Abstract
Purpose
The anatomy and neurovascular supply of the pectoralis major muscle was studied in order to establish the safe and functional muscle transfer for the reconstruction of elbow flexion in patients with arthrogryposis multiplex congenita (AMC).
Methods
Twenty pectoralis major muscles were dissected in 11 adult cadavers. The distribution of the motor end plates was studied in five pectoralis major muscles in foetuses by the detection of esterases.
Results
The pectoralis major muscle consists of clavicular, manubrial, sternocostal, costal and abdominal parts. Each part has a distinct vascular and nerve supply. The motor nerves arise from the medial and lateral pectoral nerves. The motor end plates are localised in one zone in the clavicular and manubrial parts and in two oblique zones in the distal parts of the muscle. In 15 cases, each of the muscle parts were supplied by one nerve branch. In four cases, six nerves were distinguished and the clavicular part was supplied by two nerves. In one case, four nerves were found, with the clavicular and manubrial parts supplied by one common nerve. Three branches (13 cases) or two arterial branches (seven cases) supplied the muscle, arising from thoracoacromial and lateral thoracic arteries, respectively. The superior branch supplied the clavicular and manubrial parts, whereas the dominant pectoral branch supplied the manubrial, sternocostal and costal parts of the muscle. The inferior branch of the lateral thoracic artery supplied the abdominal part in 13 cases. In seven cases, the inferior branch failed and the abdominal part was supplied from the dominant branch.
Conclusion
This study presents guidelines for the transfer of the distal parts of the pectoralis major muscle for the reconstruction of elbow flexion. The sternocostal, costal and abdominal parts of the muscle can be released as a unit from the chest wall after dissection between the second and third rib and be transferred to the brachium. They are sufficiently supplied from the dominant pectoral branch of the thoracoacromial artery in all cases and inconstantly from the inferior branch of the lateral thoracic artery and from three motor nerves.
Keywords: Pectoralis major muscle, Neurovascular supply, Arthrogryposis, Reconstruction of elbow flexion
Introduction
For various reasons, different muscles have been used to solve the reconstruction of elbow flexion [1]. In the patients with arthrogryposis multiplex congenita group I (AMC) according to Hall [2], extension contracture of the elbow joint is a common finding, and agenesis or atrophy are present in the biceps brachii and brachialis muscles, and other muscles of the shoulder girdle, respectively (Fig. 1). The pectoralis major muscle was chosen for the reconstruction of elbow flexion because this muscle is mostly unaffected in AMC and the length of the muscle fibres and muscle power are similar to the biceps brachii muscle. According to literature reports [3–11], the muscle is appropriate for segmental splitting on the basis of the segmental anatomy and neurovascular supply. The modified technique of the transfer of the distal parts of the pectoralis major muscle was developed according to Clark [12]. The vascular and nerve supply of the pectoralis major muscle has been previously studied [3–11], but the terminology and results often depend on how the muscle is used, i.e. as a free flap, a segmental flap for transfer to cover the neck or trunk wall defects or for the restoration of elbow or forearm flexors. For this reason, an anatomical study limited to the neurovascular supply of the pectoralis major muscle was conducted in order to establish the optimal part of this muscle for safe and functional transfer for the reconstruction of elbow flexion, and to retain the proximal parts of the muscle for shoulder control in patients with AMC.
Fig. 1.
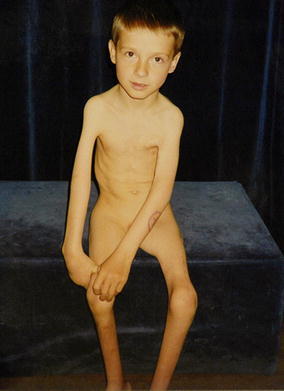
A 5-year-old boy with a tetramelic form of arthrogryposis multiplex congenita (AMC). On the right upper extremity, extension contracture of the elbow joints and severe atrophy of the muscles of the shoulder girdle are obvious. On the left side, the pectoralis major muscle was transferred to the brachium
Materials and methods
Eleven fresh adult cadavers (six women, five men, age range 50–82 years) within 48 h after death were used for this study after legal autopsy in the department of pathology. Twenty pectoralis major muscles (ten right muscles and ten left muscles) were anatomically dissected, and innervation and vascular supply were studied. The vessels and nerves were dissected and followed into muscle as far as possible. The dissections were recorded photographically and by schematic drawing. Similar to an actual surgical procedure (Fig. 2), each muscle was divided into two parts (Fig. 3), namely, the proximal one, involving the clavicular and manubruial parts of muscle, and the distal one, involving the sternocostal, costal and abdominal parts. The distal parts were rotated to the brachium (Fig. 4). The neurovascular supply of the divided parts of the muscle was particularly studied in order to establish the optimal size of the muscle for safe transfer. In addition, the distribution of the motor end plates was studied in five pectoralis major muscles in foetuses. The indigo method of Holt [13] was used for the histochemical detection of esterases for visualisation of the motor end plates. The foetuses were obtained following legal minor Caesarean sections in 9–20 weeks of gravidity. This part of the study was conducted at the department of anatomy of the medical faculty of the university.
Fig. 2.
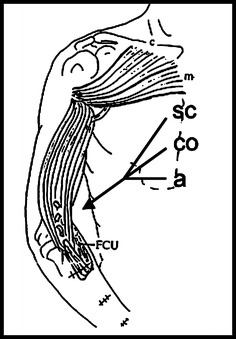
Schematic diagram of the transfer of distal parts of the pectoralis major to the brachium for the reconstruction of elbow flexion. SC sternocostal part; CO costal part, a abdominal part. Clavicular (c) and manubrial (m) parts remain intact. FCU flexor carpi ulnaris muscle
Fig. 3.
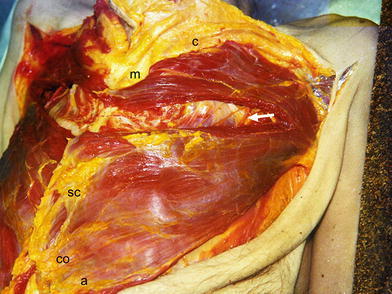
Dissection of the left pectoralis major muscle to proximal and distal parts between the second and third ribs of the cadaver. The neurovascular bundle in the lateral part is visible (arrow). c clavicular part, m manubrial part, SC sternocostal part, CO costal part, a abdominal part
Fig. 4.
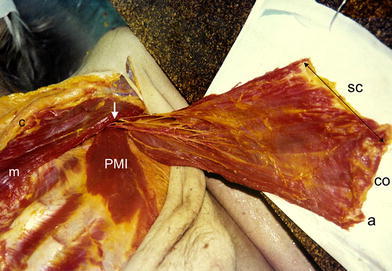
Rotation of the distal parts of the pectoralis major muscle (SC sternocostal, CO costal, a abdominal), together with the neurovascular bundle (arrow) on the same cadaver. PMI pectoralis minor muscle
Results
In concordance to known ontogeny [14, 15], we confirmed that the pectoralis major muscle consists of five segmental parts: clavicular, manubrial, sternocostal, costal and abdominal (Fig. 5). The clavicular part originates in the clavicle, the manubrial in the manubrium sterni and the medial parts of the first and second ribs, the sternocostal part originates in the corpus sterni and adjacent medial parts of the ribs from the second intercostal space to the sixth rib, and the costal part originates on the sixth rib. The anatomy of the abdominal segment is variable. This segment is primarily a separate layer that is located dorsal to the costal part, but occasionally originates from the aponeurosis of the external oblique abdominal muscle. It is possible to separate the clavicular part from the manubrial part and the manubrial part from the sternocostal part, but the costal and abdominal parts are closely connected with the sternocostal part. From known ontogenical and anatomical studies [3, 8, 14, 15], it is possible to divide the pectoralis major into two major segments: the proximal segment that contains the clavicular and manubrial parts, and the distal segment that contains the sternocostal, costal and abdominal parts. The insertion tendon on the humerus has a bilaminar U-shape [7, 8, 16]. The clavicular and manubrial parts insert into the ventral limb of the U, the sternocostal part inserts into the inferior cup of the tendon, and the costal and abdominal parts insert into the posterior limb of the tendon. Nerves and vessels run in the epimysium on the posterior surface of the pectoralis major muscle covered by the pectoralis fascia. Each part of muscle has a distinct vascular and nerve supply.
Fig. 5.
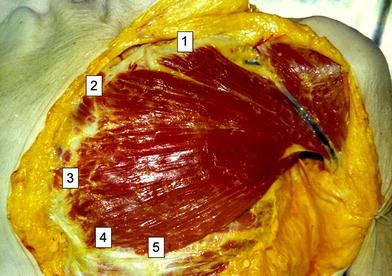
Five parts of pectoralis major muscle on the cadavers:1 clavicular,2 manubrial,3 sternocostal,4 costal,5abdominal
The motor nerves (Fig. 6) rise from the lateral and medial pectoral nerves (formerly n. thoracicus ventralis I and II). The lateral pectoral nerve originates from the lateral cord of the brachial plexus (C5, 6, 7) and enters the clavipectoral fascia medially to the tendon of the pectoralis minor muscle. The medial pectoral nerve arises from the medial cord of the brachial plexus (C8, T1) or from the anastomotic nerve loop between the medial and lateral cords. Two separate branches of the lateral pectoral nerve enter into the clavicular and manubrial parts of the pectoralis major muscle. The branches of the medial pectoral nerve divide into ventral and two dorsal branches. The ventral branch runs above the pectoralis minor muscle and supplies the sternocostal part of the muscle (17 of 20 cases) or pierces the upper part of the pectoralis minor muscle (three of 20 cases). Dorsal branches supply the costal and abdominal parts of the muscle. The nerve of the costal part pierces the pectoralis minor muscle in 15 cases (Fig. 6) or runs above the muscle (five cases). The nerve of the abdominal part arises below the pectoralis minor muscle in 13 cases (Fig. 6) or pierces the inferior part of the muscle (seven cases). Only small variations in the nerve supply were found in our study. In 15 cases, each of the five muscle parts was supplied by one nerve branch (Fig. 6). In four cases, six nerves were distinguished and two nerves supplied the clavicular parts. In one case, only four nerves were found, with the clavicular and manubrial parts supplied by one common nerve. The nerves of the clavicular and the manubrial parts enter the muscle between the medial and lateral part, and the nerves of the sternocostal, costal and abdominal parts enter the muscle between the middle and lateral third (Fig. 6). The motor end plates on the outer part of the muscle (Fig. 7, dark spots) lie in one oblique zone in the clavicular part, and in two zones in the other muscle parts, respectively. In the manubrial, sternocostal, costal and abdominal segments, the motor end plate zones lie between the lateral and middle thirds of muscle, and the middle and medial thirds of the muscle, respectively. The nerves pierce the inner surface of the muscle in the vicinity of the motor end plate zones.
Fig. 6.
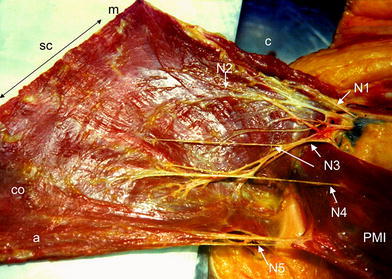
Five nerves supply the pectoralis major muscle. The nerves of the clavicular, manubrial and sternocostal parts rise above the pectoralis minor muscle (N1, N2, N3) and the nerve of costal part (N4) pierces the pectoralis minor muscle. The nerve of abdominal part 5 (N5) arises bellow the pectoralis minor muscle. The nerves enter the muscle parts between the lateral and medial third of the muscle. c clavicular part, m manubrial part, SC sternocostal part, CO costal part, a abdominal part
Fig. 7.
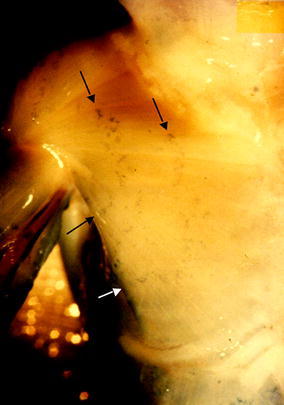
The motor end plates (dark spots, arrows) in the foetal pectoralis major muscle. The end plates lie in one zone in the clavicular part of the muscle and in two oblique zones in the remaining muscle parts between the lateral and middle, and middle and medial parts of the muscle, respectively
Vascular supply
The arteries rise from the thoracoacromial artery (a branch of the axillary artery) and the lateral thoracic artery (a branch of the subclavian artery), respectively. The committing veins follow the arteries and drain into the thoracoacromial or cephalic vein. The vessels anastomose medially with segmental perforators of the internal thoracic artery and vein in the intercostal spaces in the vicinity of the sternum. The vascular pattern is relatively constant and constitutes three main branches. The superior branch of the thoracoacromial artery (Figs. 8 and 9) pierces the fascia clavipectoralis, and supplies the clavicular and manubrial parts of the muscle, and its descending branch supplies the lateral part of the muscle near the tendon in 18 of 20 cases (Fig. 9). The dominant (pectoral) branch (Figs. 8, 9, 11 and 12) of the thoracoacromial artery supplies by one branch the manubrial segment in 12 cases (Fig. 8). Then, it runs as a main trunk and supplies the large sternocostal and costal parts of the muscle (20 cases) and the abdominal part (nine cases). From a topographical point of view, the dominant branch runs in a medio-caudal direction in a line connecting the coracoid process of the scapula and the xiphoid of the sternum. In the proximal part, the branch lies extra muscularly. Below the third rib, it pierces the muscle in the middle third. The inferior branch arises from the lateral thoracic artery below the pectoralis minor muscle in nine cases (Fig. 8) or pierces this muscle in four cases (Fig. 11), and supplies the distal part of the costal segment and the abdominal part of the muscle (13 of 20 cases). In seven cases, the inferior branch was not present, and the abdominal part of the muscle was supplied from the dominant branch (Figs. 9 and 12). Concerning the particular vascular supply of individual muscle parts, the vascular pattern was very constant. The clavicular part of the muscle was primarily supplied by a separated superior branch of the thoracoacromial artery (18 cases) or from one branch of the pectoral (dominant) branch of its artery in two remaining cases. The manubrial part was supplied mainly from one isolated branch of the dominant pectoral branch of the thoracoacromial artery (12 cases) or from the superior branch (five cases) or from an isolated branch of the thoracoacromial artery (three cases). The sternocostal and costal segments were constantly supplied from the pectoral branch of the thoracoacromial artery. The abdominal segment was primarily supplied from the branch of lateral thoracic artery (11 cases) or from the pectoral branch (seven cases). In two cases, the abdominal part was supplied by the dominant pectoral branch and also by the branch of the lateral thoracic artery.
Fig. 8.
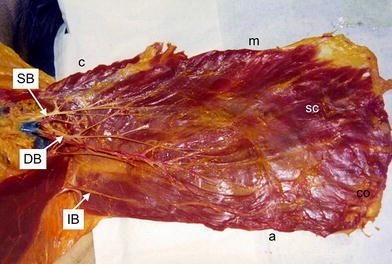
Vascular supply of the left pectoralis major muscle. Three main vascular branches supply the muscle. The superior vascular branch of the thoracoacromial artery and vein (SB) supply the clavicular part. The dominant or pectoral branch (DB) of the thoracoacromial artery and vein supplies the manubrial part and then as the main trunk supplies the sternocostal and costal parts of muscle. The inferior branches of the lateral thoracic artery and vein (IB) arise below the pectoralis minor muscle and supply the abdominal part of the muscle. c clavicular part, m manubrial part, SC sternocostal part, CO costal part, a abdominal part
Fig. 9.
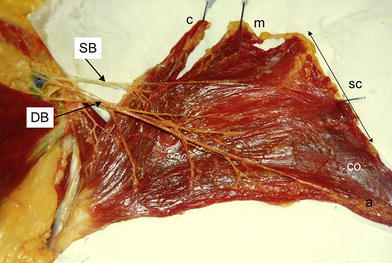
Vascular supply of the left pectoralis major muscle after particular dissection of the vessels. Superior (SB) and dominant (DB) branches supply the muscle. c clavicular part, m manubrial part, SC sternocostal part, CO costal part, a abdominal part
Fig. 11.
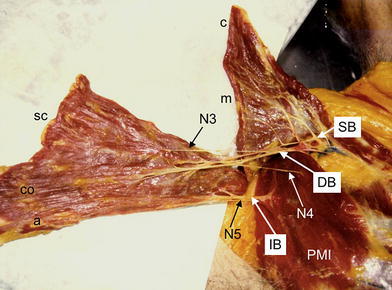
Right pectoralis major muscle dissected between the manubrial (m) and sternocostal (SC) parts of the muscle. Sternocostal, costal (CO) and abdominal (a) parts are supplied through dominant (DB) and inferior vascular branches (IB) and three motor nerves (N3, N4, N5). The inferior vascular branch and N4 and N5 pierce the pectoralis minor muscle (PMI). c clavicular part
Fig. 12.
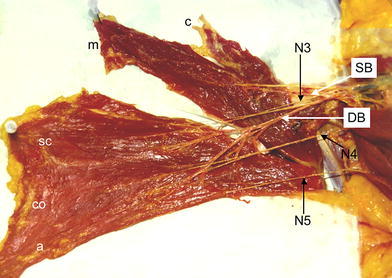
Right pectoralis major muscle dissected between the manubrial (m) and sternocostal (SC) parts of the muscle. Sternocostal, costal (CO) and abdominal (a) parts are supplied through dominant (DB) and three motor nerves (N3, N4, N5). The clavicular (c) and manubrial parts are supplied through the superior branch (SB) of the thoracoacromial artery
The neurovascular supply of the individual parts of the pectoralis major muscle is segmented; the distal parts of the muscle (sternocostal, costal and abdominal) with their neurovascular supply can be separated as a unit from the proximal parts (clavicular and manubrial). Following a blunt and sharp proximolateral dissection of the distal parts of the muscle from the chest wall and after the division of the muscle between the second and third rib (Fig. 10), the sternocostal, costal and abdominal parts are supplied in all cases through the pectoral branch of the thoracoacromial artery and inconstantly through one branch of the lateral thoracic artery and by three motor nerves (Fig. 11). In cases of a failed branch of the lateral thoracic artery (inferior branch), the distal parts of the muscle are supplied through the dominant pectoral branch only and by three motor nerves (Fig. 12). Dissection of the muscle from the chest wall must be performed carefully in the mid-clavicular line (Fig. 10) in order to avoid injuring the dominant pectoral artery running extramuscularly in the lateral part. By dividing the pectoralis major muscle between the second and third rib, the distal parts of the muscle (sternocostal, costal and abdominal) can be safely rotated to the ventral side of the arm. The dominant pectoral, as well as the inferior vessels, are clearly visible during the surgical procedure, and the lengths and diameters of the vessels allow the rotation of muscle to the brachium without vascular crisis. The insertion tendon of the transferred muscle parts and the residual clavicular and manubrial parts of the muscle remain intact.
Fig. 10.
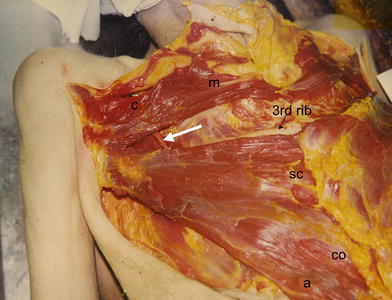
Dissection of the right pectoralis muscle between second and third rib. The dominant vascular branch (arrow) enters the muscle near to the middle clavicular line. c clavicular part, m manubrial part, SC sternocostal part, CO costal part, a abdominal part
Discussion
The transfer of different muscles has been used for the treatment of the agenesis of the biceps brachii and brachialis muscle in patients with AMC type I [1, 12, 17–20]. The small series of patients and different results described in the literature after pectoralis major transfer [1, 12, 17, 18, 21–24] led us to establish our own technique for the muscle transfer of the pectoralis major muscle and develop an algorithm for treatment in these patients. Our anatomical study of the neurovascular supply of the pectoralis major muscle was performed because there are differing descriptions of the anatomical parts and neurovascular supply of this muscle [3–10, 14, 15, 25]. According to the ontogeny, the muscle contains five separate parts [14, 15]. It is possible to surgically divide and separate these parts because each has a distinct vascular and nerve supply. We support this ontogenetical division of the muscle. Being able to transfer the distal part of muscle to the brachium and retain the proximal part for shoulder movement is quite useful for our purposes. However, there is no agreement in the literature regarding the division of the muscle into portions. Manktelow et al. [3] and Wei et al. [6] described only two parts of the muscle (clavicular and sternocostal). Tobin [7] and Smet [25] described three parts (clavicular, sternocostal and abdominal), while Moosman [4] divided the muscle into four parts (clavicular, manubrial, sternal and costoabdominal). These varying descriptions depend primarily on the clinical use of the muscle. Although segmental division of the muscle differs significantly among authors, there is agreement on the neurovascular pattern.
Regarding innervation of the muscle, we usually found five separate nerves, each for an individual part of the muscle. We agree with the opinions of Clark [12], Čihák [14], Steindler [19], Manktelow et al. [3] and Van Heest et al. [23] that the lateral pectoral nerve rises from the lateral trunk of the brachial plexus, and that it supplies the proximal parts of the muscle (clavicular and manubrial). The medial pectoral nerve rises from the medial trunk of the brachial plexus, enters the pectoralis major muscle laterally from the pectoralis minor, and supplies the sternocostal, costal and abdominal parts of the muscle. However, Moosman [4] described the names of nerves on the basis of their position to the pectoralis minor muscle and a different innervation pattern. He posited that the medial pectoral nerve rises from the lateral trunk of the brachial plexus and supplies the clavicular, manubrial and sternal part of the muscle, and that the lateral pectoral nerve rises from the medial trunk and supplies the costoabdominal part of the muscle. However, in agreement with the findings of Čihák [14], we found that the ventral branch of the medial pectoral nerve runs above the pectoralis minor muscle and supplies the sternocostal part of the muscle (17 of 20 cases) or pierces the upper part of pectoralis minor muscle (three of 20 cases). The dorsal branches supply the costal and abdominal parts of the muscle by either piercing the pectoralis minor muscle or running under it. Other authors [3, 21, 26] described only the branches that pierce the pectoralis minor muscle. Because of this, innervation of the distal parts of the muscle may be more vulnerable than the proximal parts. Although the opinions of the authors do not differ radically, differing descriptions of innervations occur primarily because of differing descriptions of the muscle parts. We found that it is important for the innervation pattern to be quite constant, and that the distal parts of the muscle used for transfer are constantly supplied by the three nerves. The length of the motor nerves and their points of entry to the muscle permit the rotation of the muscle to the brachium without damage of the nerves due to the traction.
There are very few papers in the literature [27–29] concerning the distribution of the motor end plates of the pectoralis major muscle. It is known that a single muscle fibre contains only one motor end plate [29]. Two motor end plate zones in the distal parts of the muscle probably imply that long muscle fibres running the whole length of the muscle do not exist, but they are connected to other fibres.
The vascular supply of the pectoralis muscle was constant, with only small differences. Although we did not make a perfusion study concerning the vascular territory of the muscle, there is agreement between our findings and those of other authors [3, 6, 7, 11, 21, 25] concerning the particular vascular supply of individual muscle parts. The small differences are due to the division of the muscle into muscle parts. This important concordance regards the supply of the sternocostal and costal segments from the pectoral branch of the thoracoacromial artery. We did not observe the side difference in the course of this dominant branch that is described by Nakajima et al. [9]. The most variable element in our study—and those of most of the authors—was the abdominal segment. This segment was supplied mostly from the branch of the lateral thoracic artery (11 cases) or from the pectoral branch (seven cases). In two cases, the abdominal part was supplied from both of these branches. Only the findings of Freeman et al. [5] differ, as he found the branch of the lateral thoracic artery in all specimens.
For the transfer of the muscle, it is important that the distal parts of the pectoralis major muscle are supplied by the dominant pectoral branch of the thoracoacromial artery and in the majority of cases by the lateral thoracic artery, thus, theoretically diminishing the risk of avascular necrosis of the transferred muscle parts. According to the recent perfusion study of Yang et al. [11], the pectoral branch of the thoracoacromial artery supplies 50.7% of the muscle, mainly the superior and lateral part of muscle, whereas the medial and inferior parts of muscle are supplied from the anterior intercostals perforators of the internal mammary artery. These perforators are ligated by the surgical procedure, but the medial and distal parts of the transferred muscle are still supplied through the reach anastomotic networks between these two vascular systems. The lateral thoracic artery is inconstant and supplies 6.6% of the muscle parenchyma only. It accentuates the necessity to save the pectoral branch of the thoracoacromial artery as a main supply of the transferred muscle.
Our anatomical study of the neurovascular supply illustrated the constant segmental neurovascular supply of the proximal and distal parts of the pectoralis major muscle. With careful division of the pectoralis major muscle between the second and third ribs, the sternocostal, costal and abdominal parts can be safely transferred to the ventral side of the arm. The clavicular and manubrial parts of the muscle remain functionally intact for the control of the shoulder. The transferred parts represent sufficient muscle mass for the replacement of the biceps brachii and brachialis muscle according to the cross-section and length of the muscle belly [3]. These muscle parts are available for the reconstruction of elbow flexion, even in patients with AMC type I and in other conditions, such as some types of brachial plexus palsy.
Acknowledgements
This study was sponsored by grant funds of the Ministry of Health of the Czech Republic IGA MZ CR no. 4162–3 “Muscle transfers on the upper extremity in treatment of arthrogryposis multiplex. Anatomical, electromyographic and clinical study.”
References
- 1.Atkins RM, Bell MJ, Sharrard WJW. Pectoralis major transfer for paralysis of elbow flexion in children. J Bone Joint Surg Br. 1985;67(4):640–644. doi: 10.1302/0301-620X.67B4.4030867. [DOI] [PubMed] [Google Scholar]
- 2.Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6:159–166. doi: 10.1097/01202412-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Manktelow RT, McKee NH, Vettese T. An anatomical study of the pectoralis major muscle as related to functioning free muscle transplantation. Plast Reconstr Surg. 1980;65(5):610–615. doi: 10.1097/00006534-198005000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Moosman DA. Anatomy of the pectoral nerves and their preservation in modified mastectomy. Am J Surg. 1980;139:883–886. doi: 10.1016/0002-9610(80)90403-1. [DOI] [PubMed] [Google Scholar]
- 5.Freeman JL, Walker EP, Wilson JSP, Shaw HJ. The vascular anatomy of the pectoralis major myocutaneous flap. Br J Plast Surg. 1981;34:3–10. doi: 10.1016/0007-1226(81)90086-2. [DOI] [PubMed] [Google Scholar]
- 6.Wei WI, Lam KH, Wong J. The true pectoralis major myocutaneous island flap: an anatomical study. Br J Plast Surg. 1984;37:568–573. doi: 10.1016/0007-1226(84)90151-6. [DOI] [PubMed] [Google Scholar]
- 7.Tobin GR. Pectoralis major segmental anatomy and segmentally split pectoralis major flaps. Plast Reconstr Surg. 1985;75:814–824. doi: 10.1097/00006534-198506000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Chaffaï MA, Mansat M. Anatomic basis for the construction of a musculotendinous flap derived from the pectoralis major muscle. Surg Radiol Anat. 1988;10:273–282. doi: 10.1007/BF02107898. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima K, Ide Y, Abe S, Okada M, Kikuchi A, Ide Y. Anatomical study of the pectoral branch of thoracoacromial artery. Bull Tokyo Dent Coll. 1997;38:207–215. [PubMed] [Google Scholar]
- 10.Betka I, Kacirkova J. On the vascularization of the M. Pectoralis major. Cs Otolaryng. 1988;37:188–190. [PubMed] [Google Scholar]
- 11.Yang D, Marshall G, Morris SF. Variability in the vascularity of the pectoralis major muscle. J Otolaryngol. 2003;32:12–15. doi: 10.2310/7070.2003.35357. [DOI] [PubMed] [Google Scholar]
- 12.Clark JMP. Reconstruction of biceps brachii by pectoral muscle transplantation. Br J Surg. 1946;34:180–181. doi: 10.1002/bjs.18003413408. [DOI] [PubMed] [Google Scholar]
- 13.Holt SJ. Indigogenic staining methods for esterases. In: Danielli JF, editor. General cytochemical methods 1. New York: Academic Press; 1958. pp. 375–398. [PubMed] [Google Scholar]
- 14.Čihák R. Musculus pectoralis major und seine Komponenten in der Ontogenese des Menschen. Morfologie VII. 1959;2:174–191. [Google Scholar]
- 15.Čihák R, Popelka S. Partial defects of the pectoralis major muscle. Acta Chir Orthop Traumat Cechoslovaka. 1961;28:185–194. [PubMed] [Google Scholar]
- 16.Warwick R, Williams PL (eds) (1973) Gray’s anatomy, 35th edn, Longman, Edinburgh, pp 535–536
- 17.Doyle JR, James PM, Larsen LJ, Ashley RK. Restoration of elbow flexion in arthrogryposis multiplex congenita. J Hand Surg. 1980;5:149–152. doi: 10.1016/S0363-5023(80)80146-8. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Roberts GC, Lettin AWF. Arthrogryposis multiplex congenita. J Bone Joint Surg Br. 1970;52:494–508. [Google Scholar]
- 19.Steindler A. Tendon transplantation in the upper extremity. Am J Surg. 1939;44:260–271. doi: 10.1016/S0002-9610(39)90954-2. [DOI] [Google Scholar]
- 20.Zancolli E, Mitre H. Latissimus dorsi transfer to restore elbow flexion. An appraisal of eight cases. J Bone Joint Surg Am. 1973;55:1265–1275. [PubMed] [Google Scholar]
- 21.Carroll RE, Kleinman WB. Pectoralis major transplantation to restore elbow flexion to the paralytic limb. J Hand Surg. 1979;4:501–507. doi: 10.1016/S0363-5023(79)80001-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsai T-M, Kalisman M, Burns J, Kleinert HE. Restoration of elbow flexion by pectoralis major and pectoralis minor transfer. J Hand Surg. 1983;8:186–190. doi: 10.1016/S0363-5023(83)80013-6. [DOI] [PubMed] [Google Scholar]
- 23.Van Heest A, Waters PM, Simmons BP. Surgical treatment of arthrogryposis of the elbow. J Hand Surg. 1998;23:1063–1070. doi: 10.1016/S0363-5023(98)80017-8. [DOI] [PubMed] [Google Scholar]
- 24.Lahoti O, Bell MJ. Transfer of pectoralis major in arthrogryposis to restore elbow flexion: deteriorating results in the long term. J Bone Joint Surg Br. 2005;87:858–860. doi: 10.1302/0301-620X.87B6.15506. [DOI] [PubMed] [Google Scholar]
- 25.Smet HT. Pectoralis major flaps. In: Smet HT, editor. Tissue transfers in reconstructive surgery. New York: Raven Press; 1989. pp. 74–79. [Google Scholar]
- 26.Hidalgo DA. Free muscle transplantation. In: Shaw WW, Hidalgo DA, editors. Microsurgery in trauma. New York: Futura; 1987. [Google Scholar]
- 27.Cöers C, Woolf AL. The innervation of muscle. A biopsy study. Oxford: Blackwell; 1959. pp. 1–40. [Google Scholar]
- 28.Pára F, Pařízek J (1973) Mapping of motor endplates zones in human muscles. In: Nesvadba Z (ed) Proceedings of the 3rd Symposium on Neuromuscular Disorders, Balnea, Prague, pp 28–36
- 29.Schwarzacher HG. Über die Länge und Anordnung der Muskelfasern in menschlichen Skeletmuskeln. Acta Anat (Basel) 1959;37:217–231. doi: 10.1159/000141469. [DOI] [PubMed] [Google Scholar]


