Abstract
It is known that the orbitofrontal cortex (OFC) is crucially involved in emotion regulation. However, the specific role of the OFC in controlling the behavior evoked by these emotions, such as approach–avoidance (AA) responses, remains largely unexplored. We measured behavioral and neural responses (using fMRI) during the performance of a social task, a reaction time (RT) task where subjects approached or avoided visually presented emotional faces by pulling or pushing a joystick, respectively. RTs were longer for affect-incongruent responses (approach angry faces and avoid happy faces) as compared to affect-congruent responses (approach–happy; avoid–angry). Moreover, affect-incongruent responses recruited increased activity in the left lateral OFC. These behavioral and neural effects emerged only when the subjects responded explicitly to the emotional value of the faces (AA-task) and largely disappeared when subjects responded to an affectively irrelevant feature of the faces during a control (gender evaluation: GE) task. Most crucially, the size of the OFC-effect correlated positively with the size of the behavioral costs of approaching angry faces. These findings qualify the role of the lateral OFC in the voluntary control of social–motivational behavior, emphasizing the relevance of this region for selecting rule-driven stimulus–response associations, while overriding automatic (affect-congruent) stimulus–response mappings.
Keywords: orbitofrontal cortex, approach–avoidance, motivational behavior, angry facial expression, social–emotional behavior
INTRODUCTION
Human social skills require the ability to adapt and regulate instinctive reactions to emotional signals, in particular the communicative signals of threat or appeasement conveyed by emotional facial expressions (Öhman, 1986; Blair, 2003). This ability is not trivial, as shown by the inability of non-human primates to control their approach and avoidance tendencies when engaged in collaborative activities (Melis et al., 2006), and it can be dramatically relevant, as shown by psychiatric conditions like social phobia and antisocial behaviors (e.g. Horley et al., 2004; Lewis and Lamm, 2006).
Numerous studies have addressed the neural bases of perception of social emotional signals, in particular facial expression (Adolphs, 2003), detailing the crucial role of the amygdala and other limbic structures in the automatic processing of (negative) facial expressions (Adolphs, 2002; McClure et al., 2004; Strauss et al., 2005). Here we address the cerebral and cognitive mechanisms controlling the behavior evoked by these perceptual processes. Several studies have shown that the OFC plays a crucial role in the voluntary regulation of emotions (Damasio, 1994; Rolls, 1999; Davidson et al., 2000; Ochsner and Gross, 2005) and the control of social emotional behavior (Rolls et al., 1994; Blair and Cipolotti, 2000; Veit et al., 2002; Hornak et al., 2003; Kringelbach and Rolls, 2003). In particular, the lateral OFC and the adjacent ventrolateral prefrontal cortex are involved in the selection of actions that override automatic and motivationally (reward) driven response tendencies (Elliott et al., 2000; Passingham et al., 2000; Rushworth et al., 2007). Whether this role extends to the domain of social approach–avoidance (AA) behavior remains to be studied. In the present investigation, we test the hypothesis that the contribution of the OFC—the lateral OFC in particular—to social emotional behavior predominantly consists in selecting voluntary or rule-driven behavioral responses that are different from the automatic reactions evoked by emotional stimuli.
We have tested this hypothesis in the context of an ecologically relevant emotional behavior, i.e. approach or avoidance responses to facial emotions, a common and potent social stimulus (Lang, 1990). Several studies have operationalized social AA behavior by asking human subjects to move their forearm either towards their body (approach) or away from their body (avoidance) in response to emotional face stimuli (e.g. Rotteveel and Phaf, 2004; Roelofs et al., 2005, in press; Heuer et al., 2007). Crucially, when subjects approach angry faces and avoid happy faces (affect-incongruent condition), their reaction times (RTs) are slower than when they approach happy and avoid angry faces (affect-congruent condition). This RT effect is an indication that during incongruent trials, subjects solve the task by overriding their instinctive response tendencies. This AA congruency effect is specifically linked to the generation of an explicit emotional judgment, being absent when the same stimuli, movements and stimulus–response mappings are used in a task requiring the evaluation of an affectively irrelevant feature of the same stimuli (the gender of the face, Rotteveel and Phaf, 2004). To test the involvement of the lateral OFC in the voluntary control of social AA behavior, we have adapted the AA task for fMRI. In order to isolate the specific cerebral responses that are modulated by the need to voluntary control affect-incongruent AA responses over and above the effects associated with more automated control of social AA behavior, we contrasted the AA congruency effects with effects induced by a control (gender evaluation: GE) task, in which exactly the same stimuli, joystick responses and stimulus–response mappings were applied but in which emotion evaluation was not explicitly instructed.
MATERIALS AND METHODS
Participants
Twenty-two healthy, right-handed young males [age: 21 ± 3 years (mean ± s.d.) range: 18–32 years] participated in the study after giving written informed consent according to the institutional guidelines of local ethics committee (CMO—Commissie Mensgebonden Onderzoek region Arnhem-Nijmegen, the Netherlands). All participants had normal or corrected-to-normal vision. Data from two subjects were excluded from the group analysis due to imaging artifacts related to head movements, leaving 20 subjects for the final analyses.
Experimental setup
Subjects lay supine on the MR scanner bed with their head fitted in a standard circular polarized transmitter–receiver head coil. Visual stimuli were projected onto a mirror above the subjects’ head. Stimulus presentation was controlled by a PC running Presentation software version 9.7 (http://www.nbs.neuro-bs.com). Motor responses were recorded through a MR-compatible joystick (sampling rate 250 Hz). The joystick was placed on the abdomen of the participants in such a way that the joystick could be moved in a comfortable way into both target directions (pulled towards or pushed away from themselves). We ensured that subjects performed the task by predominantly moving their right hand/wrist and by avoiding movement of the forearm as much as possible. Subjects wore MR-compatible headphones (Resonance Technology, Northridge, CA, USA) to reduce the scanner noise.
Tasks and procedure
The AA task and the control (gender evaluation: GE) task were administered in separate MR-sessions, in a counterbalanced order across subjects, with a 15 min break (outside of the scanner) between the two sessions. Each task involved an affect-congruent and an affect-incongruent response condition. During both tasks, the subjects were presented with pictures of faces displayed in the centre of the screen against a black background. The stimulus set consisted of 72 pictures taken from Ekman and Friesen (1976), Matsumoto and Ekman (1988), Martinez and Benavente (1998) and Lundqvist et al. (1998). Both the happy and angry expressions were taken from the same model (36 models in total).
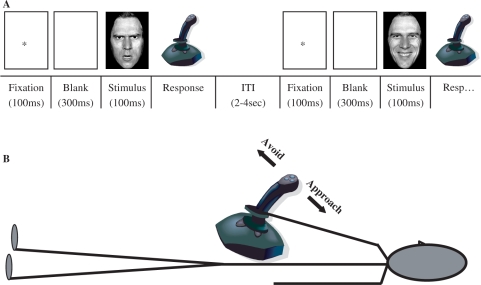
In the AA task, subjects were explicitly instructed to categorize the facial expressions. In the affect-congruent condition of the AA task, participants were instructed to pull the joystick towards their body in response to a happy face and to push the joystick away in response to an angry face. In the incongruent condition, the instructions were reversed (i.e. angry–pull; happy–push). In the GE task, participants responded to an emotionally irrelevant feature (gender) of the same visual stimuli as presented in the AA task, by means of the same joystick movements (i.e. male–push; female–pull or visa versa), resulting in the same stimulus-response contingencies. In both tasks, subjects were instructed to respond as accurately and fast as possible. For both tasks, trials were presented in instruction blocks of 12 trials, followed by a baseline period (21–24 s), for a total of 24 alternating instruction blocks per task. The order of instruction blocks in each trial was fully counterbalanced. The experiment was preceded by 24 practice trials that contained pictures that were not included in the experimental series. Each trial started with the presentation of a fixation point at the center of the screen (100 ms), followed by a blank screen (300 ms), the experimental stimulus (100 ms), and the subject's response. The inter trial interval (ITI) varied between 2000 and 4000 ms (see Figure 1 for the trial sequence and the experimental setup).
Fig. 1.
Trial sequence (A) and experimental setup (B) for the AA task and the GE task. In both tasks, subjects responded to the emotional pictures by moving the joystick either towards (approach) or away (avoid) from their body.
Physiological stress measures
Because of the known influence of the stress hormone cortisol on AA behavior (van Honk et al., 1998, 2000; Roelofs et al., 2005, in press; van Peer et al., 2007) we measured (salivary) cortisol, allowing to control for individual differences. Saliva samples were obtained using Salivette collection devices (Sarstedt, Rommelsdorf, Germany), stored at −20°C before assaying. Biochemical analysis of free cortisol in saliva was performed using a competitive electrochemiluminescence immunoassay (ECLIA, Elecsys 2010, Roche Diagnostics), as described elsewhere (Van Aken et al., 2003). Because time of the day and the subjects’ physical state can affect cortisol levels, subjects were always tested between 13.30 and 17.30 PM and were instructed to minimize physical exercise during the hour preceding the experiment and not to take large meals, coffee, drinks with low pH or cigarettes. Cortisol was measured at three time points during the experiment (before the first task, between the first and second task, and at the end of second task but before the structural MRI scan). We controlled for individual differences in cortisol levels in the behavioral and imaging analyses by adding the mean Cortisol levels as a covariate to the analyses (because the intra-individual differences over the three time points were small [T1 (6.7 nmol/l, s.d. = 3.9); T2 (6.4 nmol/l, s.d. = 3.2); T3 (6.0 nmol/l, s.d. = 3.2)], cortisol levels were averaged over time.
Image acquisition
Images were acquired on a 1.5 Tesla Sonata MRI system (Siemens, Erlangen, Germany), using a standard circular polarized head coil for radio-frequency transmission and signal reception. BOLD-sensitive functional images were acquired using a single shot gradient EPI sequence (TR/TE 2580 ms/35 ms, 35 transversal slices, interleaved acquisition, distance factor 10%, effective voxel size 3.5 × 3.5 × 3.5 mm, field of view 224 mm). Following the experimental session, high-resolution anatomical images were acquired with an MP_RAGE sequence (TE/TR 3.68/2250 ms, 176 sagittal slices, voxel size 1.0 × 1.0 × 1.0 mm, FoV 256 mm).
Image analysis
Functional data were pre-processed and analyzed using SPM2 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm). The first five volumes of each subject's data set were discarded to allow for longitudinal relaxation time equilibration. Prior to analysis, the image time series were spatially realigned using a sinc interpolation algorithm that estimates rigid body transformations (translations, rotations) by minimizing head movements between each image and the reference image (Friston et al., 1995). The time series for each voxel were temporally realigned to the middle slice in time to correct for differences in slice time acquisition. Subsequently, images were normalized onto a standard MNI-aligned EPI template using linear transformation. Finally, the normalized images were spatially smoothed using an isotropic 10 mm full-width-at-half-maximum Gaussian kernel. Each participant's structural image was spatially coregistered to the mean of the functional images (Ashburner and Friston, 1997) and spatially normalized by using the same transformation matrix applied to the functional images.
The fMRI time series were analyzed using an event-related approach in the context of the general linear model. Analysis of the imaging data considered the following effects, for the AA and GE task separately: Angry congruent, Angry incongruent, Happy congruent, Happy incongruent. Vectors describing the onsets of these trials (regressors) were convolved with the canonical haemodynamic response function and its temporal derivative.
Head movement effects were accounted for by including the six rigid body motion parameters (estimated by the spatial realignment procedure) as nuisance covariates. Three further regressors, describing the time course of signal intensities averaged over different compartments (i.e. white matter, cerebrospinal fluid and the portion of the MR image outside the skull) were added. This was done to account for image intensity shifts due to movement of the hand within or near the main magnetic field of the scanner (Culham et al., 2003; Verhagen et al., 2006). Parameter estimates for all regressors were obtained by maximum-likelihood estimation, while using a temporal high-pass filter (cut-off 60 s), and modeling temporal autocorrelation as an AR(1) process. Consistent effects across subjects were tested by using a random effects multiple regression analysis that considered, for each subject, eight contrast images [i.e. the eight conditions of the experimental design—Task (AA, GE) × Condition (Congruent, Incongruent) × Valence (Happy, Angry)]. In addition, the mean cortisol levels were included in the fMRI model as a condition-specific covariate.
The analysis was focused on testing for the relative involvement of the frontal lobe in the voluntary control of approach and avoidance behavior. This effect was operationalized as task-related differences in providing emotionally incongruent behavioral responses, i.e. a Task (AA, GE) × Condition (incongruent, congruent) interaction. When testing this interaction, we used a masking procedure to confine our search to regions that showed stronger responses during Incongruent than Congruent trials within the AA task. Therefore, this analysis isolated cerebral regions more strongly involved in voluntary emotional judgements than in the automatic generation of the same judgements (cf. Task × Condition interaction), and in which these differential effects were specifically driven by the need to voluntarily control such judgements (cf. simple main effect of Incongruent vs Congruent AA trials).
Statistical inference
The statistical significance of the estimated evoked hemodynamic responses was assessed using t-statistics in the context of a multiple regression analysis. Contrasts of the parameter estimates for each condition were calculated. Linear contrasts were used to determine the effects associated with each condition, generating t-values for each voxel in the image. Consistent effects across subjects were tested by using a random-effect group analysis with inferences drawn at the cluster level, corrected for multiple comparisons using family-wise error correction [P < 0.05 (Friston et al., 1996)]. Gaussian random field theory allowed us to make inferences corrected for the number of non-independent comparisons (Friston et al., 1995). The effective degrees of freedom of the error term took into account the temporal autocorrelation of the data (Friston et al., 1995).
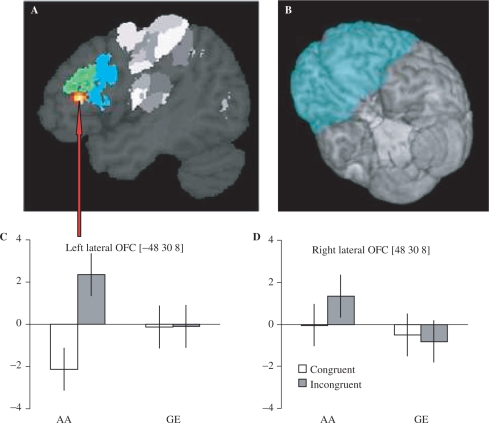
In addition to a whole-brain analysis, we focused our analyses on a search volume encompassing the OFC (bilaterally), the dorsolateral frontal cortex (bilaterally) and a 10 mm sphere centered around the coordinates (42, 0, 42—in the stereotactic space of the Montreal Neurological Institute) of a pre-central region recently shown to be involved in inhibitory control of pre-potent responses (Mars et al., 2007). The search volume was determined by selecting all voxels included in the following anatomical regions, as defined and implemented in the WFU_Pickatlas tool (http://www.fmri.wfubmc.edu/download.htm): medial, middle, and superior orbital gyrus; gyrus rectus; inferior, middle, and superior frontal gyrus (Figure 3B). Within this search volume, statistical inference (P < 0.05) was performed at the cluster-level, correcting for multiple comparisons over the search volume.
Fig. 3.
Imaging results. (B) Volume of interest (in cyan) overlayed on a 3D rendering of a structural MR-scan. (A) Cluster showing larger activity for incongruent versus congruent trails on AA task and not GE task (in red/yellow). The cluster is located in the left lateral orbitofrontal cortex (BA47/12, partially extending into BA45). To indicate the spatial relationship between the activated cluster and cytoarchitectonic maps of BA44 and BA45, the maximum probability maps of these two areas (Eickhoff et al., 2005) have been indicated in blue and green, respectively. (C and D) The effect sizes for the effects in the left (−48, 30, 8) and in the right (48, 30, 8) hemisphere, respectively. It can be seen that the Task × Congruency interaction was present in the left orbitofrontal cluster only.
Behavioral and brain-behavior analyses
Mean reaction times (RTs in milliseconds) for correct responses and error rates (percentage of trials) were calculated for each level of the three experimental factors (Task × Condition × Valence). A three-way (2 × 2 × 2) repeated measures ANOVA was carried out to examine the effects of Task (AA, GE), Condition (affect-congruent, affect-incongruent) and Valence (happy, angry) on error rates and RTs. Again, salivary cortisol was included as a covariate in the model. We also assessed the relation between condition-specific behavioral (RT) and cerebral (BOLD signal from the left OFC) by means of Pearson's correlation. The α-level was set at P < 0.05.
RESULTS
Behavioral data
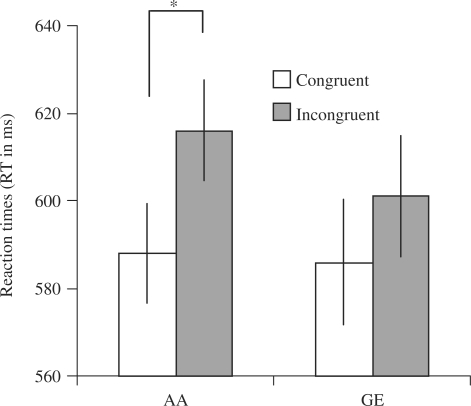
Mean RTs and error rates of the participants are indicated in Table 1. A three-way (Task × Condition × Valence) ANOVA for the RT data with cortisol as a covariate, resulted in a significant Task × Condition interaction [F(1,18) = 5.92, P = 0.026]. This finding indicates that, although there was no significant Condition effect for the GE task [F(1,18) = 0.36, P = 0.56)], there was a significant Condition effect for the AA task, with longer RTs for incongruent as compared to congruent trials [F(1, 18) = 8.43, P = 0.009]—see Figure 2. This AA Condition effect was significant for the angry face responses [F(1, 18) = 6.84, P = 0.018] and reflected a non-significant trend for happy face responses [F(1, 18) = 3.06, P = 0.097]. There were no other significant main or interaction effects in the three-way ANOVA, except for cortisol interacting significantly with the Task × Condition effect [F(1, 18) = 5.25, P = 0.034], indicating that increased AA (and not GE) congruency effects (i.e. faster RTs for congruent as compared to incongruent trials) were associated with decreased cortisol levels (r = −0.49, P = 0.026) during the course of the experiment. This relation with cortisol was largely explained by the angry face responses (r = −0.45, P = 0.044) and was not significant for happy faces (r = −0.31, ns).
Table 1.
Reaction times and error rates (mean ± SEM) for congruent and incongruent responses to happy and angry faces in the AA and GE tasks
| AA |
GE |
|||
|---|---|---|---|---|
| Happy | Angry | Happy | Angry | |
| Reaction times (in ms) | ||||
| Congruent | 560 (24) | 616 (24) | 578 (29) | 593 (29) |
| Incongruent | 592 (30) | 649 (27) | 597 (28) | 606 (28) |
| Error rates (in percentage of trials) | ||||
| Congruent | 1.53 (0.24) | 1.58 (0.14) | 1.30 (0.17) | 2.03 (0.31) |
| Incongruent | 1.51 (0.11) | 1.74 (0.22) | 2.66 (0.25) | 1.96 (0.35) |
Fig. 2.
Behavioral results. Reaction times for the AA task and the GE task (mean ± standard error of the mean). Subjects were significantly slower to provide affect-incongruent responses (approach angry faces, avoid happy faces) than affect-congruent responses in the AA task, but not in the GE task.
There were no significant main or interaction effects concerning the error rates, apart from a significant effect of Condition, indicating more errors for incongruent as compared to congruent trials [F(1,18) = 6.98, P = 0.017].
Imaging data
During the performance of the AA task, but not of the GE task, there was a significant cluster with stronger responses during incongruent than congruent trials (P = 0.048 corrected for multiple comparison; cluster size: 61 voxels; stereotactical coordinates of local maxima: −48, 30, 8)—see Figure 3A. This cluster was localized in BA47/12 (Eickhoff et al., 2005), partially extending into BA45 (Figure 3). BA47/12 (Petrides and Pandya, 2002) constitutes the posterior lateral portion of the human OFC (Kringelbach and Rolls, 2004), as well as the ventral portion of the human ventrolateral prefrontal cortex (Petrides, 2005). Post hoc analysis revealed that the increased response of this cluster during AA incongruent trials was evoked by both happy and angry faces [conjunction analysis (Nichols et al., 2005), P = 0.003]. There were no other significant differential effects when the search for this effect was extended to the whole brain, or focused on the anterior cingulate cortex (as defined through the WFU_Pickatlas tool).
Correlational analyses for AA-task
Activity in the left OFC was significantly correlated to the behavioral effects (RTs) during incongruent responses to angry faces (r = 0.46, P = 0.043), indicating that additional orbitofrontal resources were recruited as the RT costs for providing incongruent responses to angry faces increased (Figure 4). There was no such relation for congruent responses to angry faces (r = −0.24, ns), nor for incongruent and congruent responses to happy faces [(r = −0.41, ns) and (r = −0.04, ns), respectively].
Fig. 4.
Correlations between behavioral (RT) and cerebral (estimates of BOLD signal from the OFC at −48, 30, 8) effects during affect-incongruent (approach) responses to angry faces on the AA task. The positive correlation indicates that additional orbitofrontal resources were recruited as the RT costs for approaching angry faces increased.
DISCUSSION
We measured cerebral activity in a group of healthy subjects providing affective and gender evaluations of human faces with emotional expressions. Behaviorally, we confirmed that subjects are faster at approaching positive and avoiding negative social stimuli, as compared to the opposite mappings [AA congruency effect, (Rotteveel and Phaf, 2004; Roelofs et al., 2005)]. These effects were specific to affective evaluations, largely disappearing when subjects responded to an affectively irrelevant feature of the emotional faces (gender evaluation). The voluntary control of motor responses associated with these affective evaluations evoked cerebral activity in the left OFC. Subsequently, we detail and interpret these behavioral and cerebral effects.
Behavioral results
There were no significant overall differences between performance of the AA and GE tasks, indicating that the two tasks were matched for general difficulty levels and sensorimotor characteristics. Crucially, we found significant behavioral congruency effects for the AA task and not for the GE task, extending the findings of Rotteveel and Phaf (2004) to an fMRI setting. These results indicate that the present experimental set-up is suitable for the study of voluntary motivational behavior.
Imaging results
The behavioral congruency effects had a cerebral counterpart in increased metabolic activity in the left lateral OFC (BA47/12; Figure 3A). This effect was driven by the congruency of the relation between emotional valence of the faces on display and response type, over and above the main effects of perceiving faces and moving a joystick. This effect was specifically related to the voluntary control of affect–incongruent AA behavior, largely disappearing when subjects were evaluating the gender of the perceived faces rather than their emotional content. Finally, the effect was genuinely left-lateralized, being absent in the corresponding portion of the right hemisphere (Figure 3C and D).
These results emphasize the crucial contribution of the left lateral OFC in controlling voluntary AA behavior, i.e. selecting a motor response to emotional stimuli when this stimulus–response mapping is in conflict with the automatic AA reaction evoked by the emotional stimuli.
It could be argued that the present results can be explained by the inhibition of automatic emotional processing of the perceptual features of the stimuli, in line with the role of the left OFC in suppression of emotional distracters during working memory performance (Dolcos et al., 2006; Dolcos and McCarthy, 2006). However, the AA task does not evoke inhibition of emotional information per se, but rather the inhibition of the response automatically associated with the emotional stimulus and the selection of a different stimulus–response association. The virtually error-free performance obtained in the present task (Table 1), together with the known right-lateralization and dorsolateral localization of the frontal network supporting the inhibition of prepotent responses (Garavan et al., 1999; Mars et al., 2007), make it unlikely that inhibition of emotional processing can fully account for the OFC response observed in this study. Previous studies have provided examples of such emotional inhibition. For instance, when subjects generate facial expressions that are incongruent to visually presented facial expressions (i.e. frowning to happy faces and smiling to angry faces; Lee et al., 2008), the right OFC appears particularly involved in suppressing the pre-potent imitative response of expressing a facial expression congruent to the one currently perceived (Dimberg, 1982). In another study using emotional facial expressions, Hare et al. (2005) operationalized pressing a button (‘Go’) as an approach response and a lack of movement (‘No-go’) as an avoidance response. There was a correlation between amygdala activity and slowed Go responses following the presentation of fearful faces, a possible indication that this structure is involved in fear-specific inhibitory control as evoked under the inhibitory pressures of Go/No-go tasks.
In contrast to a strict inhibitory contribution, we suggest that our findings can be seen as a particular instance of the general role of the left ventral prefrontal cortex in overriding dominant stimulus–response mappings in favor of rule-driven associations (Thompson-Schill et al., 2005), as observed during the learning and performance of arbitrary stimulus–response associations (Passingham et al., 2000; Toni et al., 2001; Grol et al., 2006), in particular when there is conflict among activated action representations (Badre and Wagner, 2007). Accordingly, the present results extend the known role of the OFC in selecting the relevant stimulus–response association among a set of possibilities (Bussey et al., 2001; Rushworth et al., 2005) to the domain of social emotional responses (Rolls, 2000; Hornak et al., 2003).
Brain–behavior relationships and the control of emotional responses
It is conceivable that the size of the AA congruency effect in healthy subjects reflects the functionality of their adaptive emotional regulation (van Honk et al., 2000). For instance, the cortisol level of subjects with strong AA congruency effects for angry faces remained consistently low throughout the experiment, indicating that active emotion regulation in healthy subjects is associated with reduced basal activity of the glucocorticoid stress systems. Accordingly, it becomes relevant to explore the relationship between cerebral and behavioral effects evoked by the AA task, in particular during the presentation of angry faces. We found that the OFC contribution to the incongruent responses in the AA task was modulated by the emotional valence of the stimuli, increasing as a function of RT when subjects were asked to approach angry faces (Figure 4).
This finding helps to integrate previous accounts of emotional processing in a novel perspective focused on response control. For instance, previous studies have pointed to the involvement of the lateral OFC in evaluating and responding to threat stimuli such as angry faces (Murphy et al., 2003; Kringelbach and Rolls, 2004). Our results indicate that the lateral OFC does not respond to the stimulus emotional valence per se, but rather to an incongruence between stimulus valence and behavioral response. This interpretation complements previous findings from various human lesion and fMRI studies, indicating that lateral portions of the OFC are involved in overriding behavioral choices based on the previous reward values of stimuli and responses (for a review see Elliott et al., 2000). Considering that approaching an angry face requires the subject to override the usually rewarded tendency to avoid threat helping to diminish arousal (van Honk et al., 2000), we suggest that the lateral OFC support a control mechanism that operates in the context of monetary and accuracy-feedback rewards (Elliot et al., 2000), as well as in the context of social–motivational behavior.
Other reports have emphasized the importance of subjects’ motivation to approach or to avoid an emotional stimulus, with a left frontal dominance for approach behavior (D’Alfonso et al., 2000; van Honk et al., 2002; Harmon-Jones, 2003; Harmon-Jones et al., 2006). Although such approach-related left-hemispheric lateralization may involve the dorsolateral regions of the prefrontal cortex (DLPFC) and perhaps more anterior regions of the lateral OFC (having close connections to the DLPFC), such lateralization is less likely to involve the posterior regions of the lateral OFC implicated in the present study (BA47/12) and known to have close connections with limbic regions such as the amygdala (Elliot et al., 2000). Indeed our results indicate that the left posterior OFC does not respond to approach behavior per se, but rather to approach responses that override a different and automatic stimulus–response mapping. In this perspective, the lateralization of OFC activity observed in this study can be seen as an instance of the known left-hemispheric dominance for selecting responses in the context of arbitrary or competing sensorimotor associations (Schluter et al., 1998, 2001; Verstynen et al., 2005; Badre and Wagner, 2007).
Interpretational Limitations
The intrinsic characteristics of the AA task (i.e., a forced two-choice protocol) prevented us from introducing a control emotional category (neutral faces). This feature of the experimental design was sub-optimal for assessing the processing of the emotional stimuli irrespectively of the stimulus–response contingencies. For instance, the effects of perceiving angry faces could only be directly compared with happy faces, but we know that the amygdala is involved in detecting both angry and happy facial expressions (Fitzgerald et al., 2006). A post hoc analysis indicated that presentation of angry faces evoked stronger responses than presentation of happy faces in the left amygdala (cluster size 5 voxels; stereotactical coordinates of local maxima: −32, −4, −14), though these effects remained below statistical threshold (P < 0.05 un-corrected for multiple comparisons).
The present results have been obtained in a group of male subjects. This choice appears justified by the well established gender differences in emotion processing (Rotter and Rotter, 1988), and by the substantial fluctuations in basal cortisol levels as a function of menstrual cycle in females. Accordingly, it remains to be seen whether the present findings apply to female subjects as well.
We cannot exclude that the lateralization of OFC activity is related to a generic left-hemispheric bias associated with having studied right-handed subjects providing responses with their right hand. However, this possibility appears unlikely, given that there was no left-hemispheric bias in the GE task.
Clinical implications
This study presents a novel tool for directly assessing frontal control of overt social AA behavior, a tool that considers the behavior evoked by emotional stimuli. This appears particularly relevant given that dysfunctional AA behavior has been implicated in numerous psychiatric conditions (Gray, 1987). For instance, social phobic patients have difficulty to override their social avoidance tendencies (Horley et al., 2004, Heuer et al., 2007), whereas patients with antisocial disorders show impaired control of social approach behavior (Lewis and Lamm, 2006). Our results would predict that these disturbances involve altered responses of the left lateral orbitofrontal cortex, resulting in impaired control of the action tendencies automatically elicited by social stimuli.
CONCLUSIONS
These results extend the known role of the lateral OFC in selecting the relevant stimulus–response association among a set of possibilities to the domain of social emotional responses, demonstrating that the left OFC is particularly involved when approach reactions need to be controlled and override an automatic stimulus–response mapping, such as threat avoidance. Rather than inhibiting instinctive emotional responses, the OFC exerts executive control over social AA. These findings are particularly relevant for the study of psychiatric conditions characterized by failure to control social AA behavior, such as social anxiety disorder.
ACKNOWLEDGEMENT
The authors thank Paul Gaalman for expert assistance during scanning, Bram Daams for technical assistance, Mark Rotteveel (University of Amsterdam) for his valuable comments on the study design and Jolanda Verhagen and Hans van Pelt (Department of Clinical Chemistry, Leiden University Medical Centre) for conducting the cortisol analyses. Dutch Science Foundation (NWO VENI grant no. 451-02-115 to K.R. and NWO: VIDI grant no. 452-03-339 to I.T.); European Union's Sixth Research Framework Programme (Marie Curie EIF fellowship to R.B.M.).
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Review Neuroscience. 2003;4(3):165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning—a unified framework. Neuroimage. 1997;6(3):209–17. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philosophical Transactions of the Royal Society of London Biological Sciences. 2003;358:561–72. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal: a case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(5):971–82. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Experimental Brain Research. 2003;153(2):180–9. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- D’Alfonso AA, van Honk J, Hermans E, Postma A, de Haan E.HF. Laterality effects in selective attention to threat after rTMS at the prefrontal cortex in female subjects. Neuroscience Letters. 2000;280:195–8. doi: 10.1016/s0304-3940(00)00781-3. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error. New York: Putnam; 1994. [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Facial reactions to facial expressions. Psychophysiology. 1982;19(6):643–7. doi: 10.1111/j.1469-8986.1982.tb02516.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17(15):1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26(7):2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologist Press; 1976. [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10(3):308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–89. [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting Activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences USA. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The Psychology of Fear and Stress. 2nd. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- Grol MJ, de Lange FP, Verstraten F.AJ, Passingham RE, Toni I. Cerebral changes during performance of overlearned arbitrary visuomotor associations. The Journal of Neuroscience. 2006;26(1):117–25. doi: 10.1523/JNEUROSCI.2786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Lueck L, Fearn M, Harmon-Jones C. The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychological Science. 2006;17(5):434–40. doi: 10.1111/j.1467-9280.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Early Career Award. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40(6):838–48. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: the approach-avoidance task. Behaviour Research Therapy. 2007;45(12):2990–3001. doi: 10.1016/j.brat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research. 2004;127(1–2):43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1671–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–83. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–95. [PubMed] [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cerebral Cortex. 2008;18:104–13. doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Lamm C. Behavioral differences in aggressive children linked with neural mechanisms of emotion regulation. Annals of the New York Academy of Sciences. 2006;1094:164–77. doi: 10.1196/annals.1376.017. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. CD ROM from Department of Clinical Neuroscience. Sweden: Psychology section, Karolinska Institute; 1998. The Karolinska directed emotional faces - KDEF. [Google Scholar]
- Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cerebral Cortex. 2007;17:2972–9. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Martinez AMR. The AR face database. 1998. (CVC tech. Rep. No. 24) [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion (JACFEE) [Slides] San Francisco, CA: University of California, Human Interaction Laboratory; 1988. [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain imaging during evaluation of threat. Biological Psychiatry. 2004;55:1047–55. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Animal Behaviour. 2006;72:275–86. [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Öhman A. Face the beast and fear the face: animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–45. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Toni I, Rushworth MF. Specialisation within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Experimental Brain Research. 2000;133(1):103–13. doi: 10.1007/s002210000405. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London, Biological Sciences. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. The European Journal of Neuroscience. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Elzinga B, Rotteveel M. The effect of stress-induced cortisol on approach-avoidance behavior. Psychoneuroendocrinology. 2005;30(7):665–77. doi: 10.1016/j.psyneuen.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, De Jong P, Spinhoven PH, Elzinga B. Biological Psychiatry. HPA-axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. (in press) [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- Rolls ET. Precis of the brain and emotion. The Behavioral and Brain Sciences. 2000;23(2):177–91. doi: 10.1017/s0140525x00002429. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter NG, Rotter GS. Sex differences in encoding and decoding of negative facial emotion. Journal of Nonverbal Behavior. 1988;12:139–48. [Google Scholar]
- Rotteveel M, Phaf RH. Automatic affective evaluation does not automatically predsipose for arm flexion and extension. Emotion. 2004;4(2):156–72. doi: 10.1037/1528-3542.4.2.156. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, et al. Attentional selection and action selection in the ventral and orbital prefrontal cortex. The Journal of Neuroscience. 2005;25(50):11628–36. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences. 2007;11(4):168–76. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121(5):785–99. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39(2):105–13. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Strauss MM, Makris N, Aharon I, Vangel MG, Goodman J, Kennedy DN, et al. fMRI sensitization to angry faces. Neuroimage. 2005;26:389–413. doi: 10.1016/j.neuroimage.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Toni I, Ramnani N, Josephs O, Ashburner J, Passingham RE. Learning arbitrary visuomotor associations: temporal dynamic of brain activity. Neuroimage. 2001;14(5):1048–57. doi: 10.1006/nimg.2001.0894. [DOI] [PubMed] [Google Scholar]
- Van Aken MO, Romijn JA, Miltenburg JA, Lentjes EG. Automated measurement of cortisol. Clinical Chemistry. 2003;49(8):1408–9. doi: 10.1373/49.8.1408. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Schutter DJ, d’Alfonso AA, Kessels RP, de Haan EH. 1 hz rTMS over the right prefrontal cortex reduces vigilant attention to unmasked but not to masked fearful faces. Biological Psychiatry. 2002;52(4):312–7. doi: 10.1016/s0006-3223(02)01346-x. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijsen J, de Haan E, et al. Baseline salivary cortisol levels and preconscious selective attention for threat: a pilot study. Psychoneuroendocrinology. 1998;23:741–7. doi: 10.1016/s0306-4530(98)00047-x. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, et al. Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25:577–91. doi: 10.1016/s0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Van Peer JM, Roelofs K, Rotteveel M, van Dijk JG, Spinhoven PH, Ridderinkhof KR. The effects of cortisol administration on approach-avoidance behavior: an event-related potential study. Biological Psychology. 2007;76:135–46. doi: 10.1016/j.biopsycho.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328(3):233–6. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Verhagen L, Grol MJ, Dijkerman HC, Toni I. Studying visually-guided reach-to-grasp movements in an MR-environment. Neuroimage. 2006;31:S45. [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. Journal of Neurophysiology. 2005;93(3):1209–22. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]