Abstract
Neurobiological evidence implicates the amygdala as well as serotonergic (serotonin, 5-HT) signaling via postsynaptic 5-HT2A receptors as essential substrates of anxiety behaviors. Assuming a functional interdependence of these substrates, we hypothesized that a low-fear behavioral phenotype due to bilateral lesion of the amygdala would be associated with significant 5-HT2A receptor changes. Thus, we used [18F]altanserin positron emission tomography (PET) referenced to radioligand plasma levels and corrected for partial volume effects to quantify the spatial distribution of 5-HT2A receptor binding potential (BPP) in a rare patient with Urbach–Wiethe disease and selective bilateral amygdala calcification damage relative to 10 healthy control subjects. Consistent with our a priori hypothesis, we observed a 70% global decrease in 5-HT2A receptor BPP in the Urbach–Wiethe patient relative to controls. Thus, brain abnormalities in this patient are not restricted to the amygdala, but extend to overall 5-HT neurotransmission via 5-HT2A receptors. Our findings provide important insights into the molecular architecture of human anxiety behaviors and suggest the 5-HT2A receptor as a promising pharmacological target to control pathological anxiety.
Keywords: Amygdala, fear, anxiety, serotonin, 5-HT2A receptor, PET, Urbach-Wiethe disease
INTRODUCTION
Over the past decade, interest in the human amygdaloid complex (henceforth referred to as the amygdala) has increased tremendously, driven by the progress in animal research and functional imaging techniques. Although the amygdala has been implicated in myriad affective and motivational functions including reward–reinforced behavior, fear is still the emotion best characterized in terms of its dependence on a neural circuitry centered around the amygdala and its adaptive impact on behavior (LeDoux, 2007). However, when fear is disproportional in intensity, chronic or unrelated to genuine threats, it may become maladaptive and precipitate pathological anxiety (Millan, 2003). Anxiety spectrum disorders represent highly prevalent mental illnesses, tend to run a chronic course and are as disabling as physical diseases (Kessler et al., 1994). Consequently, there is a continuous search for novel drug targets enabling potent pharmacological control of pathological anxiety.
Serotonin [5-hydroxytryptamine (5-HT)] neurotransmission has long been implicated in the modulation of anxiety behaviors, and indeed, many anxiolytic drugs currently available interfere with 5-HT reuptake or target 5-HT receptors (Millan, 2003). Accumulating evidence points to a specific role of the postsynaptic 5-HT2A receptor (5-HT2AR) in the expression of anxiety behaviors: polymorphisms of the 5-HT2AR-coding gene (HTR2A) in humans form a haplotype that increases susceptibility for panic disorder (Unschuld et al., 2007; Yoon et al., 2008), whereas HTR2A knock-out produces a low-anxiety behavioral phenotype in mice (Weisstaub et al., 2006). Moreover, personality risk factors for anxiety disorders positively correlate with frontolimbic 5-HT2AR binding potential (BPP) in [18F]altanserin positron emission tomography (PET) (Frokjaer et al., 2008).
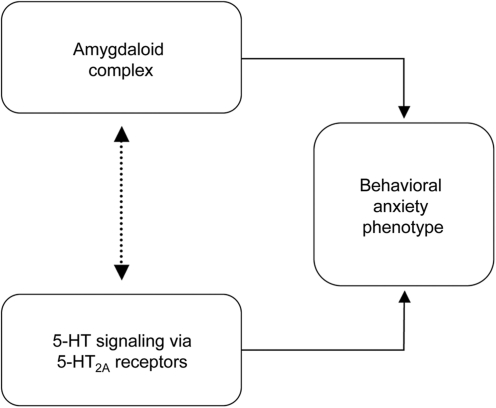
Assuming a functional relationship between the amygdala and the 5-HT2AR in the expression of anxiety behaviors, we hypothesized that a low-fear behavioral phenotype due to bilateral lesion of the amygdala would be associated with significant 5-HT2AR changes (Figure 1). Therefore, the present study is focused on a 32-year old female patient, A.M., who suffers from selective and bilaterally symmetrical calcification damage to the basolateral amygdala (BLA) as a consequence of Lipoid proteinosis of Urbach–Wiethe (LP) (Hurlemann et al., 2007). LP is a rare autosomal recessive disorder typified by cutaneous, mucosal and visceral deposits of periodic acid-Schiff (PAS)-positive hyaline (glycoprotein) material that pathognomonically presents itself in early infancy through hoarse cries due to laryngeal infiltration and follows a slowly progressive, yet often benign course. About 250 cases have been reported worldwide (Hofer, 1973). LP has been mapped to a locus on chromosome 1q21, and pathogenic loss-of-function mutations have been identified within the extracellular matrix protein 1 gene (ECM1) (Hamada et al., 2002; Chan et al., 2007).
Fig. 1.
Neurobiological evidence implicates the amygdala as well as serotonergic (serotonin, 5-HT) signaling via postsynaptic 5-HT2AR as essential substrates of anxiety behaviors. However, the functional relationship between these substrates is unclear. We hypothesized that a low-fear behavioral phenotype due to bilateral lesion of the amygdala is associated with significant changes in 5-HT2AR expression. Thus, we used [18F]altanserin PET referenced to radioligand plasma levels and corrected for partial volume effects to quantify the spatial distribution of 5-HT2AR BPP in a rare patient with Urbach–Wiethe disease and selective bilateral amygdala calcification damage relative to 10 healthy control subjects.
Selective bilateral calcification damage to the amygdala occurs in 50–75% of LP cases and has been shown to cause impaired fear recognition from facial expressions, reduced fearfulness in social contexts and a failure to acquire conditioned fear responses (Adolphs et al., 1994, 1998, 2005). These findings beg the question whether or not the documented amygdala pathology in patient A.M. is associated with localized 5-HT2AR changes consistent with the symptomatology, or alternatively, whether the symptomatology is explainable by network disruption centred on the amygdala. We therefore used [18F]altanserin PET referenced to radioligand plasma levels and corrected for partial volume effects to test whether 5-HT neurotransmission via 5-HT2AR in patient A.M. is different from 10 healthy control subjects.
METHODS
Participants
Patient A.M. and 10 healthy control subjects matched for age (age range 26.3–34.4 years; mean age 32.0 ± 3.7 years), gender and education underwent thorough psychiatric exploration by two experienced clinicians blinded for LP diagnosis to exclude either current or past Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) axis I and axis II disorders as potential confounding factors. Controls had no history of neurological or severe physical disorders and were, as A.M., naïve to neuro- and psychopharmacological treatment. Written informed consent according to the latest revision of the 1964 Declaration of Helsinki was obtained from all participants, and study protocols were approved by the ethics committees of the Medical Faculties of the Universities of Bonn and Duesseldorf, the German Federal Office for Radiation Protection (BfS) and the German Federal Institute for Drugs and Medical Devices (BfArM).
The neuroradiological assessment demonstrated that brain calcification damage in patient A.M. was amygdala-selective, with particular emphasis on the BLA and no involvement of other nearby tissues (Hurlemann et al., 2007). Consistent with observations in other LP patients (Tranel et al., 2006), A.M. denied any subjective familiarity with fear, including the feelings of anxiety, worry, fright or panic. She claimed to never have experienced such feelings and even could not imagine situations where these feelings might afflict or disturb her. She did not appear to have a normal sense of danger and would skydive from a plane without hesitation, if given the opportunity.
Interestingly, A.M. showed much less cognitive deviation from controls than initial studies of patients with this rare etiology had suggested (Siebert et al., 2003). Neuropsychological testing showed her to be of normal IQ (Wechsler Adult Intelligence Scale-III full-scale IQ 100) (Wechsler, 1981) and within normal limits for a wide range of brain functions, including linguistic, visuoperceptual, visuospatial and visuoconstructional faculties, and executive control such as planning and decision-making (Strange et al., 2003; Hurlemann et al., 2007). Declarative episodic memory was intact, however, an emotion-induced modulation of declarative episodic memory was absent (Strange et al., 2003; Hurlemann et al., 2007). The Positive and Negative Affect Schedule (Watson et al., 1988), the Hamilton Depression Rating Scale (Hamilton, 1960), the Hamilton Anxiety Rating Scale (Hamilton, 1959) and the State-Trait Anxiety Inventory for Adults (Spielberger, 1983) have been administered to A.M., all yielding no evidence of abnormal affective functioning. In brief, she had neither notable neuropsychological impairments nor notable psychopathology on conventional testing when undergoing PET scanning.
PET scanning
The [18F]altanserin PET procedure has been detailed elsewhere (Hurlemann et al., 2008).
In brief, T1-weighted cranial magnetic resonance (MR) images Magnetization Prepared Rapid Gradient Echo (MP-RAGE) were obtained on a Siemens Trio 3T scanner. Radiosynthesis of [18F]altanserin was performed with a radiochemical purity of >99%. Controls were infused 229 ± 7 MBq with a mean specific radioactivity of ≥125 ± 83 GBq/µmol; patient A.M. was infused 241 MBq with a mean specific radioactivity of 374 GBq/µmol. [18F]Altanserin was infused as a 2 min bolus followed by continuous infusion with a bolus/infusion ratio of Kbol = 2.1 h. PET measurements were performed in 3D mode on a Siemens ECAT EXACT HR+ scanner (Siemens-CTI, Knoxville, TN, USA). A 10-min transmission scan (with three 68Ge/68Ga line sources) was obtained for attenuation correction. Dynamic emission data were collected in six frames of 10 min length starting from 120 min after the start of [18F]altanserin application (zero).
PET data were corrected for randoms, scatters and attenuation; Fourier rebinned into 2D sinograms; reconstructed by filtered backprojection (Shepp filter, 2.5 mm width) with a voxel size of 2 × 2 × 2.43 mm3 [63 slices; full width of half maximum FWHM 5.8, 5.8, 6.6 mm (x, y, z) at 10 cm from the central axis], and decay-corrected. Venous blood samples were taken at 2, 5, 10, 20, 30, 45, 60, 120, 130, 140, 150, 160, 170 and 180 min p.i. The fraction of radioactive parent compound in plasma was determined by selective liquid–liquid extraction with quantification of the recovery of total radioactivity, followed by thin-layer chromatography.
Dynamic PET data obtained in ECAT7 format were converted to ‘analyze’ format using PMOD v.2.85 (PMOD Technologies Ltd., Zurich, Swizerland). Frames were realigned using SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK) to correct for head movements during the scan. MR images were oriented along the anterior commissure–posterior commissure (AC–PC) line. The realigned frames were coregistered to the individual MR images using SPM5. The cerebellar region was manually delineated on the individual MR images using PMOD v.2.85. Based on the cerebellar radioactivity concentration (CReference) and the plasma radioactivity concentration attributable to parent compound (CPPC), PET datasets containing voxel-wise radioactivity concentrations CVoxel obtained from PMOD v.2.85 were parametrized according to Equation (1) (Pinborg et al., 2003), with all variables C being averaged from 120–180 min p.i.
| (1) |
Average images were corrected for partial volume effects using the PVE2 routine implemented in PMOD v2.85. The partial volume-corrected radioactivity concentration was calculated as CGM = (Ctotal – CWM × fWM)/fGM; where CGM was the radioactivity concentration attributable to gray matter within the voxel, the result of partial volume correction; Ctotal the measured radioactivity concentration within the voxel comprised of radioactivity from gray and from white matter; CWM the white matter radioactivity concentration, which is assumed to be constant over all white matter; fWM and fGM are the fractions of white and gray matter within the voxel, respectively. Partial volume correction was performed on a voxel-wise basis. Both fWM and fGM were calculated from gray and white matter masks created by SPM5 segmentation. Missegmented voxels were manually removed using PMOD v.2.85. Masks were cut off at 0.5 and smoothed with a 5 × 5 × 5 mm3 Gaussian filter kernel implemented into the PVE2 routine. Regression start for interpolation of the ideal white matter value at 1.00 was set to 0.95. Subsequently, the partial volume-corrected images were parametrized according to equation (1). Parametric partial volume-corrected PET datasets of controls displaying 5-HT2A receptor BPP referenced to plasma were warped onto the Montreal Neurological Institute (MNI)/International Consortium for Brain Mapping (ICBM) 152 T1 template as supplied with SPM5, with individual 2 mm3 voxel size magnetic resonance imagings (MRIs) as source images and averaged using SPM5. For purposes of visualization, one of the individual MRI datasets was warped onto the same template. Both PET and MR images of patient A.M. are shown in native space (Figure 2B). The 3D surface rendering was performed with an algorithm developed inhouse.
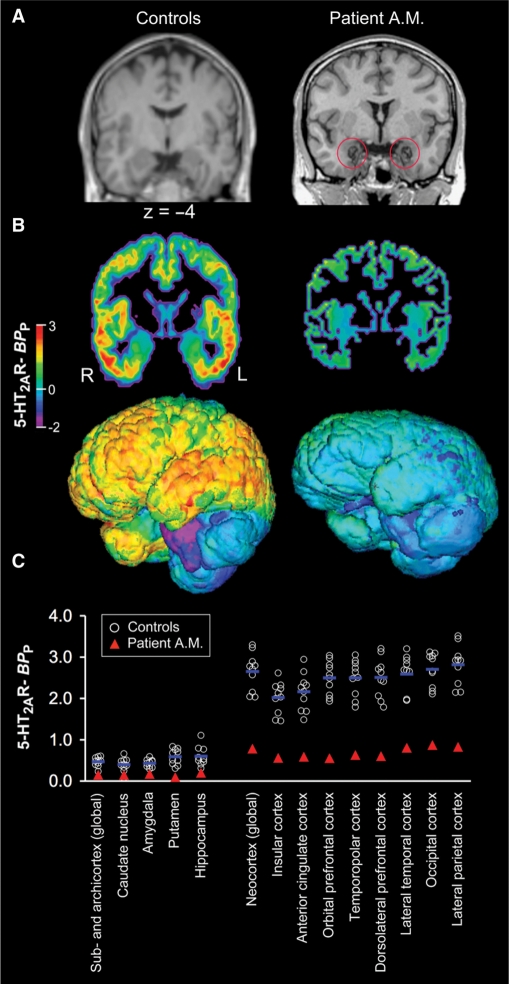
Fig. 2.
MRI and [18F]altanserin PET data obtained from LP patient A.M. and 10 healthy control subjects. (A) T1-weighted high-resolution MRI demonstrates that brain calcification damage in A.M. is amygdala-selective, with particular emphasis on the BLA and no involvement of other nearby tissues. In brief, A.M. has bilateral and relatively circumscribed destruction of the BLA. (B) Parametric maps illustrate a 70% global reduction of 5-HT2AR BPP in A.M. relative to the average of controls. Presented are coronal sections and 3D-surface renderings in true proportions. (C) Scatter plot of global and regional 5-HT2AR BPP values (averaged across hemispheres) as measured in A.M. and controls.
RESULTS
In the present study, we used [18F]altanserin PET referenced to radioligand plasma levels and corrected for partial volume effects to quantify the spatial distribution of 5-HT2AR BPP in a rare patient (A.M.) with Urbach–Wiethe disease and selective bilateral amygdala calcification damage relative to 10 healthy control subjects. We found a large global reduction to a presently unreported extent [overall reduction, 70% ± 4% (Z = 4.1); Table 1; Figure 2B and C] of 5-HT2AR BPP in cortical and subcortical regions of A.M. compared with controls. This demonstrates that brain abnormalities in A.M. are not restricted to the amygdala damage per se, but extend to a significantly decreased 5-HT2AR expression throughout the brain.
Table 1.
Synopsis of global and regional 5-HT2AR BPP values as determined in LP patient A.M. and 10 healthy control subjects
| Controls | Patient A.M. | Z score | |
|---|---|---|---|
| Sub- and archicortex (global) | 0.47 (0.1) | 0.15 | –2.8 |
| Left caudate nucleus | 0.39 (0.2) | 0.18 | –1.2 |
| Right caudate nucleus | 0.41 (0.2) | 0.11 | –1.5 |
| Left amygdala | 0.43 (0.2) | 0.15 | –1.5 |
| Right amygdala | 0.43 (0.1) | 0.19 | –1.6 |
| Left putamen | 0.69 (0.2) | 0.12 | –2.3 |
| Right putamen | 0.49 (0.3) | 0.07 | –1.6 |
| Left hippocampus | 0.57 (0.2) | 0.16 | –2.0 |
| Right hippocampus | 0.64 (0.3) | 0.24 | –1.4 |
| Neocortex (global) | 2.65 (0.5) | 0.78 | –4.1 |
| Left insular cortex | 2.02 (0.4) | 0.55 | –3.7 |
| Right insular cortex | 2.03 (0.4) | 0.57 | –3.8 |
| Left anterior cingulate cortex | 2.16 (0.5) | 0.60 | –3.6 |
| Right anterior cingulate cortex | 2.16 (0.4) | 0.55 | –3.2 |
| Left orbital prefrontal cortex | 2.54 (0.4) | 0.55 | –4.6 |
| Right orbital prefrontal cortex | 2.46 (0.4) | 0.56 | –5.0 |
| Left temporopolar cortex | 2.49 (0.4) | 0.67 | –4.2 |
| Right temporopolar cortex | 2.51 (0.4) | 0.59 | –4.3 |
| Left dorsolateral prefrontal cortex | 2.67 (0.5) | 0.84 | –3.7 |
| Right dorsolateral prefrontal cortex | 2.64 (0.5) | 0.83 | –3.4 |
| Left lateral temporal cortex | 2.57 (0.5) | 0.79 | –3.9 |
| Right lateral temporal cortex | 2.61 (0.5) | 0.83 | –3.8 |
| Left occipital cortex | 2.72 (0.4) | 0.89 | –4.2 |
| Right occipital cortex | 2.69 (0.4) | 0.86 | –4.8 |
| Left lateral parietal cortex | 2.82 (0.5) | 0.86 | –3.9 |
| Right lateral parietal cortex | 2.82 (0.5) | 0.80 | –4.4 |
Data are given as means (±SD). Abbreviations: LP, lipoid proteinosis of Urbach-Wiethe; SD, standard deviation; Z, Z score, i.e. the quotient of the difference and SD.
DISCUSSION
The capacity to experience fear is presumed to be critical to promoting survival across species. As a consequence of LP and selective bilateral calcification damage to the BLA, patient A.M. lacks this capacity, a finding which is in keeping with the neuropsychological profile reported for other LP patients (Adolphs et al., 1994, 1998, 2005; Tranel et al., 2006). Consistent with our a priori hypothesis (Figure 1), amygdala pathology in A.M. is associated with a decrease in 5-HT2AR-dependent signaling. Importantly, this decrease is global and not restricted to the amygdala.
In humans, bilateral amygdala damage is associated with impaired fear recognition from facial expressions, reduced fearfulness in social contexts and a failure to acquire conditioned fear responses (Adolphs et al., 1994, 1998, 2005). In contrast, one of the most notable findings from functional magnetic resonance imaging (fMRI) studies of anxiety disordered patients is an abnormal hyperresponsiveness of the amygdala to fear signals (Etkin and Wager, 2007). This suggests that the behavioral expression of anxiety substantially varies as a function of amygdala reactivity, ranging from absent amygdala responses and nonexpression of anxiety in amygdala-lesioned patients to exaggerated amygdala responses and overexpression of anxiety in patients with anxiety disorders.
However, the amygdala is not an island; to perceive, assess, control and adequately respond to fear signals, various brain regions must interact on electrophysiological and neurochemical levels. Quantitative analyses of amygdala connectivity have demonstrated that the amygdala is one of the most highly interconnected regions of the brain (Young et al., 1994; Pessoa, 2008). Given this empirical background, network dysfunctions, especially abnormal amygdala-prefrontal coupling, have been hypothesized as underlying anxiety disorders. These hypotheses state that due to a lack of top-down control, there is deficient inhibitory tone in the amygdala, leading to exaggerated amygdala responses to fear signals and overexpression of conditioned fear in anxiety disordered patients (Quirk and Gehlert, 2003). In addition, the amygdala receives via reciprocal projections, bottom-up modulatory input from the brainstem including the raphe nuclei, which provide the principle source of 5-HT innervation of the brain (Hornung, 2003; Hensler, 2006; Michelsen et al., 2007). Therefore, we propose that early bilateral amygdala calcification damage in patient A.M. might have resulted in a reduced formation of projections from and to the raphe nuclei, perhaps paving the way for a global underexpression of 5-HT2AR. Notwithstanding the exact etiology of this aberrance, patient A.M. had neither notable neuropsychological impairments nor notable psychopathology on conventional testing when undergoing PET scanning. This suggests that the early onset of the disease during childhood and the slowly progressive amygdala degeneration with disease duration has triggered plastic adaptations compensating potential deficits (Hurlemann et al., 2007). This hypothesis definitely merits further investigation in future studies.
The present study extends the network perspective of described neurobiological models of anxiety behaviors to the neurochemical level. While Weisstaub et al. (2006) applied a genetic knock-out strategy to demonstrate that 5-HT2AR nonexpression eliminates anxiety-like responses in mice, we used a lesion strategy to show that a homologous behavioral phenotype in humans is associated with reduced 5-HT2AR availability. Together, these studies converge on suggesting that the expression of anxiety behaviors requires integrity of a 5-HT2AR- and amygdala-dependent mechanism.
In contrast to patient A.M., anxiety disordered patients suffer from pathological fear that no longer serves adaptive functions. Traditionally, the conceptualization of the neurobiology underlying anxiety disorders has focussed on 5-HT and γ-aminobutyric acid (GABA) neurotransmission (Nutt, 2005). Indeed the hitherto most successful therapeutic approaches are based on pharmacological interventions at the 5-HT transporter (5-HTT) and the GABAA receptor (GABAAR) complex, the molecular target of the benzodiazepine class of anxiolytic drugs (Millan, 2003). Even in healthy people, multimodal imaging has shown that 5-HTT availability predicts amygdala reactivity (Rhodes et al., 2007). Imaging genetics provides consistent evidence for an association between amygdala reactivity and the 5-HTT linked polymorphic region (5-HTTLPR), with relatively heightened amygdala responses to fear signals in S allele carriers (Munafò et al., 2008). Evidence from pharmacological imaging indicates that amygdala responses to fear signals decrease after prolonged administration of the 5-HTT antagonists citalopram (Harmer et al., 2006) and escitalopram (Arce et al., 2008) as well as after single-dose administration of the potent GABAAR antagonist lorazepam (Paulus et al., 2005).
The proposed conceptualization of anxiety disorders as resulting from both neuroreceptor and network dysfunction might lead to the development of more focused treatment strategies that modulate 5-HT signaling via 5-HT2AR. There is accumulating support for this therapeutic rationale in observations of clinical populations: the 5-HT2A/2CR antagonists ritanserin and mianserin are anxiolytic in patients and effectively block the anxiogenic effects of m-chlorophenylpiperazine (Ceulemans et al., 1985; Pigott et al., 1991). The antidepressant nefazodone possesses antagonistic activity at 5-HT2AR (along with 5-HT and norepinephrine reuptake inhibition properties) and is more effective than imipramine in the therapy of anxiety disorders (Bystritsky et al., 1999). Mirtazapine is an antidepressant with anxiolytic activity; among its many effects is the ability to block 5-HT2AR (Ribeiro et al., 2001). Selective 5-HT2AR antagonists and drugs that reduce 5-HT2AR activity may have anxiolytic potential. In line with this reasoning, atypical antipsychotics with prominent 5-HT2AR blockade are being studied for their efficacy in anxiety disordered patients (Pillay and Stein, 2007; but see Griebel et al., 1997).
CONFLICT OF INTEREST
None declared.
Acknowledgments
The authors are grateful to M. X. Cohen and M. Wagner for helpful comments on an earlier version of the manuscript. In addition, the authors wish to thank H. H. Coenen, J. Ermert, S. Grafmuller, K. Hamacher, M. Lang, B. Palm, S. Rehbein, E. Wabbels (Institute of Nuclear Chemistry), D. Elmenhorst, S. Sihver, M. Vogeling (Molecular Neuroimaging Group), H. Herzog, S. Schaden, L. Tellmann, E. Theelen (PET Instrumentation Group), B. Elghahwagi, P. Engels, G. Oefler and A.-M. Oros-Peusquens (MRI Instrumentation Group, Research Center Juelich). This work was supported by the German Federal Ministry for Education and Research (BMBF) (01GI9934); Deutsche Forschungsgemeinschaft (DFG) (Klinische Forschergruppe 112 to A.B.); International Consortium for Brain Mapping (ICBM).
REFERENCES
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berlin) 2008;196:661–72. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A, Rosen R, Suri R, Vapnik T. Pilot open-label study of nefazodone in panic disorder. Depression and Anxiety. 1999;10:137–9. [PubMed] [Google Scholar]
- Ceulemans DL, Hoppenbrouwers ML, Gelders YG, Reyntjens AJ. The influence of ritanserin, a serotonin antagonist, in anxiety disorders: a double-blind placebo-controlled study versus lorazepam. Pharmacopsychiatry. 1985;18:303–5. doi: 10.1055/s-2007-1017385. [DOI] [PubMed] [Google Scholar]
- Chan I, Liu L, Hamada T, Sethuraman G, McGrath JA. The molecular basis of lipoid proteinosis: mutations in extracellular matrix protein 1. Experimental Dermatology. 2007;16:881–90. doi: 10.1111/j.1600-0625.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biological Psychiatry. 2008;63:569–76. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology. 1997;36:793–802. doi: 10.1016/s0028-3908(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Hamada T, McLean WH, Ramsay M, et al. Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1) Human Molecular Genetics. 2002;11:833–40. doi: 10.1093/hmg/11.7.833. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British Journal of Medical Psychology. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biological Psychiatry. 2006;59:816–20. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neuroscience and Biobehavioral Reviews. 2006;30:203–14. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hofer PA. Urbach-Wiethe disease (lipoglycoproteinosis; lipoid proteinosis; hyalinosis cutis et mucosae). A review. Acta Dermato-Venereologica. Supplementum. 1973;53:1–52. [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. Journal of Chemical Neuroanatomy. 2003;26:331–43. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Matusch A, Kuhn KU, et al. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berlin) 2008;195:579–90. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Wagner M, Hawellek B, et al. Amygdala control of emotion-induced forgetting and remembering: evidence from Urbach-Wiethe disease. Neuropsychologia. 2007;45:877–84. doi: 10.1016/j.neuropsychologia.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17:R868. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus–from silver stainings to a role in depression. Brain Research Reviews. 2007;55:329–42. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Millan M. The neurobiology and control of anxious states. Progress in Neurobiology. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectrums. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews. Neuroscience. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Archives of General Psychiatry. 2005;62:282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Pigott TA, Zohar J, Hill JL, et al. Metergoline blocks the behavioral and neuroendocrine effects of orally administered m-chlorophenylpiperazine in patients with obsessive-compulsive disorder. Biological Psychiatry. 1991;29:418–26. doi: 10.1016/0006-3223(91)90264-m. [DOI] [PubMed] [Google Scholar]
- Pillay NS, Stein DJ. Emerging anxiolytics. Expert Opinion on Emerging Drugs. 2007;12:541–54. doi: 10.1517/14728214.12.4.541. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Adams KH, Svarer C, et al. Quantification of 5-HT2A receptors in the human brain using [18F]altanserin-PET and the bolus/infusion approach. Journal of Cerebral Blood Flow and Metabolism. 2003;23:985–96. doi: 10.1097/01.WCB.0000074092.59115.23. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states. Annals of the New York Academy of Sciences. 2003;985:263–72. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. The Journal of Neuroscience. 2007;27:9233–7. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro L, Busnello JV, Kauer-Sant’Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Brazilian Journal of Medical and Biological Research. 2001;34:1303–7. doi: 10.1590/s0100-879x2001001000010. [DOI] [PubMed] [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–37. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13626–31. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11:219–32. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Erhardt A, et al. Polymorphisms in the serotonin receptor gene HTR2A are associated with quantitative traits in panic disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144:424–9. doi: 10.1002/ajmg.b.30412. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–40. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Yang JC, Lee HJ, Kim YK. The association between serotonin-related gene polymorphisms and panic disorder. Journal of Anxiety Disorders. 2008;22:1529–34. doi: 10.1016/j.janxdis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Young MP, Scannell JW, Burns GA, Blakemore C. Analysis of connectivity: neural systems in the cerebral cortex. Reviews in the Neurosciences. 1994;5:227–49. doi: 10.1515/revneuro.1994.5.3.227. [DOI] [PubMed] [Google Scholar]