Abstract
BACKGROUND
The ovarian follicular basal lamina underlies the epithelial membrana granulosa and maintains the avascular intra-follicular compartment. Additional layers of basal lamina occur in a number of pathologies, including pili annulati and diabetes. We previously found additional layers of follicular basal lamina in a significant percentage of healthy bovine follicles. We wished to determine if this phenomenon existed in humans, and if it was related to oocyte function in the bovine.
METHODS AND RESULTS
We examined follicles from human ovaries (n = 18) by electron microscopy and found that many follicles had additional layers of basal lamina. Oocytes (n = 222) from bovine follicles with normal or unusual basal laminas were isolated and their ability to undergo in vitro maturation, fertilization and culture to blastocyst was compared. Healthy bovine follicles with a single layer of basal lamina had oocytes with significantly (P < 0.01) greater developmental competence than healthy follicles with additional layers of follicular basal lamina (65% versus 28%).
CONCLUSIONS
These findings provide direct evidence that the phenotype of the follicular basal lamina is related to oocyte competence.
Keywords: ovary, basal lamina, oocyte competence, in vitro production
Introduction
During fetal life oogonia cease dividing and then as oocytes associated with somatic epithelial granulosa cells they form ovarian primordial follicles. These primordial follicles are encapsulated by a follicular basal lamina (Juengel et al., 2002) and can lie dormant for decades until activation. On activation, the follicular basal lamina expands as the follicle increases in size (Juengel et al., 2002). The follicular basal lamina separates the granulosa cells from the surrounding stromal elements in primordial and pre-antral follicles, or from the specialized stromal theca layers in antral follicles (Irving-Rodgers and Rodgers, 2006). The follicular basal lamina is believed to play a role in influencing granulosa cell proliferation and differentiation (Amsterdam et al., 1989; Richardson et al., 1992; Irving-Rodgers and Rodgers, 2006). Additionally, in healthy follicles it excludes capillaries, blood cells and nerve processes from the membrana granulosa until ovulation, at which time the basal lamina is degraded (Irving-Rodgers et al., 2006).
Basal laminas are important specialized sheets of extracellular matrix that underlie epithelial or endothelial cells and separate them from the adjoining stroma. Basal laminas initiate epithelial cell polarity, can control cell proliferation and differentiation, and can selectively retard the passage of cells and molecules. As such they compartmentalize tissues. A basal lamina is composed of a lattice-type network of collagen type IV intertwined with a network of laminin, stabilized by the binding of entactin/nidogen-1 or -2 (Schymeinsky et al., 2002) to the collagen and laminin.
Basal laminas are generally observed as a single layer aligned to the cell surface. However, a number of physiological and pathological conditions lead to different morphological appearances of basal laminas. In diabetes, additional layers of basal lamina are deposited in many organs and may cause many of the secondary symptoms in kidney, mircovasculature, nervous tissues and the retina of diabetic patients (Martinez-Hernandez and Amenta, 1983; Abrahamson, 1986). Folding of the basal lamina has been observed in the small intestine of tadpoles during metamorphosis (Murata et al., 1994). The folding is hypothesized to be caused by the small intestine shortening, resulting in a rapid loss of space and hence folding of the remaining excess basal lamina. Matrix vesicles associated with basal laminas have also been observed in renal tubule basal laminas (Safer and Katchburian, 1991) and basal lamina duplication has been observed in the abnormal hair shafts of pili annulati patients (Giehl et al., 2004).
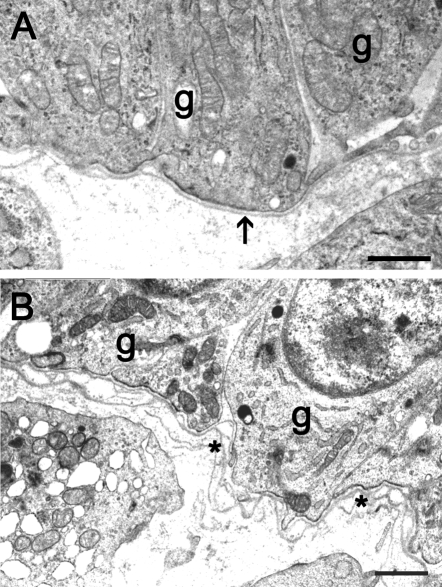
In a previous study of the bovine follicular basal lamina (Irving-Rodgers and Rodgers, 2000), we found that conventional basal laminas of a single layer aligned to the surface of the basal granulosa cells (Fig. 1A) were present in only a proportion of healthy follicles at both the early stage before (pre-antral) and at the later stage after the follicular antrum (antral) had developed. At the pre-antral stage these conventional basal laminas were thicker or even partially laminated (Irving-Rodgers and Rodgers, 2000), however, in about 50% of pre-antral follicles and antral follicles up to 4 mm diameter (Irving-Rodgers and Rodgers, 2000) the follicular basal lamina was substantially different. This second phenotype had additional layers of basal lamina. These have been called ‘loopy’ because of the appearance of additional ‘loops’ of basal lamina seen in cross sections as illustrated in Fig. 1B, in which these ‘loops’ are connected to additional layers closer to the granulosa cell surface.
Figure 1.
Electron micrographs showing a healthy antral bovine follicle with an aligned basal lamina (A; arrow) and a healthy bovine follicle with a ‘loopy’ basal lamina (B; asterisk).
g, granulosa cell. Scale bar = 1 µm.
In antral follicles, the ‘loopy’ phenotype occurred in association with basal granulosa cells that were columnar in shape, whereas other follicles with rounded basal cells had a conventional basal lamina (Irving-Rodgers and Rodgers, 2000). The appearance of the ‘loopy’ basal lamina phenotype is consistent with it being shed and replaced by new basal lamina nearer the basal surface of the adjacent granulosa cells. Cellular projections emanating from the basal surface of the basal granulosa cells and membrane-bound vesicles (often at the end of these processes and adjacent to the basal lamina) were also observed (Irving-Rodgers and Rodgers, 2000). We have speculated, since it is difficult to prove directly, that follicles with a ‘loopy’ follicular basal lamina are slower growing, producing excess basal lamina that is subsequently shed (Rodgers et al., 2001). The corollary of this hypothesis is that for the same size of follicle, those with a ‘loopy’ basal lamina are older (Rodgers et al., 2001).
To date, this observation of ‘loopy’ basal lamina has been limited to bovine follicles and is of no known function or pathology. The question remains as to the existence of the basal lamina phenotype in other species. Therefore we examined human follicles and confirmed that the ‘loopy’ basal lamina phenotype exists in human ovaries. Considering that the follicles with a ‘loopy’ basal lamina could represent older follicles (Rodgers et al., 2001) we hypothesized that they would contain oocytes of lesser quality. Using bovine ovaries we compared the quality of oocytes undergoing in vitro maturation from follicles with a ‘loopy’ basal lamina with those of an aligned basal lamina.
Materials and Methods
Light and electron microscopy
Tissues were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at 4C overnight. Following several washes with 0.1 M phosphate buffer, tissues were post-fixed in aqueous 1 or 2% osmium tetroxide for 1 h at 4C. After washes with H2O (3×5 min), tissues were dehydrated in increasing concentrations of acetone (50, 70, 90, 95 and 4 × 100%) at 4C and infiltrated with epoxy resin overnight at room temperature prior to embedding in fresh resin and polymerizing overnight at 60C. For light microscopic examination of follicular health and atresia, 1 µm thick epoxy sections were stained with 1% (w/v) aqueous methylene blue and examined using an Olympus BX50 microscope. For electron microscopy of follicular basal lamina morphology, 100 nm sections were stained with uranyl acetate and lead citrate and viewed with a Philips CM100 transmission electron microscope.
Human ovaries
Ovaries were collected from patients having elective hysterectomy and oophorectomy at the Queen Elizabeth Hospital, Adelaide, or the Royal Adelaide Hospital (13 women) and in the UK (five women) for medical conditions, including fibroids, dermoid or ovarian cysts, endometriosis and/or menorrhagia. Signed written informed consent was obtained from each patient. Ethical approval was obtained from the relevant Ethics Committees at each institution (Human Research Ethics Committee at the University of Adelaide, Women's and Children's Hospital Adelaide Research Ethics Committee, Royal Adelaide Hospital Research Ethics Committee, Wandsworth Local Research Ethics Committee and the Bromley Local Research Ethics Committee granted via The National Research Ethics Service, UK). Individual follicles were dissected from the ovarian stroma, the diameter measured and a portion of the follicle wall measuring approximately 1 × 1 × 2 mm was processed for light and electron microscopy. Polycystic ovaries were classified as having a polycystic phenotype on the basis of their ovarian morphology as described previously (Hughesdon, 1982).
Bovine follicle collection and classification
One ovary from each of 55 cows was collected within 20 min of slaughter at a local abattoir. The cows were assessed visually as non-pregnant. Ovaries were transported to the laboratory in 154 mM NaCl solution at 30–35C. Follicles 2–5 mm in diameter (n = 1–4 per ovary) were dissected from the ovarian stroma and a section through the follicle wall at the apex measuring ∼1 × 1 × 2 mm was processed for light and electron microscopy. Follicles were subsequently classified as healthy or atretic by viewing methylene blue-stained sections with a X40 objective. By standard criteria (Kruip and Dieleman, 1982; Blondin et al., 1996; Singh and Adams, 2000; Irving-Rodgers et al., 2001), atretic follicles had pyknotic nuclei and reduced numbers of granulosa cell layers (atretic 2–4 layers, healthy 6–9 layers).
Bovine oocyte collection and culture
Following removal of a portion of the apex of the follicle for classification, the oocyte was recovered and graded as either good (Grade 1 or 2) or poor (Grade 3) (Le Guienne, 1999). All media were equilibrated overnight in culture conditions at 39C in either 6% CO2 in air (for in vitro maturation and fertilization of oocytes) or 7% O2, 6% CO2, 87% N2 (for in vitro culture of blastocysts). Oocytes were matured individually for 24 h in 10 µl TCM199 supplemented with 0.05 IU/ml hCG, 10% bovine fetal calf serum (FCS), 0.1 IU/ml FSH, 100 µM cysteamine and 0.2 mM pyruvate. Mature oocytes were inseminated for a further 24 h with 2 × 104 Percoll (Amersham Biosciences, Buckinghamshire, UK)-separated motile sperm per 10 µl of Bovine In Vitro Fertilization (BIVF) Medium (Cook, Eight Mile Plains, Qld, Australia). Following insemination, putative zygotes were stripped of remaining cells and placed within individual micro-wells prepared in 1% agar in Bovine Early Cleavage Medium (BECM, Cook) and incubated at 38.5C under humidified 6% CO2, 7% O2 and 87% N2. For this, 350 µl of agar was prepared within wells of a 4-well plate and small plugs removed using a pulled glass Pasteur pipette to form micro-wells. The agar was over-layered with 450 µl of BECM and 250 µl mineral oil and equilibrated overnight. Pruvate was then added to the medium and zygotes transferred to the wells. On Day 5 following insemination, FCS (final concentration 10% v/v) was added to facilitate blastocyst development. Blastocyst formation was determined on Day 8. To provide sufficient numbers of oocytes, six separate collections of ovaries and culture of oocytes were needed to complete the experiment.
As a control for each collection 13–20 oocytes (one per ovary) were randomly chosen, pooled and cultured in a group. Pooled oocytes were matured in maturation medium for 24 h in 200 µl within a Nunc 4-well dish (Nunclon, Roskilde, Denmark), then inseminated in BIVF medium (200 µl, 2 × 106 / ml final sperm concentration). Putative zygotes were then cultured in groups of 4–5 in 20 µl microdrops of BECM in 35 mm dishes overlayered with mineral oil at 38.5C under humidified 6% CO2, 7% O2 and 87% N2 for 5 days, following which FCS (final concentration 10%) was added. Blastocyst formation was determined on Day 8.
Statistical analyses
Data on ooctye developmental competence were analysed by Chi-square test. P < 0.05 was considered statistically significant.
Results
Basal lamina morphology of human follicles
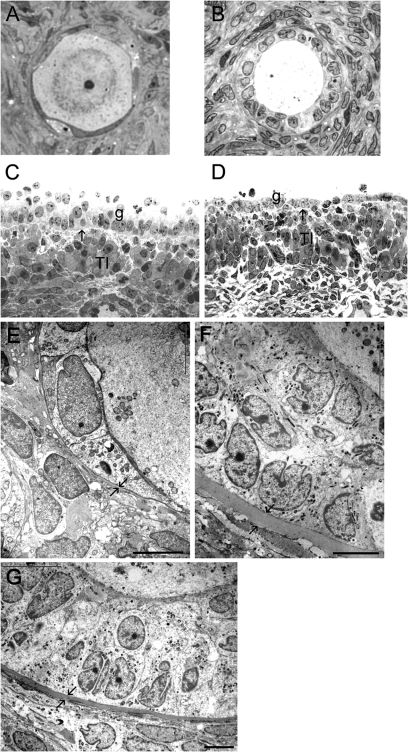
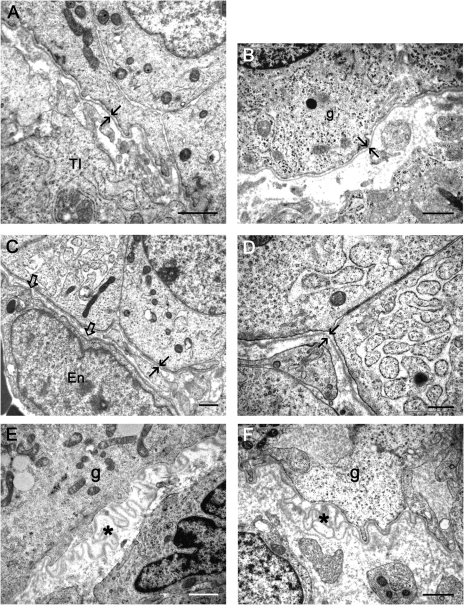
The numbers of human ovaries and follicles of each class and the size frequency distribution of healthy and atretic follicles that were observed and examined in this study are shown in Table I. Follicle stage and health was assessed by light microscopy as illustrated in Fig. 2A–D. By electron microscopy, a thin follicular basal lamina was observed to surround primordial follicles from both normal (Fig. 2E) and ovaries with a polycystic phenotype. The follicular basal lamina of primary and secondary follicles of normal ovaries (Fig. 2F and G) and those with a polycystic phenotype was substantially thicker than that of either primordial (Fig. 2E) or antral follicles (Fig. 3), as observed previously in bovine follicles (Irving-Rodgers and Rodgers, 2000). Most healthy antral follicles from both normal (Fig. 3A and B) and polycystic phenotype (Fig. 3C and D) ovaries had an aligned basal lamina, but follicles with a ‘loopy’ basal lamina were also observed (Fig. 3E and F). The numbers of follicles examined in each class were not sufficient to assign an accurate assessment of the frequency of follicles with a ‘loopy’ basal lamina.
Table I.
The numbers of human follicles examined from ovaries (n = 18) with a normal or polycystic ovarian phenotype with at least one follicle at each developmental stage
| Primordial | Primary | Pre-antral | Small antral (0.5–6 mm) | Large antral (6.5–16 mm) | Total | |
|---|---|---|---|---|---|---|
| Normal phenotype, No. of ovaries | 9 | 7 | 3 | 7 | 9 | 15 |
| Healthy follicles, No. of follicles | 12 | 13 | 6 | 11 | 8 | 50 |
| Atretic follicles, No. of follicles | 0 | 0 | 0 | 4 | 1 | 5 |
| Polycystic phenotype, No. of ovaries | 2 | 2 | 3 | 3 | 3 | 3 |
| Healthy follicles, No. of follicles | 3 | 7 | 4 | 23 | 2 | 39 |
| Atretic follicles, No. of follicles | 0 | 0 | 0 | 6 | 2 | 8 |
Figure 2.
Light (A–D) and electron micrographs (E–G) of follicles from normal human ovaries.
Healthy primordial (A), a primary (B) follicle and a healthy 4 mm (C) and an atretic 5 mm (D) follicle. Ultrastructure of the follicle wall of primordial (E) and pre-antral follicles (F, G) Arrows indicate the position of the follicular basal lamina. G, membrana granulosa; T, theca interna; O, oocyte. Scale bar = 5 µm.
Figure 3.
Electron micrographs showing aligned basal laminas (arrows) of a 6 mm (A) and 4 mm (B) follicle from human normal ovaries and of a 2 mm follicle (C, D) from an ovary with a polycystic phenotype.
Electron micrographs showing ‘loopy’ basal laminas (asterisk) of a 6 mm follicle from a normal ovary (E) and a 4 mm follicle of an ovary with a polycystic phenotype (F). Open arrows in (C) indicates a capillary sub-endothelial basal lamina, g, granulosa cell; En, endothelial cell; TI, theca interna. Scale bars A and D = 0.5 µm, B, C, E and F = 1 µm.
Since these ‘loopy’ basal laminas are a feature of both human and bovine ovarian follicles and of unknown function or pathology, we sought to examine the oocyte competence in these follicles relative to those follicles with a single layer of aligned basal lamina. We used bovine as our model as the systems for in vitro maturation and fertilization and culture to blastocyst [all referred to as in vitro production or IVP (Edwards et al., 2007)] are well advanced (Lonergan et al., 2003). In the bovine, IVP of immature bovine oocytes routinely result in 20% of oocytes developing to blastocyst and evidence suggests that the follicle size from which the oocyte is derived is a determinant in blastocyst development (Lonergan et al., 2003).
Bovine IVP
In this study, the health of follicles and their basal lamina phenotype were identified subsequently, enabling a comparison of these groups in terms of the behaviour of their oocytes during IVP. Healthy follicles had either an aligned or ‘loopy’ basal lamina (Fig. 1) and in the same proportions, as previously observed (Irving-Rodgers and Rodgers, 2000; Irving-Rodgers et al., 2002). However, nearly all atretic follicles had a ‘loopy’ basal lamina, interpreted as forming during the process of atresia as the follicular antrum is resorbed.
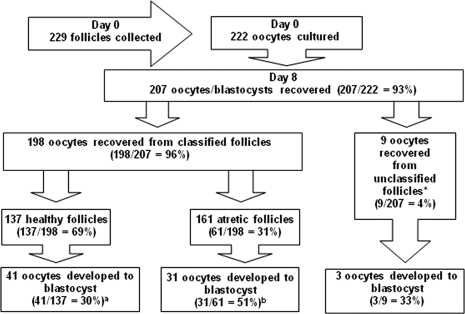
The recovery rate of oocytes from dissected bovine follicles was 97%. A total of 222 oocytes were cultured and 69% were derived from healthy follicles, 31% from atretic follicles and 4% from follicles in which the membrana granulosa was missing on histological examination (Fig. 4). The latter group was therefore excluded from further analyses. The proportion of healthy and atretic follicles in the 2–5 mm size range used in this study was equivalent to that found previously in histological sections of bovine ovary (Irving-Rodgers et al., 2001), indicating no selection bias and that the follicles used in these analyses reflect the in vivo follicle pool for this size range. Ninety percent of healthy follicles and 88% of atretic follicles had oocytes graded (Le Guienne, 1999) as 1 or 2 (good). The very high quality of recovered oocytes in the present study (∼90% grade 1, irrespective of follicle health status) compared with some other studies may be due to the method of oocyte collection used. We dissected the follicle to collect the cumulus oocyte complex and the granulosa cells, whereas the standard method is by aspiration in which the cumulus oocyte complex could be damaged.
Figure 4.
Numbers of bovine ooctyes cultured and then recovered on Day 8, and the numbers of oocytes from healthy, atretic and unclassifiable follicles that developed into blastocysts.
(Percentages or ratios are presented in brackets). abSignificantly different, P < 0.05, Chi-square test. *Follicles were not able to be classified if on histological assessment no granulosa cells were present.
In most IVP protocols for bovine oocytes, the oocytes are traditionally cultured in groups since IVP of oocytes individually has a significantly lower success rate. However, in order to subsequently relate oocyte quality to the follicular basal lamina phenotype, we had to undertake culture of individual oocytes. Therefore as a quality control, and for comparison to the individual oocyte culture, a group culture of ooctyes was undertaken for each batch of individually cultured oocytes. We found that 88% of oocytes used for this were grade 1 or 2 and following 8 days in culture, 20% had developed to the blastocyst stage. These values are in line with standard IVP in bovine, showing that the culture system used in these studies has a success rate similar to previous reports.
Seven percent (15/222) of oocytes were not recovered at Day 8 of incubation (Fig. 4). The percentage of recovered oocytes at Day 8 was similar to that reported previously following single IVP (Hagemann et al., 1999). Of oocytes recovered at Day 8, 36% (75/207) had developed to the blastocyst stage (Fig. 4). Significantly more oocytes recovered from atretic follicles (51%, 31/61) developed to the blastocyst stage than oocytes recovered from healthy follicles (30%, 41/137) (P < 0.05, Chi-square test). Of the 198 occytes recovered at Day 8 which were derived from classified follicles, 121 (61%) were further examined by electron microscopy (Table II) in order to relate the basal lamina morphology with oocyte developmental outcome. Sixty-one percent of these follicles were healthy (74/121), which did not differ significantly from the proportion of follicles assessed as healthy overall (Chi-squared test). These were derived from 55 ovaries (14 ovaries had 1 follicle examined, 21 had 2, 15 had 3 and 5 had 4). Healthy follicles had either a ‘loopy’ or an aligned basal lamina; however, most atretic follicles had a ‘loopy’ basal lamina (Table II). The important outcome was that healthy follicles with an aligned basal lamina were significantly (P < 0.01, Chi-squared test) more likely to have developmentally competent oocytes [65% (13/20) versus 28% (15/54)] than healthy follicles with a ‘loopy’ basal lamina (Table II).
Table II.
The number of ooctyes from healthy or atretic bovine follicles with either an aligned or ‘loopy’ follicular basal lamina phenotype that successfully or unsuccessfully (incompetent) completed development into blastocyst
| Healthy follicles |
Atretic follicles |
||||
|---|---|---|---|---|---|
| Follicular basal lamina |
Follicular basal lamina |
||||
| Aligneda | ‘Loopy’b | Aligned | ‘Loopy’ | ||
| Blastocyst (n = 28) | 13 | 15 | Blastocyst (n = 22) | 2 | 20 |
| Incompetent (n = 46) | 7 | 39 | Incompetent (n = 25) | 4 | 21 |
For this analysis 121 follicles of the 198 follicles whose oocytes/blastocysts were recovered on Day 8 were examined by electron microcopy for classification of their basal lamina phenotype. abSignificantly different proportion of oocytes developed into blastocysts, P < 0.01, Chi-square test.
Discussion
This study has shown that the ‘loopy’ follicular basal lamina exists in human ovaries and that, as in the bovine, it exists at both pre-antral and antral stages of development. We have also shown, using bovine ovaries, that follicles with a ‘loopy’ follicular basal lamina have oocytes which perform less well in IVP. These findings provide direct evidence that the follicular basal lamina phenotype is linked to oocyte competence, provide novel mechanistic insight into causes of infertility and have implications for assisted reproduction technologies.
Specifically, the findings have implications for both the development of IVP methods (Jurema and Nogueira, 2006) and the utilization of follicle culture as a means of obtaining mature oocytes for infertility treatment (Abir et al., 2006; Berkholtz et al., 2006). Little information exists on the influence of the follicular environment on oocyte quality (Lonergan et al., 2003). Robert et al. (2001) used a molecular approach to identify factors expressed in granulosa cells from follicles giving rise to developmentally competent oocytes, and Izumi et al. (2003) showed a marked decline in oocyte growth with increasing follicle size up to 4 mm in diameter. These and similar data have led to the important concept that oocytes acquire developmental competence as a function of follicle development. In addition, as also observed here, atretic small antral follicles have oocytes with a greater developmental capacity than healthy follicles of an equivalent size (Blondin and Sirard, 1995; Nicholas et al., 2005; Feng et al., 2007). Although at first glance, this may seem counter intuitive, atresia of antral follicles is characterized by death of granulosa cells and it is not until a very advanced stage that the culumus cells (Yang and Rajamahendran, 2000) or oocytes (Rajakoski, 1960) die, during which time the oocyte presumably continues to mature. Additionally, it has been hypothesized that in cattle atresia mimics pre-ovulatory maturation (Sirard et al., 1999). Attempts to utilize this knowledge of the association between atresia and oocyte quality by induction of apoptosis in cumulus cells did not significantly affect maturation of oocytes (Rubio Pomar et al., 2004). However, the current studies show that over and above these features of follicular development, the phenotype of the follicular basal lamina in healthy follicles is related to oocyte quality, and clearly identifies follicles with substantially different developmental capacity.
All bovine follicles larger than 5 mm have an aligned single layer of basal lamina (Irving-Rodgers and Rodgers, 2000). This suggests that either the smaller follicles with a ‘loopy’ basal lamina change phenotype as they enlarge beyond 5 mm, or that they are eliminated by atresia prior to this. If the latter occurs these follicles never ovulate during a normal cycle. However, they might be stimulated to grow by hormonal treatments in programmes for ovulation induction. It is not known whether these follicles have the capacity to respond and grow, but if they do then they may contribute poorer quality oocytes in these programmes, which could account for their low success rates. If a surrogate marker of follicles with a ‘loopy’ basal lamina could be identified in IVF aspirates, then a simple diagnostic method to detect follicles with a ‘loopy’ basal lamina could be used to exclude oocytes derived from such follicles.
The findings also raise some novel basic biological questions. How did follicles with a ‘loopy’ basal lamina evolve? What would be the evolutionary advantage of such follicles, and if none why do they exist? How does the basal lamina located some distance from oocytes and associated with granulosa cells affect the developmental capacity of the oocytes? An alternative explanation is that the oocyte is already of poor quality. Oocyte derived growth factors, such as bone morphogenetic protein 15 and growth differentiation factor-9, are known to have effects on follicular cells (Hussein et al., 2005; McNatty et al., 2006). It could be that oocytes from follicles with a ‘loopy’ follicular basal lamina might not adequately stimulate replication of granulosa cells or antrum expansion leading to the observed ‘loopy’ follicular basal lamina phenotype.
Unlike in diabetics in which the pathological changes in basal laminas appear to be widespread, these different follicular basal lamina phenotypes only exist in a proportion of ovarian follicles, suggesting the action of some intrafollicular phenomenon. In diabetics the abnormalities have been suggested to be due to advanced glycation end-products in the basal laminas (Mott et al., 1997; Gardiner et al., 2003; Goldin et al., 2006) or due to the action of reactive oxygen species (Meyer zum Gottesberge and Felix, 2005). Thus the follicles with a ‘loopy’ basal lamina could be a manifestation of some form of localized ‘environmental’ damage to the follicle. This is not likely to be due to advanced glycation end-products as these are usually caused by elevated levels of blood glucose which would potentially affect all follicles. However, reactive oxygen species could be a cause since the oxidative stress and damage may be localized. Another possible scenario is that follicles with a ‘loopy’ basal lamina represent a hitherto unrecognized intermediate or early form of follicular atresia affecting follicles <5 mm. This is supported by the present finding that 87% of atretic follicles had a ‘loopy’ basal lamina and that early stages of cell death can be detected by flow cytometry even before histological signs of atresia are evident (Blondin et al., 1996).
In summary, we have discovered in bovine follicles a characteristic that is related to the quality of their oocytes. This discovery could enable the development of diagnostic criteria for selection of better quality oocytes in assisted reproduction technology programmes, and to better understand natural follicle selection and possibly the effects of the follicular environment.
Funding
This research has been supported by the National Health and Medical Research Council of Australia, The University of Adelaide, The Wellcome Trust, UK, and the Clive and Vera Ramaciotti Foundation. Funding to Pay the Open Access Charge was provided by the National Health and Medical Research Council of Australia.
Acknowledgements
We thank Prof. Alain Gougeon of INSERM, Faculté de Médecine Lyon-Sud, for generously allowing us to histologically examine his extensive collection of human ovaries prior to undertaking these experiments, and Maglosia Krupa and Kyleen Catanzariti for their technical assistance.
References
- Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21:887–898. doi: 10.14670/HH-21.887. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR. Recent studies on the structure and pathology of basement membranes. J Pathol. 1986;149:257–278. doi: 10.1002/path.1711490402. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Rotmensch S, Furman A, Venter EA, Vlodavsky I. Synergistic effect of human chorionic gonadotropin and extracellular matrix on in vitro differentiation of human granulosa cells: progesterone production and gap junction formation. Endocrinology. 1989;124:1956–1964. doi: 10.1210/endo-124-4-1956. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24:262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- Blondin P, Dufour M, Sirard MA. Analysis of atresia in bovine follicles using different methods: flow cytometry, enzyme-linked immunosorbent assay, and classic histology. Biol Reprod. 1996;54:631–637. doi: 10.1095/biolreprod54.3.631. [DOI] [PubMed] [Google Scholar]
- Edwards R, Patrizio P, Edgar D, Field C, Brinton L. Defining IVF terminology. Reprod Biomed Online. 2007;14:553–554. doi: 10.1016/s1472-6483(10)61044-9. [DOI] [PubMed] [Google Scholar]
- Feng WG, Sui HS, Han ZB, Chang ZL, Zhou P, Liu DJ, Bao S, Tan JH. Effects of follicular atresia and size on the developmental competence of bovine oocytes: a study using the well-in-drop culture system. Theriogenology. 2007;67:1339–1350. doi: 10.1016/j.theriogenology.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Anderson HR, Stitt AW. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J Pathol. 2003;201:328–333. doi: 10.1002/path.1429. [DOI] [PubMed] [Google Scholar]
- Giehl KA, Ferguson DJ, Dean D, Chuang YH, Allen J, Berker DA, Tosti A, Dawber RP, Wojnarowska F. Alterations in the basement membrane zone in pili annulati hair follicles as demonstrated by electron microscopy and immunohistochemistry. Br J Dermatol. 2004;150:722–727. doi: 10.1111/j.0007-0963.2004.05837.x. [DOI] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Hagemann LJ, Beaumont SE, Berg M, Donnison MJ, Ledgard A, Peterson AJ, Schurmann A, Tervit HR. Development during single IVP of bovine oocytes from dissected follicles: interactive effects of estrous cycle stage, follicle size and atresia. Mol Reprod Dev. 1999;53:451–458. doi: 10.1002/(SICI)1098-2795(199908)53:4<451::AID-MRD11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118:5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Rodgers RJ. Ultrastructure of the basal lamina of bovine ovarian follicles and its relationship to the membrana granulosa. J Reprod Fertil. 2000;118:221–228. [PubMed] [Google Scholar]
- Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. Semin Reprod Med. 2006;24:195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, van Wezel IL, Mussard ML, Kinder JE, Rodgers RJ. Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction. 2001;122:761–775. [PubMed] [Google Scholar]
- Irving-Rodgers HF, Mussard ML, Kinder JE, Rodgers RJ. Composition and morphology of the follicular basal lamina during atresia of bovine antral follicles. Reproduction. 2002;123:97–106. [PubMed] [Google Scholar]
- Irving-Rodgers HF, Catanzariti KD, Aspden WJ, D'Occhio MJ, Rodgers RJ. Remodeling of extracellular matrix at ovulation of the bovine ovarian follicle. Mol Reprod Dev. 2006;73:1292–1302. doi: 10.1002/mrd.20580. [DOI] [PubMed] [Google Scholar]
- Izumi T, Sakakida S, Nagai T, Miyamoto H. Allometric study on the relation between the growth of preantral and antral follicles and that of oocytes in bovine ovaries. J Reprod Dev. 2003;49:361–368. doi: 10.1262/jrd.49.361. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, Wakefield SJ, McNatty KP. Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol Cell Endocrinol. 2002;191:1–10. doi: 10.1016/s0303-7207(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- Kruip TA, Dieleman SJ. Macroscopic classification of bovine follicles and its validation by micromorphological and steroid biochemical procedures. Reprod Nutr Dev. 1982;22:465–473. doi: 10.1051/rnd:19820403. [DOI] [PubMed] [Google Scholar]
- Le Guienne B. Atlas of the bovine oocyte. Association Europeenne de Transfert Embryonnair Newsletter. 1999;10:6–8. [Google Scholar]
- Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod Domest Anim. 2003;38:259–267. doi: 10.1046/j.1439-0531.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta PS. The basement membrane in pathology. Lab Invest. 1983;48:656–677. [PubMed] [Google Scholar]
- McNatty KP, Lawrence S, Groome NP, Meerasahib MF, Hudson NL, Whiting L, Heath DA, Juengel JL. Meat and Livestock Association Plenary Lecture 2005. Oocyte signalling molecules and their effects on reproduction in ruminants. Reprod Fertil Dev. 2006;18:403–412. doi: 10.1071/rd05104. [DOI] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Felix H. Abnormal basement membrane in the inner ear and the kidney of the Mpv17−/− mouse strain: ultrastructural and immunohistochemical investigations. Histochem Cell Biol. 2005;124:507–516. doi: 10.1007/s00418-005-0027-7. [DOI] [PubMed] [Google Scholar]
- Mott JD, Khalifah RG, Nagase H, Shield CF, 3rd, Hudson JK, Hudson BG. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int. 1997;52:1302–1312. doi: 10.1038/ki.1997.455. [DOI] [PubMed] [Google Scholar]
- Murata E, Akita M, Kaneko K, Merker HJ. Changes associated with the basal lamina during metamorphosis of Xenopus laevis. Acta Anat (Basel) 1994;150:178–185. doi: 10.1159/000147616. [DOI] [PubMed] [Google Scholar]
- Nicholas B, Alberio R, Fouladi-Nashta AA, Webb R. Relationship between low-molecular-weight insulin-like growth factor-binding proteins, caspase-3 activity, and oocyte quality. Biol Reprod. 2005;72:796–804. doi: 10.1095/biolreprod.104.036087. [DOI] [PubMed] [Google Scholar]
- Rajakoski E. The ovarian follicular system in sexually mature heifers with special reference to seasonal, cyclical, end left-right variations. Acta Endocrinol Suppl (Copenh) 1960;34(Suppl 52):1–68. [PubMed] [Google Scholar]
- Richardson MC, Davies DW, Watson RH, Dunsford ML, Inman CB, Masson GM. Cultured human granulosa cells as a model for corpus luteum function: relative roles of gonadotrophin and low density lipoprotein studied under defined culture conditions. Hum Reprod. 1992;7:12–18. doi: 10.1093/oxfordjournals.humrep.a137543. [DOI] [PubMed] [Google Scholar]
- Robert C, Gagne D, Bousquet D, Barnes FL, Sirard MA. Differential display and suppressive subtractive hybridization used to identify granulosa cell messenger rna associated with bovine oocyte developmental competence. Biol Reprod. 2001;64:1812–1820. doi: 10.1095/biolreprod64.6.1812. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Irving-Rodgers HF, van Wezel IL, Krupa M, Lavranos TC. Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol. 2001;171:41–48. doi: 10.1016/s0303-7207(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Rubio Pomar FJ, Roelen BA, Slot KA, van Tol HT, Colenbrander B, Teerds KJ. Role of Fas-mediated apoptosis and follicle-stimulating hormone on the developmental capacity of bovine cumulus oocyte complexes in vitro. Biol Reprod. 2004;71:790–796. doi: 10.1095/biolreprod.104.028613. [DOI] [PubMed] [Google Scholar]
- Safer AM, Katchburian E. Unusual membrane-bound bodies in the basal lamina of the uriniferous tubules of the camel Camelus dromedarius. Freeze-fracture and ultrathin-section study. Acta Anat (Basel) 1991;140:156–162. [PubMed] [Google Scholar]
- Schymeinsky J, Nedbal S, Miosge N, Poschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22:6820–6830. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Adams GP. Histomorphometry of dominant and subordinate bovine ovarian follicles. Anat Rec. 2000;258:58–70. doi: 10.1002/(SICI)1097-0185(20000101)258:1<58::AID-AR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sirard MA, Picard L, Dery M, Coenen K, Blondin P. The time interval between FSH administration and ovarian aspiration influences the development of cattle oocytes. Theriogenology. 1999;51:699–708. doi: 10.1016/s0093-691x(99)00019-9. [DOI] [PubMed] [Google Scholar]
- Yang MY, Rajamahendran R. Morphological and biochemical identification of apoptosis in small, medium, and large bovine follicles and the effects of follicle-stimulating hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured bovine granulosa cells. Biol Reprod. 2000;62:1209–1217. doi: 10.1095/biolreprod62.5.1209. [DOI] [PubMed] [Google Scholar]