Abstract
Although there is evidence of emotion perception deficits in autism spectrum disorder (ASD), research on this topic has been mostly confined to perception of emotions in faces. Using behavioral measures and 3T functional magnetic resonance imaging (fMRI), we examined whether such deficits extend to the perception of bodily expressed emotions. We found that individuals with ASD, in contrast to neurotypical (NT) individuals, did not exhibit a differential pattern of brain activation to bodies expressing fear as compared with emotionally neutral bodies. ASD and NT individuals showed similar patterns of activation in response to bodies engaged in emotionally neutral actions, with the exception of decreased activation in the inferior frontal cortex and the anterior insula in ASD. We discuss these findings in relation to possible abnormalities in a network of cortical and subcortical mechanisms involved in social orienting and emotion contagion. Our data suggest that emotion perception deficits in ASD may be due to compromised processing of the emotional component of observed actions.
Keywords: autism, emotion, functional magnetic resonance imaging (fMRI), bodily expression, amygdala, pulvinar, subcortical processing, mirror neurons system
INTRODUCTION
Autism spectrum disorder (ASD) is a behaviorally defined neurodevelopmental disorder that affects as many as 1 in 86 children (Baird et al., 2006). Its defining features include mild to severe impairments in communication and reciprocal social interaction as well as repetitive and stereotyped behaviors children. Difficulty in recognizing and appropriately reacting to other people's emotions, whether they are communicated by facial expressions, vocal tone, gestures or bodily postures, counts among the most frequently noted anomalies in the social-communicative skills of people with ASD.
To date, research on these issues has focused primarily on impairments in the neurofunctional processes associated with viewing facial expressions. Face perception involves a network of subcortical and cortical areas, including the superior colliculus, the pulvinar nucleus of the thalamus, the amygdala, the insula, the inferior occipital gyrus, the lateral fusiform gyrus, the superior temporal sulcus, the somato-motor cortex, the inferior frontal gyrus and the orbitofrontal cortex [for review, see Ishai (2008)]. Functional abnormalities have been found in the face perception network in ASD in response to emotionally neutral (Golarai et al., 2006; Hadjikhani et al., 2007; Kleinhans et al., 2008) as well as emotionally expressive (Dapretto et al., 2006; Hall et al., 2007; Pelphrey et al., 2007) faces, particularly in the amygdala and the mirror neuron system (MNS). Although earlier behavioral studies did not consistently find emotion perception deficits [(Hobson et al., 1988; Braverman et al., 1989; Macdonald et al., 1989; Tantam et al., 1989; Capps et al., 1992; Davies et al., 1994), but see (Ozonoff et al., 1990; Baron-Cohen et al., 1997; Grossman et al., 2000; Gepner et al., 2001; Adolphs et al., 2003)], recent studies taking a more fine-grained approach have documented emotion recognition impairments mainly in the perception of negative emotions, especially fear (Baron-Cohen et al., 2000; Dawson et al., 2004; Welchew et al., 2005; Ashwin et al., 2006, 2007; Corden et al., 2006; Gaigg and Bowler, 2007; Humphreys et al., 2007).
Emotion perception deficits in autistic individuals are not necessarily limited to the perception of faces, but may involve perception of other emotion signals abundantly available in the social environment, such as emotions expressed by the whole body. In a recent study, Hubert et al. (2007) demonstrated that ASD individuals performed significantly worse than controls in recognizing emotions from point-light displays even though they performed as well as control participants in recognizing simple actions and objects manipulations. The authors interpreted their findings as evidence that emotional perception difficulties are not restricted to faces but also affect the perception of body expression of emotion.
Investigations focusing on neutral body postures and movements have revealed some intriguing similarities between visual perception of faces and of bodies. For example, inverted presentation has been shown to have similarly disruptive effects on perception and processing of bodies and faces, suggesting that body perception, like face perception, depends on configural perceptual processes (Reed et al., 2003; Stekelenburg and de Gelder, 2004). In addition, an inversion effect has been shown in the recognition of emotions expressed by whole-body movement (Atkinson et al., 2007). A recent study by Van de Riet and colleagues (2008) that specifically compared processing of affective information from faces and bodies has further underlined the similarities between perception of emotions in faces and bodies and its neurofunctional bases. This study showed that the amygdala and the fusiform gyrus are involved in recognizing emotional signals, whether expressed via the face or the whole body, and that the extrastriate body area of the middle occipital–temporal region is not sensitive to emotion. In addition, specific parts of the superior temporal sulcus (STS), parietal lobe and subcortical structures were found to be selectively responsive to facial and body expression.
In previous studies with neurotypical (NT) individuals (Hadjikhani and de Gelder, 2003; de Gelder et al., 2004), we found that bodily expressions of emotion, in which no information was available from the face, activated a network of brain regions similar to those activated by facial expressions of emotion. These areas included the fusiform face area (FFA), the Inferior Occipital Gyrus (IOG), areas of the MNS, including the inferior frontal cortex (IFC) and the inferior parietal lobule (IPL), as well as subcortical structures, including the superior colliculus, pulvinar and amygdala. These findings suggest an overlap in the neural mechanisms subserving emotional perception whether expressed in the face or in the body. In addition, these stimuli activated areas involved in representation of movement, suggesting fear contagion and automatic preparation of the brain for action in the presence of body expression of fear.
The amygdala plays an important role in the perception of emotion, and there are indications from neuropathology, lesion and neuroimaging studies that it plays a role in the social cognition deficits in autism. Numerous studies have found abnormalities in the amygdala of autistic participants (Bauman and Kemper, 1985; Abell et al., 1999; Aylward et al., 1999; Howard et al., 2000; Pierce et al., 2001; Nacewicz et al., 2006; Schumann and Amaral, 2006) and some have suggested that amygdala dysfunction may play a causal role in autistic social impairment (Baron-Cohen et al., 1999; Adolphs et al., 2001; Schultz, 2005). Interestingly, however, prior studies (Adolphs et al., 2003; Atkinson et al., 2007) have demonstrated that the amygdala is not necessary for the normal recognition of emotions in whole bodies.
In addition, other components of the ‘structural encoding system’ for faces (Bruce and Young, 1986) are also involved in the early stages of body perception (Gliga and Dehaene-Lambertz, 2005; Johnson, 2005; Skuse, 2006; Tsuchiya and Adolphs, 2007). These include the superior colliculus and the pulvinar nucleus of the thalamus. The pulvinar plays an important role in fear recognition, as shown in lesion studies (Ward et al., 2005). It receives inputs from the superior colliculus and the retina, and has reciprocal connections with higher cortical areas, including the extrastriate cortex, the frontal cortex and the amygdala (Grieve et al., 2000). To date, only one study has examined these structures in ASD and reported decreased connectivity between the midline thalamus, the superior colliculus and the FFA during face perception (Kleinhans et al., 2008).
Here, we tested the hypothesis that abnormalities in emotional perception in ASD are not confined to faces, but that they also pertain to the perception of bodily expression. To do so, we examined behavior and brain activation in individuals viewing bodies expressing emotion, specifically fear, as compared with emotionally neutral bodies engaged in everyday actions.
MATERIALS AND METHODS
Participants
The Massachusetts General Hospital Human Studies Committee approved all procedures. After a complete description of the study was provided to the participants, written informed consent was obtained. Twelve adult high-functioning (WASI, 1999) (IQ: 126 ± 10) ASD participants (nine males, mean age 30 ± 11 years) took part in the functional magnetic resonance imaging (fMRI) study. Functional data from three ASD participants were discarded because of technical problems (excessive motion artifacts). All ASD participants were diagnosed with autism (eight participants), Asperger disorder (three participants) or pervasive developmental disorder not otherwise specified (one participant) by an experienced clinician on the basis of their current presentation and developmental history, using the Autism Diagnostic Interview—Revised (ADI-R) (Lord et al., 1994) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) (Table 1). We compared fMRI data from ASD participants with data from seven NTs who had participated in a previous study using the same paradigm and whose data were published in Hadjikhani and de Gelder (2003) and de Gelder et al. (2004) (three males, mean age 35 ± 12 years). In addition, 11 adult NT (eight males, mean age 31 ± 14 years) participated in the behavioral task only.
Table 1.
Participants’ characteristics
| ADI-R |
ADOS |
Diagnosis | |||||
|---|---|---|---|---|---|---|---|
| FSIQ | Communication | Social | Repetitive Behaviors | Communication | Social | ||
| ASD1 | 120 | 7 | 15 | 5 | 4 | 5 | Asperger |
| ASD2 | 133 | 9 | 12 | 4 | 3 | 9 | Autism |
| ASD3 | 110 | 7 | 14 | 3 | 7 | 8 | Asperger |
| ASD4* | 139 | 8 | 16 | 6 | 3 | 5 | Autism |
| ASD5 | 114 | 8 | 14 | 5 | 2 | 4 | PPS-NOS |
| ASD6 | 114 | 9 | 14 | 8 | 2 | 7 | Autism |
| ASD7 | 115 | 19 | 21 | 5 | 3 | 6 | Asperger |
| ASD8 | 139 | 14 | 14 | 5 | 3 | 8 | Autism |
| ASD9* | 136 | 20 | 18 | 7 | 3 | 7 | Autism |
| ASD10* | 127 | 16 | 22 | 8 | 3 | 10 | Autism |
| ASD11 | 128 | 12 | 17 | 5 | 2 | 7 | Autism |
| ASD12 | 117 | 18 | 18 | 2 | 6 | 13 | Autism |
*Excluded for motion.
Behavioral experiment
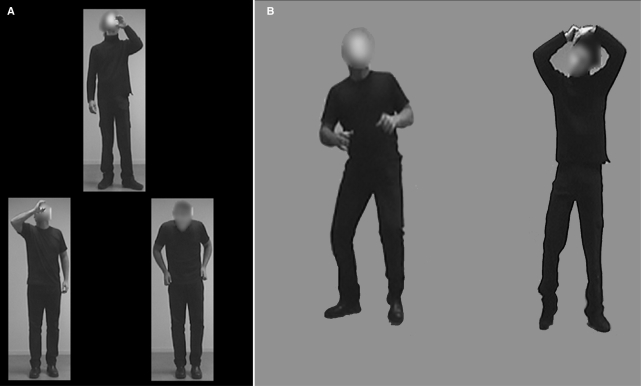
During the fMRI data acquisition, participants passively viewed the screen and no behavioral data were gathered. A separate behavioral experiment was conducted to assess an autism-specific deficit in body emotion recognition. We used a computerized, two-alternative-forced-choice, match-to-sample paradigm to test recognition of emotional bodies as compared with recognition of neutral actions in 11 ASD and 11 NT participants using a two alternative forced choice paradigm. Participants were instructed to decide which one of the two body stimuli in the bottom row matched the target stimulus on the top. The correct match among the two bottom stimuli was equivalent to the top stimulus in either action or emotion expressed, but not in the identity of the actor. The neutral action condition included actions such as opening a door, talking on the phone, pouring a drink, pulling up pants and comb hair (Figure 1A). The emotional bodies condition included bodily expressions of sadness, anger and fear in which the actor's face was obscured. Each condition included 18 stimuli presented in upright orientation and 18 stimuli presented in inverted orientation, which was expected to hinder recognition. Stimuli were presented in random order and remained on the computer screen until the participant responded. Both response accuracy and reaction time were recorded.
Fig. 1.
Stimuli. In the behavioral experiment (A), participants had to match either an action or an emotion with a pair of stimuli presented below. During the functional MRI, blocks of fearful bodies (B, left) were alternating with blocks of neutral action (B, right). Note that the face was blurred in all the stimuli.
fMRI experiment
This experiment used the same stimuli as those used in our previous studies (Hadjikhani and de Gelder, 2003; de Gelder et al., 2004) (Figure 1B). The neutral action stimuli were similar to those presented in the behavior experiment. The emotional body stimuli were limited to the expression of fear. The neutral and fearful bodies were shown in upright orientation in an AB-blocked presentation of eight cycles, each 24 s long. Within each block, an image was presented every 2 s for 300 ms with a 1700 ms blank-screen interval between stimuli, during which a fixation cross was present. Stimulus order was randomized within each block across participants.
Participants were instructed to observe the images attentively and to maintain fixation. No other task was included to avoid interference with processing of the emotion (Lange et al., 2003) and masking of stimulus-related activation in motor and premotor cortex by a motor response (de Gelder et al., 2004).
fMRI data acquisition
Anatomical and functional MR images of brain activity were collected in a 3T high-speed echoplanar-imaging device (Trio, Siemens, Erlangen, Germany) using a phased-array head coil. The scanner and the scanning sequences were identical for ASD and NT. Participants lay on a padded scanner couch in a dimly illuminated room and wore foam earplugs. Foam padding stabilized the head. The scanning acquisition parameters were the same as those used in the Hadjikhani and de Gelder's (2003) study. Two high-resolution 1.3 mm isotropic voxels structural images were obtained with a magnetization prepared rapid acquisition with gradient echoes Magnetization Prepared Rapid Gradient Echo (MP-RAGE) sequence (128 slices, 256 × 256 matrix, echo time TE = 3.44 ms; repetition time TR = 2000 ms; flip = 7°). MR images of brain activity were then collected. Functional sessions began with an initial sagital localizer scan, followed by autoshimming to maximize field homogeneity. Slices were automatically positioned using an online 3D localizer (van der Kouwe et al., 2005). To register functional data to the high resolution T1, a set of high-resolution [40 (ASD) –45 (NT) slices, Anterior commissure – posterior commissure (AC–PC), 1.5 × 1.5 mm in-plane no skip] inversion time T1-weighted echo-planar images (TE = 39 ms; TI =1200 ms; TR = 9840 ms) were acquired. The coregistered functional series (TR = 3000 ms, 40–45 AC–PC slices, 3 mm thick, 3.125 mm by 3.125 mm in plane resolution, 128 images per slice, TE = 30 ms, flip angle 90°, matrix = 64 × 64) lasted 384 s.
Data analysis
fMRI data analysis
Image analysis was conducted using the NeuroLens analysis package (Hoge and Lissot, 2004) (http://www.neurolens.org, version 1.3). All functional Echo planar imaging (EPI) and structural scans were first converted from Digital Imaging and Communications in Medicine (DICOM) to Medical Imaging NetCDF (MINC) format using NeuroLens. Functional image series were motion corrected to the third frame in each series within NeuroLens using a hardware-accelerated module based on source code from AFNI's 3dvolreg module (Cox and Jesmanowicz, 1999). Next, each image series was spatially smoothed in 3D with a 6 mm FWHM 3D Gaussian kernel. Intensity normalization was also applied to set the mean intra cranial signal of each EPI series to a standard value of 10 000. The signal at each voxel in the motion-corrected, smoothed and intensity normalized image series was then fit with a linear model consisting of a regressor representing the periods of emotional bodies presentation, plus four regressors containing the terms of a third order polynomial to represent the baseline EPI signal (in this case corresponding to fearful bodies) plus low frequency signal drift. Volumes containing the estimated effect size and associated standard error for the primary contrast (fearful vs neutral) at each voxel were then registered to a standard space based on the Montreal Neurological Institute (MNI) template (Collins et al., 1994). This spatial normalization was performed in NeuroLens by fitting the third frame of each individual's EPI series to an EPI target brain and applying the resultant transformation to the computed effect size and standard error volumes for that individual. The EPI template was generated by registering whole-brain EPI scans from 40 participants (using the same pulse sequence and parameters as the present study) to the MNI standard space and averaging them. The spatially normalized effect size and standard error volumes were input to a mixed effect group analysis in NeuroLens based on the method described by Worsley et al. (2002). This procedure combines fixed and estimated random effects variance in proportions required to achieve a user-specified number of degrees of freedom (in this case 100). The modeled group effect size and standard error were then divided to produce a volumetric map of T-statistic with 100 degrees of freedom. Based on this T-statistic volume, a map of P-values was computed based on the T value at each voxel. The computed significance values were displayed as the negative base ten logarithm of each voxel's P-value, which produces a low background value while highlighting areas of elevated significance. The map of –log(p) was then thresholded using an amplitude cutoff of 2.0 (corresponding to P = 0.01), and a cluster size threshold of 0.16 ml, which requires that 20 contiguous voxels must all exceed the specified amplitude threshold to be included. This size threshold, plus restriction of the search volume to the intracranial space, reduces the effective P-value for the minimal accepted cluster to <10–5. The thresholded P map was then sampled on the cortical surface of an individual subject using on the inverse coordinate transformation between this individual's native space and the group MNI space. Cortical surface files were generated using FreeSurfer (Dale et al., 1999; Fischl et al., 1999) (http://surfer.nmr.mgh.harvard.edu) and loaded in NeuroLens, which was then used to interpolate the values in the group T-statistic volume (transformed to the individual's space) at the vertex locations of the cortical surface.
Region of interest analysis
In addition to full brain analysis, we performed region of interest (ROI) analysis on areas that we had seen activated in our previous study comparing fearful bodies with neutral bodies in NTs (de Gelder et al., 2004, Table 1). These ROIs been computed on the cortical surfaces, where clusters of contiguous vertices with a significance of P <0.05 and covering an area of at least 185 mm3 had been identified, and Talaraich coordinates and the corresponding structure of the center of each cluster identified by visual inspection of the target individual's anatomy, and corrected for multiple comparison using Monte Carlo simulations.
Those ROIs were located in the superior colliculus, pulvinar, amygdala, accumbens, putamen, fusiform gyrus, the anterior insula and IFC. Hemodynamic time courses were extracted from each ROI. For each group and for each condition, the level of the DC normalized signal at 6 s was extracted and differences between groups were computed.
RESULTS
Behavioral results
A mixed-model Analysis of variance between groups (ANOVA) was conducted on the behavioral accuracy data with the between-subjects factor group (ASD, NT) and the within-subjects factors test condition (emotion, neutral) and orientation (upright, inverted). Main effects were found for test condition, F(1, 20) = 5.8, P < 0.05 and orientation, F(1, 20) = 16.1, P < 0.001, but not for group, F(1, 20) = 0.2, n.s. Accuracy was significantly higher in the neutral action as compared with the emotionally expressive condition, and for upright as compared with inverted orientation. There was a significant interaction effect between group and test condition, F(1, 20) = 12.6, P < 0.005. As can be seen in Table 2, the ASD group scored higher than the NT group in the action condition, but lower than the NT group in the emotion condition. ANOVAs conducted separately for each group demonstrated that whereas ASD participants were significantly more accurate in the neutral action than in the emotionally expressive condition, F(1, 10) = 9.7, P < 0.01, NT participants tended to perform better in the emotionally expressive than the neutral action condition, F(1, 10) = 4.0, P < 0.07.
Table 2.
Recognition accuracy for neutral and emotionally expressive bodies
| ASD (n = 11) M (SD) | NT (n = 11) M (SD) | |
|---|---|---|
| Neutral | ||
| Upright | 17.2 (1.2) | 16.7 (1.2) |
| Inverted | 16.7 (1.5) | 15.6 (1.5) |
| Emotional | ||
| Upright | 15.5 (2.7) | 17.3 (1.0) |
| Inverted | 14.9 (1.6) | 15.7 (1.6) |
fMRI whole brain results
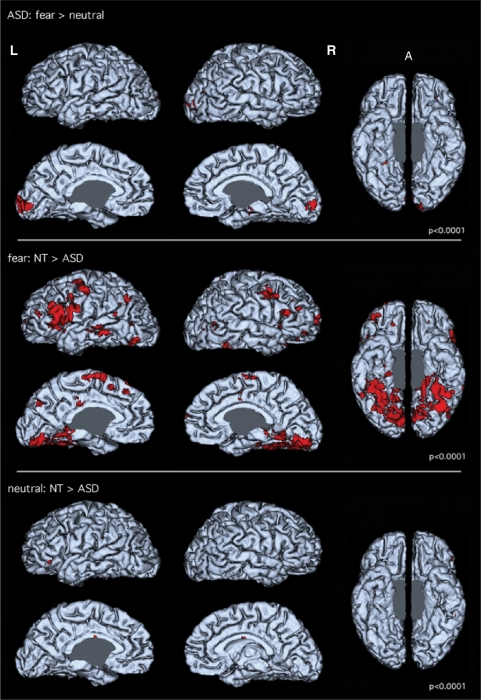
The only areas showing differential activation to fearful as compared with emotionally neutral bodies in ASD participants were the striate and extrastriate visual cortex (Figure 2, top panel).
Fig. 2.
Lateral, medial and ventral view of 3D reconstructions of a brain. Red indicates areas of significant differences (P < 10−4) between conditions (top panel) or between groups (middle and bottom panels). Top panel shows differential activation between Fearful and Neutral condition in the ASD group. Only the striate and extrastriate visual cortex show differential activation. The middle panel shows differential activation between NTs and ASD participants for the fear condition. NT participants show significantly more activation than ASD in the inferior frontal, motor, premotor, temporal and ventro occipito–temporal cortices. The bottom panel shows differential activation between NTs and ASD participants for the neutral condition. Contrary to what is seen for the emotional condition, very little difference is seen, with the presence of more activation for neutral stimuli in NT present in IFC and anterior insula only, ruling out an attentional effect in the results seen in the middle panel.
To explore whether the very limited differential activation between the fearful and neutral conditions in ASD was due to a generally lower level of activation or, consistent with our predictions, to an impaired perception of the difference between emotional and nonemotional body images, we compared groups for each condition separately. The difference of level of activation between NT and ASD participants for the emotional condition is shown in Figure 2 (middle panel), and the difference of activation between NT and ASD participants for the neutral condition is shown in Figure 2 (bottom panel).
In the emotional condition, NT participants showed higher activation than ASD participants in areas related to visual detection and observation (colliculus, pulvinar, amygdala), visual processing (extrastriate cortex, ventral temporal–occipital cortex), areas involved in emotional evaluation (amygdala, nucleus accumbens, anterior insula), preparation for action (putamen, motor and premotor cortex) and in the MNS (IFC) [see also de Gelder et al. (2004)].
In contrast, in the neutral condition, NT and ASD participants showed very similar activation, as can be seen in the bottom panel of Figure 2. This pattern of findings suggests that a lack of modulation by emotion explains the lack of differential activation between the fear and the neutral condition in ASD.
fMRI ROI-based results
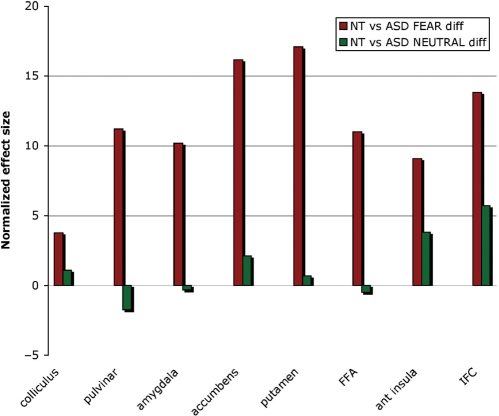
In the emotional condition, NT participants showed higher levels of activation than ASD participants in all ROIs (colliculus, pulvinar, amygdala, nucleus accumbens, putamen, FFA, anterior insula and IFC) Figure 3. In the neutral condition, areas where NT had more activation than ASD were restricted to the IFC and the anterior insula. Between-group comparisons of the differential activation for fearful vs neutral bodies is shown in Table 3.
Fig. 3.
Differences of activation in the ROIs between ASD and NT controls during the perception of fearful bodies (red bars) and neutral bodies (green bars). NT participants had more activation than ASD for the Fearful condition in all ROIs. These differences between NT and ASD were not present in the Neutral condition (green bars), except in the anterior insula and the IFC where ASD exhibited less activation than NT.
Table 3.
Peak location and T-score of differential activation in the fear vs neutral comparison between NTs and ASDs
| Tscore | Hemisphere | MNI coordinates |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Colliculus | 2.57 | rh | 10 | −31 | −2 |
| 3.19 | lh | −4 | −29 | 4 | |
| Pulvinar | 2.49 | rh | 8 | −31 | 0 |
| 3.54 | lh | −4 | −23 | 12 | |
| Amygdala | 2.09 | rh | 20 | −9 | −28 |
| 2.56 | lh | −30 | −11 | −30 | |
| Accumbens | 3.15 | rh | 20 | 1 | −10 |
| 3.43 | lh | −26 | −1 | −10 | |
| Putamen | 3.33 | rh | 32 | −7 | −2 |
| 2.42 | lh | −32 | −5 | 0 | |
| Premotor | 2.72 | rh | 40 | 15 | 38 |
| 2.91 | lh | −42 | 7 | 36 | |
| Fusiform gyrus | 7.21 | rh | 34 | −53 | −28 |
| 6.08 | lh | −34 | −55 | −26 | |
| Anterior insula | 3.19 | rh | 38 | 21 | −2 |
| 3.69 | lh | −52 | 19 | −2 | |
| IFC | 3.17 | rh | 46 | 17 | 20 |
| 4.33 | lh | −60 | 19 | 16 | |
rh = right hemisphere; lh = left hemisphere
DISCUSSION
The main finding of this study is that brain activation patterns in individuals with ASD do not show evidence of differentiation between bodily expressions of fear and bodies engaged in neutral actions. This finding suggests an abnormality in the network of brain areas that are normally engaged in the perception of bodily expressed emotions in NT individuals, and is consistent with recent behavioral findings of Hubert et al. (2007) who reported normal perception of point-light displays of neutral actions in ASD, but abnormal perception of emotions. Our previous studies (Hadjikhani and de Gelder, 2003; de Gelder et al., 2004) have shown that seeing fearful body expressions modulate activation in areas associated with emotional processing, as well as in areas linked with representation of action and movement. The coactivation of emotion- and action-related brain areas in NT individuals suggests a neural mechanism whereby observing fearful behavior in others triggers fear contagion and prepares the body for action. This kind of emotional contagion was absent in ASD participants in the present study.
A possible explanation for the lack of differential activation in response to emotional body expression could be that our ASD participants had very little activation, in general, for social stimuli, regardless of their emotional valence. A generally low level of activation could arguably result from a lack of attention in ASD participants. However, the ASD group's comparable performance with NT participants in the action condition of the behavioral task suggests that they were compliant with task instructions and attended to the stimuli in the scanner as instructed. Furthermore, the observations of similar activation in the neutral condition, except in the anterior insula and the IFC, for ASD and NT participants rule out that lower activation levels resulted from a generalized attentional effect. Our findings show that emotion perception impairments are independent of face-perception difficulties, and that they extend to the perception of bodily expression of emotion. Stimuli presented in this study had blurred faces, and no information was available from facial expression. In addition, our data show that the lack of emotional modulation by fearful bodily expression is accompanied by a lack of involvement of action-representation areas normally observed in response to bodily expression of fear, indicating an absence of emotion contagion in ASD.
Besides the cortical areas and the amygdala playing a role in emotional action observation, we also found important group differences in subcortical structures. The superior colliculus, the pulvinar and the amygdala comprise a subcortical route involved in the early detection of biologically relevant stimuli, so far mostly highlighted for its role in processing faces (Johnson, 2005; de Gelder, 2006). While these areas were activated in NT for the emotion condition, we did not observe any activation in these areas in ASD. A weaker than normal activation of structures involved in the perception of salient stimuli during emotion perception in ASD may compromise the normal recruitment of areas involved in emotional perception and preparation for action (Tsuchiya and Adolphs, 2007). These results are in agreement with a recent report of decreased connectivity between the midline thalamus, the superior colliculus and the right fusiform gyrus in ASD (Kleinhans et al., 2008). Decreased activation of the IFC in ASD is also consistent with our previous findings (Hadjikhani et al., 2006, 2007) and those of Dapretto et al.'s (2006) indicative of MNS dysfunction in ASD.
CONCLUSION
To summarize, the aim of the present study was to explore the brain mechanisms underlying the perception of expressions of emotion in the whole body in ASD. We show that emotion as expressed by the whole body and excluding facial expressions fails to activate ventral visual areas and MNS in ASD, contrary to what is found in NTs.
Our study is the first functional imaging study of emotional communication with body language in ASD. While it shows the potential of this approach, it presents several limitations. First, we only examined a small sample of participants. However, using mixed-effect analysis, we were able to show clear differences in activation between ASD and NT participants. Second, we did not collect behavioral data in the functional scans. We chose not to do so because we wanted to observe activation in premotor and motor areas, which would have been masked if a button-press component was present, and because we were collecting independent behavioral data outside the magnet.
Despite these limitations, our data do show a clear difference between NT and ASD participants during the perception of fear expressed by the body. Our results raise the possibility that an abnormal pulvinar function, by compromising social orienting, and an abnormality of the amygdala, by compromising basic stimulus-reward associations, could lead to underdevelopment of a broader brain network involved in emotional evaluation, including the MNS. An inappropriate emotional response may then fail to trigger areas of the MNS and as a consequence would lead to a lack of mirror activity that would then fail to evoke somatic markers important for the generation of the feeling of emotion (Damasio, 1999). Such a possibility could help to explain the social-emotional impairments that characterize ASD.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENT
We wish to thank Mary Foley for contributing to data collection. National Institute of Health (RO1 NS44824-01 to N.H.; K01 MH073944-01 to R.M.J.; U19 DC 03610 to H.T.F.); Swiss National Foundation (PPOOB—110741 to N.H.); Canadian Institute of Health Research (200703MOP-172783-MPI-CFCL-155844 to R.H.).
REFERENCES
- Abell F, Krams M, Ashburner J, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. Journal of Cognitive Neuroscience. 2001;13:232–40. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. Dissociable neural systems for recognizing emotions. Brain and Cognition. 2003;52:61–9. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: a test of the amygdala theory. Social Neuroscience. 2006;1:349–63. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Atkinson AP, Heberlein AS, Adolphs R. Spared ability to recognise fear from static and moving whole-body cues following bilateral amygdala damage. Neuropsychologia. 2007;45:2772–82. doi: 10.1016/j.neuropsychologia.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–50. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Baird G, Simonoff E, Pickles A, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the special needs and autism project (SNAP) Lancet. 2006;368:210–5. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a ‘language of the eyes’? Evidence from normal adults and adults with autism or Asperger syndrome. Visual Cognition. 1997;4:311–31. [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–74. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Braverman M, Fein D, Lucci D, Waterhouse L. Affect comprehension in children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1989;19:301–16. doi: 10.1007/BF02211848. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young AW. Understanding face recognition. British Journal of Psychology. 1986;77:305–27. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Capps L, Yirmiya N, Sigman M. Understanding of simple and complex emotions in non-retarded children with autism. Journal of Child Psychology and Psychiatry. 1992;33:1169–82. doi: 10.1111/j.1469-7610.1992.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- Corden B, Critchley HD, Skuse D, Dolan RJ. Fear recognition ability predicts differences in social cognitive and neural functioning in men. Journal of Cognitive Neuroscience. 2006;18:889–97. doi: 10.1162/jocn.2006.18.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–8. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens. New York: Harcourt Brace; 1999. [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Bishop D, Manstead AS, Tantam D. Face perception in children with autism and Asperger's syndrome. Journal of Child Psychology and Psychiatry. 1994;35:1033–57. doi: 10.1111/j.1469-7610.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7:340–59. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- de Gelder B. Towards the neurobiology of emotional body language. Nature Review Neuroscience. 2006;7:242–9. doi: 10.1038/nrn1872. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight. A mechanism for fear contagion when perceiving emotion expressed by a whole body. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16701–6. doi: 10.1073/pnas.0407042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Bowler DM. Differential fear conditioning in Asperger's syndrome: implications for an amygdala theory of autism. Neuropsychologia. 2007;45:2125–34. doi: 10.1016/j.neuropsychologia.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Gepner B, Deruelle C, Grynfeltt S. Motion and emotion: a novel approach to the study of face processing by young autistic children. Journal of Autism and Developmental Disorders. 2001;31:37–45. doi: 10.1023/a:1005609629218. [DOI] [PubMed] [Google Scholar]
- Gliga T, Dehaene-Lambertz G. Structural encoding of body and face in human infants and adults. Journal of Cognitive Neuroscience. 2005;17:1328–40. doi: 10.1162/0898929055002481. [DOI] [PubMed] [Google Scholar]
- Golarai G, Grill-Spector K, Reiss AL. Autism and the development of face processing. Clinical Neuroscience Research. 2006;6:145–60. doi: 10.1016/j.cnr.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends in Neurosciences. 2000;23:35–9. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Grossman JB, Klin A, Carter AS, Volkmar FR. Verbal bias in recognition of facial emotions in children with Asperger syndrome. Journal of Child Psychology and Psychiatry. 2000;41:369–79. [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Seeing fearful body expressions activates the fusiform cortex and amygdala. Current Biology. 2003;13:2201–5. doi: 10.1016/j.cub.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16:1276–82. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Human Brain Mapping. 2007;28:441–9. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GB, West CD, Szatmari P. Backward masking: evidence of reduced subcortical amygdala engagement in autism. Brain and Cognition. 2007;65:100–6. doi: 10.1016/j.bandc.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What's in a face? The case of autism. British Journal of Psychology. 1988;79:441–53. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hoge R, Lissot A. NeuroLens: an integrated visualization and analysis platform for functional and structural neuroimaging. Proceedings of the International Society for Magnetic Resonance in Medicine, 11. 2004:1096. [Google Scholar]
- Howard MA, Cowell PE, Boucher J, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–5. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Hubert B, Wicker B, Moore DG, et al. Brief report: recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:1386–92. doi: 10.1007/s10803-006-0275-y. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–95. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Ishai A. Let's face it: it's a cortical network. NeuroImage. 2008;40:415–9. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Johnson M. Subcortical face processing. Nature Neuroscience Reviews. 2005;6:2–9. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain, 131. 2008:1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, et al. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53:226–32. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macdonald H, Rutter M, Howlin P, et al. Recognition and expression of emotional cues by autistic and normal adults. Journal of Child Psychology and Psychiatry. 1989;30:865–77. doi: 10.1111/j.1469-7610.1989.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Archives of General Psychiatry. 2006;63:1417–28. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Are there emotion perception deficits in young autistic children? Journal of Child Psychology and Psychiatry. 1990;31:343–61. doi: 10.1111/j.1469-7610.1990.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Social Cognitive and Affective Neuroscience. 2007;2:140–9. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–73. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Reed CL, Stone VE, Bozova S, Tanaka J. The body-inversion effect. Psychological Science. 2003;14:302–8. doi: 10.1111/1467-9280.14431. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. Journal of Neuroscience. 2006;26:7674–9. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D. Genetic influences on the neural basis of social cognition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361:2129–41. doi: 10.1098/rstb.2006.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekelenburg JJ, de Gelder B. The neural correlates of perceiving human bodies: an ERP study on the body-inversion effect. Neuroreport. 2004;15:777–80. doi: 10.1097/00001756-200404090-00007. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children's ability to interpret faces: a research note. Journal of Child Psychology and Psychiatry. 1989;30:623–30. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Adolphs R. Emotion and consciousness. Trends in Cognitive Science, 11. 2007:158–67. doi: 10.1016/j.tics.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Van de Riet W, Grezes J, de Gelder B. Specific and common brain regions involved in the perception of faces and bodies and the representation of their emotional expressions. Social Neuroscience. 2008 doi: 10.1080/17470910701865367. in press. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Fischl B, et al. On-line automatic slice positioning for brain MR imaging. NeuroImage. 2005;27:222–30. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Bamford S. Response to visual threat following damage to the pulvinar. Current Biology. 2005;15:571–3. doi: 10.1016/j.cub.2005.01.056. [DOI] [PubMed] [Google Scholar]
- WASI. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, et al. Functional disconnectivity of the medial temporal lobe in Asperger's syndrome. Biological Psychiatry. 2005;57:991–8. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]