT, thus allowing analysis using stereological methodologies. We realize that in our phenomenological study of this patient’s growth plate, there is a limited ability to extrapolate to other human growth plates. Although not directly involved in disease, our patient and this physis experienced abnormal loading and was exposed to chemotherapy. Doxorubicin and cisplatin result in decreased growth rate and final height [13]. Therefore, the growth rate and timing of closure may have been affected by the previous treatment, although any effect on the pattern of physeal closure is unknown.
The organized cellular columns evident in an actively growing physis are disrupted in this growth plate. Furthermore, the cells in this specimen are organized into tightly packed clusters with large intervening areas of acellularity. Interestingly, the histological findings are consistent with those found in a rat physis after cessation of growth [14]. With respect to comparative biology of physes from different mammalian species, a case could be made that the “classical” proximal tibial growth plates from rabbits and rats (even mice) are extremes of growth plate structure and physiology. These growth plates have a physiological growth velocity of between 300 and 600 μm/day that depends on age, perhaps a 10–20× faster growth velocity than human growth plates. In addition, when the histological arrangement of growth plates with a slow growth velocity is observed, the trend is for chondrocytes to be less arranged in columns and more arranged in clonal clusters [15].
In this growth plate, the average hypertrophic cell volume was 5,900 μm3, with no significant difference between the different zones of the physis. In contrast, hypertrophic cell volume in adolescent rats is about 15,000 μm3. In a histological analysis of a human growth plate from a 10-year-old girl [13], Hall and Macnicol [16] found hypertrophic cell volumes from 621 to 1,572 μm3. It is hard to draw comparisons between our study and theirs. In the previous paper, their patient was [2] years younger, the cells were from the proximal tibial and distal femoral physes, and the patient had hemihypertrophy of the affected limb. Most importantly, their sample was prepared and analyzed using different techniques. Our sample was prepared with optimum chemical fixation, and a large population of hypertrophic chondrocytes from throughout the growth plate was sampled. If we measure physeal chondrocyte just proximal to the most distal 100-μm hypertrophic chondrocytes, these cells have a volume similar to that previously reported. Regardless of mammalian species, enlargement of hypertrophic chondrocyte through a process of “matrix directed cellular swelling,” is now recognized as the “chief engine of growth and the chief regulatory cell” [17–20]. As previously stated, our patient had undergone treatment with chemotherapeutic agents that slow skeletal growth, and the hypertrophic cell volume results must be interpreted in that context. The patient had not been exposed to doxorubicin and adriamycin for 13 months and the lasting effects on the hypertrophic cell volume is unknown. Despite this limitation, it is interesting to see that cessation of growth in this sample does not appear to be accompanied by decreases in cell volume in this subject; obviously, other mechanisms are needed to lead to growth cessation.
This study provided a unique opportunity to apply optimum fixation and current stereological techniques to a human distal tibial physis during the earliest phases of physiological epiphysiodesis. Using optimum fixation techniques, the human hypertrophic cell volume at this stage was found to be 5,900 μm 3. The current specimen provides quantitative support using modern stereological techniques that the distal tibial physis begins closure in the central portion.
Contributor Information
Jeremy Russell White, Email: jeremy@orthotumor.net.
Kenneth J. Noonan, Phone: +1-608-2631344, FAX: +1-608-2635631, Email: noonan@orthorehab.wisc.edu
References
- 1.Ogden JA, McCarthy SM. Radiology of postnatal skeletal development. VIII. Distal tibia and fibula. Skelet Radiol. 1983;10(4):209–220. doi: 10.1007/BF00357893. [DOI] [PubMed] [Google Scholar]
- 2.Kump WL. Vertical fractures of the distal tibial epiphysis. Am J Roentgenol Radium Ther Nucl Med. 1966;97(3):676–681. doi: 10.2214/ajr.97.3.676. [DOI] [PubMed] [Google Scholar]
- 3.Kleiger B, Mankin HJ. Fractures of the lateral portion of the distal tibial epiphysis. J Bone Joint Surg Am. 1964;46:25–32. [PubMed] [Google Scholar]
- 4.Chung T, Jarmillo D. Normal maturing distal tibia and fibula: changes with age at MR imaging. Radiology. 1995;194(1):227–232. doi: 10.1148/radiology.194.1.7997558. [DOI] [PubMed] [Google Scholar]
- 5.Love SM, Ganey R, Ogden JA. Postnatal epiphyseal development: the distal tibia and fibula. J Pediatr Orthop. 1990;10(3):299–305. doi: 10.1097/01241398-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Wilsman NJ, Farnum CE, Leiferman EM, et al. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker EB, Herrmann W, Schenk RK. Improved cartilage fixation by ruthenium hexamine trichloride (RHT) J Ultrastruct Res. 1982;81:1–12. doi: 10.1016/S0022-5320(82)90036-3. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Orive LM, Hunziker EB. Stereology for anisotrophic cells: application to growth plate cartilage. J Microsc. 1986;143:47–80. doi: 10.1111/j.1365-2818.1986.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey CD, Pittman FE. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974;49(1):9–13. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Orive LM, Hunziker EB. Stereology for anisotrophic cells: application to growth plate cartilage. J Microsc. 1986;143:47–80. doi: 10.1111/j.1365-2818.1986.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen HJG, Bendtsen TF, Korbo L et al. The new stereological tools. APMIS (1988): 96:379–394, 857–881 [DOI] [PubMed]
- 12.Noonan KJ, Hunziker EB, Nessler J, et al. Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J Orthop Res. 1998;16:500–508. doi: 10.1002/jor.1100160416. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen BL, Kamps WA, Jansen HW, et al. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev. 2000;26(5):363–376. doi: 10.1053/ctrv.2000.0180. [DOI] [PubMed] [Google Scholar]
- 14.Roach HI, Mehta G, Oreffo RO, et al. Temporal analysis of rat growth plates: cessation of growth with age despite presence of a physis. J Histochem Cytochem. 2003;51(3):373–383. doi: 10.1177/002215540305100312. [DOI] [PubMed] [Google Scholar]
- 15.Wilsman NJ, Farnum CE, Leiferman EM, et al. Growth plate biology in the context of growth by saltations and stasis. In: Lampl M, et al., editors. Saltation stasis and human growth and development: evidence, methods, and theory. Philadelphia: Smith-Gordon; 1999. pp. 71–87. [Google Scholar]
- 16.Hall AC, Macnicol MF. Looking at the human growth plate. Can Assoc Med J. 2003;168(4):459–460. [PMC free article] [PubMed] [Google Scholar]
- 17.Buckwalter JA, Mower D, Ungar R, et al. Morphometric analysis of chondrocyte hypertrophy. J Bone Joint Surg Am. 1986;68:243–255. [PubMed] [Google Scholar]
- 18.Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckwalter JA, Sjolund RD. Growth of cornstalks and long bones: does longitudinal bone growth depend on a matrix directed hydraulic mechanism? Iowa Orthop J. 1990;9:25–31. [Google Scholar]
- 20.Kronenberg HM. Insight review articles: developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]


