Abstract
Purpose
This study has evaluated the effects of immobilization versus intermittent active motion on cartilage and on antibiotic efficacy in a rabbit septic arthritis model.
Methods
Rabbits were infected and assigned to one of four groups: group 1, no treatment without immobilization (allowing intermittent active motion); group 2, cast; group 3, oxacillin without immobilization; group 4, oxacillin and cast. Animals were sacrificed 21 days later. Bacterial counts and lateral radiograms were performed. A radiological score was calculated.
Results
Immobilization had no effect on oxacillin efficacy and a deleterious effect on the radiological score.
Conclusion
Intermittent active motion has allowed a better cartilage healing during the treatment of septic arthritis.
Keywords: Child, Arthritis, Immobilization, Intermittent active motion
Introduction
Bone and joint infections due to methicillin-susceptible Staphylococcus aureus are frequent in children. Thomas Smith (in Wilson and Di Paola [1]) reported a first series of 21 cases in 1874, for which the death rate was greater than 50%. Although the development of antibimicrobial chemotherapy has improved the prognosis for survival, these infections can cause severe sequelae, which means that diagnosis and treatment are matters of urgency.
It was customary to associate antimicrobial chemotherapy (most often oxacillin with or without another antistaphylococcic drug [2]) with plaster cast immobilization, including the over- and underlying joint, for a period ranging from several days to 6 weeks [3–6]. Immobilization was justified before the development of antibiotics to ensure a good position for joint ankylosis, but is often performed today for its supposed antalgic and anti-inflammatory qualities. However, several experimental and clinical studies published since 1960 have demonstrated the deleterious effects of immobilization on cartilage in different joint diseases [7–10], including septic or inflammatory arthritis. In the 1980s, Salter et al. [9] demonstrated the interest of continuous passive motion (CPM) on cartilage healing. CPM is not often used in clinical practice to treat septic arthritides, as it is a constraining and difficult-to-use method during the first weeks of treatment. Intermittent active motion allows a voluntary mobilization of the infected joint, with the range of motion being limited by pain.
The main hypothesis in the present study was to compare intermittent active motion and immobilization on antimicrobial agent efficacy, and it was also hypothesized that immobilization during S. aureus-induced septic arthritis may worsen cartilaginous lesions.
Materials and methods
The S. aureus strain used was isolated in the joint fluid of a 12-year-old girl. The minimal inhibitory concentration (MIC) of oxacillin for this methicillin-susceptible strain was 1 mg/L, and the minimal bactericidal concentration (MBC) was 2 mg/L.
The animal model, derived from that of Salter et al. [9], used female New Zealand rabbits weighing between 1.5 and 2 kg. This choice allowed us to approximate hematogenous arthritis in the child due to methicillin-susceptible S. aureus.
The experimental protocol was as follows:
-
Day 0
General anesthesia was performed (ketamine IM, 15 mg/kg), and the right knee was shaved. After cutaneous disinfection by povidone-iodine, 1 ml of a bacterial suspension of S. aureus 108 colony forming units (CFU)/mL was injected into the knee cavity by the external parapatellar route.
-
Day 1
Twenty-four hours later, external parapatellar arthrotomy was performed under general anesthesia, and intra-articular features were noted (type of effusion, appearance of articular cartilage, appearance of growth cartilage). The joint fluid was sampled, and articular lavage was performed using 250 ml of physiological serum before the joint and skin were closed up. The animals were then randomized to four groups: group 1, no further treatment for 21 days subsequent to articular lavage, and without immobilization that allowed intermittent active motion for the infected joint; group 2, spica plaster cast immobilization; group 3, administration of oxacillin 50 mg/kg IM three times per day for 21 days without immobilization; group 4, administration of oxacillin 50 mg/kg IM three times per day plus spica plaster cast immobilization for 21 days. Infected joints in groups 1 and 3 were not immobilized, allowing normal cage activities and intermittent active motion. Fentanyl analgesia (fentanyl transdermal patch, 12 μg/h) was used for pain management prior to all experiments.
-
Day 21
Euthanasia was performed by the intravenous injection of a lethal dose (100 mg) of thiopental. Lateral radiographs of both knees of each animal were taken, and samples for bacteriological studies were obtained from each infected knee.
Judgment criteria
Clinical criteria
The ability of walking was assessed at day 1 in the four groups, and at day 7, day 14, and day 21 in groups 1 and 3 (without spica cast). Normal walking was scored 0, limping was scored 1, and the absence of weight-bearing was scored 2.
Radiological criteria
On the 21st day of treatment, lateral radiographs of both knees were read by an independent radiologist and scored on the basis of four criteria, using a system derived from Salter et al. [9] (Table 1).
Table 1.
Calculation of the radiological score
| (a) Appearance of lower femoral growth cartilage | Normal | 0 |
| Epiphysiolysis <50% of surface area | 1 | |
| Epiphysiolysis >50% of surface area | 2 | |
| (b) Appearance of articular space | Normal | 0 |
| Enlarged | 1 | |
| Narrowed | 2 | |
| (c) Condensation of subchondral bone | Absence of condensation | 0 |
| Condensation <50% of surface area | 1 | |
| Condensation >50% of surface area | 2 | |
| (d) Osteolysis | Absence of osteolysis | 0 |
| 1 or 2 punched-out lesions | 1 | |
| More than 2 punched-out lesions | 2 | |
| Major osteolysis | 3 | |
| Radiological score (a + b + c + d) | 0–9 | |
Bacteriological criteria
The surviving bacteria were counted in the joint fluid. Samples of joint fluid obtained on days 1 and 21 were weighed and mixed with 200 μl of physiological serum, and the resulting solution was seeded in pure and diluted forms at 10−2, 10−4, and 10−6 onto Chapmann gel using a Spiral seeder (Interscience®). The sensitivity threshold for this method was 20 CFU/mL.
When a culture was positive, the antibiotic sensitivity was tested on S. aureus colonies exposed to 21 days of oxacillin treatment to ensure the absence of superinfection and of any change in sensitivity to the drug.
Statistical methods
The results were expressed as the mean ± standard deviation and were compared using the appropriate parametric tests for continuous variables (comparison of surviving bacteria by analysis of variance [ANOVA] and the Bonferroni–Dunn test) and non-parametric tests for discrete variables (comparison of scores by the Kruskal–Wallis and Mann and Whitney tests). A P-value < 0.05 was considered as significant.
Results
Clinical criteria
On day 1, there was no difference between the groups: the functional score was noted as 2 for all rabbits. On day 7, there was no difference between groups 1 and 3: the functional score was noted as 2 for all rabbits. On day 14 in group 1, one rabbit recovered weight-bearing (score 1), whereas the functional score was 2 for the five remaining rabbits; in group 3, the functional score was either 1 (n = 4) or 2 (n = 4, not significant). At the end of treatment, weight-bearing was impossible for all rabbits in group 1, and for two rabbits in group 3, whereas the other six rabbits in group 3 limped (score = 1) (Mann and Whitney’s test, P = 0.0201).
Bacteriological criteria
All rabbits treated by oxacillin were “sterilized” after 21 days of treatment. There was no difference in the bacterial counts between groups 3 and 4 (with oxacillin) and groups 1 and 2 (without oxacillin) (Fig. 1). There was no difference in the bacterial counts between day 1 and day 21 for groups 1 and 2, respectively. Immobilization has no effect on the antimicrobial agent efficacy.
Fig. 1.
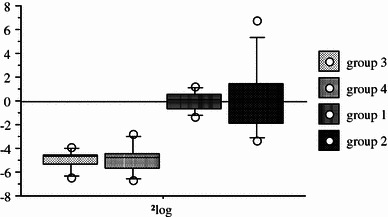
Decrease of intra-articular bacterial density after 21 days of treatment, as shown in Δlog CFU/ml (each box represents the mean ± standard deviation of the Δlog of each rabbit between days 1 and 21)
On day 21, culture of the pus of the knee joint showed no superinfection in groups 1 and 2.
Radiological criteria
There was no difference concerning the radiological score between groups 1 and 2 (groups without antimicrobial chemotherapy, respectively with no treatment and with a spica cast). The radiological score in these two groups was bad (8.2 ± 0.9 and 7.9 ± 1.2). The radiological score of group 4 (treated with oxacillin and spica cast) was poor (5.3 ± 2.3). The radiological score of group 3 (treated with oxacillin and intermittent active motion) was the lowest (1.5 ± 1); it was significantly different than the radiological scores of groups 1, 2, and 4 (Mann and Whitney’s test, P = 0.0306). The main differences between groups 4 and 3 concerned signs of deep articular lesions (subchondral bone condensation and epiphyseal osteolysis). Lesions were worse in group 2 than in group 1 (Mann and Whitney’s test, P = 0.0306). These results showed that intermittent active motion improved cartilage healing during the treatment of experimental septic arthritis.Table 2 shows the radiological scores for each group on day 21.
Table 2.
Radiological score (mean ± standard deviation) at the end of treatment. The radiological score is the sum of the four radiological criteria
| Group (number of rabbits) | Group 1 (6) | Group 2 (6) | Group 3 (8) | Group 4 (8) |
|---|---|---|---|---|
| Growth cartilage | 1.5 ± 0.5 | 1.2 ± 0.5 | 0.7 ± 0.5 | 1 ± 0.0 |
| Articular space | 1.8 ± 0.5 | 1.9 ± 0.4 | 0.3 ± 0.5 | 1.2 ± 0.8 |
| Condensation of subchondral bone | 2 ± 0.0 | 1.9 ± 0.4 | 0 ± 0.0a | 1.2 ± 0.8b |
| Osteolysis | 3 ± 0.0 | 2.9 ± 0.4 | 0.5 ± 0.5a | 2 ± 1.1b |
| Radiological score | 8.2 ± 0.9 | 7.9 ± 1.2 | 1.5 ± 1a | 5.3 ± 2.3b,c |
Mann and Whitney test after the Kruskal–Wallis test
aP ≤ 0.0306 versus groups 1, 2, and 4
bP ≤ 0.0389 versus group 1
cP = 0.0282 versus group 2
Discussion
Salter [10] was the first to assess the role of immobilization in a septic arthritis model. The animals used in his study were New Zealand rabbits at the end of the growth phase. The microorganism chosen to induce infection was penicillin-susceptible S. aureus, which is currently an exceptional susceptibility in clinical practice.
Animal models of bacterial arthritis have generally involved the rabbit (easily reproducible and inexpensive) and, less frequently, the dog, pig, mouse, rat, and hamster. Bacterial inoculation is most often performed directly by intra-articular injection [11–13]. However, few models use the hematogenous route, which, in fact, simulates best of all the physiopathology of the forms of arthritis encountered in human (particularly pediatric) disease.
The rabbit septic arthritis model chosen for the present study used a high intra-articular inoculum (108 CFU). Subsequently, only articular lavage was performed for all groups (which never prevented the development of the infection) and immobilization for two groups.
Articular lavage was performed from the 24th hour to prevent the progression of cartilaginous lesions [14]. A 24-h interval is frequent in clinical practice between the manifestation of clinical signs and articular lavage [1, 14].
In the pre-antibiotic era, septic arthritis could be cured after fistualization, and arthrodesis was then necessary because of the total disappearance of the cartilaginous layer. This meant that immobilization was required after any joint infection to allow articular ankylosis to occur in a functional position. After the discovery of antibiotics, immobilization still played an essential role in the treatment of septic arthritis.
In 1980, Salter et al. [8] compared the effects of CPM and immobilization on traumatic lesions of articular cartilage in the rabbit. They concluded that CPM was the best method for cartilaginous healing and suggested that continuous motion induces neo-chondrogenesis through the appearance of pluripotent cells at the subchondral bone level. Continuous passive motion, which has proved to be beneficial for the healing of surgically created cartilaginous defects, could help prevent cartilage degradation during septic arthritis. Salter et al. [9] subsequently showed that radiological, histological, and biochemical results were better in the group with CPM.
In our study, the radiographic results were significantly different for the two oxacillin-treated groups, with immobilization, and with intermittent active motion. Moreover, immobilization was found to be ineffective bacteriologically and to be responsible for the deleterious effect observed in radiology. Intermittent active motion prevents the joint from the deleterious effect of infection as epiphyseal osteolysis or subchondral bone condensation. In this context, several hypotheses have been put forward to account for the beneficial effects of motion. It has been suggested that intermittent active motion prevents adhesion of the synovial pannus, a condition in which synovial fluid can no longer play a nutritive role. Moreover, synovial membrane, which is rich in collagenolytic enzymes, is then in direct contact with articular cartilage. Likewise, motion might improve the diffusion and, thus, the nutritive capacity of synovial fluid and antibiotics (but without any effect on the bacteriological results). Motion might also increase the articular clearance of lysosomal enzymes and could play an inductive role in the metaplasia of pluripotent undifferentiated cells. These cells could be responsible for improved cartilaginous healing. Immobilization, on the contrary, decreases the synthesis of proteoglycans of the cartilaginous matrix [9].
Before the discovery of antibiotics, immobilization was justified to ensure a good position for healing. Since the development of antibiotics, the role of immobilization in bacteriological results has not been assessed. This study, which confirms the findings of Salter et al., shows that immobilization is ineffective against infection and can be potentially deleterious for articular cartilage, and proved that intermittent active motion is a safe and easy to use alternative to immobilization. Intermittent active motion is an easier and cheaper method than continuous active motion to use during the treatment of septic arthritis. These experimental findings must be confirmed by clinical studies.
References
- 1.Wilson NIL, Di Paola M. Acute septic arthritis in infancy and childhood. 10 years’ experience. J Bone Joint Surg Br. 1986;68:584–587. doi: 10.1302/0301-620X.68B4.3733835. [DOI] [PubMed] [Google Scholar]
- 2.Société de Pathologie Infectieuse de Langue Française Troisième conférence de consensus en thérapeutique antiinfectieuse, des infections bactériennes ostéo-articulaires en dehors des infections à mycobactéries. Med Mal Infect. 1991;21:37–44. doi: 10.1016/S0399-077X(05)80231-8. [DOI] [Google Scholar]
- 3.Barton LL, Dunkle LM, Habib FH. Septic arthritis in childhood. A 13-year review. Am J Dis Child. 1987;141:898–900. doi: 10.1001/archpedi.1987.04460080084034. [DOI] [PubMed] [Google Scholar]
- 4.Glorion C. Arthrites septiques de l’enfant [Septic arthritis in children] Rev Prat. 1994;44:2581–2586. [PubMed] [Google Scholar]
- 5.Glorion C, Palomo J, Bronfen C, Touzet P, Padovani JP, Rigault P. Les arthrites aiguës infectieuses du genou de l’enfant, pronostic et discussion thérapeutique à propos de 51 cas ayant un recul moyen de 5 ans [Acute septic arthritis of the knee in children. Prognosis and therapeutic discussion about 51 cases with mean follow-up of 5 years] Rev Chir Orthop Repar Appar Mot. 1993;79:650–660. [PubMed] [Google Scholar]
- 6.Jackson MA, Nelson JD. Etiology and medical management of acute suppurative bone and joint infections in pediatric patients. J Pediatr Orthop. 1982;2:313–323. doi: 10.1097/01241398-198208000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54:973–985. [PubMed] [Google Scholar]
- 8.Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, MacMichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62:1232–1251. [PubMed] [Google Scholar]
- 9.Salter RB, Bell RS, Keeley FW. The protective effect of continuous passive motion in living articular cartilage in acute septic arthritis: an experimental investigation in the rabbit. Clin Orthop Relat Res. 1981;159:223–247. [PubMed] [Google Scholar]
- 10.Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989;242:12–25. [PubMed] [Google Scholar]
- 11.Bremell T. Experimental models of infectious arthritis. In: Zak O, Sande MA, editors. Handbook of animal models of infection. London: Academic Press; 1999. pp. 539–547. [Google Scholar]
- 12.Crémieux AC, Carbon C. Experimental models of bone and prosthetic joint infections. Clin Infect Dis. 1997;25:1295–1302. doi: 10.1086/516135. [DOI] [PubMed] [Google Scholar]
- 13.Daniel D, Akeson W, Amiel D, Ryder M, Boyer J. Lavage of septic joints in rabbits: effects of chondrolysis. J Bone Joint Surg Am. 1976;58:393–395. [PubMed] [Google Scholar]
- 14.Shaw BA, Kasser JR. Acute septic arthritis in infancy and childhood. Clin Orthop Relat Res. 1990;257:212–215. [PubMed] [Google Scholar]


