Abstract
A goal of cartilage tissue engineering is the production of cell-laden constructs possessing sufficient mechanical and biochemical features to enable native tissue function. This study details a systematic characterization of a serum-free (SF) culture methodology employing transient growth factor supplementation to promote robust maturation of tissue-engineered cartilage. Bovine chondrocyte agarose hydrogel constructs were cultured under free-swelling conditions in serum-containing or SF medium supplemented continuously or transiently with varying doses of transforming growth factor beta 3 (TGF-β3). Constructs were harvested weekly or bi-weekly and assessed for mechanical and biochemical properties. Transient exposure (2 weeks) to low concentrations (2.5–5 ng/mL) of TGF-β3 in chemically defined medium facilitated robust and highly reproducible construct maturation. Constructs receiving transient TGF-β3 exposure achieved native tissue levels of compressive modulus (0.8 MPa) and proteoglycan content (6–7% of wet weight) after less than 2 months of in vitro culture. This maturation response was far superior to that observed after continuous growth factor supplementation or transient TGF-β3 treatment in the presence of serum. These findings represent a significant advance in developing an ex vivo culture methodology to promote production of clinically relevant and mechanically competent tissue-engineered cartilage constructs for implantation to repair damaged articular surfaces.
Introduction
Articular cartilage lines the bony surfaces of diarthrodial joints and transmits the high stresses generated with movement.1 This dense, avascular tissue consists of a proteoglycan and collagen-rich matrix maintained and continually remodeled by the chondrocytes embedded within.2 Although this tissue normally functions well over a lifetime of use, damage resulting from disease processes or trauma interrupts tissue structure and function, precipitating a degenerative cascade resulting in cartilage erosion. Because of its dense composition and scant vascularity after skeletal maturation, articular cartilage has a limited intrinsic repair capacity. To address this poor healing ability, a number of surgical and cell-based regenerative strategies have been proposed to enhance cartilage repair, including the tissue engineering of cartilage equivalents.
In the most common cartilage tissue-engineering approach, constructs for implantation are fabricated and cultured ex vivo, with their resulting functional properties and composition monitored and compared with that of native tissue. Significant progress has been made in these efforts, with some studies reporting near-native tissue properties (composition and mechanics) after long culture durations.3,4 We and others have previously shown that chondrocytes suspended in agarose culture produce a cartilaginous matrix that resembles native tissue.5–7 As with other three-dimensional (3D) culture environments, a number of factors, including cell-seeding density and medium formulations, regulate the rate of growth and ultimate properties of these hydrogel-based engineered constructs.7–9 Additional anabolic factors, including growth factor supplementation,10–13 deformation loading,14–17 and dynamic bioreactor culture,4,18,19 can further enhance the growth and maturation of tissue-engineered cartilage.
To translate these in vitro findings into clinical applications, it is of particular interest to develop serum-free (SF) culture formulations that foster construct development. Such SF formulations would overcome the regulatory barriers associated with the use of medium supplemented with serum from nonhuman sources. These defined media would also reduce variability commonly observed when studies are conducted with different serum lots. Recently, several SF medium formulations have been shown to support chondrocyte proliferation and matrix production in vitro in monolayer and 3D culture.20–23 Typically, these formulations consist of a base glucose-rich medium (Dulbecco's modified Eagle medium (DMEM) or DMEM/F12) supplemented with commercially available formulations of insulin, transferrin, and selenium (ITS). In a recent study, we demonstrated maturation of chondrocyte-seeded agarose hydrogels24 after transforming growth factor beta 3 (TGF-β3) supplementation of a chemically defined formulation commonly used to induce chondrogenesis in mesenchymal stem cells (MSCs).25 Cartilaginous growth in this SF medium was superior to that observed using common cartilage growth medium formulations containing fetal bovine serum (FBS).
To further this area of inquiry, the current work details the use of this novel SF culture methodology that employs transient supplementation of TGF-β3 to facilitate construct maturation and enhance biochemical and mechanical properties of chondrocyte-laden agarose hydrogel constructs. Using a well-characterized juvenile chondrocyte-seeded agarose system, we monitored the change in mechanical properties and biochemical content of constructs over an 8-week period. The goals of this study were to compare the effectiveness of continuous or transient TGF-β3 supplementation in a chemically defined growth medium with that of an FBS-containing medium, to determine the minimum required growth factor concentration to foster this pro-cartilaginous growth, and to determine the most effective time course over which the growth factor should be present to achieve maximal tissue growth. Findings from this study demonstrate that 2-week transient exposure to low doses (2.5–5.0 ng/mL) of TGF-β3 in a chemically defined medium elicits a robust and reproducible maturation response far superior to that observed after continuous growth factor supplementation or growth factor delivered in combination with serum. The biosynthetic enhancement after transient growth factor treatment was observed only in chemically defined medium formulations, because addition of serum appears to block the phenomenon. These findings represent a significant development in cartilage tissue engineering: promoting the maturation of constructs that achieve native tissue levels of equilibrium stiffness and proteoglycan content using a reproducible, economical, and chemically defined protocol and a short in vitro culture period.
Materials and Methods
Cell isolation and construct fabrication
Engineered cartilage hydrogels were prepared as previously described.8,14 Briefly, cartilage was isolated from six to eight forelimb carpometacarpal joints from three to four young (4–6 months old) calves for each replicate study. Cartilage fragments were pooled and sequentially digested with stirring at 37°C in 2.5 mg/mL Pronase (Calbiochem, San Diego, CA) in high-glucose DMEM (hgDMEM, 8 mL/g of tissue) for 1 h followed by treatment with 0.5 mg/mL of collagenase type II (Sigma, St. Louis, MO) in hgDMEM for 6 h. Cell suspensions were filtered, sedimented at 1000 × g for 10 min, resuspended in cell culture medium, and counted using a hemacytometer. Cell suspensions were then combined with an equivalent volume of 4% Type VII low-gelling-temperature agarose (Sigma) to produce a final cell concentration of 30 × 106 cells/mL in 2% agarose. After room-temperature gelation between two glass plates, chondrocyte-laden disks (5 mm diameter × 2.25 mm thick) were created using a tissue biopsy punch (Miltex, York, PA) and separated into 6-well plates (5–6 disks per well) in 6 mL of culture medium. Disks were subsequently cultured under free-swelling conditions in (i) a serum-containing cartilage growth medium (CM) consisting of hgDMEM supplemented with 10% FBS, amino acids (0.5× essential amino acids, 1× nonessential amino acids), buffering agents (10mM 4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid, 10mM bicarbonate of soda, 10 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid, and 10 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid), 50 μg/mL L-ascorbic acid 2- phosphate (AA), and 1% penicillin-streptomycin-Fungizone or (ii) a SF chemically defined medium (CDM) consisting of hgDMEM supplemented with ITS+ Premix (BD Biosciences, San Jose, CA), 50 μg/mL AA, 40 μg/mL L-proline, 0.1 μM dexamethasone, 110 μg/mL pyruvate, and 1% penicillin-streptomycin-Fungizone.
Construct culture and analysis
Construct maturation with transient exposure to TGF-β3 in CDM conditions was examined in a series of three related studies. In Study I, chondrocyte-laden hydrogels were cultured in the two medium formulations without growth factor supplementation (CM− or CDM−), with continuous rhTGF-β3 (R & D Systems, Minneapolis, MN) treatment at 10 ng/mL (CM+ and CDM+), or with transient TGF-β3 supplementation at 10 ng/mL for only the first 2 weeks of culture (CM + 2WT and CDM + 2WT) with subsequent medium changes in CM− or CDM−, respectively. In Study II, growth factor potency was examined under SF conditions in cultures treated continuously and transiently at growth factor concentrations of 1.0, 2.5, 5.0, and 10.0 ng/mL TGF-β3 in CDM. Finally, in Study III, TGF-β3 exposure duration in SF conditions was examined through transient culture for 2 (CDM + 2WT) or 4 weeks (CDM + 4WT) in 10 ng/mL TGF-β3 before culture in CDM− for the duration of the time course. In all studies and conditions, medium was exchanged every 3 days. Depending upon the study, constructs were harvested weekly or bi-weekly (every other week) over an 8-week time course for mechanical and biochemical analyses.
Mechanical testing
Mechanical testing was performed as previously described in unconfined compression between impermeable platens using a custom mechanical testing device.8,26 Initially, disks were equilibrated in creep (∼0.02 N tare load for 300 s) and then exposed to compressive deformation to 10% strain at a rate of 1 μm/s and allowed to stress relax to equilibrium (1200 s). All stress relaxation curves were fit to an exponential decay approaching infinity (SigmaPlot, Systat Software Inc., Richmond, CA) to determine the equilibrium force. The equilibrium compressive Young's modulus (EY) was calculated by normalizing this equilibrium force according to the original disk cross-sectional area and dividing by the equilibrium compressive strain. Dynamic testing was performed at equilibrium by applying a sinusoidal displacement equivalent to an additional 1% strain at frequencies of 0.1, 0.5, and 1.0 Hz. Dynamic moduli (G*) were determined according to the slope of the compressive stress as a function of the compressive strain at each frequency.27 After testing, specimens were stored at −80°C until they were processed for biochemical content.
Biochemical analyses
Biochemical assays were performed to assess proteoglycan, bulk collagen, and total DNA content. Samples were thawed, weighed wet, lyophilized, re-weighed dry, and digested for 16 h in sodium phosphate-buffered papain at 60°C. Aliquots were analyzed for sulfated glycosaminoglycan (sGAG) content using the 1,9-dimethylmethylene blue dye-binding assay and quantified using a chondroitin-6-sulfate standard.28 After acid hydrolysis (6 N hydrochloric acid at 110°C for 18 h), hydroxyproline content was determined according to reaction with chloramine T and dimethylaminobenzaldehyde.29,30 Hydroxyproline was converted to total collagen content at a ratio of 7.14 g collagen per g hydroxyproline (10:1 molar ratio of collagen to hydroxyproline).18,31,32 Total DNA content was assessed using the Quant-iT PicoGreen dsDNA Reagent (Invitrogen, Eugene, OR) according to the manufacturer's recommended protocol.
Histology
Constructs prepared for histological analysis were fixed overnight at 4°C in phosphate-buffered formalin, dehydrated in a graded series of ethanol, and embedded in paraffin. Samples were sectioned to 5 μm and stained with Alcian blue or Picrosirius red to assess the distribution of proteoglycan and collagen, respectively.
Statistical analyses
Data are reported as the mean and standard deviation from experiments repeated two to five times with four or five replicates per treatment group per time point per experiment. Statistical analyses were performed using analysis of variance and Tukey's post hoc tests with p < 0.05 considered significant.
Results
Study I: Transient exposure to TGF-β3 in a chemically defined medium
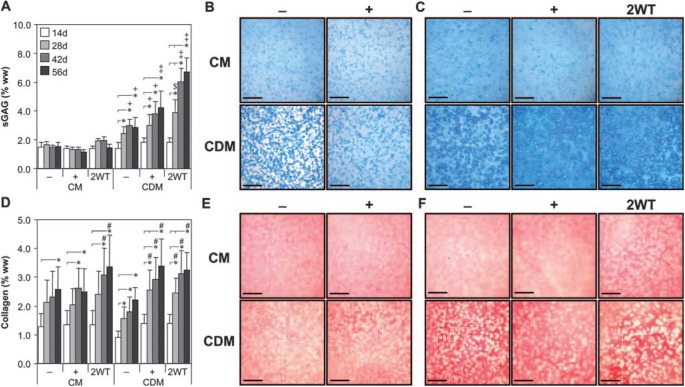
In this first series of experiments, we assessed the time-dependent maturation of chondrocyte-seeded constructs with exposure to TGF-β3 in serum-containing (CM) or chemically defined medium (CDM) formulations. Constructs increased in properties over the first 14 days of culture in CM and CDM independent of growth factor supplementation. On day 14, constructs had an EY of 40 to 50 kPa, a G* of 0.2 to 0.4 MPa, sGAG content of 1.5% wet weight (ww), and collagen content of 1.25% ww. Each parameter was significantly higher (p < 0.05) than values observed at the initiation of 3D culture (on day 0 EY = 6.5± 2.4 kPa, G* = 0.06 ± 0.01 MPa, and sGAG and collagen contents were less than 0.1% ww), and histological staining confirmed deposition of cartilaginous matrix in all treatments at this time point (Fig. 2B, E). With longer culture durations, constructs maintained in the absence of TGF-β3 (CM− and CDM− groups) or with continuous exposure to TGF-β3 (CM+ and CDM+ groups) exhibited continued yet moderate maturation by 56 days (Fig. 1 and 2). Growth was generally more robust under CM+ and CDM+ conditions over time; on day 56, EY and G* were higher for the CM+ and CDM+ than for the CM− and CDM− groups, respectively (p < 0.05, Fig. 1). After 56 days, these constructs had an EY of 100 to 200 kPa, a G* (1Hz) of 0.5 to 1.7 MPa, and sGAG and collagen contents of 2% to 3% and 2% to 3.5% ww, respectively (Fig. 1 and 2). Although the relative difference in maturation between the CM+ and CDM+ groups at day 56 was modest, higher values were observed in chemically defined medium (CDM+) for EY, G*, and sGAG content (p < 0.05 versus CM+ on day 56).
FIG. 2.
Biochemical and histological analyses of cultured chondrocyte-laden hydrogel constructs as a function of transient transforming growth factor beta 3 (TGF-β3) treatment. Constructs were cultured up to 56 days in serum-containing growth medium (CM) or a chemically defined medium (CDM) without TGF-β3 supplementation (CM– and CDM–), with continuous TGF-β3 (10 ng/mL) exposure (CM + and CDM+), or transient TGF-β3 exposure for the first 2 weeks of culture (CM + 2WT and CDM + 2WT). (A) Sulfated glycosaminoglycan (sGAG) content. Substantial increases were seen in all serum-free (SF) cultures (CDM–, CDM + , and CDM + 2WT) over time. The most pronounced enhancement was observed in CDM + 2WT cultures, correlating to the observed increase in mechanical properties. (B, C) Histology—Alcian blue. Enhanced staining was seen over time in culture under SF culture conditions. Representative micrographs are depicted at (B) 14 days and (C) 56 days (bar = 500 μm). (D) Collagen content. Time-dependent increases were seen in all culture conditions, although to a much lesser extent than observed for sGAG and at levels well below those of native cartilage. (E, F) Histology—Picrosirius red. Accumulation of a collagenous matrix was detected at (E) 14 days and (F) 56 days (bar = 500 μm). In (A) and (D), data represent the means ± standard deviations of 8 to 22 samples from two to five replicate studies, depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between time points within treatment; +versus CM–, CM + , and CM + 2WT within time point; $versus CM–, CM + , CM + 2WT, and CDM– within time point; ‡versus all other treatments within time point; #versus CDM– within time point. Color images available online at www.liebertonline.com/ten.
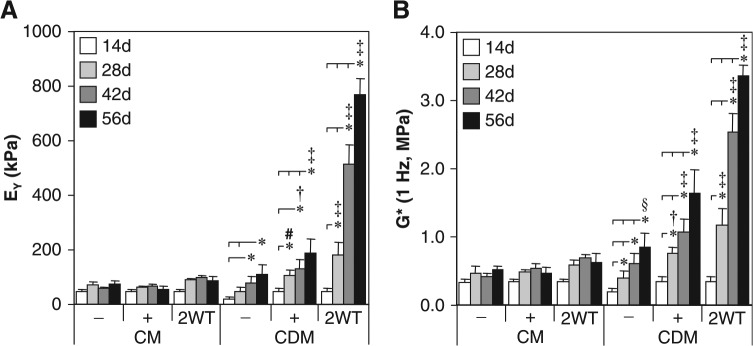
FIG. 1.
Mechanical properties of cultured chondrocyte-laden constructs as a function of transient transforming growth factor beta 3 (TGF-β3) treatment. Constructs were cultured up to 56 days in serum-containing growth medium (CM) or a chemically defined medium (CDM) without TGF-β3 supplementation (CM– and CDM–), with continuous TGF-β3 (10 ng/mL) exposure (CM + and CDM+), or transient TGF-β3 exposure for the first 2 weeks of culture (CM + 2WT and CDM + 2WT). The (A) equilibrium Young's modulus (EY) and (B) dynamic modulus (G*, 1 Hz) demonstrated robust time-dependent increases after transient TGF-β3 treatment under serum-free conditions (CDM + 2WT), whereas a similar response was not observed in serum-containing medium (CM + 2WT). Data represent means ± standard deviations of 8 to 22 samples from two to five replicate studies, depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between time points within treatment; #versus CDM– within time point; †versus CM–, CM + , and CDM– within time point; ‡versus all other treatments within time point; §versus CM– and CM + within time point.
Although these findings with continuous TGF-β3 exposure were robust relative to our previous studies using this culture system,8 still greater differences were observed after transient exposure to TGF-β3. Those groups treated initially with TGF-β3 in CDM for 2 weeks and subsequently cultured in CDM− (CDM + 2WT) showed a markedly enhanced maturation response. These effects were dependent on serum content in the underlying medium formulation (CM vs CDM). For example, CDM + 2WT constructs exhibited a dramatic culture duration–dependent increase in EY to approximately 800 kPa and sGAG content to approximately 7% ww by day 56 (Fig. 1A and 2A). G* (1 Hz) of CDM + 2WT constructs approached 3 MPa, versus approximately 1 MPa for other groups at 56 days (Fig. 1B). Although collagen content of CDM + 2WT disks increased over time (3.0% ww at 56 days, p < 0.05 vs day 0, Fig. 2D, Table 1), overall collagen content in these constructs remained low and was less than that observed in CM + 2WT disks, suggesting other factors in serum modulate collagen deposition. No differences in EY or sGAG content were observed between CM+ and CM + 2WT, suggesting that serum components abrogate the effects of transient TGF-β3 exposure observed under SF conditions. Overall, exposure to TGF-β3 appeared to increase cellularity as determined according to total DNA content (Table 1), although significant differences were not apparent because of the variability observed across study groups. Although DNA content appeared consistently higher in CM-cultured disks, histological analyses of CM−, CM+, and CM + 2WT disks revealed that multilayered cell sheets encapsulated these disks after extended culture (data not shown), which probably contributed to the observed increase in DNA content. The presence of serum proteins and adhesion molecules may have promoted cell outgrowth and subsequent proliferation at the construct periphery in the CM cultured disks, because this phenomenon was not observed for any disks maintained under SF culture conditions, independent of the TGF-β3 supplementation profile.
Table 1.
Physical and Biochemical Properties of Cultured Chondrocyte-Agarose Constructs as a Function of Transient TGF-β3 Exposure*
| Day 0 | Day 14 | Day 56 | ||||
|---|---|---|---|---|---|---|
| CM− | CM+ | CM− | CM+ | CM + 2WT | ||
| Thickness (mm) | 2.30 ± 0.04 | 2.47 ± 0.04 † | 2.38 ± 0.05 † | 2.52 ± 0.07 † | 2.64 ± 0.09 † | 2.58 ± 0.06 † |
| Diameter (mm) | 4.99 ± 0.06 | 5.13 ± 0.24 | 5.09 ± 0.07 | 5.30 ± 0.14 † | 5.46 ± 0.08 † ‡ | 5.40 ± 0.09 † ‡ |
| Wet Weight (mg) | 41.3 ± 3.8 | 57.2 ± 8.4 † | 50.3 ± 3.9 † | 59.2 ± 2.8 † ‡ | 65.5 ± 3.8 † ‡ | 63.8 ± 1.9 † ‡ |
| Dry Weight (mg) | 1.6 ± 0.3 | 3.8 ± 0.4 † | 3.6 ± 0.2 † | 4.8 ± 0.3 † | 5.0 ± 0.5 † | 5.3 ± 0.3 † |
| Water Content (%) | 96.2 ± 0.6 | 93.3 ± 0.7 † | 92.9 ± 0.5 † | 91.8 ± 0.7 † | 92.3 ± 0.7 † | 91.7 ± 0.6 † |
| sGAG (mg/disk) | 0.1 ± 0.0 | 0.9 ± 0.3 † | 0.7 ± 0.1 † | 0.9 ± 0.1 † ‡ | 0.8 ± 0.1 † ‡ | 0.9 ± 0.2 † ‡ |
| Collagen (mg/disk) | 0.0 ± 0.0 | 0.7 ± 0.3 † | 0.6 ± 0.3 † | 1.5 ± 0.5 † | 1.5 ± 0.5 † | 2.0 ± 0.6 † ‡ |
| DNA (μg/disk) | 2.9 ± 0.9 | 3.9 ± 1.1 | 4.2 ± 1.5 | 4.6 ± 1.6 | 6.4 ± 2.1 | 6.4 ± 3.7 |
| Day 14 | Day 56 | |||||
|---|---|---|---|---|---|---|
| CDM− | CDM+ | CDM− | CDM+ | CDM + 2WT | ||
| Thickness (mm) | 2.37 ± 0.04 † | 2.37 ± 0.06 † | 2.48 ± 0.05 † | 2.87 ± 0.06 † ‡ + | 2.72 ± 0.05 † ‡ + | |
| Diameter (mm) | 5.07 ± 0.10 | 5.09 ± 0.11 | 5.19 ± 0.10 † | 5.68 ± 0.12 † ‡ + | 5.60 ± 0.13 † ‡ + | |
| Wet Weight (mg) | 48.2 ± 3.2 † | 47.7 ± 5.5 † | 51.9 ± 3.8 † | 67.6 ± 4.5 † ‡ @ | 69.2 ± 3.8 † ‡ @ | |
| Dry Weight (mg) | 2.9 ± 0.4 † | 3.4 ± 0.6 † | 4.8 ± 1.2 † | 7.8 ± 1.4 † ‡ + | 9.7 ± 1.6 † ‡ + # | |
| Water Content (%) | 94.0 ± 0.7 † | 92.8 ± 0.6 † | 90.7 ± 2.5 † | 88.4 ± 2.3 † ‡ + | 85.5 ± 2.5 † ‡ + # | |
| sGAG (mg/disk) | 0.7 ± 0.2 † | 0.9 ± 0.2 † | 1.5 ± 0.3 † | 2.8 ± 0.7 † ‡ + | 4.6 ± 0.7 † ‡ + # | |
| Collagen (mg/disk) | 0.4 ± 0.1 † | 0.6 ± 0.2 † | 1.1 ± 0.2 † | 2.2 ± 0.6 † ‡ | 2.3 ± 0.3 † ‡ | |
| DNA (μg/disk) | 2.2 ± 0.6 | 3.2 ± 1.0 | 3.3 ± 1.6 | 4.3 ± 1.4 | 3.4 ± 1.2 | |
Constructs were examined at days 0, 14, or 56 following culture in serum-containing growth medium (CM) or a chemically defined medium (CDM) without TGF-β3 (CM− and CDM−), with continuous TGF-β3 (10 ng/mL) exposure (CM+ and CDM+), or transient TGF-β3 (10 ng/mL) exposure for the first two weeks of culture (CM+2WT and CDM+2WT). Data represent the mean ± S.D. of 8–22 samples from 2–5 replicate studies depending on treatment. Pairwise comparisons (statistical significance set at p < 0.05) are reported for all groups versus Day 0 and between treatments within the Day 14 and Day 56 timepoints: † versus Day 0; ‡ versus Day 56 CDM−; + versus Day 56 CM−, CM+, and CM+2WT; @ versus Day 56 CM−; # versus Day 56 CDM+.
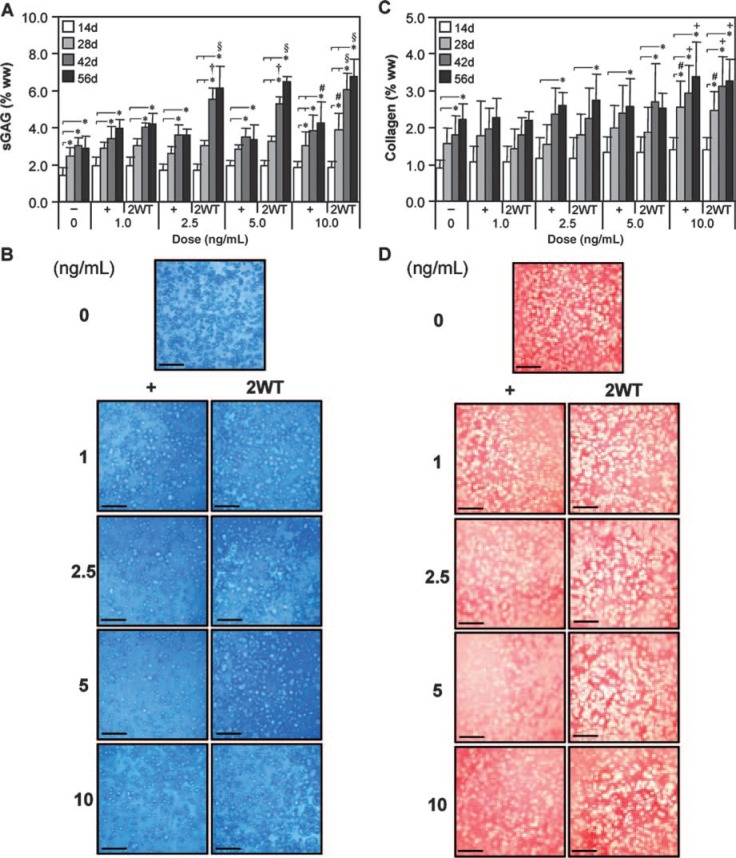
Study II: Dose-response of transient TGF-β3 exposure
Based on the effect of transient TGF-β3 exposure observed under SF conditions, additional experiments were performed to evaluate the effect of varying growth factor doses in combination with transient exposure on construct maturation. For these studies, a range of TGF-β3 concentrations were applied in CDM− continuously (CDM+) and transiently (CDM + 2WT). Physical and biochemical properties are presented in Table 2 and Figures 3 and 4. Continuous treatment with TGF-β3 resulted in comparable but moderate enhancement of disk maturation at any of the doses examined (1–10 ng/mL), suggesting that TGF-β3 has a broad effective dose range under SF conditions. Notably, a dose-dependent effect on the maturation characteristics after transient exposure was observed. All CDM + 2WT groups cultured in more than 2.5 ng/mL of TGF-β3 achieved maximal biochemical growth (Fig. 4 and Table 2), whereas groups treated with more than 5 ng/mL attained maximal biomechanical properties (Fig. 3A, B). At 1 ng/mL, a slightly diminished response was observed; however, underscoring the potency of this phenomenon, an order of magnitude reduction in growth factor dose (from 10 to 1 ng/mL) supplied during the transient exposure period only reduced the EY by a factor of two (from ∼800 to ∼350 kPa, p < 0.05) at 56 days (Fig. 3A). Similar dose-dependent reductions were observed for G* (Fig. 3B) and sGAG content (Fig. 4A) in this concentration range. As in Study I, transient supplementation did not alter collagen accumulation in these constructs (Fig. 4C). Histological staining confirmed the persistent deposition of high levels of sGAG and collagen even at lower TGF-β3 concentrations (Fig. 4B, D).
Table 2.
Physical and Biochemical Properties of Cultured Chondryocyte-Agarose Constructs as a Function of TGF-β3 Treatment Dose*
| 1 ng/mL TGF-β3 | 2.5 ng/mL TGF-β3 | ||||
|---|---|---|---|---|---|
| CDM− | CDM+ | CDM + 2WT | CDM+ | CDM + 2WT | |
| Thickness (mm) | 2.48 ± 0.05 | 2.89 ± 0.16 † ‡ | 2.61 ± 0.05 † * | 2.95 ± 0.13 † ‡ | 2.67 ± 0.06 † * |
| Diameter (mm) | 5.19 ± 0.10 | 5.59 ± 0.09 † | 5.34 ± 0.17 * + | 5.72 ± 0.14 † | 5.50 ± 0.15 † |
| Wet Weight (mg) | 51.9 ± 3.8 | 67.1 ± 3.3 † | 59.9 ± 2.2 † * + | 69.6 ± 3.6 † | 63.4 ± 4.3 † |
| Dry Weight (mg) | 4.8 ± 1.2 | 6.7 ± 0.4 † + | 6.1 ± 0.6 @ # + | 7.0 ± 0.6 † + | 8.0 ± 0.5 † + |
| Water Content (%) | 90.7 ± 2.5 | 90.0 ± 0.4 + | 89.8 ± 0.7 + | 89.9 ± 0.8 + | 87.4 ± 0.9 † |
| sGAG (mg/disk) | 1.5 ± 0.3 | 2.7 ± 0.4 † | 2.5 ± 0.4 † ‡ | 2.5 ± 0.2 † | 3.9 ± 0.6 † * |
| Collagen (mg/disk) | 1.1 ± 0.2 | 1.4 ± 0.3 # + | 1.2 ± 0.2 # + | 1.7 ± 0.2 † | 1.6 ± 0.4 † |
| DNA (μg/disk) | 3.3 ± 1.6 | 5.4 ± 1.8 | 4.9 ± 2.7 | 5.5 ± 2.5 | 4.7 ± 2.2 |
| 5 ng/mL TGF-β3 | 10 ng/mL TGF-β3 | ||||
|---|---|---|---|---|---|
| CDM+ | CDM + 2WT | CDM+ | CDM + 2WT | ||
| Thickness (mm) | 2.85 ± 0.12 † ‡ | 2.69 ± 0.06 † * | 2.87 ± 0.06 † ‡ | 2.72 ± 0.05 † * | |
| Diameter (mm) | 5.70 ± 0.15 † | 5.50 ± 0.12 † | 5.68 ± 0.12 † | 5.60 ± 0.13 † | |
| Wet Weight (mg) | 67.8 ± 5.1 † | 66.6 ± 4.4 † | 67.6 ± 4.5 † | 69.2 ± 3.8 † | |
| Dry Weight (mg) | 6.7 ± 0.8 † + | 8.6 ± 0.5 † | 7.8 ± 1.4 † + | 9.7 ± 1.6 † | |
| Water Content (%) | 90.2 ± 1.0 + | 87.1 ± 0.8 † | 88.4 ± 2.3 † + | 85.5 ± 2.5 † | |
| sGAG (mg/disk) | 2.3 ± 0.6 | 4.1 ± 0.5 † * | 2.8 ± 0.7 † | 4.6 ± 0.7 † * | |
| Collagen (mg/disk) | 1.8 ± 0.7 † | 1.6 ± 0.3 † | 2.2 ± 0.6 † | 2.3 ± 0.3 † | |
| DNA (μg/disk) | 5.2 ± 2.3 | 4.7 ± 2.1 | 4.3 ± 1.4 | 3.4 ± 1.2 | |
Constructs were examined at day 56 following culture in chemically defined medium without growth factor supplementation (CDM−) or with continuous (CDM+) or two-week transient (CDM + 2WT) TGF-β3 exposure at varying doses of TGF-β3 (1, 2.5, 5, and 10 ng/mL). Data represent the mean ± S.D. of 8–22 samples from 2–5 replicate studies depending on treatment. Pairwise comparisons (statistical significance set at p < 0.05): † versus CDM−; * versus all doses of CDM+; ‡ versus all doses of CDM−2WT; # versus 10 ng/mL CDM+; @ versus 5 ng/mL CDM+2WT; + versus 10 ng/mL CDM + 2WT.
FIG. 3.
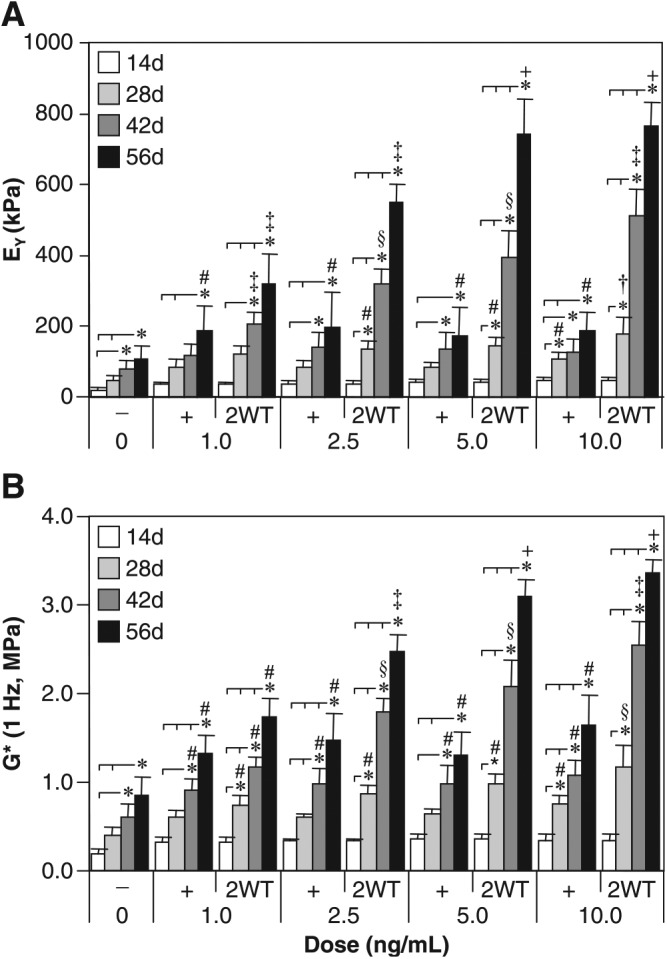
Mechanical properties of cultured chondrocyte-laden constructs as a function of transforming growth factor beta 3 (TGF-β3) dose. Constructs were cultured up to 56 days in chemically defined medium (CDM) without TGF-β3 supplementation (CDM–) or with continuous (CDM+) or 2-week transient (CDM + 2WT) exposure to varying doses of TGF-β3 (1.0, 2.5, 5.0, and 10.0 ng/mL). All groups receiving continuous doses of TGF-β3 demonstrated comparable enhancement of (A) equilibrium Young's moduli (EY) and (B) dynamic moduli (G*, 1 Hz) with time in culture; however, a robust dose-dependence was observed in groups receiving transient TGF-β3 exposure. Maximal response in EY and G* were achieved with a transient dose of at least 5.0 ng/mL. Data represent the means ± standard deviations of 8 to 22 samples from two to five replicate studies, depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between time points within treatment; #versus CDM– within time point; ‡versus all other treatments within time point; †versus CDM– and all doses of CDM + within time point; §versus CDM–, all doses of CDM + , and 1 ng/mL of CDM + 2WT within time point; +versus CDM–, all doses of CDM + , and 1.0 and 2.5 ng/mL of CDM + 2WT within time point.
FIG. 4.
Biochemical and histological analyses of cultured chondrocyte-laden constructs as a function of transforming growth factor beta 3 (TGF-β3) dose. Constructs were cultured up to 56 days in chemically defined medium (CDM) without TGF-β3 supplementation (CDM−) or with continuous (CDM+) or 2-week transient (CDM + 2WT) exposure to varying doses of TGF-β3 (1.0, 2.5, 5.0, and 10.0 ng/mL). (A) Sulfated glycosaminoglycan (sGAG) content. Transient TGF-β3 exposure resulted in a dose-dependent increase in sGAG content, with peak levels attained at 2.5 ng/mL of CDM + 2WT at 56 days. (B) Histology—Alcian blue. Robust staining indicating sGAG accumulation was seen across all doses of constant and transient TGF-β3 treatment after 56 days of culture (bar = 500 μm). (C) Collagen content. Unlike with sGAG, collagen content increased only moderately over time, with the highest dose providing the most-significant collagen accumulation. (D) Histology—Picrosirius red. Staining at 56 days confirmed accumulation of a collagenous matrix (bar = 500 μm). In (A) and (C), data represent the means ± standard deviations of 8 to 22 samples from two to five replicate studies, depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between time points within treatment; #versus CDM− within time point; †versus CDM− and all doses of CDM + within time point; §versus CDM−, all doses of CDM+, and 1 ng/mL of CDM + 2WT within time point; +versus CDM−, 1 ng/mL of CDM + , and 1 ng/mL of CDM + 2WT within time point. Color images available online at www.liebertonline.com/ten.
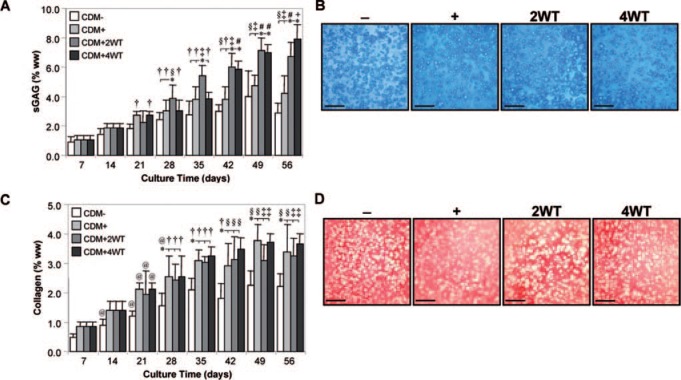
Study III: Duration of transient exposure to TGF-β3
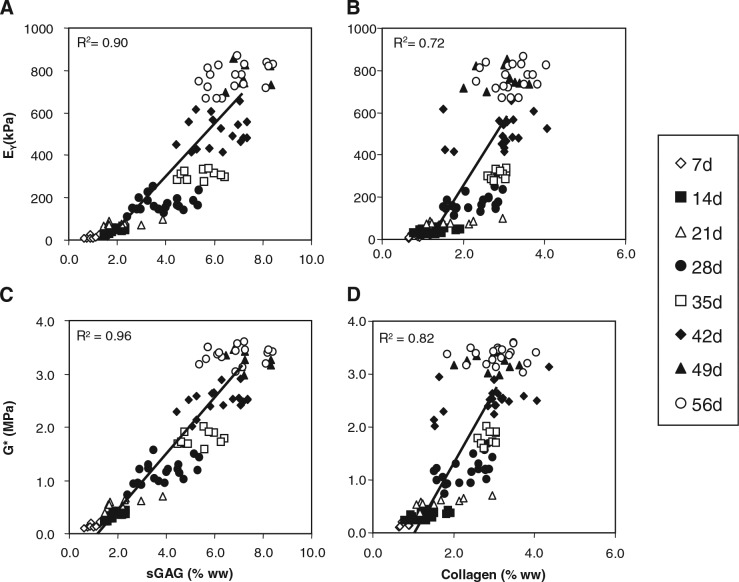
As a final characterization of this novel culture system, we explored the influence of transient exposure duration on the maturation response. For this study, constructs were cultured in the presence of 10 ng/mL of TGF-β3 in CDM medium for 2 (CDM + 2WT) or 4 (CDM + 4WT) weeks before culture in CDM− for the duration of the time course. After transient growth factor treatment, constructs were cultured through day 56, with weekly determination of physical characteristics, biochemical content, and mechanical properties (Table 3, Fig. 5 and 6). As detailed in Study I, CDM + 2WT conditions led to a 20-fold increase in compressive stiffness (from ∼40 to ∼800 kPa, Fig. 5A), an approximately 10-fold increase in dynamic modulus (from 0.3 to 3.0 MPa, Figure 5B), an approximately 3.5-fold increase in sGAG content (from ∼ 2 to 7% ww, Fig. 6A), and little effect on collagen content (Fig. 6C) during the 6 weeks of culture after 2 weeks of transient TGF-β3 treatment. The weekly time course in the CDM + 2WT group revealed an approximate 2-week delay after growth factor removal before indications of robust growth (relative to CDM+) were apparent. When TGF-β3 was delivered transiently for 4 weeks (CDM + 4WT), the ultimate modulus, dynamic stiffness, and sGAG content attained by day 56 were comparable with those of CDM +2WT disks (Fig. 5A, B, 6A, C). Histological analysis confirmed these findings (Fig. 6B, D). Similar to the CDM +2WT group, a 2-week delay was observed before robust biochemical and biomechanical maturation in CDM + 4WT cultured disks. The CDM + 2WT disks required 5 weeks of culture after growth factor removal on day 14 to attain maximal biomechanical values; however, the maturation phase proceeded faster in the CDM + 4WT specimens, with similar peak biomechanical properties attained by day 49, or just 3 weeks after TGF-β3 removal from the culture medium. Biomechanical data from this study were further analyzed by plotting EY and G* from the CDM + 2WT group for each time point as a function of sGAG and collagen content. In agreement with the robust increase in sGAG content observed after “removal of TGF-β3,” linear correlations of this data suggest that sGAG content is a better predictor of compressive stiffness (sGAG coefficient of determination (R2) = 0.90 versus collagen R2 = 0.72, Fig. 7A, B) and dynamic modulus (sGAG R2 = 0.96 versus collagen R2 = 0.82, Fig. 7C, D). This observation appeared to be unique to groups experiencing transient TGF-β3 exposure, because CDM + disks revealed comparable correlation coefficients for EY (R2 = 0.86) and G* (R2 = 0.89) as a function of sGAG or collagen content (data not shown).
Table 3.
Physical and Biochemical Properties of Cultured Chondrocyte-Agarose Constructs as a Function of TGF-β3 Treatment Duration*
| CDM + 2WT | ||||
|---|---|---|---|---|
| Day 14 (CDM+) | Day 28 | Day 42 | Day 56 | |
| Thickness (mm) | 2.37 ± 0.06 | 2.59 ± 0.10† | 2.65 ± 0.06† | 2.72 ± 0.05* |
| Diameter (mm) | 5.09 ± 0.11 | 5.29 ± 0.12† | 5.43 ± 0.08† | 5.60 ± 0.13* |
| Wet Weight (mg) | 47.7 ± 5.5 | 54.1 ± 4.4 | 62.0 ± 4.7* | 69.2 ± 3.8‡ |
| Dry Weight (mg) | 3.4 ± 0.6 | 5.3 ± 0.8† | 8.1 ± 1.3* | 9.7 ± 1.6‡ |
| Water Content (%) | 92.8 ± 0.6 | 90.1 ± 1.5† | 86.8 ± 2.4* | 85.5 ± 2.5* |
| sGAG (mg/disk) | 0.9 ± 0.2 | 2.1 ± 0.4† | 3.7 ± 0.4* | 4.6 ± 0.7‡ |
| Collagen (mg/disk) | 0.6 ± 0.2 | 1.2 ± 0.3† | 2.0 ± 0.3* | 2.3 ± 0.3* |
| DNA (μg/disk) | 3.2 ± 1.0 | 3.8 ± 1.0 | 3.5 ± 0.6 | 3.4 ± 1.2 |
| CDM + 4WT | ||||
|---|---|---|---|---|
| Day 14 (CDM+) | Day 28 (CDM+) | Day 42 | Day 56 | |
| Thickness (mm) | 2.37 ± 0.06 | 2.63 ± 0.07† | 2.70 ± 0.06† | 2.87 ± 0.11‡+ |
| Diameter (mm) | 5.09 ± 0.11 | 5.35 ± 0.14† | 5.50 ± 0.09† | 5.72 ± 0.14‡ |
| Wet Weight (mg) | 47.7 ± 5.5 | 51.6 ± 4.2 | 59.3 ± 4.3 | 73.3 ± 5.7‡ |
| Dry Weight (mg) | 3.4 ± 0.6 | 4.6 ± 0.6 | 8.1 ± 0.9* | 12.4 ± 2.4‡ |
| Water Content (%) | 92.8 ± 0.6 | 91.1 ± 1.3 | 86.2 ± 2.2* | 83.2 ± 2.3* |
| sGAG (mg/disk) | 0.9 ± 0.2 | 1.5 ± 0.3† | 3.6 ± 0.5* | 5.8 ± 0.5‡+ |
| Collagen (mg/disk) | 0.6 ± 0.2 | 1.4 ± 0.1† | 1.9 ± 0.1* | 2.5 ± 0.3* |
| DNA (μg/disk) | 3.2 ± 1.0 | 4.0 ± 1.1 | 4.9 ± 1.3 | 4.2 ± 1.8 |
Constructs were examined at days 14, 28, 42, and 56 following culture in a chemically defined medium with transient TGF-β3 (10 ng/mL) supplementation for the first two (CDM + 2WT) or four (CDM + 4WT) weeks of culture. Time points with all prior medium changes containing TGF-β3 are also labeled (CDM+) in the table. Data represent the mean ± S.D. of 10–22 samples from 2–5 replicate studies depending on treatment. Pairwise comparisons (statistical significance set at p < 0.05):† versus all Day 14 treatment groups; * versus all Day 14 and 28 treatment groups; ‡ versus all Day 14, 28, and 42 treatment groups; + versus Day 56 CDM + 2WT.
FIG. 5.
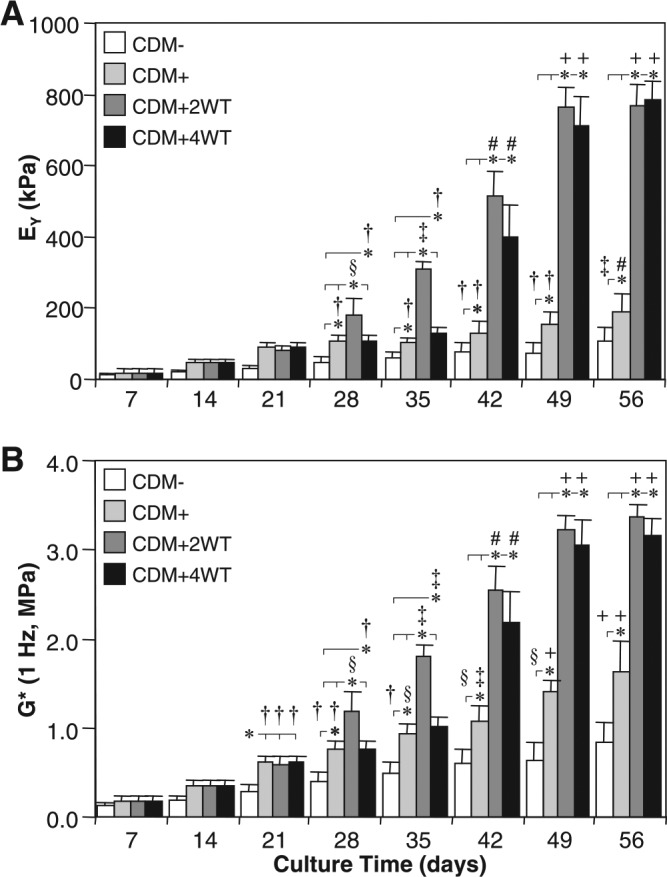
Mechanical properties of cultured chondrocyte-laden constructs as a function of transforming growth factor beta 3 (TGF-β3) treatment duration. Constructs were harvested weekly up to 56 days after culture in chemically defined medium (CDM) without TGF-β3 supplementation (CDM–) or with continuous (CDM+) or transient TGF-β3 exposure for the first 2 weeks (CDM + 2WT) or 4 weeks (CDM + 4WT) of culture. Both groups receiving transient growth factor treatment exhibited robust time-dependent increases in (A) equilibrium Young's moduli (EY) and (B) dynamic moduli (G*, 1 Hz) and achieved comparable equilibrium values at 56 days. Data represent the means ± standard deviations of 10 to 22 samples from two to five replicate studies depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between treatments within time point; †versus day 14 and all prior time points within treatment; §versus day 21 and all prior time points within treatment; ‡versus day 28 and all prior time points within treatment; #versus day 35 and all prior time points within treatment; +versus day 42 and all prior time points within treatment.
FIG. 6.
Biochemical and histological analyses of cultured chondrocyte-laden constructs as a function of transforming growth factor beta 3 (TGF-β3) treatment duration. Constructs were harvested weekly up to 56 days after culture in chemically defined medium (CDM) without TGF-β3 supplementation (CDM–) or with continuous (CDM+) or transient TGF-β3 exposure for the first 2 weeks (CDM + 2WT) or 4 weeks of culture (CDM + 4WT). (A) Sulfated glycosaminoglycan (sGAG) content. All treatment groups exhibited time-dependent increases in sGAG content, with the transient exposure groups attaining the highest levels in correlation to the observed mechanical properties for these culture conditions. (B) Histology—Alcian blue. Robust staining indicating accumulation of sulfated proteoglycan-rich extracellular matrix was seen in all treatments at 56 days (bar = 500 μm). (C) Collagen content. Similar to sGAG content, collagen content demonstrated time-dependent increases for all treatment groups, although transient TGF-β3 exposure did not enhance collagen deposition more than continuous growth factor supplementation. (D) Histology—Picrosirius red. Staining at 56 days demonstrated accumulation of a collagenous matrix (bar = 500 μm). In (A) and (C), data represent the means ± standard deviations of 10 to 22 samples from two to five replicate studies depending on treatment. Statistical differences (p < 0.05) based on pairwise comparisons are depicted within treatment over time and between treatments within time: *between treatments within time point; @versus day 7 within treatment; †versus day 14 and all prior time points within treatment; §versus day 21 and all prior time points within treatment; ‡versus day 28 and all prior time points within treatment; #versus day 35 and all prior time points within treatment; +versus day 42 and all prior time points within treatment. Color images available online at www.liebertonline.com/ten.
FIG. 7.
Correlation of equilibrium Young's moduli (EY) and dynamic moduli (G*) with sulfated glycosaminoglycan (sGAG) or collagen contents of cultured chondrocyte-laden constructs. (A) EY with sGAG, (B) EY with collagen, (C) G* with sGAG, and (D) G* with collagen. A linear correlation was seen with all parameters in constructs examined from day 7 to day 56 after culture in a chemically defined medium containing transiently supplemented transforming growth factor beta 3 (10 ng/mL) for the first 2 weeks of culture. Coefficient of determination values ranged from 0.72 to 0.96.
Discussion
This study details a novel culture methodology that promotes robust maturation of tissue-engineered cartilage constructs under free-swelling conditions. With short-term, transient exposure to TGF-β3 in a CDM, constructs achieved native tissue levels of compressive modulus (0.8 MPa) and proteoglycan content (6–7% ww) in less than 2 months of in vitro culture. Under these SF culture conditions, short-term exposure (2 weeks) to low doses (2.5–5.0 ng/mL) of TGF-β3 resulted in a reproducible and robust maturation response after growth factor was removed from the culture medium (or growth factor “release”), and constructs matured to levels far greater than were achieved with continuous exposure to the same dose of TGF-β3 (Fig. 1 and 2). Mechanical and biochemical properties achieved in constructs receiving transient growth factor exposure were significantly higher than we have previously reported using this juvenile bovine chondrocyte-agarose hydrogel culture system under SF24 and high-serum conditions.8 This growth factor “release” phenomenon was observed only under SF culture conditions, because no such response was observed with temporal TGF-β3 exposure in standard serum-containing medium formulations. This finding represents a significant step forward in the production of clinically relevant and mechanically competent tissue-engineered cartilage constructs for implantation to repair damaged articular surfaces.
The current study employing transient growth factor exposure aligns with the reports by Gray and coworkers, who studied the effect of transient mechanical compression on matrix biosynthesis in cartilage explants.33 In their studies, proline incorporation, an indicator of collagen production, rebounded from diminished levels with static compression to levels greater than free-swelling controls soon after the removal of the static load. In our system, transient growth factor supplementation similarly resulted in higher levels of matrix production than in controls. The kinetics of the transient physical compression were observed in hours, whereas the response to transient TGF-β3 exposure became distinguishable from constant TGF-β3 supplementation only after weeks of subsequent culture in growth factor–free conditions. Furthermore, 2- or 4-week transient exposure resulted in a comparable 2-week delay before the profound maturation phase was observed, suggesting that internal cell processes and perhaps signaling cascades elicited upon growth factor removal require substantial time to cause functionally detectable outcomes.
It remains undetermined whether matrix components existing before growth factor removal are merely supplemented by newly synthesized moieties or are turned over during the observed lag phase in favor of species providing more-robust biomechanical integrity, such as larger proteoglycan molecules. The origin of the rapidly accumulated proteoglycan (which leads to the improved compressive properties observed in this study) does not appear to result from increased cellularity, because DNA content was not markedly different between CDM+ and CDM + 2WT conditions (Tables 1–3). Our findings show that inclusion of serum in the culture medium abrogates the robust maturation response, suggesting that biologic factors in serum block the mechanism of the response. It is unclear whether this results from modulation of TGF-β3–mediated signaling during the initial exposure period or whether serum factors during culture after growth factor removal from the medium attenuate the effects.
Previous studies have employed sequential growth factor addition with the goal of first increasing construct cellularity (fibroblast growth factor-2/TGF-β1) followed by enhancing cartilaginous growth (with insulinlike growth factor-1).34 Unlike the hydrogel used in our work, this previous study was carried out on a fibrous scaffold, and growth factors were added in the context of a serum-containing medium, making direct comparisons challenging. It has also been reported that chemically defined formulations containing TGF-β1 are able to enhance the re-expression of the cartilage phenotype in pellet cultures of aged human chondrocytes that have de-differentiated during culture expansion.35 Other studies report better matrix deposition in pellet cultures of juvenile human chondrocytes in SF medium formulations than in serum-containing medium.23 In 3D culture using agarose and self-assembling peptide gels, low-serum formulations typically result in better maturation than serum-containing medium.21,22
Together, the present results and previous studies suggest that TGF-β3 treatment may have two divergent effects on fully differentiated chondrocytes. First, this factor enhances retention or assumption of the chondrocyte phenotype by activating the cellular machinery for rapid production of sGAGs and sulfated proteoglycan. This activity is likely to be critical in the chondroinductive effects of TGF-β3 on MSCs.25 However, a muted anabolic response is seen with constant TGF-β3 exposure, suggesting that TGF-β3 may act to “prime the pump” and thereby enable the robust and rapid accumulation of these molecules after its effects have dissipated. Such a temporal profile of TGF-β3 is reminiscent of the spatiotemporally specific pattern of TGF-β3 regulation of chondrogenesis in the developing embryonic limb.36 Whether such a prochondrogenic response is an intrinsic feature of cellular response to transient TGF-β3 exposure or is dependent upon the involvement of other secondary factors remains to be determined. Based on this highly regulated, spatiotemporal expression pattern during development, it is likely that sustained exposure of a single growth factor, such as TGF-β3, or a combination of factors, such as those present in serum, significantly affects the homeostasis of ex vivo–cultured constructs. Continuous treatment for prolonged time periods could attenuate cellular anabolic response or even elicit more-aggressive matrix turnover by triggering catabolic pathways that promote release of matrix components to the culture media. Either mechanism could severely restrict peak equilibrium levels of accumulated extracellular matrix, as observed in this study for the CM + , CM + 2WT, and CDM + groups but not in the CDM + 2WT specimens. As a result, further studies are required to assess matrix synthesis, accumulation, and release after continuous and transient growth factor exposure to elucidate the mechanism of this robust maturation phenomenon. Additionally, it is plausible that this maturation response is not unique to TGF-β3, because other TGF-β isoforms, members of the TGF-β superfamily, or even unrelated growth factor families may exhibit a comparable response after transient exposure under SF culture conditions.
The culture methodology and prochondrogenic medium formulation provides significant advantages to cartilage tissue engineering ex vivo. First, this new formulation is SF and chemically defined, obviating concerns over contaminating protein byproducts and facilitating passage through regulatory barriers. Furthermore, the chemically defined nature of this medium allows for better quality control of the constituent components to enhance consistency and reproducibility, as is demonstrated by the findings of this study, which used multiple donors and experimental replicates. If adopted as a positive control or included as a reference standard, this culture methodology should permit direct comparison of findings from different laboratories. For example, our collaborators have already independently reproduced these findings and have initiated studies on the role of mechanical preconditioning in this response.37 Finally, that low levels of TGF-β3 applied for only a short period of time are sufficient to elicit a robust chondrogenic maturation response resulting in native tissue properties improves the cost-effectiveness of the medium formulation. In the present study, the shortest duration of TGF-β3 exposure was 2 weeks, and whether even shorter exposure is sufficient to direct comparable chondrogenic maturation remains to be investigated.
In future studies, several issues should be addressed. First, the mechanism or pathways of this phenomenon should be elucidated to further optimize culture conditions or tailor the outcome and identify any adverse changes in phenotype that might occur. Next, the response of chondrocytes attained in other 3D substrates should be examined. Recent studies have shown a dependence on small levels of serum in self-assembling peptide gels that was not apparent in agarose.22 The dependence of this response on the age of the target cells also deserves analysis. The present study was performed using juvenile chondrocytes, yet it has been clearly demonstrated that chondrocytes lose matrix-forming potency with age.38 As a result, they may well be limited in their reversion to phenotype and responsiveness to this type of transient growth factor culture methodology if derived from aged or diseased human tissues. Finally, although the compressive equilibrium and sGAG contents achieved in this study were as high as or higher than native levels, the collagen content and the corresponding dynamic compressive modulus remained well below those of native tissue (∼25% of native levels). This deficiency was unrelated to time in culture, because most parameters appeared to approach equilibrium by day 56. Further modes of stimulation, such as mechanical preconditioning or alternative growth factor supplementation, may be required to overcome this limitation.
The findings of this study may be useful in combination with other tissue engineering modalities. For example, TGF-β3 may modulate the maturation of engineered constructs produced with MSCs in a similar manner. Indeed, recent studies in our laboratory suggest that a single high dose of TGF-β1 delivered to an alginate/poly-L-lactide acid foam amalgam seeded with human MSCs is sufficient to induce and maintain the chondrocyte phenotype over a 21-day culture period.39 Similar studies in agarose gels have noted continuing chondrogenic phenotype and a limited response to transient growth factor treatment in bovine MSCs seeded in agarose culture.40 Additional delivery modalities for exploration include controlled-release, cell–gel implants that transiently deliver TGF-β3 to co-implanted chondrocytes to enhance maturation post-implantation. Alternatively, gene transduction of cells to express TGF-β3 or comparable pro-chondrogenic morphogen(s) may also be considered to tailor transient growth factor delivery in an autocrine fashion.41
Conclusions
This work describes a chemically defined, reproducible, and cost-effective culture protocol to promote the maturation of chondrocyte-based, tissue-engineered cartilage with mechanical and biochemical properties comparable with those of native tissue. The mechanical properties achieved using this free-swelling, static culture system are three to four times as high as previously attained in an optimized deformational loading study using the same cell source and hydrogel carrier.8 In addition, the timed treatment with growth factors addresses current limitations of cartilage tissue engineering using a clinically relevant, SF culture system. Our findings are directly applicable to cartilage tissue engineering for the fabrication of mechanically robust constructs suitable for cartilage repair.
Acknowledgments
This work was supported by the Intramural Research Program of NIAMS, NIH (ZO1 AR41131).
References
- 1.Ateshian G.A. Hung C.T. Patellofemoral joint biomechanics and tissue engineering. Clin Orthop Relat Res. 2005:81. doi: 10.1097/01.blo.0000171542.53342.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilak F. Sah R.L. Setton L.A. Physical Regulation of Cartilage Metabolism, in Basic Orthopaedic Biomechanics. In: Mow V.C., editor; Hayes W.C., editor. Lippincott-Raven; Philadelphia: 1997. pp. 179–207. [Google Scholar]
- 3.Vunjak-Novakovic G. Obradovic B. Martin I. Bursac P.M. Langer R. Freed L.E. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 4.Freed L.E. Langer R. Martin I. Pellis N.R. Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc Natl Acad Sci U S A. 1997;94:13885. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108((Pt 4)):1497. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 7.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 8.Mauck R.L. Wang C.C. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Chang S.C. Rowley J.A. Tobias G. Genes N.G. Roy A.K. Mooney D.J. Vacanti C.A. Bonassar L.J. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001;55:503. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Blunk T. Sieminski A.L. Gooch K.J. Courter D.L. Hollander A.P. Nahir A.M. Langer R. Vunjak-Novakovic G. Freed L.E. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8:73. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 11.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 12.Gooch K.J. Blunk T. Courter D.L. Sieminski A.L. Bursac P.M. Vunjak-Novakovic G. Freed L.E. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 13.Gooch K.J. Blunk T. Courter D.L. Sieminski A.L. Vunjak-Novakovic G. Freed L.E. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8:591. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 14.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 15.Seidel J.O. Pei M. Gray M.L. Langer R. Freed L.E. Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445. [PubMed] [Google Scholar]
- 16.Kisiday J.D. Jin M. DiMicco M.A. Kurz B. Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Waldman S.D. Spiteri C.G. Grynpas M.D. Pilliar R.M. Kandel R.A. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 18.Vunjak-Novakovic G. Martin I. Obradovic B. Treppo S. Grodzinsky A.J. Langer R. Freed L.E. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 19.Saini S. Wick T.M. Concentric cylinder bioreactor for production of tissue engineered cartilage: effect of seeding density and hydrodynamic loading on construct development. Biotechnol Prog. 2003;19:510. doi: 10.1021/bp0256519. [DOI] [PubMed] [Google Scholar]
- 20.Chua K.H. Aminuddin B.S. Fuzina N.H. Ruszymah B.H. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cell Mater. 2005;9:58. doi: 10.22203/ecm.v009a08. [DOI] [PubMed] [Google Scholar]
- 21.Kisiday J. Jin M. Kurz B. Hung H. Semino C. Zhang S. Grodzinsky A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:99961. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisiday J.D. Kurz B. DiMicco M.A. Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11:141. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 23.Adkisson H.D. Gillis M.P. Davis E.C. Maloney W. Hruska K.A. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001:S280. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 24.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Barry F. Boynton R.E. Liu B. Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 26.Soltz M.A. Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 27.Park S.Y. Hung C.T. Ateshian G.A. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. J Biomech. 2003;12:391. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 29.Woessner J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 30.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 31.Nimni M.E. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum. 1983;13:1. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- 32.Hollander A.P. Heathfield T.F. Webber C. Iwata Y. Bourne R. Rorabeck C. Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray M.L. Pizzanelli A.M. Lee R.C. Grodzinsky A.J. Swann D.A. Kinetics of the chondrocyte biosynthetic response to compressive load and release. Biochim Biophys Acta. 1989;991:415. doi: 10.1016/0304-4165(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 34.Pei M. Seidel J. Vunjak-Novakovic G. Freed L.E. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294:149. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg A.J. Lee D.A. Bader D.L. Bentley G. Autologous chondrocyte implantation. Culture in a TGF-beta-containing medium enhances the re-expression of a chondrocytic phenotype in passaged human chondrocytes in pellet culture. J Bone Joint Surg Br. 2005;87:128. [PubMed] [Google Scholar]
- 36.Merino R. Ganan Y. Macias D. Economides A.N. Sampath K.T. Hurle J.M. Morphogenesis of digits in the avian limb is controlled by FGFs, TGFbetas, and noggin through BMP signaling. Dev Biol. 1998;200:35. doi: 10.1006/dbio.1998.8946. [DOI] [PubMed] [Google Scholar]
- 37.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran-Khanh N. Hoemann C.D. McKee M.D. Henderson J.E. Buschmann M.D. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J Orthop Res. 2005;23:1354. doi: 10.1016/j.orthres.2005.05.009.1100230617. [DOI] [PubMed] [Google Scholar]
- 39.Caterson E.J. Nesti L.J. Li W.J. Danielson K.G. Albert T.J. Vaccaro A.R. Tuan R.S. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Mauck R.L. Byers B.A. Yuan X. Rackwitz L. Tuan R.S. Cartilage tissue engineering with MSC-laden hydrogels: effect of seeding density, exposure to chondrogenic medium, and dynamic loading. Proceedings of the 52nd Annual Orthopaedic Research Society Meeting; Chicago, IL. 2006. [Google Scholar]
- 41.Palmer G.D. Steinert A. Pascher A. Gouze E. Gouze J.N. Betz O. Johnstone B. Evans C.H. Ghivizzani S.C. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12:219. doi: 10.1016/j.ymthe.2005.03.024. [DOI] [PubMed] [Google Scholar]