Abstract
Programmed cell death (apoptosis) is a prominent feature in human and experimental sepsis, especially as it involves the lymphoid system with resulting immunoparalysis. In addition, sepsis is associated with strong activation of the complement system, resulting in generation of the powerful anaphylatoxin, C5a, as well as the upregulation of the C5a receptor (C5aR) in a variety of different organs. The consequences of C5a interactions with C5aR can be directly linked to apoptosis of thymocytes and adrenal medullary cells after cecal ligation and puncture (CLP) -induced sepsis in rodents, as well as with other accompanying complications of CLP: cardiac dysfunction, consumptive coagulopathy, organ dysfunction, and lethality. This communication reviews the evidence for the adverse roles of C5a and C5aR in the setting of experimental sepsis and linkages to the various complications of sepsis, especially apoptosis as well as the roles of the two C5a receptors (C5aR AND C5L2) in experimental sepsis.
Keywords: Apoptosis, thymocytes, adrenal medullary cells, C5a, C5aR, C5L2, sepsis
Sepsis is a major cause of mortality in humans, resulting in >200,000 fatalities in the U.S., a number close to the number of deaths from acute myocardial infarction (1,2). Septic shock is a major complication of sepsis, usually requiring vasopressor support in order to main vascular perfusion (3–5), although the reason for this complication is poorly understood. The inability of the heart during sepsis to maintain adequate cardiac output and blood pressure has been referred to as the “cardiomyopathy of sepsis” (6). In addition to inadequate cardiac function during sepsis, it is well known in both human and experimental sepsis that a rapid caspase-dependent development of apoptosis of both T and B cells occurs at an early stage, leading to immunosuppression (7). In rodent sepsis occurring after CLP, we have shown that robust complement activation occurs, resulting in signaling paralysis of blood neutrophils (PMNs) and loss of their innate immune functions (phagocytosis, chemotaxis, respiratory burst), together with profound apoptosis of thymocytes (8–12). Treatment of rodents with blocking antibodies either to the powerful complement-derived anaphylatoxin, C5a, or to its receptor, C5aR, is highly protective, resulting in greatly improved survival (9, 11), reduced thymocyte apoptosis (11), retention of innate immune functions of PMNs (8), and attenuated consumptive coagulopathy after CLP (13). In this report we will emphasize linkages between C5a, C5aR and development of apoptosis of thymocytes as well as onset of other complications (listed above) of experimental sepsis. As will be described below, during sepsis C5a is generated, upregulation of C5aR occurs, there is loss of innate immune functions of PMNs, contractility defects in cardiomyocytes develops (14), apoptosis of thymocytes (11) and adrenal medullary cells (15) are prominent, and lethality is high (9), all of which can be linked to C5a and its interactions with receptors during sepsis. As will be emphasized below, a link has now been established between catecholamine release, adrenal medullary cell apoptosis, and septic shock of sepsis (15). The development of apoptosis after CLP appears to be linked to appearance of C5a and its interaction with the two C5a receptors (C5aR, C5L2). In the setting of endotoxemia, the use of the inhibitor of C1 esterase (C1 INH) was protective in the setting of lethal endotoxemia (16), although treatment with C1 INH did not reduce mortality in human sepsis (17). This raises the question as to whether blockade of the early steps in the complement activation cascade is desirable, since most downstream products, especially those related to C3-derived opsonic (phagocytosis-promoting) products, would be curtailed in production.
1. Complement Activation After CLP
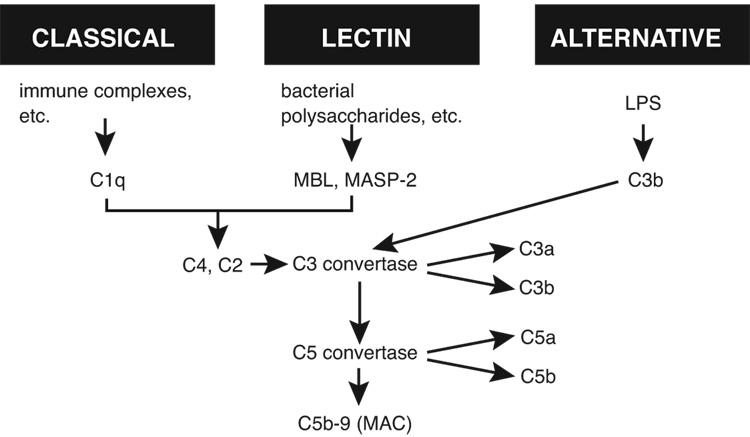
As in many types of sepsis, both in humans and in animals, CLP triggers activation of all three complement pathways (Figure 1), with evidence for engagement of all pathways (classical, alternative, lectin) of complement activation (18). Precisely how sepsis triggers this complex and reinforcing pattern of activation is not understood. It seems clear that, if bacterial lipopolysaccharide plays a role in human sepsis, its participation is probably minor. CLP-induced sepsis is polymicrobial (involving both gram positive and gram negative bacteria) and, as such, features in plasma and in lymphoid tissues draining the peritoneal cavity both aerobic and anaerobic bacteria translocated from the gut. It should also be noted that approximately 50% of humans with sepsis have gram positive bacterial pneumonia (1–3). While lipopolysaccharide (LPS) has been speculated to cause harmful outcomes, there is other evidence (in TLR4−/−, CD14−/−, and LPS-binding protein−/− mice) suggesting that, at least in the setting of CLP, LPS may not be a major determinant in the adverse outcomes (19–21).
Figure 1.
The three pathways of complement activation, collectively resulting in biologically active split products of C3 and C5.
All three pathways of complement activation converge to generate the “C3 convertase”, which cleaves C3 into a small (C3a) and a large (C3b) fragmentation product (Figure 1). C3b is known to be a vital opsonic product that coats bacteria and other microbes, resulting in uptake by phagocytic cells (neutrophils [PMNs] and macrophages), followed by oxygen-dependent intracellular killing of bacteria (22). Mice deficient in C3 are incredibly sensitive to sepsis and die very quickly (9,23). Downstream activation of complement results in C5 cleavage, productive of C5a and C5b. C5a is an extremely active proinflammatory peptide, reacting with high affinity receptors (C5aR and C5L2) on phagocytic cells at low nM concentrations, resulting in the case of C5aR in cell activation which causes generation of reactive oxygen species (O2•, H2O2, HO•) that are toxic to other cells, connective tissue constituents and microbes. In addition, C5a causes enzyme secretion from phagocytic cells, which results in damage of nearby cells and matrix (24). C5a is highly chemotactic for phagocytic cells, especially neutrophils. Collectively, the outcome of C5a generation in vivo is induction of the acute inflammatory response characterized by increased vascular permeability and accumulation (and activation) of neutrophils and tissue macrophages. In CLP mice, C5a can be detected in plasma in levels that are nearly 5-fold above those in sham sera, and by 24 hr the levels have risen by at least 10-fold (15). The reason for this lag in plasma C5a after CLP is probably related to the fact that C5aRs on blood PMNs likely have to be saturated before plasma C5a can be detected. The other C5 activation product, C5b, interacts with C6, C7, C8 and C9 to form the membrane attack complex (MAC) (C5b-9) which causes pore formation in target cells (including bacteria as well as nucleated and non-nucleated cells), resulting in cell lysis (25). In sub-lytic concentrations, C5b-9 (MAC) can activate endothelial cells to release proinflammatory chemokines such as IL-8 and chemokines (26).
2. C5a, C5aR and Thymocyte Apoptosis
It is now recognized that C5aR binds C5a with high affinity and is present on a variety of myeloid cells (phagocytes and dendritic cells) as well as non-myeloid cells (endothelial cells, cardiomyocytes, alveolar epithelial cells, to name just a few examples). C5aR on phagocytes is present in very high levels (circa 50,000 binding sites/cell), but on non-myeloid cells, such as endothelial cells, the levels are much lower (<1,000 binding sites/cells). However, it is now clear that CLP-induced sepsis causes an abrupt increase in C5aR levels in lung, spleen, liver, thymus, and heart, indicating that C5aR is in a very dynamic state during sepsis (27). In the case of thymocytes, this increased level of C5aR can be linked to IL-6 (28) which was originally described to be released from Kupffer cells, causing increased C5aR content on hepatocytes (29). Table 1 summarizes changes in rodent thymocytes after CLP. There is upregulation of mRNA and protein for C5aR on thymocytes within the first few hours after CLP (11). Correlating with this is increased binding of 125I-C5a, which leads to apoptosis as defined by increased binding of annexin V binding to thymocytes, a strong indicator of apoptosis due to expression on the outer cell membrane of phosphatidyl serine, which has an affinity for annexin V. Correlative changes include DNA laddering, which is reflective of double strand breaks in DNA. The increased binding of C5a and annexin V to thymocytes after CLP as well as the DNA laddering are C5a-dependent, as shown by the ability of intravenously administered blocking if IgG antibody to C5a to greatly attenuate these indicators of apoptosis (12).
TABLE 1.
Changes in rodent thymocytes after CLP
| 1. | Increased mRNA for C5aR |
| 2. | Increased C5aR protein |
| 3. | Increased binding of C5a |
| 4. | Induction of apoptosis as reflected by: |
| a) increased binding of annexin V | |
| b) DNA laddering |
Other evidence of thymic apoptosis after CLP has been shown by the evaluation of thymic extracts, which show increases in catalytic activity for caspases 3, 6 and 9, but not caspase 8, as shown by functional assays for caspase activities (Table 2 and ref 12). The 10-fold increase in caspase 3 activity after CLP was reduced to a 3-fold increase in rodents treated with anti-C5a at the time of CLP. The 5-fold increase in caspase 6 activity fell to < 2-fold in anti-C5a treated rodents, while the increased activity of caspase 8 was only modestly reduced. Finally, the 30-fold increase in caspase 9 activity fell to a 13-fold increase when C5a was blocked in vivo at the time of CLP. This pattern suggests that CLP induces the intrinsic pathway of mitochondrial-dependent apoptosis. When in vitro binding of annexin V to thymocytes was quantitated by flow cytometry, treatment of the CLP rodents with anti-C5a reduced annexin V binding by 80% (12), indicating that availability of C5a is linked to caspase activation in thymocytes, increased binding of annexin V and apoptosis. As well, intervention with anti-C5a caused greatly reduced laddering of DNA (12).
TABLE 2.
Patterns of thymocyte activation of caspases after CLP
| fold increase relative to sham cells | ||
|---|---|---|
| Caspase | CLP + IgG | CLP + anti-C5a IgG |
| 3 | 10 ± 2 | 3 ± 0.5 |
| 6 | 5 ± 1 | < 2 |
| 8 | 8 ± 3 | 6 ± 1 |
| 9 | 30 ± 4 | 13 ± 2 |
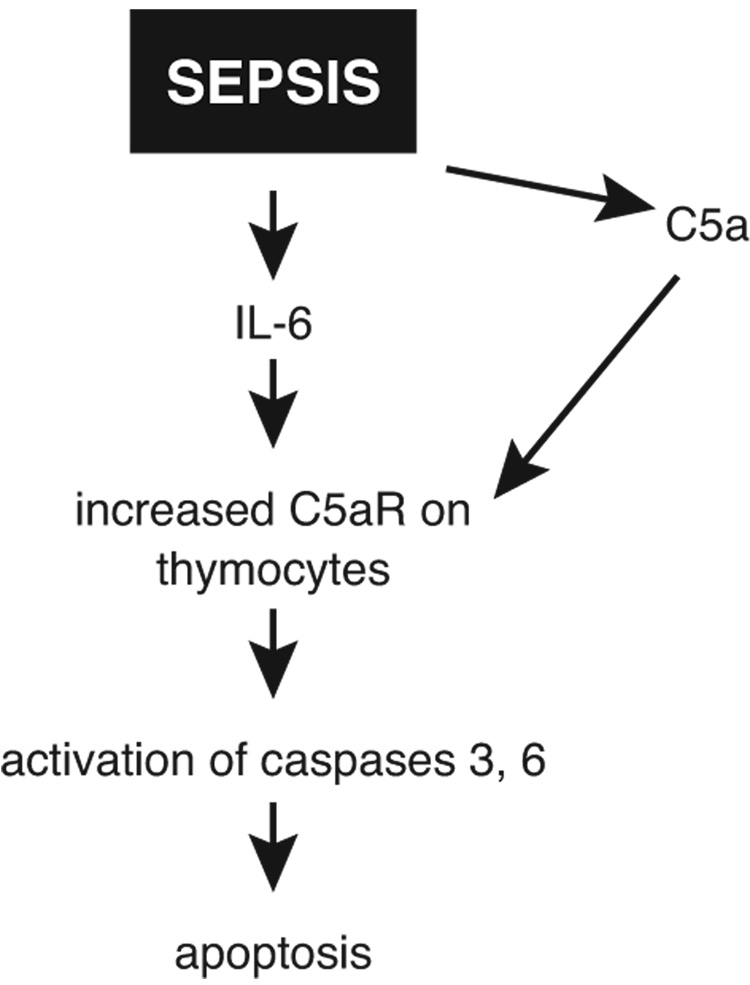
Our current concept of thymocyte apoptosis after CLP is shown in Figure 2. As already indicated, CLP causes an increase in C5aR on thymocytes as well as in a variety of organs, such as lung, liver, kidney, and heart and blockade of C5aR is protective (31). It appears that the increase in C5aR is driven by IL-6, since in vivo treatment of CLP mice with anti-IL-6 greatly reduces the sepsis-induced increase in C5aR protein in various organs (28), although the effects on thymocyte C5aR under the same conditions have not yet been determined. C5a generated after CLP then interacts with C5aR, leading to activation of caspases 3, 6 and 9, resulting in thymocyte apoptosis, as described above. Although we have not yet determined if similar events occur on T cells and B cells after CLP, we hypothesize that this is likely the case during sepsis. If so, this could link the well established apoptosis of lymphoid cells during sepsis (7, 30) with interaction of C5a with C5aR on lymphoid cells. When C5aR was blocked in vivo after CLP with either anti-C5aR (27) or with a peptide antagonist to C5aR (31), survival was greatly improved, again incriminating the adverse role of C5aR in sepsis.
Figure 2.
Role of IL-6 and C5a in apoptosis of thymocytes in rodents after sepsis induced by cecal ligation and puncture (CLP).
3. Apoptosis of Adrenal Medullary Cells in Experimental Sepsis
As indicated above, septic shock often develops in the setting of human and experimental sepsis (4–6, 14), usually requiring use of vasopressor drugs to maintain tissue perfusion. The reasons for development of septic shock are poorly understood but might be related to inadequate availability of catecholamines (epinephrine, norepinephrine) which tend to maintain vascular tone and pressure. Catecholamines are produced in relatively large amounts and released from adrenal medullary cells. In addition, they are released from presynaptic neurons and, in very small quantities, from activated T cells. Recently, we described that phagocytic cells (PMNs and macrophages) have the synthetic machinery to locally produce and release norepinephrine and epinephrine, which in a positive feedback loop, bind to α2 adrenergic receptors on the same cells to greatly reinforce the release of proinflammatory mediators (32). As a result of these observations, it seems clear that phagocytes represent an important source of catecholamines that locally may greatly influence the inflammatory response.
In the setting of sepsis, a rapid buildup of norepinephrine occurs in plasma 6 hr after CLP (15). This may represent the “fight or flight” response of the autonomic nervous system in order to cope with the onset of a life-threatening situation, via release of large amounts of catecholamine from the adrenal medulla. When frozen sections of rat adrenal medulla regions were obtained and stained for evidence of apoptosis (by TUNEL staining), extensive evidence of apoptosis was found (15). If animals had been treated with sera rich in blocking antibodies to C5aR and to C5L2, nearly all evidence of adrenal medullary apoptosis disappeared. In another set of experiments, a rat pheochromocytoma cell line (PC12) was used. After in vitro incubation with rat recombinant C5a (0.01–100 nM), PC12 cells showed a dramatic reduction in release of norepinephrine and dopamine (a precursor of norepinephrine) when compared to PC12 cells maintained in tissue culture fluid during which time the cells constitutively released norepinephrine and dopamine. PC12 cells incubated with 10 nM C5a had striking evidence of apoptosis as shown by annexing V binding as well as binding of propidium iodide-rhodamine, indicating that C5a induces apoptosis of PC12 cells (15). Collectively, these data suggest that C5a may cause apoptosis of intrinsic adrenal medullary cells via engagement of C5aR and C5L2 as well as causing apoptosis of the rat pheochromocytoma cell lines, PC12. Thus, the 6 hr. peak in noradrenaline after CLP in rats may represent rapid apoptosis of adrenal medullary cells, followed by greatly reduced ability of the adrenal medulla to respond to stressful stimuli. Perhaps, at least in part, this contributes to the septic shock occurring in rodents after CLP and in septic humans.
4. Protective Effects of Anti-C5a in CLP
In Table 3, we summarize how in vivo blockade of C5a greatly attenuates many of the complications that develop following CLP. Firstly, survival in CLP rodents is greatly enhanced (for example, survival that is approximately 20% rises to 60%) after in vivo blockade of C5a when anti-C5a is given at the time of CLP (9). Secondly, blockade of C5a at the time of CLP protects against the loss of innate immune functions of blood neutrophils (10). In the setting of CLP, neutrophils lose their phagocytic ability and become globally refractory to chemotactic factors (C5a and N-formyl-Met-Leu-Phe). At this point, PMNs have a greatly reduced respiratory burst (production of H2O2) after cell stimulation with phorbol ester and show impairment of chemotaxis and phagocytosis. The loss of innate immune functions in neutrophils is associated with signaling paralysis in these cells, as measured by the inability of stimulated neutrophils to demonstrate activation of Erk1/2 (phosphorylation) after CLP (10). C5a blockade or blockade of C5aR in vivo preserves this signaling pathway (reviewed, 33). A third effect of C5a blockade at the time of CLP is reversal in the loss of cardiac function. In the usual course of CLP, left ventricular output diminishes and left ventricular pressures fall both during systole and diastole. In addition, cardiomyocytes isolated from the left ventricular wall of CLP rats show that contractility in vitro is greatly reduced. Another feature of changes in cardiomyocytes in CLP-induced sepsis is the presence of substantially increased C5aR content on the surfaces of these cells (14). Cardiomyocyte contractility defects, which are associated with “cardiomyopathy of sepsis”, are totally reversed in the presence of C5a blockade in vivo (14). Fourthly, as described above, C5a blockade in CLP rodents greatly diminishes apoptosis of thymocytes. In vitro addition of C5a to these cells results in the development of apoptosis as measured by increased binding of the annexin V. Blockade of C5a during sepsis largely reverses the indicators of consumptive coagulopathy as measured by reduced platelet levels in blood and the presence in plasma of activation products of the clotting system (such as thrombin-anti-thrombin complexes, various defects in clotting times, fibrin degradation products, etc.) (13).
TABLE 3.
In vivo blockade of C5a after CLP
| 1. | Improved survival |
| 2. | Reduced loss of innate immune function of blood PMNs |
| 3. | Alleviation of the cardiomyopathy of sepsis |
| 4. | Greatly diminished apoptosis of thymocytes |
| 5. | Attenuation of consumptive coagulopathy of sepsis |
In the context of apoptosis after CLP in mice, there are several published reports regarding the development and role of apoptosis in CLP-induced sepsis in mice (Table 4). Apoptosis of lymphoid cells in spleen and in Peyer’s patches of the small intestine has been documented both in septic humans and mice (7,30). Mice with overexpression of BCL2 (a well known natural inhibitor of apoptosis, functioning to prevent the loss of mitochondrial membrane potential and release of cytochrome C into the cytosol), demonstrate resistance to CLP-induced apoptosis and show improved survival (30). In addition, the use of chemical inhibitors of activated caspases protects against the apoptosis of CLP-induced sepsis (30). Finally, there are also suggestions that the extrinsic pathway of caspase activation may also be involved in the apoptosis of sepsis, since blockade of either Fas or Fas ligand have led to reduced apoptosis of lymphoid tissues after CLP and improved survival (34). Collectively, it appears that lymphoid apoptosis in sepsis causes immunosuppression and inability to contain bacteria.
TABLE 4.
Evidence for lymphoid apoptosis in experimental sepsis
| 1. | Apoptotic lymphocytes in CLP mice |
| 2. | Resistance of mice overexpressing Bcl2 |
| 3. | Protective effect of chemical inhibitors (Z-VAD) of caspases in CLP mice |
| 4. | Protective effect of Fas−/− or FAS ligand−/− mice after CLP |
5. Roles of C5a receptors in sepsis
With the recognition and structural definition of C5aR many years ago, it was quickly shown that this receptor bound C5a with high affinity. Mutational alteration of C5aR also demonstrated the important regions of the receptor necessary for high affinity binding of C5a and for signaling responses of this G-protein dependent receptor. The recent recognition of the second C5a receptor, C5L2, demonstrated that ligan (C5a or C5a des Arg) binding did not result in an intracellular Ca2+ signal (35). The functional responses in PMNs (chemotaxis, enzyme release, respiratory burst) occurring with C5a/C5aR interaction were not found with C5a/C5L2 interaction, leading to the designation of C5L2 as a “default” or “decoy” receptor (35), suggesting that C5L2 competes with C5aR for C5a binding, with the cell responses being determined by the balance of C5a binding to c54ar and C5L2 (reviewed, 36). Recently, however, it has been shown that in CLP-induced sepsis in mice, both C5aR and C5L2 contribute to the harmful outcomes of sepsis, since absence of receptor or blockade of either receptor attenuated the end results (lethality and the “cytokine storm”) (37). It was also demonstrated that C5L2 contributes in vitro and in vivo to the release of HMGB1, a transcription factor that is secreted and binds to phagocytic cells to cause elaboration of pro-inflammatory mediators from PMNs, macrophages and peripheral blood mononuclear cells (monocytes). C5L2−/− mouse macrophages or PMNs stimulated with LPS ± C5a would not release HMGB1, whereas C5aR−/− or wild type phagocytic cells released equivalent amounts of HMGB1 (37). Since HMGB1 has been shown to play a harmful role in experimental sepsis (38), there is now a direct linkage between C5L2 and HMGB1 (38).
6. Concluding Remarks
The development of experimental sepsis in CLP rodents unleashes a large number of changes which collectively define a state of unregulated inflammation, together with development of adverse changes in blood PMNs and cardiomyocytes as well as apoptosis of T and B cells, thymocytes and adrenal medullary cells. There is an accompanying intense consumptive coagulopathy. In addition, there is surge of proinflammatory cytokines appearing in the plasma (systemic inflammatory response syndrome, SIRS). All of these changes in rodents can be greatly mitigated by in vivo blockade of C5a, C5a receptors or caspases. Understanding in greater detail the molecular mechanisms involved in these adverse outcomes could set the stage for more effective treatment in vivo of septic patients.
ACKNOWLEDGEMENTS
This work was supported by NIH grants GM-29507, HL-31963 and GM-61656.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current endemiology of septic shock: the CUB-Rea Network. Am J Respi Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 3.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 4.Rabuel C, Mebazaa A. Septic shock: a heart story since the 1960s. Intensive Care Med. 2006;32:799–807. doi: 10.1007/s00134-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 5.MacLean LD, Mulligan WG, McLean AP, Duff JH. Patterns of septic shock in man: a detailed study of 56 patients. Ann Surg. 1967;166:543–562. doi: 10.1097/00000658-196710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellrodt AG, et al. Left ventricular performance in septic shock: reversible segmental and global abnormalities. Am Heart J. 1985;110:402–409. doi: 10.1016/0002-8703(85)90163-2. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 8.Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Younkin EM, Laudes IJ, Riedemann NC, Younger JG, Ward PA. Protective effects of anti-C5a peptide antibodies in experimental sepsis. FASEB J. 2001;15(3):568–570. doi: 10.1096/fj.00-0653fje. [DOI] [PubMed] [Google Scholar]
- 9.Czermak BJ, Sarma JV, Pierson CL, Warner RL, Huber-Lang MS, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nature Med. 1999;5(7):788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 10.Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Curnutte JT, Erickson R, Ward PA. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002;169(6):3223–3231. doi: 10.4049/jimmunol.169.6.3223. [DOI] [PubMed] [Google Scholar]
- 11.Riedemann NC, Guo RF, Laudes IJ, Keller K, Sarma VJ, Padgaonkar V, Ward PA. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 2002;16(8):887–888. doi: 10.1096/fj.02-0033fje. [DOI] [PubMed] [Google Scholar]
- 12.Guo RF, Huber-Lang MS, Wang X, Sarma JV, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–1280. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laudes IJ, Chu JC, Sikranth S, Huber-Lang MS, Guo RF, Riedemann N, Sarma JV, Schmaier AH, Ward PA. Anti-C5a ameliorates coagulation/fibrinolytic protein changes in a rat model of sepsis. Am J Pathol. 2002;160:1867–1875. doi: 10.1016/S0002-9440(10)61133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederbichler AD, Hoesel LM, Westfall MV, Gao H, Ipaktchi KR, Sun L, Zetoune FS, Su GL, Arbabi S, Sarma JV, Wang SC, Hemmila MR, Ward PA. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Experimental Medicine. 2005;203(1):53–61. doi: 10.1084/jem.20051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flierl MA, Rittirsch D, Chen AJ, Nadeau BA, Day DE, Sarma JV, Huber-Lang MS, Ward PA. The complement anaphylatoxin C5a induces apoptosis in adrenomedullary cells during experimental sepsis. PLoS ONE. 2008 Jul 2;3(7):e2560. doi: 10.1371/journal.pone.0002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Cai S, Gu X, Scafidi J, Wu X, Davis AE., III C1 inhibitor prevents endotoxin shock via a direct interaction with lipopolysaccharide. J Immunology. 2003;171:2594–2601. doi: 10.4049/jimmunol.171.5.2594. [DOI] [PubMed] [Google Scholar]
- 17.Caliezi C, Zeerleder S, Redondo M, Regli B, Rothen HU, Zurcher-Zenklusen R, Rieben R, Devay J, Hack CE, Lammle B, Wuillemin WA. C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit Care Med. 2002;30(8):1722–1728. doi: 10.1097/00003246-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Stöve S, Welte T, Wagner TO, Kola A, Klos A, Bautsch W, Köhl J. Circulating complement proteins in patients with sepsis or systemic inflammatory response syndrome. Clin Diagn Lab Immunol. 1996;3(2):175–183. doi: 10.1128/cdli.3.2.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med. 2006;34(2):461–470. doi: 10.1097/01.ccm.0000198527.71819.e1. [DOI] [PubMed] [Google Scholar]
- 20.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69(4):2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Echtenacher B, Urbaschek R, Weigl K, Freudenberg MA, Männel DN. Treatment of experimental sepsis-induced immunoparalysis with TNF. Immunobiology. 2003;208(4):381–389. doi: 10.1078/0171-2985-00282. [DOI] [PubMed] [Google Scholar]
- 22.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 23.Barrington R, Zhang M, Fischer M, Carroll MC. The role of complement in inflammation and adaptive immunity. Immunol Rev. 2001;180:5–15. doi: 10.1034/j.1600-065x.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- 24.Schröder JM, Christophers E. Transient absence of C5a-specific neutrophil function in inflammatory disorders of the skin. J Invest Dermatol. 1985;85(3):194–198. doi: 10.1111/1523-1747.ep12276664. [DOI] [PubMed] [Google Scholar]
- 25.Drogari-Apiranthitou M, Kuijper EJ, Dekker N, Dankert J. Complement activation and formation of the membrane attack complex on serogroup B Neisseria meningitidis in the presence or absence of serum bactericidal activity. Infect Immun. 2002;70(7):3752–3758. doi: 10.1128/IAI.70.7.3752-3758.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149(3):953–961. [PMC free article] [PubMed] [Google Scholar]
- 27.Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma JV, Markiewski MM, Mastellos D, Strey CW, Pierson CL, Lambris JD, Zetoune FS, Ward PA. Increased C5a receptor expression in sepsis. J. Clin. Invest. 2002;110:101–108. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170(1):503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 29.Schieferdecker HL, Schlaf G, Koleva M, Götze O, Jungermann K. Induction of functional anaphylatoxin C5a receptors on hepatocytes by in vivo treatment of rats with IL-6. J Immunol. 2000;164(10):5453–5458. doi: 10.4049/jimmunol.164.10.5453. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci U S A. 1999;96(25):14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber-Lang MS, Riedemann NC, Sarma JV, Younkin EM, McGuire SR, Laudes IJ, Lu KT, Guo RF, Neff TA, Padgaonkar VA, Lambris JD, Spruce L, Mastellos D, Zetoune FS, Ward PA. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- 32.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 33.Ward PA. The dark side of C5a in sepsis. Nature Reviews-Immunology. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 34.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. Leukoc Biol. 2003;74(3):344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 35.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280(48):39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 36.Johswich K, Klos A. C5L2--an anti-inflammatory molecule or a receptor for acylation stimulating protein (C3a-desArg)? Adv Exp Med Biol. 2007;598:159–180. doi: 10.1007/978-0-387-71767-8_12. Review. [DOI] [PubMed] [Google Scholar]
- 37.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang MS, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Koehl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Medicine. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]