Abstract
Despite increases in the human life span, people have not increased their rate of saving. In a phenomenon known as ‘temporal discounting’, people value immediate gains over future gains. According to a future self-continuity hypothesis, individuals perceive and treat the future self differently from the present self, and so might fail to save for their future. Neuroimaging offers a novel means of testing this hypothesis, since previous research indicates that self- vs other-judgments elicit activation in the rostral anterior cingulate (rACC). Using event-related functional magnetic resonance imaging, we predicted and found not only individual differences in rACC activation while rating the current vs future self, but also that individual differences in current vs future self activation predicted temporal discounting assessed behaviorally a week after scanning. In addition to supporting the future self-continuity hypothesis, these findings hold implications for significant financial decisions, such as choosing whether to save for the future or spend in the present.
Keywords: temporal discounting, saving behavior, self vs other differences, cortical midline structures
Around the world, the average human life span is increasing, and with it, the number of years that individuals can expect to spend in retirement. For instance, although the average retirement age in the United States has remained at 65 for the past 60 years (International Monetary Fund, 2004), life expectancy over the same period has increased dramatically (Arias, 2007). Similarly, life savings have not risen substantially in the face of an increasing life span. Economists have estimated that the average American who is now within 15 years of retirement saves at only one-third of the rate necessary for maintaining pre-retirement levels of consumption (Bernheim et al., 2000). While estimates of an adequate level of saving vary, people save at rates lower than what most experts recommend (Li, 1996; Bernheim et al., 2000).
Theorists from many fields including economics, philosophy and psychology have characterized saving as an ‘intertemporal choice’ problem involving a decision between benefits that occur now vs in the future (Parfit, 1971; Mischel, 1974; Schelling, 1982; Laibson, 1997; Laibson et al., 1998; Frederick et al., 2003). Research shows that people often care less about future outcomes than they do about present ones, a phenomenon known as temporal discounting (Chapman and Elstein, 1995; Chapman, 1996; Frederick, 1999, 2003; Frederick et al., 2003). According to an early model of temporal discounting, while people devalue future gains as a function of temporal distance from the present, individuals vary in the degree to which they devalue future gains. This tradeoff between time and magnitude was originally described with an exponential function (Samuelson, 1937), but is better fit by a hyperbolic or quasi-hyperbolic function (Strotz, 1956; Kirby and Marakovic, 1996; Laibson, 1997; Laibson et al., 1998).1
Theorists have argued that temporal discounting might emerge from conflicts of interest between temporally different selves (Parfit, 1971, 1987; Schelling, 1984). According to this view, psychological connectedness of the present to the future self varies as a function of time, such that people feel more connected to their potential self of 5 years than their potential self of 50 years. Thus, people might care less about more temporally distant future selves to the point at which an extremely distant future self may seem like a different person altogether (Parfit, 1971; Pronin and Ross, 2006; Pronin et al., 2008). This ‘multiple selves’ view has implications for financial saving. If people consider the future self as a stranger, then they may rationally have no more reason to save money for themselves than to give the money to a stranger. Critically, this account predicts that the degree to which an individual feels disconnected from his or her future self should correlate with the degree to which that individual discounts future rewards. For the purposes of this article, we will call this the ‘future self-continuity hypothesis’.
Currently, only one behavioral study has examined whether future self-continuity predicts temporal discounting. Frederick (1999) asked subjects to rate the degree to which they thought themselves to be similar to their past and future selves at various intervals. In addition, subjects completed a standard hypothetical discounting choice task (e.g. ‘Would you prefer seven dollars now or ten dollars in 60 days?’). Neither predicted nor reported future self-continuity correlated significantly with subjects’ discount rates. However, since this study used self-report measures and hypothetical scenarios to estimate self-continuity across time, behavioral and neuroimaging methods might allow a more complete test of the future self-continuity hypothesis.
Previous neuroimaging research suggests that people show decreased activation in cortical midline structures when considering information about others vs the self (Craik et al., 1999; Kelley et al., 2002; Northoff and Bermpohl, 2004; Amodio and Frith, 2006; Heatherton et al., 2006; Moran et al., 2006; Northoff et al., 2006) and increased activation when engaging in self-reflection or introspection (Gusnard et al., 2001; Raichle et al., 2001; Johnson et al., 2002). Kelley et al. (2002), for example, scanned subjects with event-related fMRI as they made judgments about three attributes of trait adjectives: self-relevance, other-relevance, or letter case (i.e. whether or not the word was printed in upper- or lower-case letters). The investigators found that judgments of self-relevance selectively maintained activation in the mesial prefrontal cortex (MPFC) at a baseline rate, while judgments of other-relevance or case decreased MPFC activation below baseline. Similarly, in a subsequent meta-analysis of related studies, Northoff et al. (2006) found that processing other- vs self-relevant information elicited decreased activation in a broad swath of cortical midline structures, including the MPFC and rostral anterior cingulate (rACC). Furthermore, a recent study found that processing past self vs current self-relevant information decreased activation in these cortical midline structures (D'Argembeau et al., 2008).
Research on past selves can be extended to test the future self-continuity hypothesis. If people effectively consider their future selves as others, judgments about the future vs current self should elicit reduced activation in cortical midline structures. Furthermore, individuals with greater decreases in activation for the future vs current self should more steeply discount future rewards. The goal of this experiment was to determine whether neural indices of future self-continuity could predict temporal discounting. To test these hypotheses, subjects were scanned with event-related fMRI while making judgments about the extent to which trait adjectives applied to their current self, a future self, a current other, or a future other. A week later, subjects completed a temporal discounting task that yielded an estimate of the degree to which each individual discounted future rewards. Analyses focused on changes in activation in the MPFC and rACC during current vs future self-ratings. First, we predicted that rating the self vs another person would increase activation in the MPFC and rACC (Kelley et al., 2002), consistent with previous findings. Second, we predicted that overall, rating the current vs future self would increase MPFC and rACC activation. Finally, based on the future self-continuity hypothesis, we predicted that individual differences in current vs future self rating elicited MPFC and rACC activation would predict individual differences in temporal discounting, tested behaviorally at least a week later. This research represents the first attempt to link a neural index of future self-continuity to temporal discounting.
METHODS
Subjects
Eighteen subjects between the ages of 18 and 23 (10 men, 8 women) were recruited from the Stanford University community. Subjects were screened for typical magnetic resonance exclusions (e.g. metal in the body) prior to collecting informed consent. For the scanning session, subjects received $20.00 an hour for their participation. For the behavioral session 2 weeks later, subjects received $15.00 an hour for their participation, in addition to payment for whichever trial was drawn at random from the temporal discounting task (see below). In addition to the 18 subjects who were included in the analysis, five subjects were excluded due to excessive head motion (i.e. more than 2 mm from one whole brain acquisition to the next during the scanning session).
Self-reference task
Upon completing a consent form and a standard imaging screening form, subjects were instructed in a practice version of the task that they would perform in the scanner, consisting of eight trials. The practice session was repeated until subjects felt comfortable with the task. During the task, which was adapted from Kelley et al. (2002), subjects made judgments about trait adjectives.
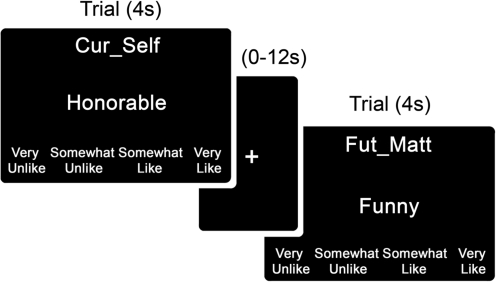
Judgments were one of five types, which subjects were trained to associate with target person task prompts: CUR_SELF (‘Does this word describe yourself now?’), FUT_SELF (‘Does this word describe yourself in the future, i.e. ten years from now?’), CUR_MATT (for men) or CUR_NAT (for women)2 (‘Does this word describe Matt Damon/Natalie Portman now?’), FUT_MATT or FUT_NAT (‘Does this word describe Matt Damon/Natalie Portman in the future, i.e. ten years from now?’) and UPP_CASE (‘Is this word in all capital letters?’)3. Subjects judged whether the trait word applied to the target person using a four-point scale (Very Unlike, Somewhat Unlike, Somewhat Like, Very Like). Each trial lasted 4000 ms and began with a target person prompt at the top of the screen (Figure 1). A unique trait word (e.g. ‘Honorable’) appeared in the middle of the screen. The four-point rating scale appeared at the bottom of the screen, and subjects responded using a four-button button box. Between each trial, a fixation cross appeared in the center of the screen for a variable interval (0–12 s). These fixation trials were included to introduce ‘jitter’ into the time series so that unique estimates of the hemodynamic responses for the trial types of interest could be generated (Ollinger et al., 2001).
Fig. 1.
Self-reference task trial structure and timing.
Words for the task were selected from a pool of normalized trait words which had previously been rated on valence and arousal dimensions (Anderson, 1968). An equal number of positive and negative4 words were chosen for each trial type, which produced ten different conditions: positive current self, negative current self, positive future self, negative future self, positive current other, negative current other, positive future other, negative future other, positive upper case and negative upper case. A total of 18 positive and 18 negative words were chosen for the task, and lists were equated for number of letters, syllables and arousal scores. The OptSeq program (Greve, 2002) was used to generate a randomized order of trials in which each trial type followed every other trial type an equal number of times. Each participant saw the same order of trials, but a randomized order of the 18 positive or 18 negative words within those trials. Thus, subjects made 18 judgments for each of the 10 conditions, totaling 180 trials. The task was divided into two functional blocks that lasted approximately 10 min and contained 90 trials each.
Temporal discounting task
Approximately 1 week after the scanning session, subjects returned to the laboratory for a half-hour follow-up session in which they participated in a temporal discounting task. During each trial, subjects indicated which of two options they preferred (Mitchell, 1999). A delayed gain featured $10.00 available after one of six delays (0, 7, 30, 90, 180 or 365 days), vs an immediate and variable gain (consisting of 23 increments ranging from $0.01 to $10.50) available at the conclusion of the experimental session. Crossing the six standard with 23 alternative options yielded 137 questions (omitting the redundant choice between $10 dollars now or $10 dollars now). For each trial, a delayed gain (e.g. $10 in 7 days) and an immediate gain (e.g. $1.50 at end of the experiment) were presented in a random order, without replacement. Based on subjects’ choices during the task, we estimated a discounting rate (k) for each subject (see Supplementary data for details).
fMRI acquisition
Images were acquired with a 1.5-T General Electric MRI scanner using a standard birdcage quadrature head coil. Twenty-four 4 mm-thick slices (in-plane resolution 3.75 × 3.75 mm2, no gap) extended axially from the mid-pons to the top of the skull, providing whole-brain coverage and adequate spatial resolution of regions of interest (e.g. MPFC, anterior cingulate cortex). Whole-brain functional scans were acquired with a T2*-sensitive spiral in-/out-pulse sequence (TR = 2 s, TE = 40 ms, flip = 90°) designed to minimize signal dropout at the base of the brain (Glover and Law, 2001). High-resolution structural scans were also acquired to facilitate localization and coregistration of functional data using a T1-weighted spoiled grass sequence (TR = 100 ms, TE = 7 ms, flip = 90°).
fMRI analysis
Analyses modeled changes in activation specifically during stimulus presentation periods for all trial types (i.e. when subjects saw the target person and cue word, and subsequently judged applicability of the trait word). Analyses were conducted using Analysis of Functional Neural Images (AFNI) software (Cox, 1996). For preprocessing, voxel time series were sinc interpolated to correct for non-simultaneous slice acquisition within each volume, concatenated across runs, slightly spatially smoothed (full width at half maximum 4 mm) to minimize the effects of anatomical variability, corrected for motion, high-pass filtered (admitting frequencies with period <90 s) and normalized to percent signal change with respect to the voxel mean for the entire task. Visual inspection of motion correction estimates confirmed that no subject's head moved more than 2 mm in any dimension from one volume acquisition to the next.
Analyses progressed through three stages: localization, verification and individual differences. The goal of the localization analysis was to identify candidate regions whose activity maximally correlated with the three orthogonal regressors derived from the interaction model including main effects of Person (self vs other) and Time (current vs future), as well as their interaction. These regressors of interest were convolved with a gamma-variate function approximating a canonical hemodynamic prior to inclusion in regression models (Cohen, 1997). Regressors of no interest indexed residual motion (n = 6) and baseline, linear and quadratic trends for the two runs (n = 6). Coefficients for regressors of interest were coregistered with structural maps, spatially normalized by warping to Talairach space, and collectively submitted to a one-sample t-test against the null hypothesis of no activation to test for a group difference while controlling for random effects. Foci activation thresholds for a priori regions of interest (MPFC and rACC) were identified (Z > 2.81, p < 0.005, Bonferroni-corrected for the volumes of interest), requiring a minimum cluster of two contiguous 64 mm3 voxels. For the more exploratory whole-brain localization, activation thresholds were set at a higher threshold (Z > 3.28; p < 0.001, uncorrected) and required a minimum cluster of two contiguous 64 mm3 voxels.
Verification analyses were conducted to establish the direction and significance of localization results within each identified region. Volumes of interest (VOIs) were specified by imposing 8 mm diameter spheres at peak activation foci in regions of interest in the MPFC and rACC, ensuring that equal amounts of data were extracted for each subject in each region. Spatially averaged percent signal change time courses were extracted from each region of interest. Time courses were then averaged for each trial type (e.g. current self trials, current other trials, etc.) within subject. Peak activation values at a 4 s lag (i.e. trial TRs 4 and 5, to account for the delay in hemodynamic response) were then submitted to a repeated-measures ANOVA that included two within-subjects factors (Person: self, other; Time: current, future). These region of interest percent signal change data were also submitted to individual differences analyses.
Individual differences analyses utilized individual differences in peak percent signal change for each region of interest to predict each subject's temporal discounting rates, collected during a behavioral test 1 week post-scan. To examine whole-brain activation correlations with individual differences in temporal discounting, we computed a neural measure of current-future self difference by subtracting percent signal change in the conjoined regions of interest for future self trials from percent signal change for current self trials. This value was correlated with each individual's rate of temporal discounting (k) using multiple regression.
RESULTS
Behavior
Reaction time
To normalize the skewed distribution of reaction times, we log-transformed each subject's reaction times. A repeated-measures ANOVA with two within-subjects factors (Person: self, other; Time: current, future) analyzed the effect of trial type on reaction time. Results indicated that there was no main effect for Person [F(1,17) = 0.01, p = 0.95], no main effect for Time [F(1,17) = 0.12, p = 0.73] and no significant interaction of Person × Time for reaction time data [F(1,17) = 2.63, p = 0.12].
Endorsement
A repeated-measures ANOVA with two within-subjects factors (Person: self, other; Time: current, future) examined whether trial type influenced endorsement of trait words. A main effect of Person indicated that subjects endorsed trait words that applied to the self more than trait words that applied to the other, F(1,17) = 10.71, p < 0.005. A main effect of Time revealed that subjects endorsed trait words that applied to future targets more than words that applied to current targets, F(1,17) = 4.84, p < .05. There was no significant interaction [F(1,17) = 0.18, p = 0.67; Table 1].
Table 1.
Reaction time (in milliseconds) and rating means and standard deviations for the four conditions
| Reaction time |
Rating (Scale 1–4) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Self |
Other |
Self |
Other |
|||||
| M | (s.d.) | M | (s.d.) | M | (s.d.) | M | (s.d.) | |
| Current | 2277.68 | (228.96) | 2236.81 | (224.08) | 2.74 | (0.22) | 2.54 | (0.15) |
| Future | 2230.58 | (230.60) | 2268.22 | (230.13) | 2.78 | (0.28) | 2.61 | (0.19) |
Brain activation
Localization
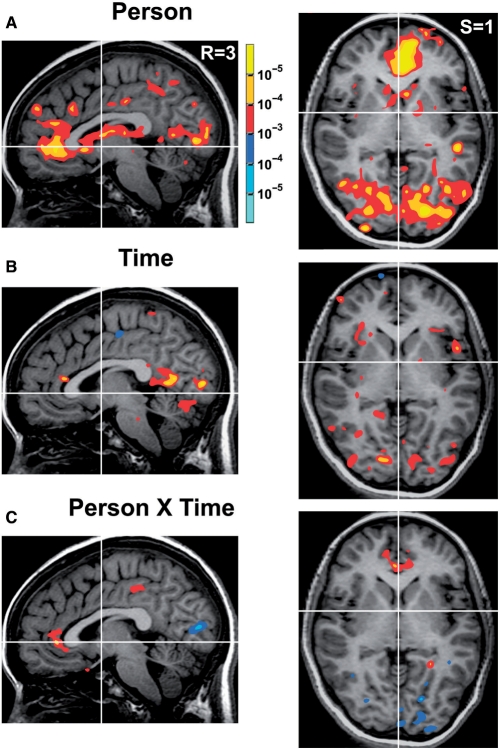
A predicted main effect of Person (self vs other) indicated correlated activation in the left and right MPFC and rostral anterior cingulate (z = 5.39; −4, 38, −3; z = 3.83; 3, 44, −3; Figure 2A and Supplementaary Table 1), replicating and extending prior findings (Kelley et al., 2002), as well as other cortical and subcortical regions (Supplementary Table 1). A main effect of Time (current vs future) did not correlate with activation in the regions of interest, but did correlate with activation in other cortical and subcortical regions (Figure 2B and Supplementary Table 1). The predicted interaction of Person by Time (which included the critical contrast of current self vs future self) correlated with activation in the bilateral rACC (z = 3.04; −9, 37, 1; z = 3.41; 3, 37, 0; Figure 2C and Supplementary Table 1). Conjunction analysis revealed that this rACC region overlapped with the region activated by the main effect of Person (Figure 3A, activated regions thresholded at p < 0.005).
Fig. 2.
Brain regions correlated with the model main effects and interaction. (A) Brain regions correlated with Person (self > other), including the MPFC and rACC. (B) Time (current > future), including the posterior cingulate. (C) Person × Time, selectively activating the rACC; threshold p < 0.01 uncorrected.
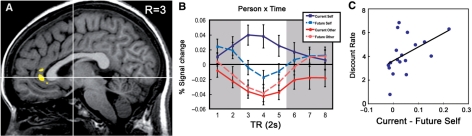
Fig. 3.
Neural activation differences between current self and future self trials correlate with discounting rates. (A) Conjunction showing that the medial prefrontal cortex (MPFC) and rostral anterior cingulate cortex (rACC) are selectively activated by both Person (self vs other) and Person × Time (current vs future self); threshold p < 0.005, uncorrected. (B) Activation time courses for each condition in the right rACC volume of interest. The white section represents predicted peak signal change related to person judgment. Error bars indicate standard error of the mean. (C) Scatterplot of individual differences in discount rates [log(k) + 10] and individual differences between peak current self and future self activation in the rACC volume of interest (r = 0.47, p < 0.05). Note: For display purposes and ease of interpretation, a constant of 10 was added to the log(k) values.
Verification
Analysis of peak activation timecourse data extracted from the right MPFC region of interest showed a main effect of Person [F(1,17) = 4.72, p < 0.05], such that self trials showed greater activation than other trials, replicating prior findings (Figure 2A). There was no main effect of Time in the right MPFC VOI [F(1,17) = 2.04, p = 0.17], nor was there a significant Person × Time interaction, [F(1,17) = 0.00, p = 0.98]. Similar results were obtained in the left MPFC VOI, which showed a main effect of Person [F(1,17) = 22.42, p < 0.001], but no main effect of Time (F(1,17) = 2.02, p = 0.17) and no Person × Time interaction [F(1,17) = 1.30, p = 0.27].
Analysis of activation in the right rostral anterior cingulate cortex (right rACC) VOI yielded a main effect of Person [F(1,17) = 10.00, p < 0.01], such that self-relevant trials showed greater activation than other trials. In line with the future self-continuity hypothesis, this main effect of Person was qualified by a significant and predicted Person × Time interaction [F(1,17) = 6.24, p < 0.05]. Pairwise comparisons indicated that right rACC activation was greater for current self trials than for future self trials [t(17) = 2.94, p < 0.01), but no different for current other trials compared to future other trials [t(17) = −0.61, p = 0.55) (Figure 3B). There was not a significant main effect of Time in the right rACC[(F(1,17) = 3.10, p = 0.10].
Analysis of peak activation in the left rostral anterior cingulate cortex (left rACC) VOI yielded similar results. There was a main effect of person [F(1,17) = 15.12, p < 0.001], such that self-relevant trials showed greater activation than other trials. Again, this main effect of Person was qualified by a significant and predicted Person × Time interaction [F(1,17) = 6.17, p < 0.05]. Pairwise comparisons indicated that left rACC activation was significantly greater for current self trials than for future self trials [t(17) = 2.47, p < 0.05], but not different for current other trials compared to future other trials [t(17) = −1.13, p = 0.28] (Figure 3B). [In fact, in an exploratory whole-brain analysis of current vs future self activation, the only other region than the left and right rACC that showed significant activation was the right superior frontal gyrus (z = 4.12; 26, −1, 53)].There was not a significant main effect of Time in the left rACC, [F(1,17) = 1.44, p = 0.25)].
Individual differences
Individual estimates of temporal discounting (k) were derived for the behavioral task performed at least 1 week post-scan. Higher k values indicated a greater propensity for an individual to discount future rewards. k values were log transformed to normalize their positively skewed distribution. Although subjects were sampled from a relatively homogenous group of undergraduates, they showed marked individual variation in temporal discounting, with discounting rates similar to those reported in similar (Kirby and Marakovic, 1996; Mitchell, 1999). To assess the relationship between brain activation and subsequent behaviorally assessed discounting rates, we used a neural measure of current-future self activation differences for each region of interest (i.e. MPFC and rACC). For purposes of comparison, we also computed difference scores for ‘other’ trials. To establish robustness against outliers, difference scores were correlated with discounting rates using both parametric (Pearson's r) and non-parametric methods (Spearman's ρ).
Results revealed a significant positive correlation between current self vs future self difference scores in the right rACC and discounting rates [r(16) = 0.47, p < 0.05; ρ = 0.54, p < 0.055] (Figure 3C). To statistically decompose this result, we separately correlated current self activation levels with discounting rates, as well as future self activation levels with discounting rates. Results indicated that current self activation was correlated with discounting [r(16) = 0.59, p < 0.05], while future self activation was not significantly correlated with discounting [r(16) = 0.06, p = 0.81], suggesting the level of activation during current self ratings could primarily account for the association of future self-continuity with temporal discounting. There were no other correlations between current vs future self difference scores and discounting rates in the other regions of interest (all r's < 0.32, all ρ's < 0.37, all p's > 0.12). Furthermore, as predicted, there were no positive correlations between current other vs future other activation differences and discounting rates in any of the regions of interest. In an exploratory regression of discounting scores on the current vs future self coefficient, the right rACC was the only region of interest where activation showed significant correlation with discounting rates (z = 3.10; 0, −33, 15).
DISCUSSION
Why don't people save for the future? According to a future self-continuity account, if one views the future self as a stranger, she should be no more motivated to save for her future self than to give money to a stranger (Parfit, 1971, 1987). This account implies that an individual's perceived similarity to her future self should relate to her preference for future vs immediate rewards. We tested these predictions in the present study with event-related fMRI and found not only that current self- vs future self-relevant information activated a portion of the anterior cingulate cortex, but also that individual differences in the magnitude of this effect predicted the tendency to devalue future rewards.
These findings add to a growing body of research suggesting that specific neural circuits represent self- vs other-relevant information (Craik et al., 1999; Kelley et al., 2002; Northoff and Bermpohl, 2004; Moran et al., 2006; Northoff et al., 2006; D'Argembeau et al., 2008). As in previous research, consideration of self- vs other-relevant information elicited activation in medial prefrontal regions extending from the MPFC to rostral ACC. The present findings also provide the first demonstration that within these medial prefrontal regions, current self- vs future self-relevant information increases rostral ACC activation. While previous findings primarily suggested that activation stayed at baseline during consideration of self-relevant material, but decreased from baseline during consideration of other-relevant material, in the present study, we instead observed significantly increased activation during consideration of self-relevant information. Methodological differences between studies might help account for the presently observed increase in self-relevant activation. While most studies of self-relevance have employed block designs, the present study employed a pseudorandomly ordered event-related design, obviating potential confounds related to anticipation and habituation, and facilitating temporal isolation of changes in neural activation that occurred prior to and during each rating. In the regions of interest, averaging over more extended timescales may hinder investigators’ abilities to detect transient and temporally specific increases in activation (Knutson et al., 2003).
Investigators have proposed several distinct accounts for increased neural activation to self- vs other-relevant information. These accounts variously invoke perceived lack of similarity with others (Mitchell et al., 2005), perceived distinctness from others (Kelley et al., 2002), more positive valence for the self than others (Moran et al., 2006; Harris et al., 2007), deeper levels of processing for the self than others (Northoff et al., 2006) and an inability to ‘mentalize’ or infer the other's thoughts (Fletcher et al., 1995). Thus, the precise psychological mechanisms underlying differences in neural activation for self- vs other-relevant information remain unclear. From the vantage point of the future self-continuity hypothesis, this explanatory ambiguity extends to differences in neural responses to presentation of current self- vs future self-relevant information. Since, in the present study, neural activation elicited by consideration of self- vs other-relevant information overlapped with activation elicited by consideration of current self- vs future self-relevant information in the anterior cingulate cortex, a common mechanism might account for both effects. Future studies, however, will need to disentangle which psychological mechanisms most powerfully modulate neural activation elicited by current self- vs future self-representations.
Beyond increased activation during consideration of self- vs other-relevant information (Northoff et al., 2006), several studies have demonstrated that conflict detection and monitoring can also increase rostral anterior cingulate activation (Botvinick et al., 1999). These findings imply that if information relevant to the current self elicits more conflict (e.g. due to greater richness) than information relevant to the future self, then rostral anterior cingulate activation might reflect this conflict (Pronin and Ross, 2006). While previous research suggests that increased conflict corresponds with increased reaction times (Eriksen and Eriksen, 1974), the present findings yielded no evidence of increased reaction time when subjects rated information relevant to the current self vs the future self, inconsistent with such an increased conflict interpretation.
By linking self-relevant anterior cingulate activation to temporal discounting, these findings provide initial empirical support for a future self-continuity account. Although early tests of this hypothesis with self-report measures did not yield significant results (Frederick, 1999), neural activation may provide a more sensitive and unobtrusive measure of peoples’ perceived similarity of present and future self-representations. Interestingly, neural responses to current self- rather than future self-relevant information primarily predicted temporal discounting. This finding might be consistent with an effect in which current emotional arousal both distinguishes the current self from the future self and makes the (less emotional) future self seem less similar (Ariely and Loewenstein, 2006). According to one alternative account, a general inability to envision the future can promote temporal discounting (Klineberg, 1968), regardless of the future object of representation (e.g. self vs other). However, neural measures of future self-continuity but not future other-continuity specifically predicted reductions in temporal discounting, suggesting that consideration of the future self, and not just the future in general, is associated with discounting future rewards. Since animals and children show greater discounting of future rewards than adults, medial prefrontal regions may primarily act to minimize temporal discounting by extending the time horizon of the current self into the indefinite future (Fellows and Farah, 2005; Sharot et al., 2007).
The present study offers a number of advances over previous research. The randomized and event-related fMRI design ensured that neural activation occurred in response to self- or other-descriptive stimuli, rather than as a result of attentional or anticipatory confounds. Use of an incentive-compatible measure of temporal discounting (i.e. subjects made decisions with real monetary consequences) ensured that stakes were consequential for subjects (both in the present and in the future). Additionally, the measure of temporal discounting used in this study has been widely replicated and correlated with individual differences outside the laboratory for impulse control disorders including smoking (Mitchell, 1999), gambling (MacKillop et al., 2006) and alcoholism (Mitchell et al., 2005). Future research might, however, profitably explore the connection between future self-representations and actual saving decisions (e.g. for retirement). Additionally, while the present study examined neural responses to a self representation 10 years in the future, further research might vary temporal distance of self-representations to determine exactly how increasing temporal distance influences future self-continuity.
In conclusion, these findings provide initial empirical support for a future self-continuity account of temporal discounting. Specifically, the extent to which individuals neurally distinguish between the current and future self predicts their tendency to devalue future gains in a subsequent behavioral task. If individual differences in savings partially depend upon future self-continuity, then savings behavior might be modified either by altering perceptions of the future self or by projecting the current self into the future. The findings thus may hold implications both for understanding and encouraging saving for the future self.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Samuel McClure, Jeffrey Cooper, Kacey Ballard, Gregory Samanez-Larkin, Matthew Lieberman and an anonymous reviewer for helpful comments. This research was funded in part by Center on Advancing Decision Making in Aging Grant AG024957.
Footnotes
In the original Kelley et al. (2002) study, the ‘other’ was George W. Bush. Their study was run in July of 2001 before public opinion of Bush became more polarized. We felt it necessary to use target ‘others’ who were less controversial. Accordingly, in a pilot study, we asked 60 people to list the most well-known, least controversial public figures. Matt Damon and Natalie Portman were listed most often. We used these two people as the ‘other’ target for male and female subjects, respectively.
Trials on which subjects were asked to judge the case of the trait adjective were included to provide a task baseline and were not analyzed further.
It was necessary to include positive and negative words because valenced words are more applicable to subjects’ self-perceptions than neutral words. However, minimal effects of word valence were observed (both behavioral and neuroimaging). Thus, further analyses focus on person and time factors. See Online Supplementary data for further details.
For this correlation analysis, there was one outlier on the self-difference score dimension (2.73 s.d. above the mean). When removing this outlier, the parametric correlation dropped to non-significance [r(15) = 0.35, p = 0.16], but the non-parametric correlation remained significant (ρ = 0.51, p < 0.05).
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Arias E. United States Life Tables, 2004. National Vital Statistics Reports. 2007;56:1–40. [PubMed] [Google Scholar]
- Ariely D, Loewenstein G. The heat of the moment: The effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making. 2006;19:87–98. [Google Scholar]
- Bernheim BD, Forni L, Gokhale J, Kotlikoff LJ. How much should Americans be saving for retirement? American Economic Review. 2000;90(2):288–292. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Chapman GB. Temporal discounting and utility for health and money. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22:771–791. doi: 10.1037//0278-7393.22.3.771. [DOI] [PubMed] [Google Scholar]
- Chapman GB, Elstein AS. Valuing the future: Temporal discounting of health and money. Medical Decision Making. 1995;15:373–386. doi: 10.1177/0272989X9501500408. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D'Argembeau A, Feyers D, Majerus S, et al. Self-reflection acros time: Cortical midline structures differentiate between present and past selves. Social Cognitive and Affective Neuroscience. 2008;3:244–252. doi: 10.1093/scan/nsn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Fellows LK, Farah MJ. Dissociable elements of human foresight: a role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005;43:1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frederick S. Discounting, Time Preference, and Identity: A Dissertation Submitted to the Department of Social and Decision Sciences. Carnegie Mellon University: 1999. [Google Scholar]
- Frederick S. Time preference and personal identity. In: Loewenstein G, Read D, Baumeister RF, editors. Time and Decision: Economic and Psychological Perspectives on Intertemporal Choice. New York: Russell Sage Foundation; 2003. p. xiii, 569. [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: A critical review. In: Loewenstein G, Read D, Baumeister RF, editors. Time and Decision: Economic and Psychological Perspectives on Intertemporal Choice. New York: Russell Sage Foundation; 2003. p. xiii, 569. [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Greve DN. 2002 http://surfer.nmr.mgh.harvard.edu/optseq (last accessed 10 February 2008)
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, McClure SM, van den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cognitive Affective and Behavioral Neuroscience. 2007;7:309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Monetary Fund. World Economic Outlook: The Global Demographic Transition. Washington, DC: International Monetary Fundo; 2004. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Marakovic N. Modeling myopic decisions: Evidence for hyperbolic delay-discounting within subjects and amounts. Organizational Behavior and Human Decision Processes. 1996;64:22–30. [Google Scholar]
- Klineberg SL. Future time perspective and preference for delayed reward. Journal of Personality and Social Psychology. 1968;8:253–257. doi: 10.1037/h0025581. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CS, Hommer D. A region of mesial prefrontal coretex tracks monetarily rewarding outcomes: Characterization with rapid event-related FMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Laibson D. Golden eggs and hyperbolic discounting. Quarterly Journal of Economics. 1997;112:443–477. [Google Scholar]
- Laibson D, Repetto A, Tobacman J. Self-control and saving for retirement. Brookings Papers on Economic Activity. 1998;1:91–196. [Google Scholar]
- Li JP. Intended Retirement and Wealth Adequacy. Unpublished Dissertation: The Ohio State University, Columbus, OH; 1996. [Google Scholar]
- MacKillop J, Anderson EJ, Castelda BA, Mattson RE, Donovick PJ. Divergent validity of measures of cognitive distortions, impulsivity, and time perspective in pathological gambling. Journal of Gambling Studies. 2006;22:339–354. doi: 10.1007/s10899-006-9021-9. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons J, Mazur JE, Nevin JA, Rachlin H, editors. The Effect of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mischel W. Processes in delay of gratification. Advances in Experimental Social Psychology. 1974;7:249–291. [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism-Clinical and Experimental Research. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Parfit D. Personal Identity. Philosophical Review. 1971;80:3–27. [Google Scholar]
- Parfit D. Reasons and Persons (Repr. with corrections. ed.) Oxford: Clarendon Press; 1987. [Google Scholar]
- Pronin E, Olivola CY, Kennedy KA. Doing unto future selves as you would do unto others: psychological distance and decision making. Personality and Social Psychology Bulletin. 2008;34:224–236. doi: 10.1177/0146167207310023. [DOI] [PubMed] [Google Scholar]
- Pronin E, Ross L. Temporal differences in trait self-ascription: When the self is seen as an other. Journal of Personality and Social Psychology. 2006;90:197–209. doi: 10.1037/0022-3514.90.2.197. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson PA. A note on the measurement of utility. The Review of Economic Studies. 1937;4:155–161. [Google Scholar]
- Schelling TC. Ethics, Law, and the Exercise of Self-Command. Boston, MA: Harvard Institute of Economic Researcho; 1982. [Google Scholar]
- Schelling TC. Self-command in practice, in policy, and in a theory of rational choice. The American Economic Review. 1984;74:1–11. [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Strotz RH. Myopia and inconsistency in dynamic utility maximization. The Review of Economic Studies. 1956;23:165–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.