Abstract
Biochemical networks are perturbed both by fluctuations in environmental conditions and genetic variation. These perturbations must be compensated for, especially when they occur during embryonic pattern formation. Complex chemical reaction networks displaying spatiotemporal dynamics have been controlled and understood by perturbing their environment in space and time1–3. Here, we apply this approach using microfluidics to investigate the robust network in Drosophila melanogaster that compensates for variation in the Bicoid morphogen gradient. We show that the compensation system can counteract the effects of extremely unnatural environmental conditions—a temperature step—in which the anterior and posterior halves of the embryo are developing at different temperatures and thus at different rates. Embryonic patterning was normal under this condition, suggesting that a simple reciprocal gradient system is not the mechanism of compensation. Time-specific reversals of the temperature step narrowed down the critical period for compensation to between 65 and 100 min after onset of embryonic development. The microfluidic technology used here may prove useful to future studies, as it allows spatial and temporal regulation of embryonic development.
Although rates of production and degradation of morphogens are affected by genetic and environmental variations, the mechanisms of embryo patterning have evolved to compensate for these variations. In Drosophila melanogaster, morphogens such as Bicoid protein act early on in the genetic hierarchy that patterns the Drosophila embryo along the antero-posterior axis. Altering the copy number of bicoid genes shifts the Bicoid expression profile and results in shifting of the expression pattern of a direct downstream target gene, hunchback, and subsequent expression of pair-rule and segment polarity genes4. The expression profile of the maternal Bicoid protein gradient, however, varies measurably between individual wild-type embryos, and is particularly affected by variations in temperature5. This naturally occurring variation does not result in altered expression of hunchback5, suggesting that there must be a compensatory mechanism to filter noise from the initial maternal Bicoid gradient. This compensation is important because the correct expression of gap and pair-rule genes is required to relay positional information for determining cell fate, although later compensation for shifts in patterning is also possible4. It has recently been suggested that anterior shifts in gap gene expression may be due to dynamic establishment of the Bicoid gradient in addition to regulation among the gap genes6. However, even in these analyses, the Hunchback protein boundary was stable6.
Although the exact nature of compensation is unknown, we wanted to test the limits of compensation, to determine whether compensation occurs by a simple reciprocal posterior gradient system, and to determine precisely when the compensatory mechanism functions during early development. We developed a microfluidic apparatus to precisely control the temperature of different parts of the embryo both spatially and temporally, thus differentially controlling the rate of development in the anterior and posterior halves of the embryo. In the natural world, a developing Drosophila embryo is unlikely to experience conditions that maintain the two halves of the embryo at different temperatures. Thermal diffusion on the scale of a Drosophila embryo (500 µm) is rapid and would equalize the temperatures in the embryo’s environment and the two halves of the embryo within seconds.
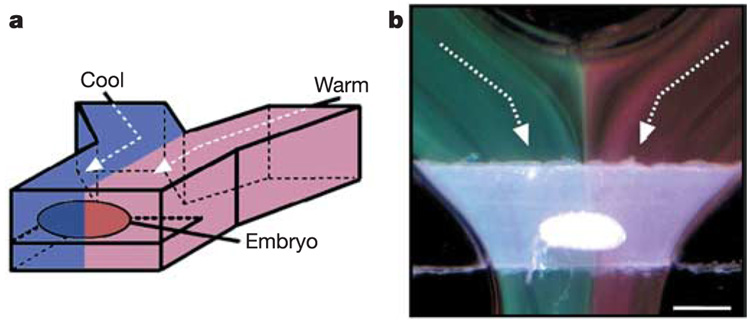
We used microfluidic laminar flow7–10 to create temperature differences by flowing two converging aqueous streams around an embryo, each at a controlled temperature, to provide rapid supply and removal of heat. The apparatus consists of two asymmetric moulds with alignment posts and holes11; moulds were made in polydimethylsiloxane (PDMS)12, which has low thermal conductivity. The microfluidic device can be assembled in one minute around a live embryo, with the result that the embryo is suspended in the cross-section of the channel (Fig. 1a) (see Supplementary Information for details on microfabrication, assembly of the microfluidic device and characterization of the flow).
Figure 1.
Experimental set up. a, A schematic drawing of a PDMS microfluidic device with a D. melanogaster embryo developing in a temperature-step (T-step). b, A microphotograph illustrating the T-step around the embryo, visualized using a suspension of thermochromic liquid crystals (Image Therm Engineering) flowing at a flow rate of 50 mm s−1. The temperature of the green stream is 21 °C and the temperature of the red stream is 24 °C. Scale bar, 400 µm.
We first demonstrated that development of the embryo was not adversely affected by flow at uniform temperature. Shear rate at the embryo was ~700 s−1, which was within biological range13, and dissolved oxygen in the flow was within the range of normoxia14. Embryos kept under flow in control experiments, where both streams were 20 °C or both streams were 27 °C, developed normally (see Supplementary Information).
A temperature step (T-step) was created by separately heating one laminar stream of flow and cooling the other. The difference in temperature was visualized using thermochromic liquid crystals15 (Fig. 1b). The response time15 of thermochromic liquid crystals is ~10 ms, and thus the colour at a given point in the channel is representative of the temperature 500 µm upstream of that point. Across the embryo, thermal diffusion between the two streams was calculated to be ~50 µm, which corresponds to ~10% of the egg length (EL). We calculated that the timescale of heat transfer was ~10 µs through the ~1.5-µm thick eggshell, much shorter than the timescale of heat transfer of ~1 s across the ~500-µm long embryo (see Supplementary Information).
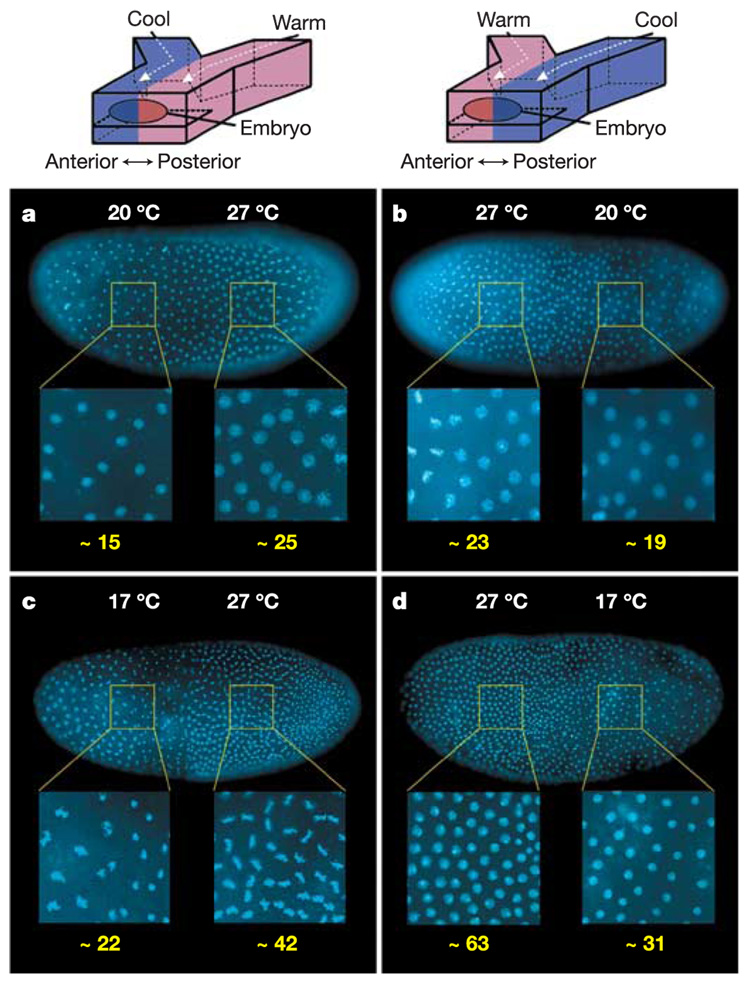
Differences in the density of nuclei in the two halves of the embryo showed experimentally that development was affected by the T-step. In normal embryonic development, 13 cycles of nuclear division are followed by cellularization of the embryo during the fourteenth cycle16. Although there are transient waves of mitosis, the cycles occur nearly simultaneously across the embryo and the density of nuclei stays relatively uniform.
Embryos developing in a T-step had a higher density of nuclei in the warmer half, confirming that the warmer half was developing more rapidly (Fig. 2). Embryos showed a greater difference when exposed to a larger T-step for a longer time (17 °C/27 °C for 150 min; Fig. 2c, d) than when exposed to a smaller T-step for a shorter time (20 °C/27 °C for 140 min; Fig. 2a, b). Embryos exposed to a T-step of 17 °C/27 °C showed a difference of two cell cycles (a fourfold difference in nuclear density) between the anterior and posterior halves, with the cool half closest to cycle 11 and the warm half closest to cycle 13 in control embryos. Therefore, the difference in nuclear density was more than a mitotic wave. Embryos exposed to a T-step of 20 °C/27 °C showed a difference of one cell cycle (a twofold difference in nuclear density) between the anterior and posterior halves, with the cool half and warm half closest to cycle 11 and 12 in controls, respectively (see Supplementary Information). Notably, the boundary in nuclear density was relatively sharp, not only confirming a tight thermal boundary, but demonstrating that cell cycle regulation could occur fairly independently between neighbouring nuclei in the syncytial blastoderm. Wasp embryos set in an agar block over heating elements have previously been exposed to temperature gradients17. Notably, these experiments resulted in normal patterning late in development, but as no molecular markers were assayed, it was unclear at what point compensation occurred. Furthermore, the morphogen gradients involved in patterning in wasps are unknown.
Figure 2.
The rate of development in each half of the embryo exposed to a T-step is affected by temperature. Each half of the embryo is in a different cell cycle, as demonstrated by difference in nuclear density. Number of nuclei in enlarged areas shown underneath in yellow numbering. a, b, Embryos exposed to a T-step of 20 °C/27 °C for 140 min. a, Anterior half 20 °C, posterior half 27 °C. b, Anterior half 27 °C, posterior half 20 °C. c, d, Embryos exposed to a T-step of 17 °C/27 °C for 150 min. c, Anterior half 17 °C, posterior half 27 °C. d, Anterior half 27 °C, posterior half 17 °C. In all images, higher nuclear density was observed in the warmer half of the embryo.
Our initial presumption was that Drosophila embryos developing in the unusual environment established within our microfluidic apparatus would have obvious defects in patterning. It has been shown that the shape of the Bicoid gradient, which is determined by a combination of production, degradation and diffusion of Bicoid, is strongly affected by temperature5. In our experiments, these processes will occur at different rates in the two halves of embryos. To our surprise, embryos allowed to develop in a temperature step (either 17 °C/27 °C or 20 °C/27 °C) for 150 min and then left at room temperature developed into normal larvae with the correct number and pattern of segments (data not shown). The normal appearance of larvae suggests that the embryo compensates for the unnatural temperature environment.
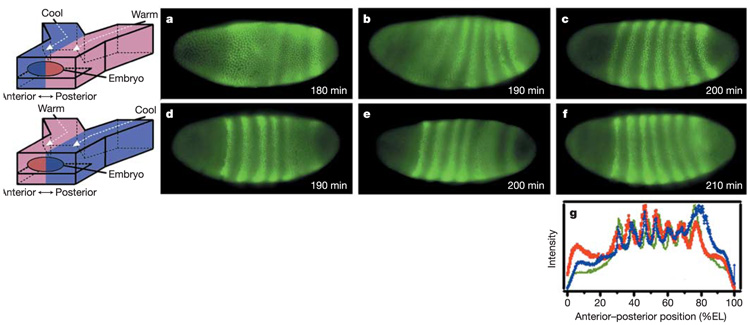
We used the microfluidic system to test whether patterning of Even-skipped18 remained robust in embryos exposed to the T-step. Normally, Even-skipped resolves into seven stripes in a specific order. First, stripes 1 and 2 resolve, and then stripe 7 resolves. Stripes 3, 4, 5, and 6 resolve next, more or less simultaneously18. Once resolved (by late cycle 14), the stripes are positioned at ~31, 40, 48, 55, 62, 69, and 78% EL19. The pattern of Even-skipped expression was monitored at the T-step of 20 °C/27 °C (Fig. 3), which was chosen so that we could observe the entire embryo within cycle 14. A temperature difference greater than this causes gastrulation and germband extension to begin in the warmer half before Even-skipped stripes in the cooler half resolve (see Supplementary Information), making measurement of stripe positions inaccurate, but illustrating that the two halves still develop at different rates. Embryos exposed to the T-step (20 °C/27 °C) clearly formed Even-skipped stripes out of order. When the anterior half of the embryo was warmer, Even-skipped stripes 1, 2 and 3 consistently resolved first (Fig. 3d, e).When the posterior half of the embryo was warmer, Even-skipped stripes 5, 6 and 7 consistently resolved first (Fig. 3a, b); this was particularly striking because in normal development stripes 5 and 6 resolve last.
Figure 3.
Even-skipped expression in embryos exposed to a T-step of 20 °C/27 °C. a–c, Embryos with a cool anterior half and warm posterior half. d–f, Embryos with a warm anterior half and cool posterior half. Even-skipped stripes were consistently expressed in the warm half first (a and b, d and e), but resolved in the correct positions (c, f). g, Intensity profile of Even-skipped expression in embryos exposed to the T-step in panels c (red) and f (blue), compared to an average intensity profile (green) of Even-skipped expression in four control embryos that developed at room temperature.
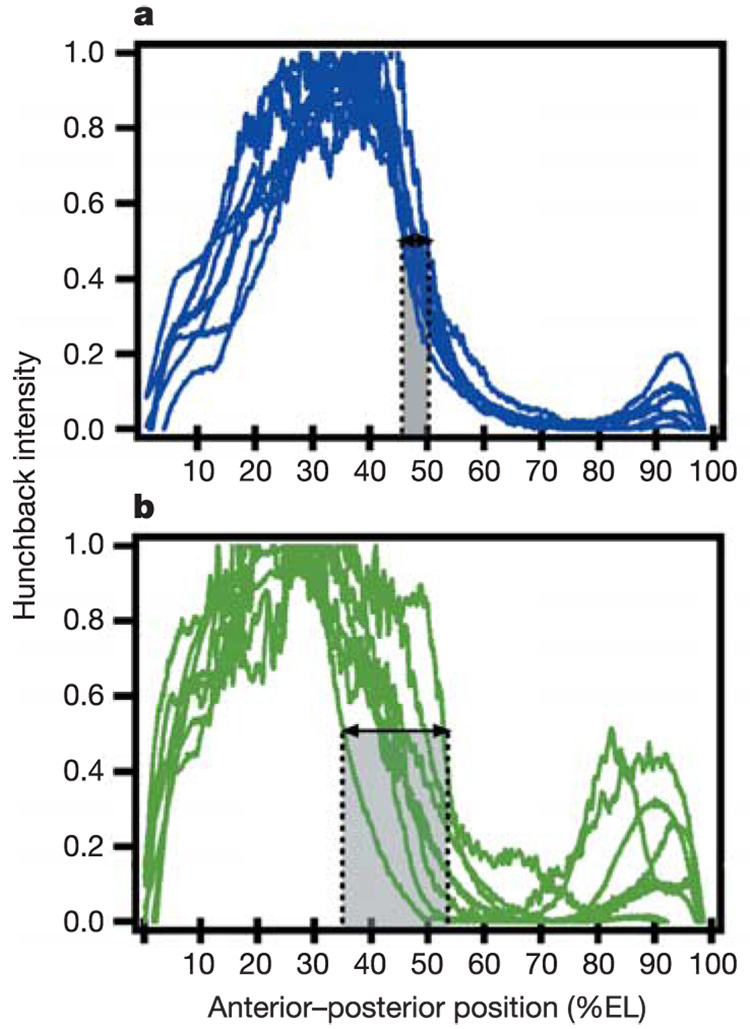
Surprisingly, Even-skipped stripe positions were precise, despite the difference in developmental rate in the two halves of the embryo (Fig. 3c, f). Even-skipped stripes 1 through 7 resolved at 30, 38, 48, 54, 62, 68, and 78 ± 1% EL, respectively, for embryos exposed to a T-step of 20 °C/27 °C (with either half being warmer) (Fig. 3g). Embryos exposed to the T-step of 20 °C/27 °C also compensated for the unnatural environment at the level of Hunchback expression, which is a more direct readout of the maternal Bicoid morphogen gradient. For embryos with anterior 27 °C/posterior 20 °C (Fig. 4a) and embryos with anterior 20 °C/posterior 27 °C (data not shown), the Hunchback boundary remained spatially precise (46–51% EL), comparable to control embryos5.
Figure 4.
Hunchback expression in embryos exposed to a T-step and time-dependent T-step. a, Hunchback intensity profiles of embryos with anterior half at 27 °C and posterior half at 20 °C. Hunchback position was normal, varying over 5% EL (46–51% EL). b, Hunchback intensity profiles of embryos exposed to a time-dependent T-step with anterior half at 27 °C and posterior half at 20 °C, with the exception of a brief temperature reversal (anterior 20 °C/posterior 27 °C) between 65 and 100 min. The position of Hunchback was variable over 18% EL (35–53% EL).
We determined the critical period during which the compensation mechanism functions by setting up a temperature regimen as follows: embryos developed with anterior at 27 °C/posterior at 20 °C with the exception of a brief 35-min period during which the orientation of the T-step was transiently reversed (anterior 20 °C/posterior 27 °C at t 1) before being reversed back (anterior 27 °C/posterior 20 °C at t 2). Embryos exposed to a brief temperature reversal (t 1 = 65 min and t 2 = 100 min) showed a marked increase in variability of the Hunchback boundary (35–53% EL) with anterior bias (Fig. 4b), resembling the embryos carrying the stauHL or the stau r9 allele5, which also showed variability with anterior bias. Corresponding nuclear images stained with 4,6-diamidino-2-phenylindole (DAPI) do not support the possibility that the increased variability of the Hunchback boundary position is simply due to differences in the ages of the embryos in cycle 14 (see Supplementary Information). Other earlier (t 1 = 35 min, t 2 = 70 min) or later (t 1 = 95 min, t 2 = 130 min) transient reversals of the T-step resulted in relatively low variability in the Hunchback boundary (42–49% and 43–50% EL, respectively) (see Supplementary Information). These results show that a key part of the compensatory mechanism is established between 65 and 100 min. By extension, we suggest that normally occurring variations are ‘measured’ and possibly compensated for during this stage of development.
To test the possible polarity of the correction mechanism, we inverted the temperature regimen: embryos developed with anterior at 20 °C and posterior at 27 °C, with the exception of a brief 35-min period during which the orientation of the T-step was transiently reversed (anterior 27 °C/posterior 20 °C at t 1) before being reversed back (anterior 20 °C/posterior 27 °C at t 2). Embryos exposed to later temperature reversals (t 1 = 65 min, t 2 = 100 min or t 1 = 95 min, t 2 = 130 min) showed low variability in the Hunchback boundary (43–48% or 42–49% EL, respectively). Embryos exposed to an earlier temperature reversal (t 1 = 35 min, t 2 = 70 min) also showed low variability in the Hunchback boundary (51–55% EL), but in this case with a slight posterior bias (see Supplementary Information).
Using microfluidics, we changed the relative rate of development of the two halves of the embryo. Interestingly, Even-skipped expression in Fig. 3d and e bears resemblance to the pattern seen in beetle embryos20, in that Even-skipped stripes do not all appear more or less simultaneously. Despite our ability to change the temporal order in which Even-skipped stripes form, the spatial precision of the pattern remained intact. Thus, the low variability of the Hunchback boundary and Even-skipped pattern in these embryos suggests that the compensatory mechanism of the embryo can allow for correct patterning even in extremely unnatural environments.
A plausible compensatory mechanism for maintaining a stable Hunchback boundary during normal development could involve an opposing gradient system using the anterior Bicoid gradient plus an unknown posterior gradient. Previous work has ruled out many genetic candidates for the possible posterior gradient (including nanos and caudal), but found that certain staufen alleles affected precision, suggesting that the compensatory system might involve the localization of an uncharacterized messenger RNA5. Our results suggest that the compensation system is not simply a reciprocal posterior gradient, as such a system would have failed when the two halves of the embryo developed at two different temperatures. Temperature reversal experiments lead us to suggest that the compensation mechanism has a critical establishment phase corresponding to 65–100 min of development.
Understanding the dynamics of a complex system by perturbing its environment in space and time does not require a priori knowledge of the system’s components. This approach has been used to understand and control biological systems (such as appearance of fibrillations in a mammalian heart21) and chemical reaction networks (such as formation of patterns in the Belousov-Zhabotinsky reaction3 and in surface-catalysed CO oxidations1). We believe that the microfluidic methods we have established here will prove to be an experimentally powerful approach that will allow the environment of the embryos to be spatiotemporally controlled. Perturbing the environment is a complementary approach to perturbing the molecular components of the network, as it might provide information on where and when events occur, rather than which molecules are involved. A combination of these two approaches might prove especially useful for identifying correction mechanisms responsible for the robustness of developmental and other bio-chemical networks.
Methods
Fly stocks, fabrication of the microfluidic device, and experimental set up
All embryos were collected using wild-type Oregon R. Drosophila. Multilevel PDMS moulds were made using rapid prototyping. Embryos were collected at 23 °C for 2–5 min, placed on double-sided tape and assembled in the microfluidic device. Embryos were exposed to flow within 10 min of the start of collection. Syringe pumps (KdScientific) were used to control volumetric flow rate (2 ml min−1 total). Temperature of each laminar stream was maintained using a double chilling/heating plate (Echo therm, Torrey Pines Scientific).
Immunostaining
After removal from flow, embryos were dechorionated, fixed in 3% formaldehyde in PEM buffer and immunostained using standard methods with anti-Even-skipped (mouse monoclonal 2B8) and anti-Hunchback (mouse monoclonal 1G10) antibodies, and goat anti-mouse IgG (H + L) AlexaFluor 488 conjugated secondary antibody (Molecular Probes).
Image acquisition and analysis
Images were acquired using a Leica DM IRE2 inverted microscope with a ×20 0.7 NA objective and cooled CCD camera ORCA ERG 1394 (12-bit, 1344 × 1024 resolution, Hamamatsu Photonics). Even-skipped and Hunchback expression intensity profiles were analysed using MetaMorph Imaging System (Universal Imaging Corp).
See Supplementary Information for details regarding Methods.
Supplementary Material
Supplementary Information accompanies the paper on www.nature.com/nature.
Acknowledgements
This work was supported by the Searle Scholars Program and was performed at the Chicago MRSEC microfluidic facility funded by the NSF. N.H.P. is an Investigator of the Howard Hughes Medical Institute. We thank J. B. Brokaw, C. A. Macrander and M. Giorgianni for preliminary experiments. We thank D. Bilder and I. Hariharan for discussions and comments on the manuscript.
Footnotes
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Wolff J, et al. Spatiotemporal addressing of surface activity. Science. 2001;294:134–137. doi: 10.1126/science.1063597. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai T, Mihaliuk E, Chirila F, Showalter K. Design and control of wave propagation patterns in excitable media. Science. 2002;296:2009–2012. doi: 10.1126/science.1071265. [DOI] [PubMed] [Google Scholar]
- 3.Vanag VK, et al. Oscillatory cluster patterns in a homogeneous chemical system with global feedback. Nature. 2000;406:389–391. doi: 10.1038/35019038. [DOI] [PubMed] [Google Scholar]
- 4.Driever W, Nusslein Volhard C. The Bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 5.Houchmandzadeh B, Wieschaus E, Leibler S. Establishment of developmental precision and proportions in the early Drosophila embryo. Nature. 2002;415:798–802. doi: 10.1038/415798a. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger J, et al. Dynamic control of positional information in the early Drosophila embryo. Nature. 2004;430:368–371. doi: 10.1038/nature02678. [DOI] [PubMed] [Google Scholar]
- 7.Kenis PJA, Ismagilov RF, Whitesides GM. Microfabrication inside capillaries using multiphase laminar flow patterning. Science. 1999;285:83–85. doi: 10.1126/science.285.5424.83. [DOI] [PubMed] [Google Scholar]
- 8.Takayama S, et al. Subcellular positioning of small molecules. Nature. 2001;411:1016. doi: 10.1038/35082637. [DOI] [PubMed] [Google Scholar]
- 9.Hatch A, et al. A rapid diffusion immunoassay in a T-sensor. Nature Biotechnol. 2001;19:461–465. doi: 10.1038/88135. [DOI] [PubMed] [Google Scholar]
- 10.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JR, et al. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal. Chem. 2000;72:3158–3164. doi: 10.1021/ac9912294. [DOI] [PubMed] [Google Scholar]
- 12.McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Accounts Chem. Res. 2002;35:491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 13.Furlong EEM, Profitt D, Scott MP. Automated sorting of live transgenic embryos. Nature Biotechnol. 2001;19:153–156. doi: 10.1038/84422. [DOI] [PubMed] [Google Scholar]
- 14.DiGregorio PJ, Ubersax JA, O’Farrell PH. Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 2001;276:1930–1937. doi: 10.1074/jbc.M003911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stasiek JA, Kowalewski TA. Thermochromic liquid crystals applied for heat transfer research. Opto-Electron. Rev. 2002;10:1–10. [Google Scholar]
- 16.Foe VE, Odell GM, Edgar BA. In: The Development of Drosophila melanogaster. Bate M, Martinez Arias A, editors. New York: Cold Spring Harbor Press; 1993. [Google Scholar]
- 17.Niemuth J, Wolf R. Developmental asynchrony caused by steep temperature-gradients does not impair pattern-formation in the wasp, Pimpla turionellae L. Roux’s Arch. Dev. Biol. 1995;204:444–452. doi: 10.1007/BF00360852. [DOI] [PubMed] [Google Scholar]
- 18.Frasch M, et al. Characterization and localization of the Even-skipped protein of Drosophila. EMBO J. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myasnikova E, et al. Registration of the expression patterns of Drosophila segmentation genes by two independent methods. Bioinformatics. 2001;17:3–12. doi: 10.1093/bioinformatics/17.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Patel NH, Condron BG, Zinn K. Pair-rule expression patterns of Even-skipped are found in both short-germ and long-germ beetles. Nature. 1994;367:429–434. doi: 10.1038/367429a0. [DOI] [PubMed] [Google Scholar]
- 21.Witkowski FX, et al. Spatiotemporal evolution of ventricular fibrillation. Nature. 1998;392:78–82. doi: 10.1038/32170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on www.nature.com/nature.